Abstract
Background: The mechanism of ferroptosis is primarily driven by the iron-dependent lethal accumulation of membrane lipid peroxidation. Bavachin has been found to exacerbate lipid peroxidation in cancer cells; however, whether it hinders hepatocellular carcinoma (HCC) progression by way of ferroptosis remains unknown. Methods: Cell counting kit-8 (CCK-8) assay was used to measure the effect of Bavachin on the viability of HCC cells, so as to determine the appropriate drug concentration for subsequent experiments. Combining molecular biology experimental techniques such as CCK-8, flow cytometry, western blotting, wound-healing assay, DCFH-DA (2’,7’-dichlorodihydrofluorescein diacetate) fluorescent probe and a variety of biological kits, the effects of Bavachin on HCC cell malignant phenotype progression and ferroptosis were investigated. Results: Bavachin significantly induced cytotoxicity of Huh-7 and HepG2 cells at concentrations of 20 and 40 μM, respectively. Bavachin intervention prominently reduced cell proliferation and migration, and enhanced cell apoptosis in HCC. Also, Bavachin enhanced the process of lipid peroxidation, as indicated by increased reactive oxygen species (ROS), lipid peroxidation (LPO) and malondialdehyde (MDA) production, and decreased superoxide dismutase (SOD) and glutathione (GSH) production. Ferrostin-1, a ferroptosis inhibitor, reduced the Bavachin-induced cell survival rate. Bavachin intervention induced ferroptosis by enhancing iron ion concentration, acyl-CoA synthetase long-chain family member 4 (ACSL4) expression, and reducing glutathione peroxidase-4 (GPX4) expression. Bavachin exerted anti-cancer effects though inducing ferroptosis by activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/Heme oxygenase-1 (HO-1) pathway. Conclusion: Bavachin acted as a ferroptosis inducer, promoted ROS release, enhanced lipid peroxidation, and inhibited HCC cell malignant phenotype progression by modulating the Nrf2/HO-1 pathway.
Keywords: Bavachin, lipid peroxidation, hepatocellular carcinoma, ferroptosis
Introduction
Primary liver malignant tumors originate from epithelial or mesenchymal tissues of the liver, among which hepatocellular carcinoma (HCC) is the main form of primary liver cancer [1]. HCC has a complex etiology, insidious pathogenesis, aggressiveness, and strong ability for tumor cell proliferation, invasion, and metastasis, which often leads to incomplete surgical resection and poor selection of postoperative radiotherapy, which worsens outcome [2-4]. Therefore, it is urgent to explore the pathogenesis and progression of HCC and to find drugs that can safely and effectively treat HCC.
Iron is a critical element in numerous biologic processes, but it can also be toxic when present in excess. In ferroptosis, iron catalyzes the formation of lipid reactive oxygen species (L-ROS), which is particularly damaging to the cell membrane due to their ability to peroxidize lipids [5]. The mechanism underlying ferroptosis involves the disruption of the balance between the production and clearance of reactive oxygen species (ROS), with a particular focus on the lipid peroxidation pathway. One of the key players in ferroptosis is the enzyme glutathione peroxidase 4 (GPX4), which is responsible for reducing lipid hydroperoxides and thus preventing their accumulation [6]. When GPX4 activity is compromised, lipid peroxidation can spiral out of control, leading to ferroptosis. ACSL4, or long-chain Acyl-CoA Synthetase 4, is a key regulator in ferroptosis, which is defined as a cell death process caused by iron-dependent peroxidation of lipids [7]. It is involved in the activation of long-chain fatty acids by converting them into their corresponding CoA esters, a crucial step in lipid metabolism. The overexpression of ACSL4 has been linked to sensitivity to ferroptosis, as it contributes to the biosynthesis of arachidonoyl-CoA, which in turn triggers phospholipid peroxidation, an event central to the execution of ferroptosis.
Bavachin is a naturally occurring flavonoid, derived from the plant Psoralea corylifolia. It is also known as 7-hydroxy-2-(4-hydroxyphenyl)-6-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one, with molecular formula C20H20O4 and molecular weight 324.14 (Figure 1A). The compound’s multifaceted pharmacological profile includes antioxidant activity, anti-inflammatory effects, and immunomodulatory properties, which position it as a candidate for treating a spectrum of conditions. Studies have highlighted its ability to modulate immune response, suggesting a role in autoimmune diseases and inflammatory disorders [8,9]. Its antioxidant capabilities are also being explored for their potential in mitigating oxidative stress-related diseases, such as complications of diabetes, neurodegenerative disorders, and cardiovascular diseases [10-12]. A study indicated that it might reduce the risk of non-alcoholic fatty liver disease by inhibiting de novo lipogenesis and reducing hepatic inflammation [13]. Interestingly, it has been reported that Bavachin can inhibit the proliferation of cancer cells and induce apoptosis, and its anti-tumor effects may be mediated by the same or different mechanisms [14-16]. However, whether Bavachin has a functional role in regulating ferroptosis in HCC cells has not been explored in depth.
Figure 1.
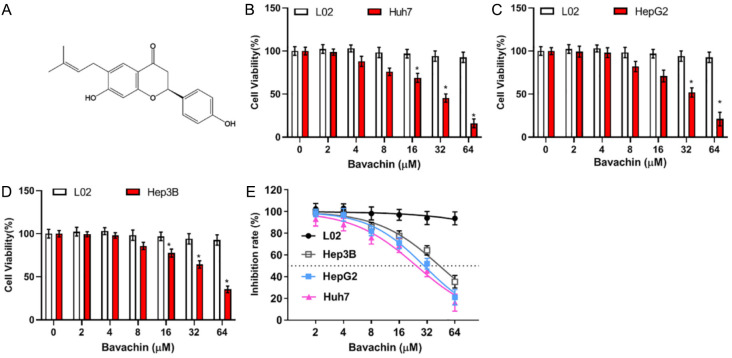
Inhibiting effect of Bavachin on hepatocellular carcinoma (HCC) cell vitality. A: Chemical structure of Bavachin. B-D: Cell counting kit-8 (CCK-8) results of Huh7, HepG2, Hep3B, and L02 cells incubated with various concentrations of Bavachin (0, 2, 4, 8, 16, 32, 64 μM) for 24 h. E: Effect of Bavachin on IC50 of Huh7, HepG2, Hep3B, and L02 cells. Compared to the L02 group, *P < 0.05.
The nuclear factor erythroid 2-related factor 2 (Nrf2) signaling cascade operates as a primary responder to ROS, governing the transcription of genes controlled by the antioxidant response element (ARE) [17]. This regulation is essential for counteracting oxidative stress and preserving the cell’s redox equilibrium. Upon activation, the Nrf2/Kelch-like ECH-associated protein 1 (Keap1)/ARE pathway boosts the production of enzymes that neutralize ROS, including Heme oxygenase-1 (HO-1) and NAD(P)H Quinone Dehydrogenase 1 (NQO1). This activation enhances the levels of glutathione (GSH) and superoxide dismutase (SOD) while reducing the concentration of malondialdehyde (MDA), thereby mitigating the effects of excessive oxidative stress. Additionally, Nrf2 has been recognized for its role in shielding cancer cells from ferroptosis, a type of cell death triggered by agents such as erastin or RSL3 [18]. This protective function of Nrf2 suggests its value as a target for modulating cancer cell vulnerability to oxidative stress. Nonetheless, to harness the Nrf2/HO-1 axis for therapeutic intervention in cancer treatment, especially in the context of Bavachin’s impact on cancer progression, more research is warranted to elucidate the underlying regulatory mechanisms and its interplay with ferroptosis.
In this study, Bavachin was applied to reduce HCC cell viability, and the effective working concentration was determined. We found that Bavachin exhibited potent anti-cancer effects by inducing lipid peroxidation to enhance ferroptosis sensitivity of HCC cells. This knowledge can contribute to the development of new clinical therapies for HCC treatment.
Materials and methods
Cell culture and treatment
The HCC cells and L02 cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences, and all cell lines were cultured in complete medium (DEME containing 10% fetal bovine serum, 1% penicillin/streptomycin). The above (70-80% density) were cultured using different methods: (1) Bavachin concentration gradients (0, 2, 4, 8, 16, 32 and 64 μM) for 24 h; (2) Bavachin (20 and 40 μM) with or without Z-Val-Ala-Asp(OMe)-Fluoromethylketone (Z-VAD), necrostatin-1 (Nec-1) or ferrostatin-1 (Fer-1) for 12 h, respectively [19]. This study was approved by the Ethics Committee of The First Affiliated Hospital of Naval Medical University.
Cell viabilit
HCC cells were seeded in 96-well plates. Following this, the cells were treated with Bavachin for an additional 24 hours. Thereafter, the cells were rinsed with phosphate buffered saline (PBS) and then incubated with chemical reagents from cell counting kit-8 (CCK-8) (10 μL, Solaibio, Beijing, China) for one hour. The optical density was subsequently measured at a wavelength of 450 nm using a microplate reader (Thermo, USA) to determine the number of viable cells.
Western blot analysis
The total protein of the HCC cells was extracted using radioimmunoprecipitation assay (RIPA) buffer. Protein samples (equal amount) was separated by 8-12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene fluoride (PVDF) membranes, blocked with 5% skim milk, and incubated with primary antibodies overnight at 4°C. The following antibodies were used: ACSL4 (1:50000, ab155282, Abcam), GPX4 (1:1000, ab125066, Abcam), Nrf2 (1:500, ab137550, Abcam), HO-1 (1:1000, ab68477, Abcam), and GAPDH (1:1000, ab9485, Abcam). This was followed by HRP-conjugated secondary antibody incubation, chemiluminescence solution, and an X-ray machine was used for blot detection.
Flow cytometry
Apoptosis Detection Kit combined with Flow Cytometer™ system (BD Bioscience, San Jose, CA, USA) was used to analyze the apoptotic rate. The corresponding Bavachin treatment cells were harvested after 24 hours, followed by the addition of 1× Binding buffer. Then, 100 μL of the cell suspension was taken, and Annexin V-FITC staining solution (5 μL) and propidium iodide (PI, 5 μL) were added. After a thorough mix, the solution was incubated at room temperature in dark. Each tube was added with 1× Binding buffer (400 μL), filtered, and analyzed.
Detection of iron content
The cellular iron levels were determined using the ab83366 Iron Assay Kit from Abcam (Cambridge, UK). After treatment with Bavachin for 24 hours, the cells were lysed, and the lysates were collected. Then, a 50 μL aliquot of each sample was transferred into 96-well plates, and the volume was adjusted to 100 μL using assay buffer. To detect ferrous iron, 5 μL of assay buffer was added, while for total iron, 5 μL of reducer was used. After the addition of 100 μL of iron probe, the samples were incubated for 60-minute, and absorbance was then measured at 593 nm.
Determination of the levels of oxidative stress
HCC cells were seeded in 6-well plates and cultured with Bavachin for 24 hours. Subsequently, cells were harvested and washed twice with PBS. The 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA,10 μM) was purchased from Beyotime (Shanghai, China) to measure the ROS level in cells under fluorescence microscope (Olympus, Tokyo, Japan). Besides, after treatment with Bavachin for 24 hours, the cells were lysed, and the lysates were collected. Then, the concentration of total SOD, GSH, LPO, and MDA in cells was determined by an SOD assay kit, GSH assay kit, LPO assay kit, and MDA assay kits (Beyotime, Shanghai, China), respectively.
RNA stability and reverse transcription quantitative polymerase chain reaction (RT-qPCR)
HCC cells (1×103) were treated with 1 mL of TRIzol reagent (TakaRa, Japan). Using the mRNA reverse transcription kit (Thermo Fisher, Waltham, MA, USA), mRNA was subsequently reverse transcribed into cDNA. qPCR was performed on Step One Plus real-time PCR systems (Applied Biosystems, Shanghai, China), in which β-actin was used as a reference gene. The 2-∆∆Ct method was used to calculate the relative transcription level of the target gene.
Wound-healing assay
The cell suspension, once gathered, was plated in a 6-well culture dish and allowed to grow to nearly 90% confluence. A sterile 200 μL pipette tip was used to etch a line through the cell layer, marking the area of interest. The dishes underwent three PBS rinses and were then placed in a serum-free environment for culture. At 0 and 24 hours post-incubation, images were captured using a light microscope. The distance between the edges of the etched line at varying time intervals was assessed with Image J software (National Institutes of Health, Bethesda, MA, USA) to determine the rate of cellular migration.
Statistical analysis
Statistical processing was performed using GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA, USA). Data were expressed as mean ± standard deviation (SD), and differences between two groups were identified using the t-test. A p-value of less than 0.05 was set as the threshold for significance.
Results
Bavachin reduced cell viability of HCC
To investigate the inhibitory effect of Bavachin on HCC cells, three common HCC cell lines Huh7, HepG2, Hep3B and normal liver cell line L02 were selected and different concentration gradients of Bavachin (0, 2, 4, 8, 16, 32, 64 μM) were set for further study. CCK-8 results showed that compared with L02 cells, the cell viability of HepG2, Huh7, and Hep3B was decreased with an increasing concentration of Bavachin, and the inhibitory effect was significant when the concentration was greater than 40 μM (Figure 1B-D). In addition, based on the half-maximal inhibitory concentration (IC50) of Bavachin at 24 h, we found that Huh7 (IC50: 24.54 μM) and HepG2 (IC50: 29.75 μM) were more sensitive to Bavachin than Hep3B cells (IC50: 43.72 μM) (Figure 1E). Therefore, we finally selected Huh7 and HepG2 cells for subsequent experiments.
Bavachin inhibited HCC cell proliferation and migration and promoted apoptosis
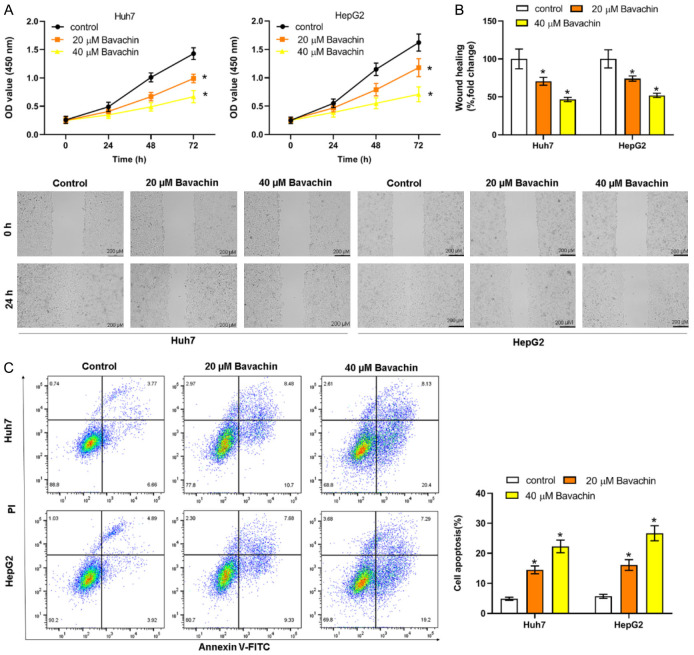
Huh7 and HepG2 cells were then treated with two specific concentrations of Bavachin (20 and 40 μM) to determine the biological function of Bavachin. The incubation of Bavachin with Huh7 and HepG2 cells resulted in a significant inhibition of cell proliferation in comparison with control (Figure 2A). Wound-healing assay results indicated that the cell migration efficiency was markedly reduced by treatment with 20 and 40 μM of Bavachin (Figure 2B). We then proceeded to evaluate the impact of Bavachin on apoptosis in the aforementioned cell lines using flow cytometry analysis. The results indicated that treatment with Bavachin led to a significant rise in the proportion of apoptotic cells, as illustrated in Figure 2C.
Figure 2.
Bavachin regulates the malignant behavior of hepatocellular carcinoma (HCC) cells. Huh7 and HepG2 cells were treated with 20 and 40 μM of Bavachin for 24 h. A: Elevation of HCC cell proliferation (cell counting kit-8; CCK-8). B: Elevation of HCC cell migration (wound-healing assay). C: Elevation of HCC cell apoptosis (flow cytometry). Compared to the control group, *P < 0.05.
Bavachin enhanced the process of lipid peroxidation induced by ROS
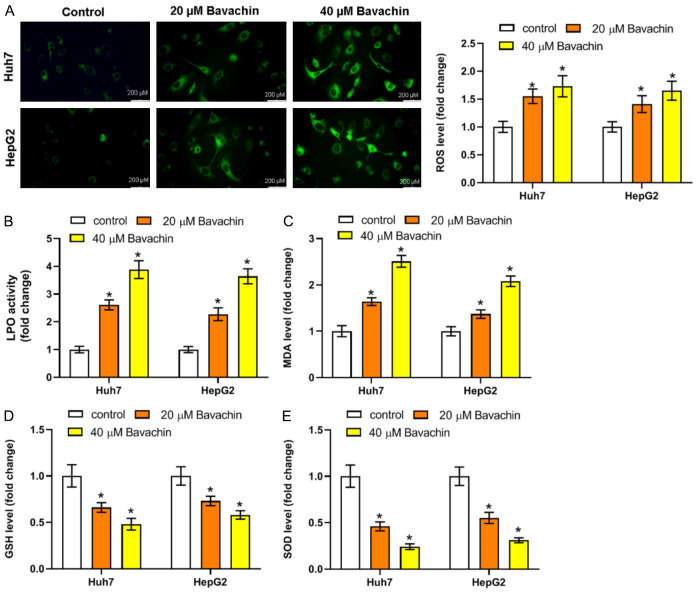
We next investigated the effect of Bavachin on intracellular ROS levels using a DCFH-DA fluorescent probe and fluorescence microanalysis. The results reflected that the ROS level was reduced by Bavachin treatment (Figure 3A). The levels of LPO and MDA were observably increased in Huh7 and HepG2 cells through treatment with Bavachin (Figure 3B and 3C). In comparison, the levels of GSH and SOD exhibited notable reductions in the groups treated with Bavachin (20 and 40 μM) (Figure 3D and 3E).
Figure 3.
Ferroptosis is involved in Bavachin-induced hepatocellular carcinoma (HCC) cell death. A: Fluorescence probe results of reactive oxygen species (ROS) level in HCC cell incubated with Bavachin. B-E: Results of lipid peroxidation (LPO), malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) levels in HCC cells incubated with Bavachin. Compared to the control group, *P < 0.05.
Bavachin promoted lipid peroxidation-mediated ferroptosis
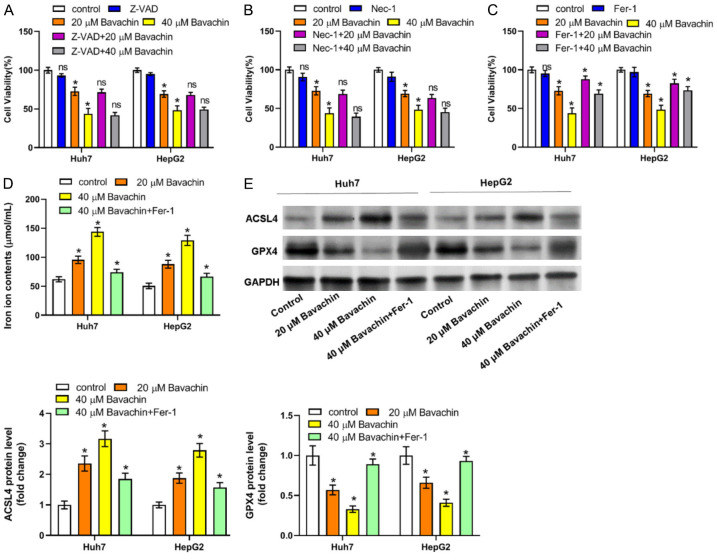
We made a preliminary determination of the form of cell death in which Bavachin induces a decrease in cell number by using several known inhibitors of the cell death mode, such as the apoptosis inhibitor Z-VAD-FMK, the necroptosis inhibitor necrostatin-1, and the ferroptosis inhibitor ferrostin-1. After co-treatment of Bavachin with the above cell death inhibitors, cell survival was increased, but the increase in cell survival was more pronounced after the action of ferrostin-1, a ferroptosis inhibitor (Figure 4A-C). Therefore, we found that Bavachin may induce ferroptosis in HCC cells. Further, we revealed that Bavachin enhanced the ferroptosis process, as indicated by increased iron and ACSL4 levels and decreased GPX4 expression, while co-treatment of Bavachin (40 μM) and ferrostin-1 (1 μM) reversed these changes (Figure 4D and 4E).
Figure 4.
Bavachin contributes to hepatocellular carcinoma (HCC) cell death by inducing ferroptosis. A-C: Effect on HCC cell viability of Bavachin alone or combined with necrostatin-1 (Nec-1; 10 μM), Z-Val-Ala-Asp(OMe)-Fluoromethylketone (Z-VAD; 10 μM), or ferrostatin-1 (Fer-1; 1 μM). Compared to the control group, *P < 0.05; compared to the Bavachin group (20 or 40 μM), nsP > 0.05. D: Iron levels in HCC cells incubated with Bavachin. E: Quantification of western blot results of acyl-CoA synthetase long-chain family member 4 (ACSL4) and glutathione peroxidase-4 (GPX4). Compared to the control group, *P < 0.05.
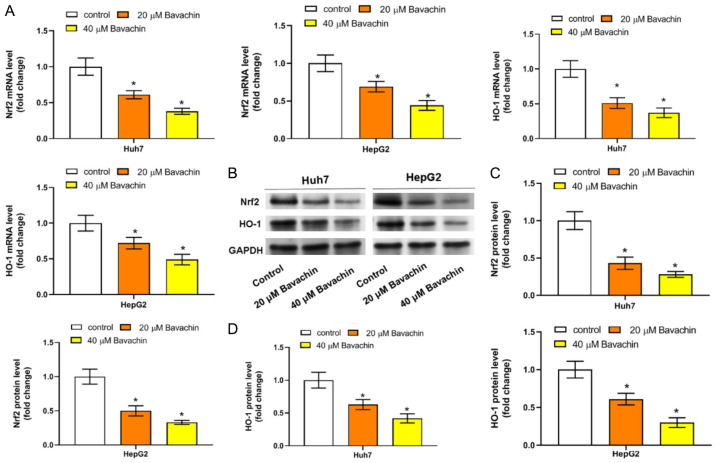
Bavachin reduced lipid peroxidation and ferroptosis in HCC by activating the Nrf2/HO-1 axis
Nrf2 is one of the most important transcription factors regulating ferroptosis, and HO-1 as its downstream target gene has the ability to resist ferroptosis in cancer. Therefore, we further explored whether Bavachin is involved in Nrf2/HO-1 signal pathway mediated-ferroptosis in HCC cells. RT-qPCR results revealed that Bavachin treatment led to significantly reduced mRNA levels of both Nrf2 and HO-1 (Figure 5A). Western blotting results further revealed a significant reduction in both Nrf2 and HO-1 protein levels after Bavachin treatment (Figure 5B-D).
Figure 5.
Bavachin promotes ferroptosis by inactivation of the nuclear factor erythroid 2-related factor 2 (Nrf2)/Heme oxygenase-1 (HO-1) pathway. A: Representative RNA stability and reverse transcription quantitative polymerase chain reaction (RT-qPCR) results of the mRNA levels of Nrf2 and HO-1 in hepatocellular carcinoma (HCC) cells incubated with Bavachin. B: Representative western blot results of the protein levels of Nrf2 and HO-1 in HCC cells incubated with Bavachin. C and D: Quantification of western blot results of Nrf2 and HO-1. Compared to the control group, *P < 0.05.
Discussion
Ferroptosis is widely found in various cancers and participates in the malignant proliferation and metastasis of cancer cells. The liver is the primary organ involved in iron storage and regulation of normal iron metabolism in the body. Abnormalities in iron metabolism play an important role in the process of liver diseases [20-23]. Iron overload, lipid peroxidation, and oxidative stress have all been implicated in the pathogenesis of HCC. Induction of ferroptosis in HCC cells can significantly slow down the progression of HCC. Some prospective observational study on the predictive value of ferroptosis-related biomarkers for HCC found that GPX4, ACSL4 and transferrin Receptor-1 (TFR-1) might have a good predictive value for HCC [24-26]. Ferroptosis holds great promise for research and clinical application in the clinical treatment of tumors, with the potential to significantly impact targeted therapy for HCC and improve clinical outcomes.
In the absence of ideal therapeutic means, traditional Chinese medicine (TCM) plays a unique role in the treatment of HCC and is an important research direction for the prevention and treatment of HCC. Bavachin, a flavonoid, has shown promising results in inhibiting the growth of cancer cells and inducing apoptosis. It has been shown that Bavachin can inhibit the growth of human placental choriocarcinoma, multiple myeloma, osteosarcoma, and other tumors. Bavachin was reported to promote apoptosis in human placental choriocarcinoma cells by inducing caspase-3-dependent apoptotic pathway, and induce mitochondrial dysfunction through modulation of the electron transport chain [14]. In multiple myeloma cells, it induced apoptosis by repressing nuclear factor kappa-B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) activation [16]. In osteosarcoma, it reduced endomitochondrial cristae and thus induced ferroptosis via the STAT3/P53/SLC7A11 pathway [15]. Surprisingly, a large amount of ROS was present in HCC cells HepG2 after Bavachin treatment and subsequently led to a significant increase in the apoptosis rate [27]. However, it had remained unknown whether Bavachin induces ferroptosis in HCC cells by regulating the production of ROS. In this experiment, we revealed that Bavachin was a candidate drug for HCC treatment. To demonstrate this, we included normal (non-tumor) control cells, L02, in our experiments to compare with HCC cells. Bavachin showed almost no cytotoxicity and inhibitory effect on L02 cells. This may be due to the fact that cancer cells usually require increased iron to promote their proliferation compared to non-cancerous cells. This iron dependence also makes cancer cells more sensitive to ferroptosis [28]. Then, we examined the malignant behavior and lipid peroxidation level of HCC cells and found that the proliferation and invasion of HCC cells were significantly reduced after Bavachin administration, and the lipid peroxidation level was significantly reduced. We also found that cell death was primarily ferroptosis, characterized by elevated iron levels and ferroptosis markers. This suggests that Bavachin may act as a tumor suppressor by mediating ferroptosis through lipid peroxidation pathways.
Nrf2 regulates multiple processes of ferroptosis, including its involvement in mediating iron metabolism, catabolism of reactive intermediates, and GSH synthesis and metabolism. Nrf2 is a transcription factor that mediates the upregulation of HO-1 to reduce iron accumulation and ROS production, thereby hindering cellular ferroptosis. Targeting Nrf2/HO-1-mediated ferroptosis may be a promising anti-tumor strategy. In metastatic colorectal cancer research, researchers have found that down-regulation of the Nrf2/HO-1 pathway to promote ferroptosis in tumor cells is negatively correlated with anti-cancer effects. Also, enhancing the ferroptosis process - by increasing iron levels, ROS, and promoting lipid peroxidation, while inhibiting the Nrf2/HO-1 pathway - has been shown to sensitize tumor cells to cetuximab, thereby enhancing its effectiveness in the treatment of colorectal cancer [29]. Polyphyllin I was found to induce ferroptosis in HCC cells by inactivating the Nrf2/HO-1 signaling pathway, leading to increased iron accumulation, along with a down-regulation of GPX4 expression, which promoted lipid peroxidation [30]. Sun et al. found that activation of the Nrf2 pathway prevented ferroptosis in HCC cells [31]. Consistent with previous reports, this study found that the Nrf2/HO-1 pathway was activated in HCC, and Bavachin could act as a ferroptosis inducer to inhibit the expression of Nrf2 and HO-1, hindering the activation of the Nrf2/HO-1 pathway, leading to lipid peroxidation and cellular iron accumulation.
In conclusion, Bavachin may promote an increase in ROS content by inhibiting the Nrf2/HO-1 pathway. ROS can further increase the content of MDA and decrease the content of GSH and SOD in HCC cells, which promotes the occurrence of lipid peroxidation and ferroptosis, and inhibits cellular malignant behavior in HCC cells.
Acknowledgements
This study was funded by the Project on the Development of Medicine and Health and Information Technology Construction (ZHGC102767), and Shanghai Clinical Key Specialty Project (shslczdzk02402).
Disclosure of conflict of interest
None.
References
- 1.Sidali S, Trépo E, Sutter O, Nault JC. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. 2022;10:765–774. doi: 10.1002/ueg2.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwamoto H, Shimose S, Shirono T, Niizeki T, Kawaguchi T. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in the era of chemo-diversity. Clin Mol Hepatol. 2023;29:593–604. doi: 10.3350/cmh.2022.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. Biosci Trends. 2021;15:138–141. doi: 10.5582/bst.2021.01094. [DOI] [PubMed] [Google Scholar]
- 4.Xie D, Shi J, Zhou J, Fan J, Gao Q. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Chinese perspective. Clin Mol Hepatol. 2023;29:206–216. doi: 10.3350/cmh.2022.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–170. doi: 10.1007/82_2016_508. [DOI] [PubMed] [Google Scholar]
- 7.Huang Q, Ru Y, Luo Y, Luo X, Liu D, Ma Y, Zhou X, Linghu M, Xu W, Gao F, Huang Y. Identification of a targeted ACSL4 inhibitor to treat ferroptosis-related diseases. Sci Adv. 2024;10:eadk1200. doi: 10.1126/sciadv.adk1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung YL, Wang SC, Suzuki K, Fang SH, Chen CS, Cheng WC, Su CC, Yeh HC, Tu HP, Liu PL, Huang MY, Li CY. Bavachin attenuates LPS-induced inflammatory response and inhibits the activation of NLRP3 inflammasome in macrophages. Phytomedicine. 2019;59:152785. doi: 10.1016/j.phymed.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Yang Z, Wang Q, Ren Y, Wang Q, Li Z. Bavachin exerted anti-neuroinflammatory effects by regulation of A20 ubiquitin-editing complex. Int Immunopharmacol. 2021;100:108085. doi: 10.1016/j.intimp.2021.108085. [DOI] [PubMed] [Google Scholar]
- 10.Park J, Seo E, Jun HS. Bavachin alleviates diabetic nephropathy in db/db mice by inhibition of oxidative stress and improvement of mitochondria function. Biomed Pharmacother. 2023;161:114479. doi: 10.1016/j.biopha.2023.114479. [DOI] [PubMed] [Google Scholar]
- 11.Xu QX, Hu Y, Li GY, Xu W, Zhang YT, Yang XW. Multi-target anti-alzheimer activities of four prenylated compounds from psoralea fructus. Molecules. 2018;23:614. doi: 10.3390/molecules23030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He HQ, Law BYK, Zhang N, Qiu CL, Qu YQ, Wu AG, Han Y, Song Q, Zheng WL, Liu Y, He YZ, Wong VKW. Bavachin protects human aortic smooth muscle cells against β-glycerophosphate-mediated vascular calcification and apoptosis via activation of mTOR-dependent autophagy and suppression of β-catenin signaling. Front Pharmacol. 2019;10:1427. doi: 10.3389/fphar.2019.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Lin L, Yuan QQ, Wang XY, Zhang Q, Zhang XM, Tang KC, Guo MY, Dong TY, Han W, Huang DK, Qi YL, Zhang M, Zhang HB. Bavachin protects against diet-induced hepatic steatosis and obesity in mice. Acta Pharmacol Sin. 2023;44:1416–1428. doi: 10.1038/s41401-023-01056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Lim W, Song G. Bavachin suppresses human placental choriocarcinoma cells by targeting electron transport chain complexes and mitochondrial dysfunction. Free Radic Biol Med. 2020;156:26–35. doi: 10.1016/j.freeradbiomed.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Gao X, Zou L, Lei M, Feng J, Hu Z. Bavachin induces ferroptosis through the STAT3/P53/SLC7A11 axis in osteosarcoma cells. Oxid Med Cell Longev. 2021;2021:1783485. doi: 10.1155/2021/1783485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda T, Tsubaki M, Tomonari Y, Kawashima K, Itoh T, Imano M, Satou T, Nishida S. Bavachin induces the apoptosis of multiple myeloma cell lines by inhibiting the activation of nuclear factor kappa B and signal transducer and activator of transcription 3. Biomed Pharmacother. 2018;100:486–494. doi: 10.1016/j.biopha.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Wu Z, Liu Y, Zhan Z, Yang L, Wang C, Jiang Q, Ran H, Li P, Wang Z. ROS-responsive liposomes as an inhaled drug delivery nanoplatform for idiopathic pulmonary fibrosis treatment via Nrf2 signaling. J Nanobiotechnology. 2022;20:213. doi: 10.1186/s12951-022-01435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen P, Wu Q, Feng J, Yan L, Sun Y, Liu S, Xiang Y, Zhang M, Pan T, Chen X, Duan T, Zhai L, Zhai B, Wang W, Zhang R, Chen B, Han X, Li Y, Chen L, Liu Y, Huang X, Jin T, Zhang W, Luo H, Chen X, Li Y, Li Q, Li G, Zhang Q, Zhuo L, Yang Z, Tang H, Xie T, Ouyang X, Sui X. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct Target Ther. 2020;5:51. doi: 10.1038/s41392-020-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu A, Feng B, Yu J, Yan L, Che L, Zhuo Y, Luo Y, Yu B, Wu D, Chen D. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 2021;46:102131. doi: 10.1016/j.redox.2021.102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y, Jiao H, Yue Y, He K, Jin Y, Zhang J, Zhang J, Wei Y, Luo H, Hao Z, Zhao X, Xia Q, Zhong Q, Zhang J. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. 2022;29:1705–1718. doi: 10.1038/s41418-022-00957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He F, Zhang P, Liu J, Wang R, Kaufman RJ, Yaden BC, Karin M. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J Hepatol. 2023;79:362–377. doi: 10.1016/j.jhep.2023.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamane D, Hayashi Y, Matsumoto M, Nakanishi H, Imagawa H, Kohara M, Lemon SM, Ichi I. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem Biol. 2022;29:799–810. e794. doi: 10.1016/j.chembiol.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei W, Jiang M, Liu H, Song J, Hu J. The prognostic and antitumor roles of key genes of ferroptosis in liver hepatocellular cancer and stomach adenocarcinoma. Cancer Biomark. 2024;39:335–347. doi: 10.3233/CBM-230114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi M, Kai K, Yamaji K, Ide T, Noshiro H, Kawaguchi A, Aishima S. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology. 2019;75:63–73. doi: 10.1111/his.13847. [DOI] [PubMed] [Google Scholar]
- 26.Feng J, Lu PZ, Zhu GZ, Hooi SC, Wu Y, Huang XW, Dai HQ, Chen PH, Li ZJ, Su WJ, Han CY, Ye XP, Peng T, Zhou J, Lu GD. ACSL4 is a predictive biomarker of sorafenib sensitivity in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:160–170. doi: 10.1038/s41401-020-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Tang X, Hao F, Ma Z, Wang Y, Wang L, Gao Y. Bavachin induces apoptosis through mitochondrial regulated ER stress pathway in HepG2 cells. Biol Pharm Bull. 2018;41:198–207. doi: 10.1248/bpb.b17-00672. [DOI] [PubMed] [Google Scholar]
- 28.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–849. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X, Jiang L, Ye L. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12:1079. doi: 10.1038/s41419-021-04367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang R, Gao W, Wang Z, Jian H, Peng L, Yu X, Xue P, Peng W, Li K, Zeng P. Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine. 2024;122:155135. doi: 10.1016/j.phymed.2023.155135. [DOI] [PubMed] [Google Scholar]
- 31.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]