Abstract
Objective: To explore prognostic differences in breast cancer (BC) recurrence risk across estrogen receptor (ER) and progesterone receptor (PR) defined subtypes, stratified by body mass index (BMI) categories, aiming to identify potential mechanisms. Methods: A cohort of 358 breast cancer patients provided data on height, weight, menopausal status, and receptor profiles for analysis. Results: Our findings highlighted that BMI’s impact on BC prognosis was significantly influenced by ER/PR tumor status. In premenopausal women, BMI notably affected recurrence rates, especially in patients with ER-positive and PR-positive subtypes. Conclusions: Adjusting treatment strategies based on BMI across different menopausal statuses and tumor subtypes could improve outcome for patients with ER-positive/PR-positive tumors.
Keywords: Height, body mass index, breast cancer recurrence, estrogen receptor, progesterone receptor
Introduction
Breast cancer (BC) is a complex and heterogeneous disease influenced by various interrelated factors in its development [1]. Among these, height and weight play crucial roles [2]. Previous studies indicate that both tall height and obesity are associated with poorer prognosis in BC patients [3]. The elevated risk of recurrence may stem from the association between taller stature and increased cellular division and proliferation, possibly leading to abnormal modifications within breast tissue and a higher likelihood of cancer recurrence [4]. Furthermore, taller height has been linked to faster tumor growth rate and an increased risk of metastasis.
In addition, adult body mass index (BMI) - a measure of relative body weight-correlates with BC prognosis in both premenopausal and postmenopausal women [5]. Higher BMI, often classified as overweight or obese, is associated with reduced treatment response and lower survival rates following a BC diagnosis. This may be due to excess adipose tissue, which produces estrogen that can stimulate the growth and progression of estrogen-receptor-positive tumors. Concurrently, higher body weight can impede the absorption, metabolism, and overall efficacy of therapeutic drugs, potentially raising the risk of recurrence [6,7].
BC is now recognized as a heterogeneous tumor with distinct subtypes that carry varied risk factors [8]. The prognostic relationship between height, weight, and BC has garnered significant research interest, with studies suggesting that estrogen receptor (ER) and progesterone receptor (PR) status may mediate these associations [9,10]. However, the role of ER and PR in BC prognosis remains complex and not fully understood. Under normal physiologic conditions, these receptors regulate the female reproductive system and help maintain hormonal balance [11]. Abnormal activation or imbalance of these receptors can lead to malignant traits like excessive cell proliferation and metastasis.
In postmenopausal women, a correlation exists between BMI and steroid/estrogen-related hormone pathways in ER-positive tumors, with higher BMI significantly raising the recurrence risk. In contrast, ER-negative tumors appear less dependent on estrogen levels [12]. These findings may help explain inconsistent results in studies linking BMI and BC prognosis, possibly due to confounding factors [12]. Limited research has explored early adult BMI, adult weight gain, height, and their association with ER/PR subtype and BC subtypes [13].
To further investigate the interplay of height, weight, and BC prognosis, we conducted a comprehensive analysis, considering ER status, PR status, combined ER/PR status, and various BC subtypes. By synthesizing data across BC cohorts and examining anthropometric indicators’ influence on BC risk and prognosis, our goal is to elucidate how height and weight impact treatment outcomes. Ultimately, we aim to refine BC management and personalize treatment strategies, tailored to ER/PR status and tumor type, while addressing holistic health factors like mental well-being and nutrition to optimize prognosis.
Subjects and methods
Study population
BC patients diagnosed by pathology at Dongyang City People’s Hospital between 2010 and 2020 were retrospectively identified using the inpatient electronic medical record system. BC subtypes were classified based on receptor profiles, including steroid hormones, ER status, and Ki-67 (KI67) receptor status.
Study protocol
In this retrospective analysis, we identified BC patients with well-defined receptor profiles and documented their height, weight, and menstrual status at diagnosis. Medical records were reviewed thoroughly, with follow-up efforts by the research team to accurately record and validate each patient’s post-treatment and recovery status. Patients lacking baseline data, such as height, weight, and menstrual status, or with indeterminate ER/PR or KI67 receptor status, were excluded to ensure the study’s accuracy and reliability.
Statistical analysis
Participants with a history of cancers other than breast cancer at baseline, missing height or weight data, or incomplete BC receptor testing were excluded. Cox proportional hazards models were applied to assess relative risks (RR) for specific subtypes, including ER-positive, ER-negative, PR-positive, PR-negative, and combined ER/PR subtypes. Follow-up duration was recorded from initial BC diagnosis until recurrence, death, loss to follow-up, or study end. Random-effects models combined study-specific RRs, and heterogeneity was assessed using Q statistics [14]. Body measurement categories were analyzed by assigning medians to each category and treating the variable as continuous variables for trend modeling. Contrast tests were conducted to examine BC subtypes by hormone receptor status (ER-negative vs ER-positive; PR-negative vs PR-positive) and clinical type variations. Statistical significance was set at P<0.05 (two-sided).
Restricted cubic spline regression was used to evaluate the association between height, BMI, and BC subtype. Analyses were stratified by menstrual status and BC type, with adjustment for confounding variables. Extreme outliers (top and bottom 1%) were excluded, and the distribution was truncated at 1% and 99%. The approximate median of the reference category was used as a baseline for spline analysis. For linear relationships between anthropometric variables and BC recurrence risk, further analysis was conducted using these variables as continuous predictors.
Results
Patient characteristics by hormone receptor status
In a cohort of 538 patients, a total of 358 valid cases were included. Among these, the ER-positive group constituted 69.55% (249/358), while the ER-negative group made up 30.47% (109/358). The PR-positive group comprised 57.54% (206/358), with the PR-negative group at 42.46% (152/358). By subtype, Luminal A represented 19.27%, pure human epidermal growth factor receptor 2 (Her-2) positive type 16.20%, Luminal B (Her-2 positive) type 17.60%, Luminal B (Her-2 negative) type 21.79%, triple-negative BC (TNBC) 10.06%, and in situ carcinoma 7.26%.
Premenopausal women represented 51.12% (183/358) of the sample, while postmenopausal women made up 48.88% (175/358). The age range at menopause ranged from 37 to 60 years, with most between 45 and 55 years old. Among premenopausal women, the ER-positive group constituted 81.14% (142/175) and the ER-negative group 18.86% (33/175). The PR-positive group included 75.43% (132/175), and the PR-negative group 24.57% (43/175). Subtype distribution in premenopausal women was as follows: Luminal A, 25.14%; pure Her-2 positive, 13.14%; Luminal B (Her-2 positive), 17.71%; Luminal B (Her-2 negative), 25.71%; TNBC, 4.57%; and in situ carcinoma, 9.71%.
For postmenopausal women, the ER-positive group accounted for 51.17% (107/183), and the ER-negative group for 41.53% (76/183). PR-positive cases constituted 40.44% (74/183), while PR-negative cases were 59.56% (109/183). The subtype breakdown in postmenopausal women was as follows: Luminal A, 13.66%; pure Her-2 positive, 19.13%; Luminal B (Her-2 positive), 17.49%; Luminal B (Her-2 negative), 18.03%, TNBC, 15.30%, and in situ carcinoma, 4.92% (Tables 1, 2).
Table 1.
| Breast cancer subtype | Number of cases | Percentage |
|---|---|---|
| Premenopausal | ||
| Overall | 175 | 48.88% |
| ER*-positive | 142 | 81.14% |
| ER-negative | 33 | 18.86% |
| PR*-positive | 132 | 75.43% |
| PR-negative | 43 | 24.57% |
| Postmenopausal | ||
| Overall | 183 | 51.17% |
| ER-positive | 107 | 58.47% |
| ER-negative | 76 | 41.53% |
| PR-positive | 74 | 40.44% |
| PR-negative | 109 | 59.56% |
ER, estrogen receptor; PR, progesterone receptor.
Table 2.
Number of BC* patients with different BC subtypes
| Breast cancer subtype | Number of cases | Percentage |
|---|---|---|
| Premenopausal | ||
| Luminal A | 44 | 25.14% |
| Her-2* positive | 23 | 13.14% |
| Luminal B (Her-2 positive) | 31 | 17.71% |
| Luminal B (Her-2 negative) | 45 | 25.71% |
| Triple-Negative Breast Cancer | 8 | 4.57% |
| In Situ Carcinoma | 17 | 9.71% |
| Postmenopausal | ||
| Luminal A | 25 | 13.66% |
| Her-2 positive | 35 | 19.13% |
| Luminal B (Her-2 positive) | 32 | 17.49% |
| Luminal B (Her-2 negative) | 33 | 18.03% |
| Triple-Negative Breast Cancer | 28 | 15.30% |
| In Situ Carcinoma | 9 | 4.92% |
Her-2, human epidermal growth factor receptor 2; BC, breast cancer.
Correlation between height and BC prognosis
As shown in Figure 1, an inverse correlation existed between height and BC recurrence rates among postmenopausal women, suggesting that taller postmenopausal women may have a more favorable prognosis. A segmented analysis by menopausal status supports this observation, with taller postmenopausal women demonstrating improved prognostic outcomes. Statistical analysis yielded p-values of 0.0714 for premenopausal women and 0.0040 for postmenopausal women, indicating a significant relationship between height and prognosis within the postmenopausal cohort. This finding suggests potential heterogeneity in BC prognosis across different menopausal stages.
Figure 1.
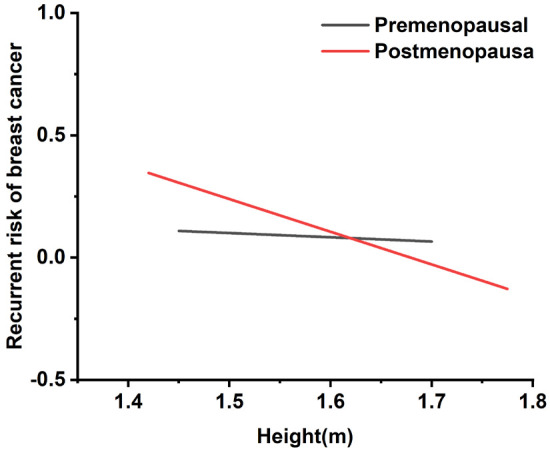
Regression curves for the association between height and breast cancer recurrence risk.
A robust association was also identified between patient height and hormone receptor subtypes, affecting prognosis for both premenopausal and postmenopausal women. Figure 2 highlights this correlation, showing that among premenopausal patients, height was negatively correlated with tumor recurrence, with a more pronounced effect in receptor-negative cases. In postmenopausal patients, a strong negative correlation between height and recurrence rate was observed regardless of ER or PR status. Postmenopausal women over 1.75 meters showed a 40-50% better prognosis compared to those between 1.55 and 1.60 meters, as detailed in Tables S1, S2. These findings suggest that ER/PR status significantly influences post-treatment prognosis, with taller postmenopausal women-particularly those with specific hormone receptor profiles-achieving more favorable outcomes.
Figure 2.
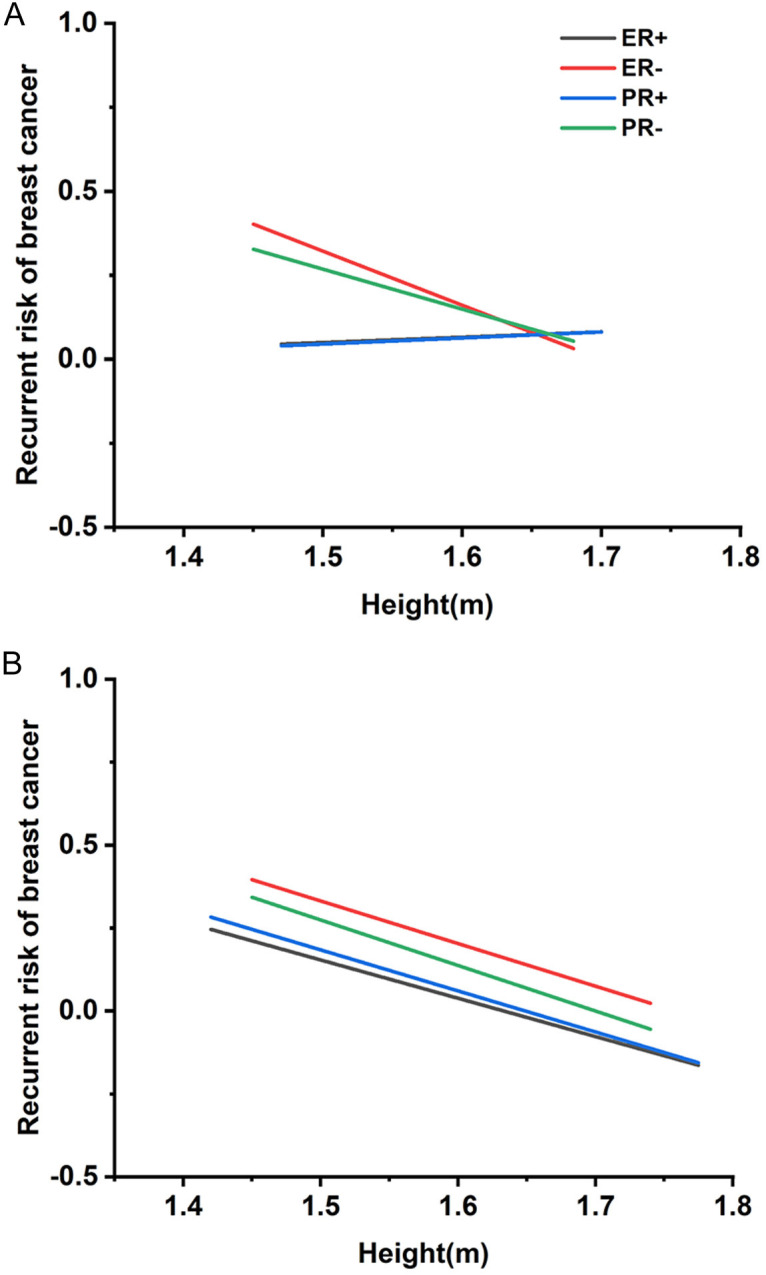
Regression curves between height and distinct estrogen receptor (ER)/progesterone receptor (PR) breast cancer (BC) recurrence rates. A. Regression curves between premenopausal women height and various ER/PR BC recurrence rates. B. Regression curve between postmenopausal women height and different ER/PR BC recurrence rates.
Expression levels of ER, PR, Her-2, and the proliferation marker Ki-67 play a pivotal role in post-treatment prognosis and BC classification into five distinct subtypes. To further investigate, we conducted a statistical evaluation of BC subtypes relative to patient height. Figure 3 illustrates the correlation between height and recurrence rates across different menopausal statuses and BC subtypes. Among premenopausal patients, height was inversely correlated with recurrence rates in pure Her-2 positive and TNBC subtypes, while Luminal A and Luminal B (Her-2 positive) subtypes exhibited a positive correlation between height and recurrence rates. In postmenopausal patients, recurrence rates for pure Her-2 positive, Luminal B (Her-2 positive and negative), and TNBC subtypes were inversely correlated with height. However, no significant prognostic differences were observed for the Luminal A subtype or carcinoma in situ based on height.
Figure 3.
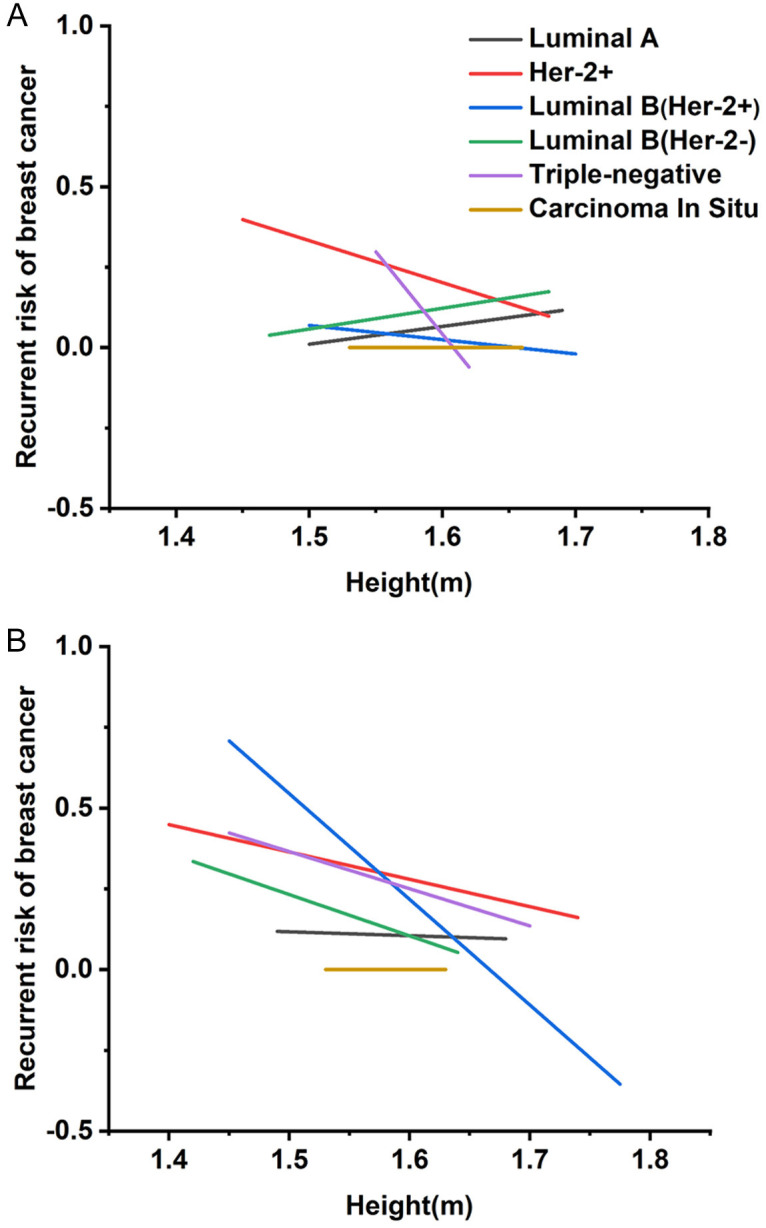
Regression curves between height and recurrence rates of different subtypes of breast cancer (BC) are presented. A. Regression curves between premenopausal women height and recurrence rates of different BC subtypes. B. Regression curves between postmenopausal height and recurrence rates of different BC subtypes.
Detailed analyses among postmenopausal BC patients revealed significant prognostic differences, particularly in Luminal B (Her-2 positive) and TNBC subtypes (P<0.05). Postmenopausal patients with the Luminal B (Her-2 positive) subtype and a height of 1.70 meters or taller demonstrated a substantially improved prognosis-approximately 40-50% - compared to those with heights between 1.55 and 1.60 meters, as further outlined in Tables S3, S4.
Impact of BMI on BC recurrence
Our research demonstrates that height can influence the post-treatment prognosis of cancer patients. We now turn to another health indicator, BMI, to assess its impact on BC prognosis [15]. Figure 4 illustrates a positive correlation between adult BMI and BC recurrence rates in both premenopausal and postmenopausal women. Notably, women with a BMI over 30 kg/m2 face a higher recurrence risk, especially in postmenopausal BC, with a relative risk of 1.6000 (95% CI: 1.1940-1.9320), compared to a relative risk of 0.7140 (95% CI: 0.3590-0.9570) in premenopausal BC. For those with an intermediate BMI (21-23 kg/m2), recurrence risk is 1.0909 (95% CI: 1.0002-1.1816) in premenopausal BC and 1.2157 (95% CI: 1.0963-1.3350) in postmenopausal BC. These findings suggest that while elevated BMI increases recurrence risk across both groups, its impact is more significant in postmenopausal women.
Figure 4.
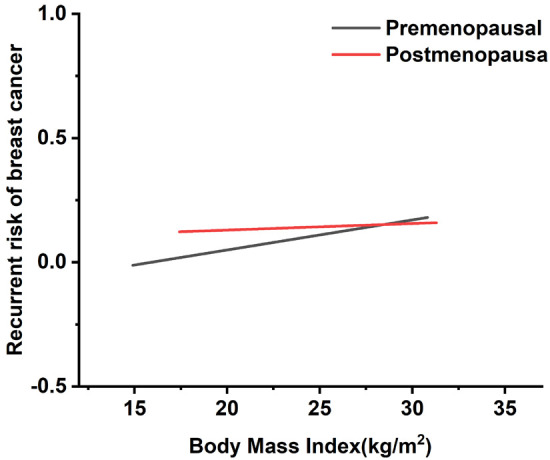
Regression curve relating body mass index to breast cancer recurrence rate.
Additionally, regression analysis reveals a linear trend: increasing BMI in premenopausal women correlates with progressively worse prognosis, highlighting BMI’s role in influencing treatment and management strategies. BMI correlates positively with recurrence rates in ER-positive premenopausal BC (Figure 5). To further examine the relationship between adult weight changes and BC prognosis, we conducted a stratified analysis using a BMI threshold of 21 kg/m2, aligning with the median BMI in our cohort. Results show that a BMI of 25 kg/m2 or higher correlates with worse prognosis in premenopausal BC patients, especially in those with ER-positive and PR-positive subtypes, indicating a complex association where lower BMI corresponds to better outcome.
Figure 5.
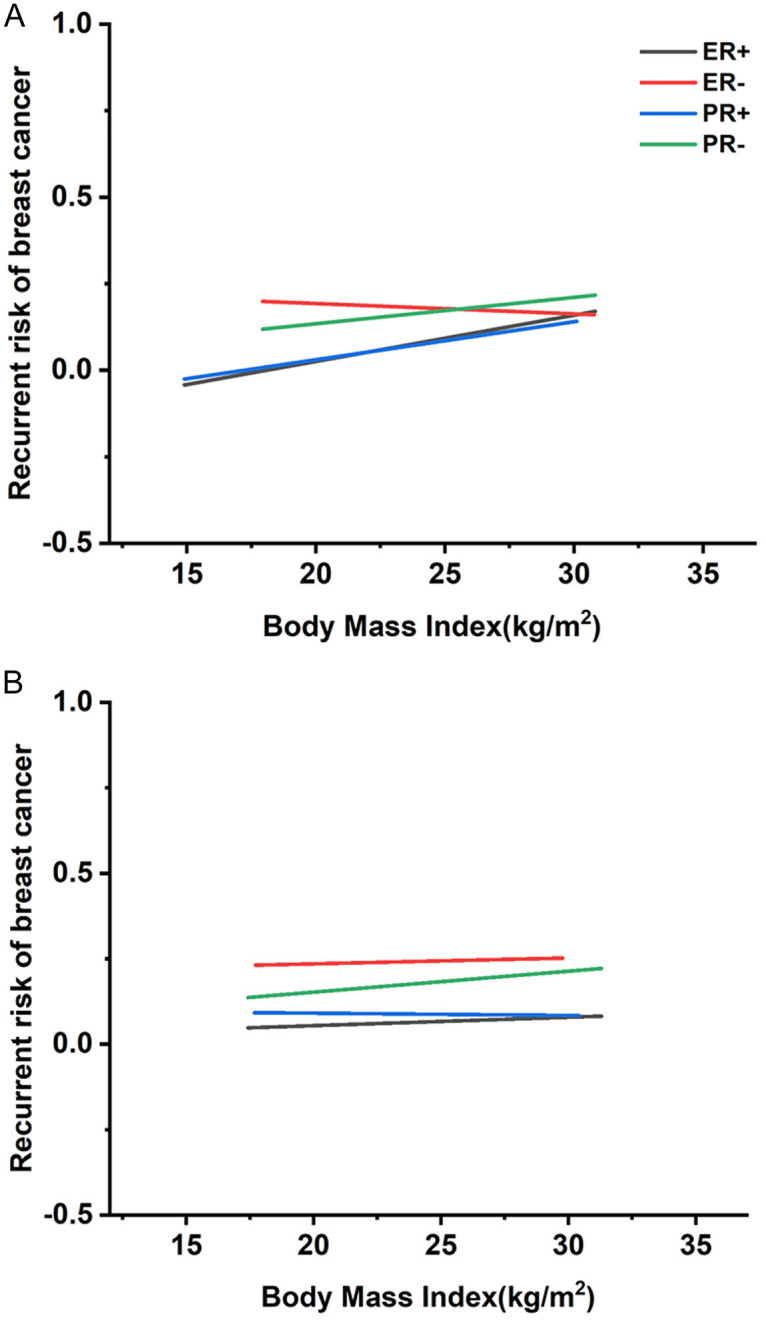
Regression curves between body mass index (BMI) and distinct rates of estrogen receptor (ER)/progesterone receptor (PR) breast cancer (BC) recurrence rare. A. Regression curve between premenopausal BMI and various rates of ER/PR breast cancer recurrence risk. B. Regression curve between postmenopausal BMI and different rates of ER/PR breast cancer recurrence risk.
In particular, lean women (BMI 18-21 kg/m2) exhibit lower recurrence rates. However, in this demographic, higher BMI is significantly associated with increased BC recurrence risk in ER-positive and PR-positive subtypes. Women with a BMI of 25 kg/m2 or above experience a 30% increased recurrence risk. For PR-positive BC, women with lower BMI show a reduced recurrence risk by approximately 20-35%, whereas women of normal weight show only a marginal reduction (<10%). By contrast, ER-negative BC demonstrates an inverse correlation between BMI and recurrence risk. No significant association between BMI and prognosis was observed in postmenopausal patients, as detailed in Tables S5, S6.
Figure 6 shows the correlation between BMI and prognosis rates across BC subtypes in premenopausal and postmenopausal women. In premenopausal patients, higher BMI correlates with increased recurrence rates in Luminal A, Luminal B, and TNBC subtypes, suggesting a poorer outcome. Interestingly, the pure Her-2 positive subtype with higher BMI associates with a lower recurrence rate and better prognosis. BMI could serve as a preliminary indicator for prognosis assessment in premenopausal BC subtypes, including Luminal A, pure Her-2 positive, Luminal B, and TNBC. For postmenopausal women with pure Her-2 positive subtype, higher BMI also correlates with improved prognosis, paralleling the trend observed in premenopausal pure Her-2 positive patients.
Figure 6.
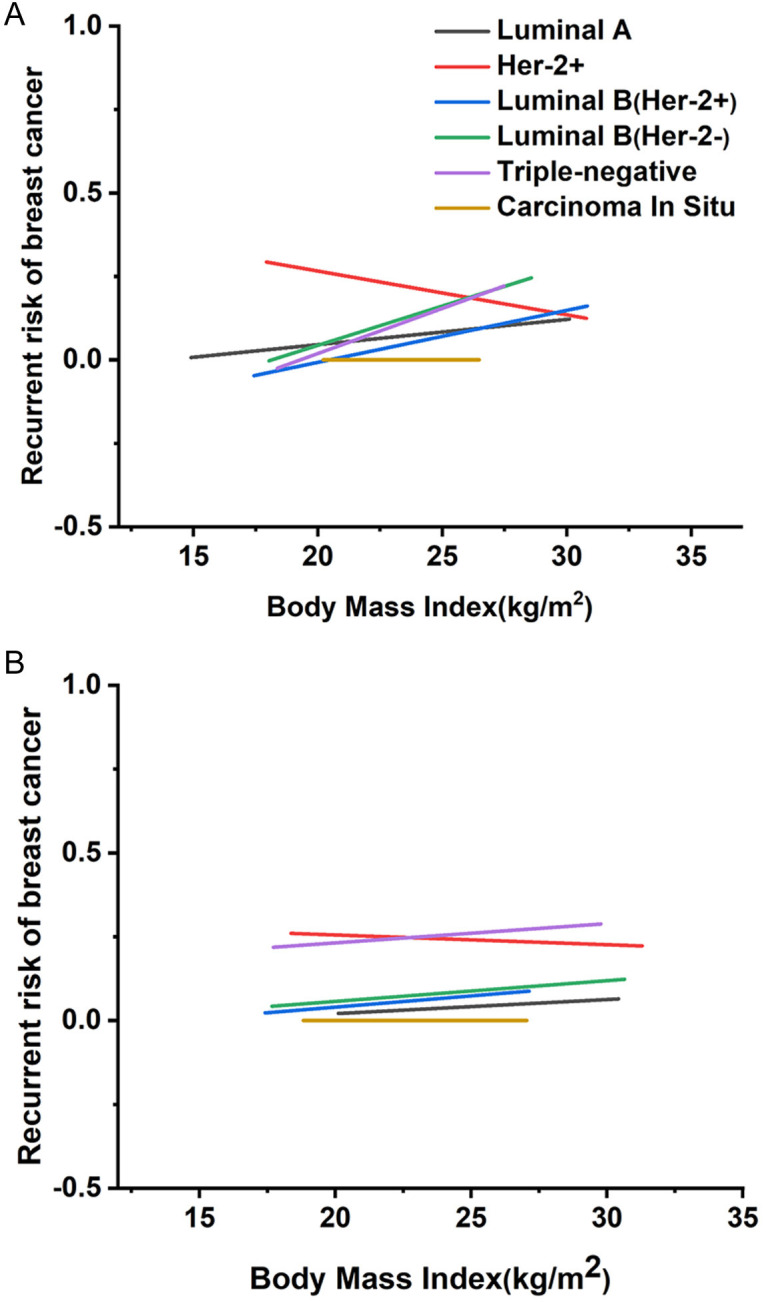
Regression curves between body mass index (BMI) and recurrence rates for various subtypes of breast cancer recurrence risk. A. Regression curve between premenopausal BMI and recurrence rates for different subtypes of breast cancer. B. Regression curve between postmenopausal BMI and recurrence rates for different subtypes of breast cancer.
To further explore BMI’s impact on BC prognosis, we conducted a targeted analysis of recurrence rates in pure Her-2 positive BC among premenopausal women. Findings indicate that patients with a BMI of 25 or higher have a 10-20% reduction in recurrence rates compared to those with BMI below 21. These results underscore the variability in BC outcomes across subtypes, suggesting that BMI plays a significant role in modulating recurrence rates in premenopausal BC patients. Conversely, in postmenopausal patients, no significant trend emerged linking BMI to prognosis across BC subtypes, as further detailed in Tables S7, S8.
Discussion
This study, encompassing 358 BC patients, provides a comprehensive analysis of the relationship between body mass index (BMI), hormone receptor status, and BC prognosis. Our findings reveal a complex distribution of hormone receptor statuses: 69.55% of patients were ER-positive, and 30.47% were ER-negative. Similarly, 57.54% were PR-positive, while 42.46% were PR-negative. These proportions align with the broader oncological literature, highlighting the higher prevalence of ER- and PR-positive cases [16,17].
In terms of BC subtypes, our results are consistent with extensive epidemiological research. The Luminal A subtype, often associated with favorable prognosis due to hormone sensitivity, was identified in 19.27% of cases. The pure human epidermal growth factor receptor 2 (Her-2) positive subtype constituted 16.20%, while the Luminal B (Her-2 positive) and Luminal B (Her-2 negative) subtypes accounted for 17.60% and 21.79%, respectively. TNBC, known for its aggressive nature and lack of ER, PR, and Her-2 expression, represented 10.06% of cases, while ductal carcinoma in situ, indicative of early-stage disease, comprised 7.26%. These data underscore BMI’s substantial influence on BC prognosis, which appears dependent on ER/PR status [18,19]. This finding is significant for personalized medicine, suggesting that BMI’s impact on prognosis varies across BC subtypes and is particularly influenced by hormone receptor status. This builds upon prior research estimating BC incidence based on BMI [20].
Height also emerged as a significant prognostic marker, showing a negative linear correlation with prognosis in both pre- and postmenopausal BC patients [21]. This association was particularly pronounced among postmenopausal women and may be related to postmenopausal hormonal shifts, where reduced estrogen levels could modulate tumor growth and recurrence risk. Additionally, the higher adipose tissue levels in taller women, often associated with increased estrogen, may influence BC prognosis, consistent with prior findings that greater height offers a protective effect against BC development [22,23].
In the molecular classification of BC our analysis of 358 patients reveals how BMI and hormone receptor status intersect with BC prognosis [24]. Notably, height was negatively correlated with recurrence rate in pure Her-2 positive and TNBC subtypes, possibly due to these tumors’ heightened sensitivity to hormonal fluctuations. In postmenopausal women, lower estrogen levels may affect tumor behavior across different height categories. Conversely, recurrence rates in Luminal A and Luminal B (Her-2 negative) subtypes were positively correlated with height, suggesting that distinct biological factors related to height may influence tumor growth and recurrence in these subtypes.
Previous research has consistently shown that height, BMI, lifestyle factors, and dietary habits contribute to BC risk. These findings underscore the importance of considering BMI and hormone receptor status in BC prognosis, especially in the development of personalized treatment strategies [25].
BMI, as a notable prognostic factor, shows a positive correlation with the recurrence rate of premenopausal BC, particularly in receptor-positive subtypes. This association may arise from hormonal variations in individuals with elevated BMI, as adipose tissue serves as a significant source of estrogen production. Among premenopausal patients with Luminal A, Luminal B, and TNBC subtypes, an increased BMI is linked to a higher recurrence risk and worse prognosis. In contrast, for the pure Her-2 positive subtype, a negative correlation between BMI and recurrence rate is observed, suggesting that BMI may influence tumor biology differently in this subtype. Consistent with relevant research, BMI similarly affects the recurrence risk profile in Her-2 positive BC cases [26].
These findings have important implications for tailoring BC treatment strategies. For instance, postmenopausal patients with greater height may benefit from more frequent surveillance and potentially more intensive therapeutic regimens to reduce recurrence risk and improve outcomes [27]. In premenopausal individuals with elevated BMI, treatment adjustments to address their higher recurrence risk may be warranted to enhance survival and quality of life [28].
Further investigation into the biological mechanisms linking height, BMI, and BC prognosis is essential. Future research should also examine how these factors interact with other clinical and molecular tumor features to support more precise prognostic assessments and therapy guidance. Insights into these relationships could lead to novel therapeutic targets, such as hormones or metabolic pathways, which could be exploited to enhance treatment efficacy in specific patient groups. Most current research focuses on the effects of height and BMI on BC incidence, with limited exploration of these factors within different hormone receptor contexts [29]. While some studies have addressed various BC subtypes, they have primarily emphasized incidence rates over recurrence risk [30].
Our study distinguished itself by using a more nuanced approach, analyzing the interaction between height, BMI, and hormone receptor diversity in shaping recurrence risk among BC patients. Our goal was to clarify the complex interactions between these variables and the potential for cancer relapse, thus enhancing the precision of clinical interventions and preventive strategies. Through this comprehensive analysis, we aimed to uncover novel biological insights and to tailor treatment strategies more precisely to the unique needs of BC patients.
In summary, building upon prior research, our study provides a more detailed analysis of prognostic patterns among BC patients, specifically exploring correlations between height, BMI, and biomarker-defined subtypes of tumor [31]. Our findings highlight significant prognostic disparities associated with height and BMI, particularly between premenopausal and postmenopausal BC cohorts. This study sets itself apart by employing advanced statistical techniques to investigate the links between anthropometric measurements and BC prognosis, thereby enhancing the rigor and precision of our analysis.
However, we recognize certain limitations. The reliance on self-reported anthropometric data may have introduced bias, and the study’s relatively small sample size and limited racial/ethnic diversity are acknowledged constraints. Despite these challenges, our research offers valuable insight for clinical applications, emphasizing the potential of using anthropometric indicators-particularly emenopausal history. Our findings support the importance of preventive strategies, such as weight management, to reduce BC recurrence risk.
Looking forward, future studies should address these limitations by incorporating objective anthropometric measurements and expanding to include more diverse racial and ethnic groups. Additionally, examining additional biomarkers and clinical factors associated with BC prognosis will be essential to develop more personalized approaches to treatment and management.
In conclusion, the relationship between height, BMI, and BC recurrence rate varies with menopausal status and BC subtype. This association generally follows a linear trend, exerting a more significant effect on hormone receptor-positive subtypes in both premenopausal and postmenopausal patients. Height shows a negative linear correlation with recurrence rates in hormone receptor-negative BC, while BMI demonstrates a positive correlation with recurrence rates in both premenopausal and postmenopausal receptor-positive BC. Further research is needed to clarify the mechanisms by which BMI influences BC recurrence rates in these groups. Moreover, height and BMI values may serve as preliminary prognostic indicators for premenopausal patients with pure human epidermal growth factor receptor 2 (Her-2) positive subtype BC.
Acknowledgements
This work was supported by the Contributing to the National Public Hospital Research Capacity Enhancement Public Welfare Program (No. KM-ZLGJ-057).
Disclosure of conflict of interest
None.
Abbreviations
- ER
estrogen receptor
- PR
progesterone receptor
- BC
breast cancer
- BMI
body mass index
- KI67
marker of proliferation Ki-67
- Her-2
human epidermal growth factor receptor 2
- RR
relative risks
- TNBC
triple-negative breast cancer
Supporting Information
References
- 1.Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2021;11:632079. doi: 10.3389/fphar.2020.632079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaikh H, Bradhurst P, Ma LX, Tan SYC, Egger SJ, Vardy JL. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. 2020;12:CD012110. doi: 10.1002/14651858.CD012110.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devericks EN, Carson MS, McCullough LE, Coleman MF, Hursting SD. The obesity-breast cancer link: a multidisciplinary perspective. Cancer Metastasis Rev. 2022;41:607–625. doi: 10.1007/s10555-022-10043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, Zaguia A, Koundal S, Belay A. Global increase in breast cancer incidence: risk factors and preventive measures. Biomed Res Int. 2022;2022:9605439. doi: 10.1155/2022/9605439. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.García-Estévez L, Cortés J, Pérez S, Calvo I, Gallegos I, Moreno-Bueno G. Obesity and breast cancer: a paradoxical and controversial relationship influenced by menopausal status. Front Oncol. 2021;11:705911. doi: 10.3389/fonc.2021.705911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The impact of obesity on breast cancer diagnosis and treatment. Curr Oncol Rep. 2019;21:41. doi: 10.1007/s11912-019-0787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JC, Sarwer DB, Troxel AB, Sturgeon K, DeMichele AM, Denlinger CS, Schmitz KH. A randomized trial of exercise and diet on body composition in survivors of breast cancer with overweight or obesity. Breast Cancer Res Treat. 2021;189:145–154. doi: 10.1007/s10549-021-06284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel) 2021;13:4287. doi: 10.3390/cancers13174287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oudanonh T, Nabi H, Ennour-Idrissi K, Lemieux J, Diorio C. Progesterone receptor status modifies the association between body mass index and prognosis in women diagnosed with estrogen receptor positive breast cancer. Int J Cancer. 2020;146:2736–2745. doi: 10.1002/ijc.32621. [DOI] [PubMed] [Google Scholar]
- 10.Chauhan R, Trivedi V, Rani R, Singh U. A comparative analysis of body mass index with estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 status in pre- and postmenopausal breast cancer patients. J Midlife Health. 2020;11:210–216. doi: 10.4103/jmh.JMH_97_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu K, Huang ZY, Xu XL, Li J, Fu XW, Deng SL. Estrogen receptor function: impact on the human endometrium. Front Endocrinol (Lausanne) 2022;13:827724. doi: 10.3389/fendo.2022.827724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang Y, Wei Y, Kartsonaki C. Associations of adiposity and weight change with recurrence and survival in breast cancer patients: a systematic review and meta-analysis. Breast Cancer. 2022;29:575–588. doi: 10.1007/s12282-022-01355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Li J, Hu Q, Zhang X, Chen F. Association of physical weight statuses defined by body mass index (BMI) with molecular subtypes of premenopausal breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2024;203:429–447. doi: 10.1007/s10549-023-07139-z. [DOI] [PubMed] [Google Scholar]
- 14.Davis PM. Statistics for describing populations. Handbook of sampling methods for arthropods in agriculture. CRC Press; 2020. pp. 33–54. [Google Scholar]
- 15.Davies A, Wellard-Cole L, Rangan A, Allman-Farinelli M. Validity of self-reported weight and height for BMI classification: a cross-sectional study among young adults. Nutrition. 2020;71:110622. doi: 10.1016/j.nut.2019.110622. [DOI] [PubMed] [Google Scholar]
- 16.Sleightholm R, Neilsen BK, Elkhatib S, Flores L, Dukkipati S, Zhao R, Choudhury S, Gardner B, Carmichael J, Smith L, Bennion N, Wahl A, Baine M. Percentage of hormone receptor positivity in breast cancer provides prognostic value: a single-institute study. J Clin Med Res. 2021;13:9–19. doi: 10.14740/jocmr4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson ALV, Trewin CB, Fredriksson I, Reinertsen KV, Russnes H, Ursin G. In modern times, how important are breast cancer stage, grade and receptor subtype for survival: a population-based cohort study. Breast Cancer Res. 2021;23:17. doi: 10.1186/s13058-021-01393-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzenios N, Tazanios ME, Chahine M. The impact of BMI on breast cancer - an updated systematic review and meta-analysis. Medicine (Baltimore) 2024;103:e36831. doi: 10.1097/MD.0000000000036831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy AM, Friebel-Klingner T, Ehsan S, He W, Welch M, Chen J, Kontos D, Domchek SM, Conant EF, Semine A, Hughes K, Bardia A, Lehman C, Armstrong K. Relationship of established risk factors with breast cancer subtypes. Cancer Med. 2021;10:6456–6467. doi: 10.1002/cam4.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bissell MCS, Kerlikowske K, Sprague BL, Tice JA, Gard CC, Tossas KY, Rauscher GH, Trentham-Dietz A, Henderson LM, Onega T, Keegan THM, Miglioretti DL Breast Cancer Surveillance Consortium. Breast cancer population attributable risk proportions associated with body mass index and breast density by race/ethnicity and menopausal status. Cancer Epidemiol Biomarkers Prev. 2020;29:2048–2056. doi: 10.1158/1055-9965.EPI-20-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swaminathan H, Saravanamurali K, Yadav SA. Extensive review on breast cancer its etiology, progression, prognostic markers, and treatment. Med Oncol. 2023;40:238. doi: 10.1007/s12032-023-02111-9. [DOI] [PubMed] [Google Scholar]
- 22.Si S, Tewara MA, Ji X, Wang Y, Liu Y, Dai X, Wang Z, Xue F. Body surface area, height, and body fat percentage as more sensitive risk factors of cancer and cardiovascular disease. Cancer Med. 2020;9:4433–4446. doi: 10.1002/cam4.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lubián López DM, Butrón Hinojo CA, Castillo Lara M, Sánchez-Prieto M, Sánchez-Borrego R, Mendoza Ladrón de Guevara N, González Mesa E. Relationship of breast volume, obesity and central obesity with different prognostic factors of breast cancer. Sci Rep. 2021;11:1872. doi: 10.1038/s41598-021-81436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruan GT, Xie HL, Hu CL, Liu CA, Zhang HY, Zhang Q, Wang ZW, Zhang X, Ge YZ, Lin SQ, Tang M, Song MM, Zhang XW, Liu XY, Zhang KP, Yang M, Yu KY, Wang KH, Hu W, Deng L, Cong MH, Shi HP. Comprehensive prognostic effects of systemic inflammation and Insulin resistance in women with breast cancer with different BMI: a prospective multicenter cohort. Sci Rep. 2023;13:4303. doi: 10.1038/s41598-023-31450-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantini L, Pistelli M, Merloni F, Fontana A, Bertolini I, De Angelis C, Bastianelli L, Della Mora A, Santinelli A, Savini A, Maccaroni E, Diodati L, Falcone A, Berardi R. Body mass index and hormone receptor status influence recurrence risk in HER2-positive early breast cancer patients. Clin Breast Cancer. 2020;20:e89–e98. doi: 10.1016/j.clbc.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Ligorio F, Zambelli L, Bottiglieri A, Castagnoli L, Zattarin E, Lobefaro R, Ottini A, Vingiani A, Pupa SM, Bianchi GV, Capri G, Pruneri G, de Braud F, Vernieri C. Hormone receptor status influences the impact of body mass index and hyperglycemia on the risk of tumor relapse in early-stage HER2-positive breast cancer patients. Ther Adv Med Oncol. 2021;13:17588359211006960. doi: 10.1177/17588359211006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen K, Zhang J, Beeraka NM, Tang C, Babayeva YV, Sinelnikov MY, Zhang X, Zhang J, Liu J, Reshetov IV, Sukocheva OA, Lu P, Fan R. Advances in the prevention and treatment of obesity-driven effects in breast cancers. Front Oncol. 2022;12:820968. doi: 10.3389/fonc.2022.820968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeVee A, Mortimer J. The challenges of treating patients with breast cancer and obesity. Cancers (Basel) 2023;15:2526. doi: 10.3390/cancers15092526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohmann AE, Soldera SV, Pimentel I, Ribnikar D, Ennis M, Amir E, Goodwin PJ. Association of obesity with breast cancer outcome in relation to cancer subtypes: a meta-analysis. J Natl Cancer Inst. 2021;113:1465–1475. doi: 10.1093/jnci/djab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atoum MF, Alzoughool F, Al-Hourani H. Linkage between obesity leptin and breast cancer. Breast Cancer (Auckl) 2020;14:1178223419898458. doi: 10.1177/1178223419898458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarighati E, Keivan H, Mahani H. A review of prognostic and predictive biomarkers in breast cancer. Clin Exp Med. 2023;23:1–16. doi: 10.1007/s10238-021-00781-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


