Abstract
Objectives: Cholecystectomy is noted for potentially impacting blood lipid/glucose levels, yet causal links remain unclear. Methods: Cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) 2017-2018 were employed to explore the relationship between cholecystectomy and blood lipid/glucose traits. Propensity-score matching (PSM) was performed to equalize baseline differences. Genome-wide association study (GWAS) data from the UK Biobank, FinnGen, Global Lipids Genetics Consortium (GLGC), and the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) were analyzed by Mendelian randomization (MR) to infer causality. Combination of MR results was achieved with meta-analysis. Results: Based on the NHANES database, significantly decreased levels of total cholesterol (TC) (P = 0.021), low-density lipoprotein cholesterol (LDL-C) (P = 0.036), high-density lipoprotein cholesterol (HDL-C) (P = 0.017) and augmented triglyceride (TG) (P = 0.021) were found in patients with gallbladder removal after PSM. No difference was observed in fasting glucose, fasting insulin and hemoglobin A1c (HbA1c). In MR analysis, significant associations were found between cholecystectomy and lower TC (P = 0.002), especially LDL-C (P = 0.002) and HDL-C (P = 0.044). No significant associations were observed with TG, fasting glucose, fasting insulin or HbA1c. Conclusions: Cholecystectomy has specific impacts on serum lipid profiles instead of glucose traits.
Keywords: Cholecystectomy, cross-sectional study, Mendelian randomization, blood lipids, blood glucose, causality analysis
Introduction
Cholecystectomy is the gold-standard treatment for gallbladder diseases [1-3]. It has become one of the most frequently performed abdominal surgeries worldwide, with millions undergoing the procedure annually [4,5]. In addition, cholecystectomy has attracted attention for its potential influence on lipid and glucose metabolism [6].
Several contradictory studies have suggested associations between cholecystectomy and changes in lipid and glucose traits. A longitudinal study conducted in South Korea found that patients who underwent cholecystectomy had a 21% higher risk of developing incident metabolic syndrome (as indicated by hyperlipidemia) and hyperglycemia, compared with those who did not receive the surgery [7]. However, other observational studies [8-10] reported a reduction in cardio-cerebrovascular diseases among patients who underwent cholecystectomy, which was associated with improvements in lipid or glucose metabolism. These conflicting studies have suggested uncertain associations between cholecystectomy and metabolic profiles, especially lipid and glucose metabolism. Additionally, the coexistence of dyslipidemia and hyperglycemia in individuals predisposed to gallbladder diseases complicates the elucidation of the causal relationship between cholecystectomy and metabolic outcomes [11]. Investigating the impact is crucial for enhancing our understanding of the metabolic repercussions of cholecystectomy, potentially altering the indications of cholecystectomy in patients with dyslipidemia and pathoglycemia.
Mendelian randomization (MR) has been considered as a powerful tool for inferring causal correlations between risk factors and health outcomes [12,13]. In contrast to conventional observational studies, which are susceptible to confounding and reverse causation biases, MR employs genetic variants as instrumental variables to infer causality more robustly. By leveraging germline genetic variants randomly apportioned during meiosis, the MR design effectively minimizes confounding components and remains unaffected by environmental or self-adopted factors, thereby enhancing causal inference [14,15]. By utilizing selected genetic variants associated with the exposure (cholecystectomy) but unaffected by confounders (blood lipid and glucose traits), MR facilitates the emulation of randomized controlled trials in observational settings, thereby providing valuable insights into causal associations.
In this study, we firstly analyzed cross-sectional data from the United States National Health and Nutrition Examination Survey (NHANES) to explore the associations between cholecystectomy and blood lipid/glucose traits. To balance the baseline parameters, propensity score matching (PSM) analyses were conducted to ensure changes in serum lipids/glucose after cholecystectomy. Furthermore, unlike previous observational studies which were only able to infer ambiguous correlations, MR analyses were applied in this study to address whether cholecystectomy has causal effects on blood lipids/glucose by utilizing genome-wide association study (GWAS) data from the Global Lipids Genetics Consortium (GLGC), the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC), as well as the UK Biobank and FinnGen.
Materials and methods
Study design
In the present study, we investigated the impact of cholecystectomy on the blood lipid and glucose traits in a cross-sectional study based on the NHANES database. PSM analyses were performed to adjust the observed bias due to the baseline differences. We then performed two-sample MR analyses to evaluate the causal relationships. The results of the MR analyses from the different databases were combined in a meta-analysis.
Cross-sectional study
Data source
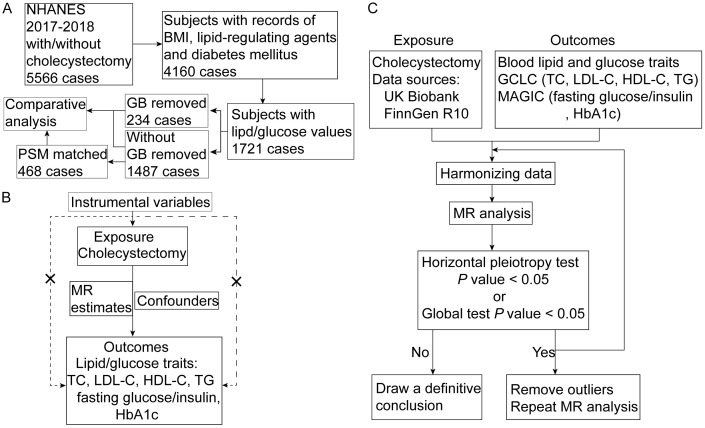
The data for the observational cross-sectional study were from the 2017-2018 NHANES. A total of 5,566 participants with complete data regarding whether they received cholecystectomy or not were included. Ultimately, after removing the patients with incomplete records for blood lipid/glucose, body mass index (BMI) data, diabetes or cholesterol-regulating agent usage, a total of 1,721 patients were enrolled (Figure 1A). Informed consents were acquired from all the individuals analyzed in this study, and ethical approval was awarded by the National Center for Health Statistics (NCHS) Ethical Review Board.
Figure 1.
Graphical overview of the whole study design. A. Workflow of propensity score matching (PSM) analysis using National Health and Nutrition Examination Survey (NHANES) database; B. Assumptions of MR analysis; C. Flowchart of the MR analysis. Abbreviations: BMI, body mass index; GB, gallbladder; PSM, propensity score matching; MR, Mendelian randomization; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HbA1c, hemoglobin A1c; GLGC, Global Lipids Genetics Consortium; MAGIC, Meta-Analyses of Glucose and Insulin-related traits Consortium.
Statistical analysis
Continuous variables were recorded as means ± standard deviations or medians with interquartile ranges (IQRs). Student’s t-test or the Mann-Whitney U test was utilized for comparisons of two groups of continuous variables. The Chi-squared test or the Kruskal-Wallis test was applied to compare categorical variables that were reported as weighted counts and percentages. PSM was performed using the MatchIt package to balance baseline factors between the patients who underwent cholecystectomy and those who did not. We matched the patients for age, sex, BMI, history of diabetes, and lipid-regulating agent usage, which were predisposed to affect the blood lipid/glucose traits. The PSM analysis employs a nearest-neighbor method with a no-replacement strategy at a 1:2 ratio and a caliper width of 0.2, using logit distance to estimate the matching extent. R software version 4.3.2 was applied.
Mendelian randomization
Cholecystectomy was used as the exposure factor and blood lipids and glucose were used as the outcome factors. The prerequisite for conducting the two-sample MR analysis is to meet three core assumptions: (1) the selected single-nucleotide polymorphisms (SNPs) as instrument variables are significantly related with exposure (i.e., cholecystectomy); (2) the selected SNPs are independent to confounding factors; and (3) the selected SNPs should be connected to the outcome only through exposure (Figure 1B). The flowchart of the MR analyses is shown in Figure 1C. Summary data from public databases (NHANES, UK Biobank, FinnGen, GLGC and MAGIC) which had acquired individual consent and ethical approval were analyzed.
Data sources
SNPs associated with cholecystectomy were extracted from the UK Biobank dataset, which consisted of 18,319 cases and 444,614 controls (total sample size: 462,933) of European ancestry, as well as from the FinnGen R10 dataset, which included 29,157 cases and 383,024 controls. SNPs for blood lipid traits (high-density lipid cholesterol [HDL-C], low-density lipid cholesterol [LDL-C], total cholesterol [TC], and triglycerides [TG]) were extracted from a GWAS dataset in the GLGC database that included 1.32 million cases of European ancestry [16]. Summary statistic data for blood glucose were obtained from the MAGIC database of European ancestry [17,18]. Specific brief information is exhibited in Table 1.
Table 1.
Characteristics of data used in the Mendelian randomization study
| Exposure/Outcome | Participants | Resource |
|---|---|---|
| Cholecystectomy | 18,319 cases and 444,614 non-cases of European ancestry | UK Biobank |
| Cholecystectomy | 29,157 cases and 383,024 non-cases of European ancestry | FinnGen R10 |
| TC | 1.32 million individuals of European ancestry | GLGC |
| LDL-C | 1.32 million individuals of European ancestry | GLGC |
| HDL-C | 1.32 million individuals of European ancestry | GLGC |
| TG | 1.32 million individuals of European ancestry | GLGC |
| Fasting Glucose | 58,074 individuals of European ancestry | MAGIC |
| Fasting Insulin | 51,750 individuals of European ancestry | MAGIC |
| HbA1c | 46,368 individuals of European ancestry | MAGIC |
Abbreviations: TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HbA1c, hemoglobin A1c; GLGC, Global Lipids Genetics Consortium; MAGIC, Meta-Analyses of Glucose and Insulin-related traits Consortium.
Selection criteria for instrumental variables (IVs)
The following criteria were adopted to screen independent and significant SNPs as IVs for exposure factors (cholecystectomy): (1) SNPs were considered significant if they met the genome-wide association threshold of P < 5 × 10-6. (2) All selected SNPs were required to be independent, with a linkage disequilibrium (LD) threshold of r2 < 0.01, using a clumping window of 10,000 kb. (3) SNPs with F-statistics below 10 were excluded to minimize weak instrument bias. The F-statistic was calculated using the formula F = [(N-K-1)/K] × [R2/(1-R2)], where R2 represents the cumulative variance explained by the selected SNPs for the exposure, N is the sample size of the exposure dataset, and K is the number of SNPs included in the analysis. An F-statistic greater than 10 indicates a reduced risk of weak instrument bias. (4) SNPs containing palindromic sequences were excluded from the analysis. (5) SNPs that showed significant associations (P < 1 × 10-5) with known confounding factors, such as lipid and glucose metabolism, were also removed.
Sensitivity analysis
Heterogeneity was detected using Cochran’s Q-test [20], applying the inverse-variance weighted (IVW) method [21]. A significance threshold of P < 0.05 was interpreted as evidence of heterogeneity. In the presence of heterogeneity, the random-effects IVW method was employed. The MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) method was employed to mitigate the influence of horizontal pleiotropy [22]. If the MR-PRESSO global test or the pleiotropy test indicated significance, it suggested the presence of horizontal pleiotropy; therefore, all outlier IVs were removed before conducting further MR analysis. Additionally, a leave-one-out sensitivity analysis was performed to validate the robustness of the results by systematically removing each SNP with iterations. Scatter and funnel plots were created to visually assess the outcomes of the MR analyses.
MR analyses
Two-sample MR analyses were conducted to investigate the potential causal associations between cholecystectomy and blood lipid and glucose traits. A variety of statistical methods were employed, including MR Egger (MRE), IVW, Weighted Median (WMed), weighted Mode (WMod), and Simple Mode (SMod) methods. The main results were based on the IVW analysis. Common-effect or random-effect models were conducted to combine MR estimates from different data sources based on heterogeneity testing. The heterogeneity test results were evaluated according to I2 statistic or the Q statistic. TwoSampleMR [23] and MR-PRESSO [22] packages were used to perform all the analyses. If the horizontal pleiotropy was detected, all the outliers were removed using the RadialMR package, and further repeated MR analyses were performed. R software version 4.3.2 was utilized.
Results
Effects of cholecystectomy on serum lipids and glucose before and after PSM analyses by using NHANES data
As analyzed in Table 2, before PSM, patients who underwent cholecystectomy were more likely to be older (55.1% vs. 38.7%, P < 0.001), female sex (76.1% vs. 49.6%, P < 0.001), and have higher BMI (33.2 ± 8.2 vs. 29.6 ± 7.2, P < 0.001) compared with those who did not undergo cholecystectomy. Moreover, subjects with cholecystectomy had higher comorbidity rates of hypertension (57.3% vs. 41.6%, P < 0.001), diabetes mellitus (32.5% vs. 18.2%, P < 0.001), and usage of lipid-regulating agents (45.7% vs. 38.1%, P < 0.001) and reduced TC (median, 177.0 vs. 184.0, P = 0.020), LDL-C (median, 101.0 vs. 109.0, P = 0.006), and TG (median, 129.0 vs. 110.0, P < 0.001) levels. Elevated levels of fasting glucose (median, 110.0 vs. 105.0, P < 0.001), fasting insulin (median, 12.8 vs. 9.7, P < 0.001), and HbA1c (median, 5.8% vs. 5.6%, P < 0.001) were observed in cholecystectomized patients. No changes in HDL-C (median, 50.0 vs. 52.0, P = 0.212) were detected after cholecystectomy.
Table 2.
Overview of demographic parameters from NHANES database before/after PSM analyses
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| With cholecystectomy | Without cholecystectomy | P | With cholecystectomy | Without cholecystectomy | P | |
| (n = 234) | (n = 1487) | (n = 234) | (n = 468) | |||
| Age (> 60, (%)) | 129 (55.1) | 575 (38.7) | < 0.001* | 129 (55.1) | 244 (52.1) | 0.504 |
| Sex (Female, (%)) | 178 (76.1) | 737 (49.6) | < 0.001* | 178 (76.1) | 365 (78.0) | 0.633 |
| Education level (%) | 0.058 | 0.418 | ||||
| < 9th grade | 20 (8.5) | 105 (7.1) | 20 (8.5) | 40 (8.5) | ||
| 9-11th grade | 29 (12.4) | 155 (10.4) | 29 (12.4) | 50 (10.7) | ||
| High school | 64 (27.4) | 329 (22.1) | 64 (27.4) | 103 (22.0) | ||
| Some college or AA degree | 79 (33.8) | 492 (33.1) | 79 (33.8) | 172 (36.8) | ||
| College graduate or above | 42 (17.9) | 405 (27.2) | 42 (17.9) | 103 (22.0) | ||
| Don’t Know | 0 (0.0) | 1 (0.1) | / | / | ||
| BMI (mean (SD)) | 33.2 (8.2) | 29.6 (7.2) | < 0.001* | 33.2 (8.2) | 32.7 (8.8) | 0.406 |
| Hypertension (%) | 134 (57.3) | 618 (41.6) | < 0.001* | 134 (57.3) | 231 (49.4) | 0.058 |
| Diabetes mellitus (%) | 76 (32.5) | 271 (18.2) | < 0.001* | 76 (32.5) | 139 (29.7) | 0.506 |
| Lipid-regulating agents (%) | 107 (45.7) | 566 (38.1) | 0.031* | 107 (45.7) | 201 (42.9) | 0.536 |
| TC (mg/dL, median [IQR]) | 177.0 [152.2, 207.5] | 184.0 [160.0, 215.0] | 0.020* | 177.0 [152.3, 207.5] | 184.0 [161.0, 217.0] | 0.021* |
| LDL-C (mg/dL, median [IQR]) | 101.0 [78.0, 127.8] | 109.0 [87.0, 135.0] | 0.006* | 101.0 [78.0, 127.8] | 108.0 [86.8, 136.0] | 0.036* |
| HDL-C (mg/dL, median [IQR]) | 50.0 [42.0, 61.0] | 52.0 [43.0, 63.0] | 0.212 | 50.0 [42.0, 61.0] | 53.0 [44.0, 65.0] | 0.017* |
| TG (mg/dL, median [IQR]) | 129.0 [91.0, 167.8] | 110.0 [78.0, 155.0] | < 0.001* | 129.0 [91.0, 167.8] | 114.0 [84.0, 158.0] | 0.021* |
| Fasting glucose (mg/dL, median [IQR]) | 110.0 [100.0, 129.0] | 105.0 [97.0, 117.0] | < 0.001* | 110.0 [100.0, 129.0] | 107.0 [97.8, 127.0] | 0.061 |
| Fasting insulin (uU/dL, median [IQR]) | 12.8 [8.8, 20.4] | 9.7 [6.1, 15.8] | < 0.001* | 12.8 [8.8, 20.4] | 11.7 [7.3, 20.2] | 0.071 |
| HbA1c (%, median [IQR]) | 5.8 [5.4, 6.4] | 5.6 [5.3, 6.0] | < 0.001* | 5.8 [5.4, 6.4] | 5.8 [5.4, 6.5] | 0.860 |
P < 0.05.
Abbreviations: BMI, body mass index; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HbA1c, hemoglobin A1c; IQR, interquartile range.
Given the baseline difference, we performed PSM to match age, sex, BMI, history of diabetes, and lipid-regulating agent usage, which was predisposed to affect the blood lipid/glucose traits. After balancing baseline values by PSM, no differences in age, sex, BMI values, hypertension rate, diabetes mellitus rate, or use of lipid-regulating agents were found in the matched patients. Notably, decreased concentrations of TC (median, 177.0 vs. 184.0, P = 0.021), LDL-C (median, 101.0 vs. 108.0, P = 0.036), and HDL-C (median, 50.0 vs. 53.0, P = 0.017) but increased levels of TG (median, 129.0 vs. 114.0, P = 0.021) were noted in those with cholecystectomy. Although exhibiting increasing trends, no significance was detected for fasting glucose (median, 110.0 vs. 107.0, P = 0.061), fasting insulin (median, 12.8 vs. 11.7, P = 0.071), or HbA1c (median, 5.8% vs. 5.8%, P = 0.860) between individuals with and without cholecystectomy. To clarify the effects of cholecystectomy on serum lipid and glucose traits, we further conducted MR analyses to simulate random clinical trials to explore the causality.
Primary MR results of lipid and glucose traits
In the primary MR analysis, 52 genome-wide significant SNPs from UK Biobank and 108 SNPs from FinnGen were selected as IVs, excluding palindromic SNPs and those associated with cholesterol, lipid, and glucose metabolism. All selected SNPs had F-statistics greater than 10, demonstrating the strong validity of the genetic instruments. Comprehensive details of the SNPs employed as instrumental variables can be found in Tables S1 and S2.
Based on the data from the UK Biobank, we observed a decreasing trend towards a lower odds ratio (odds ratio [OR]: 0.635, 95% confidence interval [CI]: 0.408-0.988, P = 0.044) between cholecystectomy and LDL-C. No significant associations were found with other lipid or glucose parameters (Table 3). In the analysis of the FinnGen data, cholecystectomy demonstrated a significant association with TC levels (OR: 0.983, 95% CI: 0.972-0.994, P = 0.002). Furthermore, regarding specific cholesterol types, cholecystectomy showed significant associations with LDL-C levels (OR: 0.983, 95% CI: 0.972-0.994, P = 0.004). A significant association was observed between cholecystectomy and HDL-C levels (OR: 0.990, 95% CI: 0.982-0.999, P = 0.022). There were no significant associations between cholecystectomy and TG, fasting glucose, fasting insulin, and HbA1c levels. Heterogeneity was detected in the analysis of lipid traits and fasting insulin; therefore, the random-effect IVW method was performed. However, in the MR test of the lipid traits, significant heterogeneity and horizontal pleiotropy were observed, suggesting that the causal relationship may lack robustness (Table S3). Therefore, we removed outlier SNPs using RadialMR, and performed a further two-sample MR for the lipid traits.
Table 3.
Primary MR analyses of cholecystectomy and blood lipid/glucose traits before outlier removal
| Source | UK Biobank | FinnGen | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| TC | GLGC | 0.767 | (0.490; 1.201) | 0.246# | 0.983 | (0.972; 0.994) | 0.002*,# |
| LDL-C | GLGC | 0.635 | (0.408; 0.988) | 0.044*,# | 0.983 | (0.972; 0.994) | 0.004*,# |
| HDL-C | GLGC | 0.628 | (0.339; 1.161) | 0.138# | 0.990 | (0.982; 0.999) | 0.022*,# |
| TG | GLGC | 1.912 | (0.890; 4.107) | 0.097# | 1.006 | (0.997; 1.015) | 0.182# |
| Fasting Glucose | MAGIC | 1.658 | (0.804; 3.417) | 0.171 | 1.023 | (0.999; 1.048) | 0.066 |
| Fasting Insulin | MAGIC | 1.123 | (0.454; 2.778) | 0.802# | 1.006 | (0.982; 1.031) | 0.607 |
| HbA1c | MAGIC | 0.970 | (0.490; 1.921) | 0.930 | 1.001 | (0.972; 1.032) | 0.929 |
P < 0.05;
calculated by random-effect IVW method.
Abbreviations: TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HbA1c, hemoglobin A1c; GLGC, Global Lipids Genetics Consortium; MAGIC, Meta-Analyses of Glucose and Insulin-related traits Consortium; OR, odds ratio; CI, confidence interval.
Replicated MR analysis of lipid traits
As significant heterogeneity and horizontal pleiotropy were detected in the primary lipid-associated MR analysis, replicated MR analyses were performed after excluding all outlier SNPs (see Tables S4 and S5). In the replicated MR analysis, neither the MR-PRESSO global test nor the pleiotropy tests showed evidence of horizontal pleiotropy (Table S6). Moreover, no heterogeneity was observed in the subsequent MR analysis (Table S6).
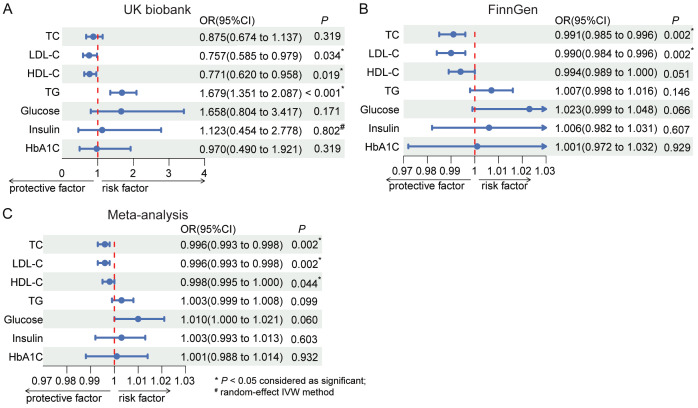
In the repeated MR analysis, genetically predicted cholecystectomy was significantly associated with lower TC levels in the FinnGen data (OR: 0.991, 95% CI: 0.985-0.996, P = 0.002). While not statistically significant, a consistent trend for total cholesterol (TC) was noted in the UK Biobank dataset (OR: 0.875, 95% CI: 0.674-1.137, P = 0.319). Regarding specific cholesterol levels, genetically predicted cholecystectomy was significantly associated with lower LDL-C levels in the FinnGen cohort (OR: 0.990, 95% CI: 0.984-0.996, P = 0.002), and a similar trend was observed in the UK Biobank dataset (OR: 0.757, 95% CI: 0.585-0.979, P = 0.034). However, there was no significant association between genetically predicted cholecystectomy and HDL-C levels in the FinnGen data (OR: 0.994, 95% CI: 0.989-1.000, P = 0.051), and lower HDL-C levels were observed in the UK Biobank database (OR: 0.771, 95% CI: 0.620-0.958, P = 0.019). TG levels showed inconsistency between the UK Biobank (OR: 1.679, 95% CI: 1.351-2.087, P < 0.001) and FinnGen datasets (OR: 1.007, 95% CI: 0.998-1.016, P = 0.146) (Figure 2A and 2B). The results of the IVW, MRE, WMed, WMod, and SMod methods for the lipid traits (repeated MR results) and glucose traits (primary MR results) are shown in Tables S7, S8, S9, S10. Scatter plots, funnel plots and forest plots of these outcomes are presented in Figures S1, S2, S3. Sensitivity analysis using the leave-one-out method demonstrated the robustness of the results (see Figure S4).
Figure 2.
Forest plots of associations between cholecystectomy and blood lipid/glucose traits by replicated MR analyses after outlier removal. A. MR results from UK biobank dataset; B. MR results from FinnGen dataset; C. Meta-analysis combining the MR results of UK biobank and FinnGen. Abbreviations: TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; HbA1c, hemoglobin A1c; IVW method, Inverse Variance Weighted method; OR, odds ratio; CI, confidence interval.
Combined results of lipid and glucose traits by meta-analysis
Based on the repeated MR analyses in both the UK Biobank and FinnGen cohorts, we conducted a meta-analysis to combine the two sets of results and derive a more generalized conclusion. Specific model selection of the meta-analysis was based on the I2 statistic and Q statistics generated by the heterogeneity test (Table S11). The meta-analysis of the UK Biobank and FinnGen data (see Figure 2C) demonstrated a significant reduction in TC levels associated with cholecystectomy (OR: 0.996, 95% CI: 0.993-0.998, P = 0.002). Similarly, the meta-analysis revealed significant decreases in LDL-C levels (OR: 0.996, 95% CI: 0.993-0.998, P = 0.002) and HDL-C levels (OR: 0.998, 95% CI: 0.995-1.000, P = 0.044) levels associated with cholecystectomy. However, there were no significant associations found between genetically predicted cholecystectomy and TG levels (P = 0.099), fasting glucose (P = 0.060), fasting insulin (P = 0.603), and HbA1c (P = 0.932).
Discussion
In this study, we first employed population-based cross-sectional data from NHANES to infer associations between cholecystectomy and serum lipid/glucose levels. Then, we conducted PSM analyses to avoid baseline differences. Moreover, we performed MR analyses on multiple large-sample cohorts to investigate the associations between cholecystectomy and blood lipid/glucose profiles. Our findings revealed the causal relationship between cholecystectomy and reduced TC levels, specifically LDL-C and HDL-C, with no notable changes in TG and glucose metabolic indices.
Previous studies have reported varied findings regarding the impact of cholecystectomy on blood lipid profiles. Malik et al. found significant reductions in serum TC and LDL-C among patients who underwent cholecystectomy [24], in agreement with our results. Similarly, Walmsley et al. reported a maximum reduction of 30-36% in serum cholesterol levels [25]. Conversely, although TC and LDL-C levels declined on the 3rd day after gallbladder removal, Juvonen et al. reported that these values quickly returned to preoperative levels [26]. Gill et al. described increased HDL-C concentrations and stable LDL-C concentrations after cholecystectomy [27]. Nervi revealed that serum levels of TC, LDL-C, HDL-C, and TG remained unchanged. However, an accumulation of apolipoprotein B (apoB) lipoprotein, which is the main component of LDL, was observed in cholecystectomized patients compared with controls [28]. Discrepancies among these findings may be attributed to factors such as preoperative lipid status and dietary habits [29,30]. Additionally, predisposed disorders in lipid metabolism leading to gallbladder disease and dietary restrictions, particularly fat intake post-operation, may contribute to the diverse findings. Advantageously, our study effectively utilized GWAS data from large cohorts and applied MR analysis, providing fresh perspectives on the metabolic effects of cholecystectomy. By judiciously excluding lipid/glucose-related SNPs during instrumental variable selection, we ensured a robust reduction of potential biases, thereby enhancing our understanding of the metabolic implications of cholecystectomy.
As antecedently reported [11,25], cholecystectomy alters the storage and reabsorption of bile acids (BAs). These acids dissolve triglycerides, thereby ameliorating the predisposition to cholesterol accumulation. Additionally, this process affects metabolites such as bile salts, which play a crucial role in lowering serum cholesterol levels. Cholecystectomy exonerates the concentrated effect of the gallbladder on BAs and promotes BA entering the intestine rapidly, thereby impairing BA homeostasis. BA, as hormonal signaling molecules, can interact with nuclear farnesoid X receptor (FXR) through activating small heterodimer partner (SHP) and peroxisome proliferator-activated receptor α (PPARα) to decrease lipogenesis and to increase lipolysis. Also, the BA-FXR axis is associated with reduced accumulation of intrahepatic cholesterol [31], whose main effect is transporting intrinsic cholesterol. Meanwhile, BA-FXR axis activates fibroblast growth factor 19 (FGF19) and the downstream receptor FGFR4 inhibits lipogenesis by suppressing synthesis of fatty acids and sterol and activities of lipogenic enzymes [31,32]. Moreover, cholecystectomy also disturbs gut microbiota homeostasis, which in turn affects generation and reabsorption of secondary BA, exerting a regulatory impact on lipid metabolism [33,34]. These results could elucidate our findings from a mechanistic perspective.
No significant differences were observed in fasting blood glucose, fasting insulin levels, or HbA1c levels between individuals who underwent cholecystectomy and those who did not in either the cross-sectional study or the MR analysis. Similar to our findings, Park et al. reported no significant changes in serum fasting serum glucose concentrations in patients who underwent cholecystectomy compared with those who did not undergo gallbladder resection [10]. However, in a pilot study assessing Hispanic patients, serum insulin levels increased from 8.1 ± 0.7 to 10.0 ± 1.9 μU/ml 24 months after cholecystectomy in non-obese patients [28]. Moreover, cholecystectomized patients exhibited elevated serum fasting glucagon and postprandial glucose levels compared with controls [35]. The risk of higher blood glucose increased by 1.21-fold in individuals who underwent cholecystectomy compared with those who did not [7]. Nevertheless, our study did not find comparable effects on insulin sensitivity after cholecystectomy. The findings of previous studies may be influenced by dietary control after the operation, resulting in improved blood glucose management, and surgical stress, which could lead to insulin resistance following cholecystectomy [36-38]. Importantly, our study mitigated these biases by excluding glucose- and insulin-related SNPs during instrumental variable selection, potentially explaining the biases of previous studies. Our findings enrich the existing literature by offering insights into the glucose metabolic consequences of cholecystectomy and underscore the necessity for additional studies to clarify the underlying mechanisms.
The present study possesses several advantages, including the utilization of MR analysis and large-scale GWAS data. MR analysis allows us to infer causal relationships by leveraging genetic variants as instrumental variables, mimicking a randomized controlled study. This methodological approach bolsters the robustness and validity of our findings minimizing the bias from the observational cross-sectional study. Despite these strengths, we should acknowledge several limitations. First, our study predominantly focused on populations of European ancestry, thereby limiting the generalizability of our findings to other ethnic groups. In particular, Asian populations may present different etiologies of cholelithiasis, as these populations have less predominant lipid metabolism disorder compared with Western populations. Second, the retrospective nature of the GWAS data introduced selection bias, as individuals who undergo cholecystectomy may systematically differ from those who do not.
In conclusion, our study elucidated the association between cholecystectomy and blood lipid and glucose profiles. The cross-sectional study with PSM showed significant associations between cholecystectomy and decreased TC, LDL-C, and HDL-C levels and increased TG levels. The meta-analysis combining the MR results of the UK Biobank and FinnGen cohorts unveiled the causal relationship between cholecystectomy and lower TC levels, especially lower LDL-C levels. No causal impacts of cholecystectomy on HDL-C and TG levels and glucose metabolic indices were observed. Therefore, blood cholesterol levels in patients who have undergone cholecystectomy should be monitored diligently; thus, the indications for cholecystectomy in individuals with high cholesterol levels might be expanded.
Acknowledgements
We thank the generous investigators who provided summary data to build the National Health and Nutrition Examination Survey (NHANES) 2017-2018 database and the Global Lipids Genetics Consortium (GLGC), the Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC), UK Biobank and FinnGen datasets. This work was supported by grants from the Clinical Research Project of Shanghai Municipal Health Commission (20224Y0148).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Di Martino M, Ielpo B, Pata F, Pellino G, Di Saverio S, Catena F, De Simone B, Coccolini F, Sartelli M, Damaskos D, Mole D, Murzi V, Leppaniemi A, Pisanu A, Podda M MANCTRA-1 Collaborative Group. Timing of cholecystectomy after moderate and severe acute biliary pancreatitis. JAMA Surg. 2023;158:e233660. doi: 10.1001/jamasurg.2023.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed I, Hudson J, Innes K, Hernandez R, Gillies K, Bruce R, Bell V, Avenell A, Blazeby J, Brazzelli M, Cotton S, Croal B, Forrest M, MacLennan G, Murchie P, Wileman S, Ramsay C C-GALL Study Group. Effectiveness of conservative management versus laparoscopic cholecystectomy in the prevention of recurrent symptoms and complications in adults with uncomplicated symptomatic gallstone disease (C-GALL trial): pragmatic, multicentre randomised controlled trial. BMJ. 2023;383:e075383. doi: 10.1136/bmj-2023-075383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammert F, Gurusamy K, Ko CW, Miquel JF, Mendez-Sanchez N, Portincasa P, van Erpecum KJ, van Laarhoven CJ, Wang DQ. Gallstones. Nat Rev Dis Primers. 2016;2:16024. doi: 10.1038/nrdp.2016.24. [DOI] [PubMed] [Google Scholar]
- 4.Kalata S, Thumma JR, Norton EC, Dimick JB, Sheetz KH. Comparative safety of robotic-assisted vs laparoscopic cholecystectomy. JAMA Surg. 2023;158:1303–1310. doi: 10.1001/jamasurg.2023.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kharazmi E, Scherer D, Boekstegers F, Liang Q, Sundquist K, Sundquist J, Fallah M, Lorenzo Bermejo J. Gallstones, cholecystectomy, and kidney cancer: observational and mendelian randomization results based on large cohorts. Gastroenterology. 2023;165:218–227. e218. doi: 10.1053/j.gastro.2023.03.227. [DOI] [PubMed] [Google Scholar]
- 6.Xu F, Chen R, Zhang C, Wang H, Ding Z, Yu L, Tian F, Chen W, Zhou Y, Zhai Q. Cholecystectomy significantly alters gut microbiota homeostasis and metabolic profiles: a cross-sectional study. Nutrients. 2023;15:4399. doi: 10.3390/nu15204399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huh JH, Lee KJ, Cho YK, Moon S, Kim YJ, Han KD, Kang JG, Lee SJ, Ihm SH. Cholecystectomy increases the risk of metabolic syndrome in the Korean population: a longitudinal cohort study. Hepatobiliary Surg Nutr. 2023;12:523–533. doi: 10.21037/hbsn-22-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shabanzadeh DM, Skaaby T, Sorensen LT, Jorgensen T. Screen-detected gallstone disease and cardiovascular disease. Eur J Epidemiol. 2017;32:501–510. doi: 10.1007/s10654-017-0263-x. [DOI] [PubMed] [Google Scholar]
- 9.Park SM, Kim HJ, Kang TU, Swan H, Ahn HS. Cholecystectomy reduces the risk of myocardial and cerebral infarction in patients with gallstone-related infection. Sci Rep. 2022;12:16749. doi: 10.1038/s41598-022-20700-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S, Jeong S, Park SJ, Song J, Kim SM, Chang J, Choi S, Cho Y, Oh YH, Kim JS, Park YJ, Son JS, Ahn JC, Park SM. Associations of cholecystectomy with metabolic health changes and incident cardiovascular disease: a retrospective cohort study. Sci Rep. 2024;14:3195. doi: 10.1038/s41598-024-53161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu F, Yu Z, Liu Y, Du T, Yu L, Tian F, Chen W, Zhai Q. A high-fat, high-cholesterol diet promotes intestinal inflammation by exacerbating gut microbiome dysbiosis and bile acid disorders in cholecystectomy. Nutrients. 2023;15:3829. doi: 10.3390/nu15173829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Went M, Sud A, Mills C, Hyde A, Culliford R, Law P, Vijayakrishnan J, Gockel I, Maj C, Schumacher J, Palles C, Kaiser M, Houlston R. Phenome-wide Mendelian randomisation analysis of 378,142 cases reveals risk factors for eight common cancers. Nat Commun. 2024;15:2637. doi: 10.1038/s41467-024-46927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson MJ, Lawlor DA, Millard LAC. Identifying the potential causal role of insomnia symptoms on 11,409 health-related outcomes: a phenome-wide Mendelian randomisation analysis in UK Biobank. BMC Med. 2023;21:128. doi: 10.1186/s12916-023-02832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julian TH, Cooper-Knock J, MacGregor S, Guo H, Aslam T, Sanderson E, Black GCM, Sergouniotis PI. Phenome-wide Mendelian randomisation analysis identifies causal factors for age-related macular degeneration. Elife. 2023;12:e82546. doi: 10.7554/eLife.82546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue Z, Cheng Y, Yu Q, Wang T, Fan C, Zou Z, Zhang X. Assessment of causal factors for Parkinson’s disease in European populations: a phenome-wide Mendelian randomisation analysis. Asia Pac J Clin Nutr. 2021;30:329–339. doi: 10.6133/apjcn.202106_30(2).0018. [DOI] [PubMed] [Google Scholar]
- 16.Graham SE, Clarke SL, Wu KH, Kanoni S, Zajac GJM, Ramdas S, Surakka I, Ntalla I, Vedantam S, Winkler TW, Locke AE, Marouli E, Hwang MY, Han S, Narita A, Choudhury A, Bentley AR, Ekoru K, Verma A, Trivedi B, Martin HC, Hunt KA, Hui Q, Klarin D, Zhu X, Thorleifsson G, Helgadottir A, Gudbjartsson DF, Holm H, Olafsson I, Akiyama M, Sakaue S, Terao C, Kanai M, Zhou W, Brumpton BM, Rasheed H, Ruotsalainen SE, Havulinna AS, Veturi Y, Feng Q, Rosenthal EA, Lingren T, Pacheco JA, Pendergrass SA, Haessler J, Giulianini F, Bradford Y, Miller JE, Campbell A, Lin K, Millwood IY, Hindy G, Rasheed A, Faul JD, Zhao W, Weir DR, Turman C, Huang H, Graff M, Mahajan A, Brown MR, Zhang W, Yu K, Schmidt EM, Pandit A, Gustafsson S, Yin X, Luan J, Zhao JH, Matsuda F, Jang HM, Yoon K, Medina-Gomez C, Pitsillides A, Hottenga JJ, Willemsen G, Wood AR, Ji Y, Gao Z, Haworth S, Mitchell RE, Chai JF, Aadahl M, Yao J, Manichaikul A, Warren HR, Ramirez J, Bork-Jensen J, Karhus LL, Goel A, Sabater-Lleal M, Noordam R, Sidore C, Fiorillo E, McDaid AF, Marques-Vidal P, Wielscher M, Trompet S, Sattar N, Mollehave LT, Thuesen BH, Munz M, Zeng L, Huang J, Yang B, Poveda A, Kurbasic A, Lamina C, Forer L, Scholz M, Galesloot TE, Bradfield JP, Daw EW, Zmuda JM, Mitchell JS, Fuchsberger C, Christensen H, Brody JA, Feitosa MF, Wojczynski MK, Preuss M, Mangino M, Christofidou P, Verweij N, Benjamins JW, Engmann J, Kember RL, Slieker RC, Lo KS, Zilhao NR, Le P, Kleber ME, Delgado GE, Huo S, Ikeda DD, Iha H, Yang J, Liu J, Leonard HL, Marten J, Schmidt B, Arendt M, Smyth LJ, Canadas-Garre M, Wang C, Nakatochi M, Wong A, Hutri-Kahonen N, Sim X, Xia R, Huerta-Chagoya A, Fernandez-Lopez JC, Lyssenko V, Ahmed M, Jackson AU, Yousri NA, Irvin MR, Oldmeadow C, Kim HN, Ryu S, Timmers P, Arbeeva L, Dorajoo R, Lange LA, Chai X, Prasad G, Lores-Motta L, Pauper M, Long J, Li X, Theusch E, Takeuchi F, Spracklen CN, Loukola A, Bollepalli S, Warner SC, Wang YX, Wei WB, Nutile T, Ruggiero D, Sung YJ, Hung YJ, Chen S, Liu F, Yang J, Kentistou KA, Gorski M, Brumat M, Meidtner K, Bielak LF, Smith JA, Hebbar P, Farmaki AE, Hofer E, Lin M, Xue C, Zhang J, Concas MP, Vaccargiu S, van der Most PJ, Pitkanen N, Cade BE, Lee J, van der Laan SW, Chitrala KN, Weiss S, Zimmermann ME, Lee JY, Choi HS, Nethander M, Freitag-Wolf S, Southam L, Rayner NW, Wang CA, Lin SY, Wang JS, Couture C, Lyytikainen LP, Nikus K, Cuellar-Partida G, Vestergaard H, Hildalgo B, Giannakopoulou O, Cai Q, Obura MO, van Setten J, Li X, Schwander K, Terzikhan N, Shin JH, Jackson RD, Reiner AP, Martin LW, Chen Z, Li L, Highland HM, Young KL, Kawaguchi T, Thiery J, Bis JC, Nadkarni GN, Launer LJ, Li H, Nalls MA, Raitakari OT, Ichihara S, Wild SH, Nelson CP, Campbell H, Jager S, Nabika T, Al-Mulla F, Niinikoski H, Braund PS, Kolcic I, Kovacs P, Giardoglou T, Katsuya T, Bhatti KF, de Kleijn D, de Borst GJ, Kim EK, Adams HHH, Ikram MA, Zhu X, Asselbergs FW, Kraaijeveld AO, Beulens JWJ, Shu XO, Rallidis LS, Pedersen O, Hansen T, Mitchell P, Hewitt AW, Kahonen M, Perusse L, Bouchard C, Tonjes A, Chen YI, Pennell CE, Mori TA, Lieb W, Franke A, Ohlsson C, Mellstrom D, Cho YS, Lee H, Yuan JM, Koh WP, Rhee SY, Woo JT, Heid IM, Stark KJ, Volzke H, Homuth G, Evans MK, Zonderman AB, Polasek O, Pasterkamp G, Hoefer IE, Redline S, Pahkala K, Oldehinkel AJ, Snieder H, Biino G, Schmidt R, Schmidt H, Chen YE, Bandinelli S, Dedoussis G, Thanaraj TA, Kardia SLR, Kato N, Schulze MB, Girotto G, Jung B, Boger CA, Joshi PK, Bennett DA, De Jager PL, Lu X, Mamakou V, Brown M, Caulfield MJ, Munroe PB, Guo X, Ciullo M, Jonas JB, Samani NJ, Kaprio J, Pajukanta P, Adair LS, Bechayda SA, de Silva HJ, Wickremasinghe AR, Krauss RM, Wu JY, Zheng W, den Hollander AI, Bharadwaj D, Correa A, Wilson JG, Lind L, Heng CK, Nelson AE, Golightly YM, Wilson JF, Penninx B, Kim HL, Attia J, Scott RJ, Rao DC, Arnett DK, Hunt SC, Walker M, Koistinen HA, Chandak GR, Yajnik CS, Mercader JM, Tusie-Luna T, Aguilar-Salinas CA, Villalpando CG, Orozco L, Fornage M, Tai ES, van Dam RM, Lehtimaki T, Chaturvedi N, Yokota M, Liu J, Reilly DF, McKnight AJ, Kee F, Jockel KH, McCarthy MI, Palmer CNA, Vitart V, Hayward C, Simonsick E, van Duijn CM, Lu F, Qu J, Hishigaki H, Lin X, Marz W, Parra EJ, Cruz M, Gudnason V, Tardif JC, Lettre G, t Hart LM, Elders PJM, Damrauer SM, Kumari M, Kivimaki M, van der Harst P, Spector TD, Loos RJF, Province MA, Psaty BM, Brandslund I, Pramstaller PP, Christensen K, Ripatti S, Widen E, Hakonarson H, Grant SFA, Kiemeney L, de Graaf J, Loeffler M, Kronenberg F, Gu D, Erdmann J, Schunkert H, Franks PW, Linneberg A, Jukema JW, Khera AV, Mannikko M, Jarvelin MR, Kutalik Z, Cucca F, Mook-Kanamori DO, van Dijk KW, Watkins H, Strachan DP, Grarup N, Sever P, Poulter N, Rotter JI, Dantoft TM, Karpe F, Neville MJ, Timpson NJ, Cheng CY, Wong TY, Khor CC, Sabanayagam C, Peters A, Gieger C, Hattersley AT, Pedersen NL, Magnusson PKE, Boomsma DI, de Geus EJC, Cupples LA, van Meurs JBJ, Ghanbari M, Gordon-Larsen P, Huang W, Kim YJ, Tabara Y, Wareham NJ, Langenberg C, Zeggini E, Kuusisto J, Laakso M, Ingelsson E, Abecasis G, Chambers JC, Kooner JS, de Vries PS, Morrison AC, North KE, Daviglus M, Kraft P, Martin NG, Whitfield JB, Abbas S, Saleheen D, Walters RG, Holmes MV, Black C, Smith BH, Justice AE, Baras A, Buring JE, Ridker PM, Chasman DI, Kooperberg C, Wei WQ, Jarvik GP, Namjou B, Hayes MG, Ritchie MD, Jousilahti P, Salomaa V, Hveem K, Asvold BO, Kubo M, Kamatani Y, Okada Y, Murakami Y, Thorsteinsdottir U, Stefansson K, Ho YL, Lynch JA, Rader DJ, Tsao PS, Chang KM, Cho K, O’Donnell CJ, Gaziano JM, Wilson P, Rotimi CN, Hazelhurst S, Ramsay M, Trembath RC, van Heel DA, Tamiya G, Yamamoto M, Kim BJ, Mohlke KL, Frayling TM, Hirschhorn JN, Kathiresan S VA Million Veteran Program; Global Lipids Genetics Consortium. Boehnke M, Natarajan P, Peloso GM, Brown CD, Morris AP, Assimes TL, Deloukas P, Sun YV, Willer CJ. The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600:675–679. doi: 10.1038/s41586-021-04064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators. Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, Bouatia-Naji N, Langenberg C, Prokopenko I, Stolerman E, Sandhu MS, Heeney MM, Devaney JM, Reilly MP, Ricketts SL, Stewart AF, Voight BF, Willenborg C, Wright B, Altshuler D, Arking D, Balkau B, Barnes D, Boerwinkle E, Bohm B, Bonnefond A, Bonnycastle LL, Boomsma DI, Bornstein SR, Bottcher Y, Bumpstead S, Burnett-Miller MS, Campbell H, Cao A, Chambers J, Clark R, Collins FS, Coresh J, de Geus EJ, Dei M, Deloukas P, Doring A, Egan JM, Elosua R, Ferrucci L, Forouhi N, Fox CS, Franklin C, Franzosi MG, Gallina S, Goel A, Graessler J, Grallert H, Greinacher A, Hadley D, Hall A, Hamsten A, Hayward C, Heath S, Herder C, Homuth G, Hottenga JJ, Hunter-Merrill R, Illig T, Jackson AU, Jula A, Kleber M, Knouff CW, Kong A, Kooner J, Kottgen A, Kovacs P, Krohn K, Kuhnel B, Kuusisto J, Laakso M, Lathrop M, Lecoeur C, Li M, Li M, Loos RJ, Luan J, Lyssenko V, Magi R, Magnusson PK, Malarstig A, Mangino M, Martinez-Larrad MT, Marz W, McArdle WL, McPherson R, Meisinger C, Meitinger T, Melander O, Mohlke KL, Mooser VE, Morken MA, Narisu N, Nathan DM, Nauck M, O’Donnell C, Oexle K, Olla N, Pankow JS, Payne F, Peden JF, Pedersen NL, Peltonen L, Perola M, Polasek O, Porcu E, Rader DJ, Rathmann W, Ripatti S, Rocheleau G, Roden M, Rudan I, Salomaa V, Saxena R, Schlessinger D, Schunkert H, Schwarz P, Seedorf U, Selvin E, Serrano-Rios M, Shrader P, Silveira A, Siscovick D, Song K, Spector TD, Stefansson K, Steinthorsdottir V, Strachan DP, Strawbridge R, Stumvoll M, Surakka I, Swift AJ, Tanaka T, Teumer A, Thorleifsson G, Thorsteinsdottir U, Tonjes A, Usala G, Vitart V, Volzke H, Wallaschofski H, Waterworth DM, Watkins H, Wichmann HE, Wild SH, Willemsen G, Williams GH, Wilson JF, Winkelmann J, Wright AF Wtccc. Zabena C, Zhao JH, Epstein SE, Erdmann J, Hakonarson HH, Kathiresan S, Khaw KT, Roberts R, Samani NJ, Fleming MD, Sladek R, Abecasis G, Boehnke M, Froguel P, Groop L, McCarthy MI, Kao WH, Florez JC, Uda M, Wareham NJ, Barroso I, Meigs JB. Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–3239. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgess S, Thompson SG CRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–764. doi: 10.1093/ije/dyr036. [DOI] [PubMed] [Google Scholar]
- 20.Bowden J, Spiller W, Del Greco M F, Sheehan N, Thompson J, Minelli C, Davey Smith G. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol. 2018;47:1264–1278. doi: 10.1093/ije/dyy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. 2017;26:2333–2355. doi: 10.1177/0962280215597579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik AA, Wani ML, Tak SI, Irshad I, Ul-Hassan N. Association of dyslipidaemia with cholilithiasis and effect of cholecystectomy on the same. Int J Surg. 2011;9:641–642. doi: 10.1016/j.ijsu.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Walmsley MJ, Waddecar J, Schofield PF. Serial serum cholesterol estimations after biliary-tract surgery. Br J Surg. 1970;57:829–831. doi: 10.1002/bjs.1800571109. [DOI] [PubMed] [Google Scholar]
- 26.Juvonen T, Kervinen K, Kairaluoma MI, Kesaniemi YA. Effect of cholecystectomy on plasma lipid and lipoprotein levels. Hepatogastroenterology. 1995;42:377–382. [PubMed] [Google Scholar]
- 27.Gill GS, Gupta K. Pre- and post-operative comparative analysis of serum lipid profile in patients with cholelithiasis. Int J Appl Basic Med Res. 2017;7:186–188. doi: 10.4103/2229-516X.212968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortes V, Quezada N, Uribe S, Arrese M, Nervi F. Effect of cholecystectomy on hepatic fat accumulation and insulin resistance in non-obese Hispanic patients: a pilot study. Lipids Health Dis. 2017;16:129. doi: 10.1186/s12944-017-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yazdankhah Kenary A, Yaghoobi Notash A Jr, Nazari M, Yaghoobi Notash A, Borjian A, Afshin N, Khashayar P, Ahmadi Amoli H, Morteza A. Measuring the rate of weight gain and the influential role of diet in patients undergoing elective laparoscopic cholecystectomy: a 6-month follow-up study. Int J Food Sci Nutr. 2012;63:645–648. doi: 10.3109/09637486.2011.644767. [DOI] [PubMed] [Google Scholar]
- 30.Ali RB, Cahill RA, Watson RG. Weight gain after laparoscopic cholecystectomy. Ir J Med Sci. 2004;173:9–12. doi: 10.1007/BF02914515. [DOI] [PubMed] [Google Scholar]
- 31.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–2603. doi: 10.1210/en.2003-1671. [DOI] [PubMed] [Google Scholar]
- 32.Bhatnagar S, Damron HA, Hillgartner FB. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Xu J, Ren X, Zhang Y, Ke Z, Zhou J, Wang Y, Zhang Y, Liu Y. Cholecystectomy-induced secondary bile acids accumulation ameliorates colitis through inhibiting monocyte/macrophage recruitment. Gut Microbes. 2022;14:2107387. doi: 10.1080/19490976.2022.2107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Zhang T, Yue Y, Wu X. Effects of bile acid modulation by dietary fat, cholecystectomy, and bile acid sequestrant on energy, glucose, and lipid metabolism and gut microbiota in mice. Int J Mol Sci. 2022;23:5935. doi: 10.3390/ijms23115935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonne DP, Hare KJ, Martens P, Rehfeld JF, Holst JJ, Vilsboll T, Knop FK. Postprandial gut hormone responses and glucose metabolism in cholecystectomized patients. Am J Physiol Gastrointest Liver Physiol. 2013;304:G413–419. doi: 10.1152/ajpgi.00435.2012. [DOI] [PubMed] [Google Scholar]
- 36.Blixt C, Ahlstedt C, Ljungqvist O, Isaksson B, Kalman S, Rooyackers O. The effect of perioperative glucose control on postoperative insulin resistance. Clin Nutr. 2012;31:676–681. doi: 10.1016/j.clnu.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Ljungqvist O. Insulin resistance and outcomes in surgery. J Clin Endocrinol Metab. 2010;95:4217–4219. doi: 10.1210/jc.2010-1525. [DOI] [PubMed] [Google Scholar]
- 38.Yadav K, Prakash R, Singh GP, Gautam S, Arshad Z, Singh BP. Effect of carbohydrate loading in diabetic patients undergoing laparoscopic cholecystectomy: a randomized controlled trial. Cureus. 2023;15:e44570. doi: 10.7759/cureus.44570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.