Abstract
Objective: To investigate the effects of graded exercise rehabilitation training tailored to pulmonary function classification on dyspnea, pulmonary function, and exercise capacity during postoperative rehabilitation in elderly patients following lung cancer surgery. Methods: A retrospective analysis was conducted on clinical data from 168 elderly patients undergoing postoperative rehabilitation after lung cancer surgery at Panjin Liaohe Oilfield Gem Flower Hospital from January 2021 to December 2022. Patients were divided into two groups based on the rehabilitation received: the control group (n=71), receiving standard rehabilitation, and the study group (n=97), receiving additional graded exercise rehabilitation based on pulmonary function classification. Outcomes were compared before and after a 12-week intervention, including psychological status (Hamilton Anxiety Scale (HAMA) and Hamilton Depression Rating Scale (HAMD)), symptom scores, dyspnea (Modified Medical Research Council (mMRC) and St. George’s Respiratory Questionnaire (SGRQ) scores), pulmonary function (Forced Expiratory Volume in 1 Second (FEV1), Forced Vital Capacity (FVC), Peak Expiratory Flow (PEF), Maximum Voluntary Ventilation (MVV), and respiratory muscle strength), inflammatory markers (Interleukin-8 (IL-8)), tumor markers (Carcinoembryonic Antigen (CEA) and Cytokeratin-19 Fragment antigen 21-1 (CYFRA21-1)), exercise capacity (6-minute walk test (6MWT) distance, Maximum Oxygen Consumption (VO2max), Maximum Workload (MWL), and Anaerobic Threshold (AT)), sleep quality (Pittsburgh Sleep Quality Index (PSQI)), and quality of life (World Health Organization Quality of Life-BREF (WHOQOL-BREF)). Results: After 12 weeks, both groups exhibited significant reductions in HAMA, HAMD, cough, sputum production, chest pain, shortness of breath, mMRC, SGRQ, and PSQI scores, with the study group showing more pronounced decreases (all P < 0.05). FEV1, FVC, PEF, 6MWT distance, and WHOQOL-BREF scores increased significantly in both groups, with greater improvements in the study group (all P < 0.05). IL-8, CEA, and CYFRA21-1 levels decreased significantly in both groups, with IL-8 levels lower in the study group (all P < 0.05); however, no significant differences were observed in CEA or CYFRA21-1 between groups post-intervention (both P > 0.05). Conclusion: Graded exercise rehabilitation based on pulmonary function classification effectively enhances pulmonary function, relieves symptoms, improves sleep quality, and supports recovery in elderly patients post-lung cancer surgery.
Keywords: Pulmonary function classification, exercise rehabilitation training, elderly lung cancer, dyspnea, exercise capacity
Introduction
Lung cancer is a prevalent malignancy with high incidence and mortality rate globally. The disease originates from the bronchial mucosa or lung glandular structures, with primary symptoms such as cough, sputum production, chest pain, and dyspnea, all significantly worsening patient health and survival [1]. Surgical resection remains a key treatment option, and the adoption of video-assisted thoracoscopic surgery (VATS) for lung cancer resection has increased due to its minimally invasive nature, therapeutic effectiveness, and quicker recovery time, contributing to extended patient survival [2,3]. In elderly patients, while surgery improves survival, clinical research increasingly emphasizes postoperative management to enhance lung function, exercise capacity, and reduce dyspnea [4]. Evidence suggests that exercise rehabilitation training can significantly improve pulmonary function and quality of life in post-lung cancer surgery patients [5]. Graded exercise rehabilitation, customized according to pulmonary function classification, offers a targeted approach that accommodates different lung function levels, thus enhancing pulmonary function, exercise capacity, and quality of life [7]. This approach has shown efficacy ifor managing respiratory conditions such as chronic obstructive pulmonary disease (COPD), asthma, and interstitial lung disease, though its application in lung cancer rehabilitation remains limited.
In light of these benefits, this study aims to bridge the gap by implementing graded exercise rehabilitation training, tailored to lung function classification, for the postoperative recovery of elderly lung cancer patients. To evaluate the effects of this training, we assessed various indicators, including psychological well-being, dyspnea, pulmonary function, inflammatory markers, tumor markers, exercise capacity, sleep quality, and overall quality of life. This retrospective analysis includes clinical data from 168 elderly patients in the postoperative rehabilitation phase following lung cancer surgery, assessing the effect of graded exercise rehabilitation training based on pulmonary function classification.
Materials and methods
General information
This retrospective study analyzed clinical data from 168 elderly patients undergoing postoperative rehabilitation following primary lung cancer surgery at Panjin Liaohe Oilfield Gem Flower Hospital from January 2021 to December 2022. The cohort included 98 males and 70 females, aged 61-83 years, with a mean age of 72.54±4.31 years. Pathologic types included adenocarcinoma (78 cases), squamous cell carcinoma (65 cases), and adenosquamous carcinoma (25 cases). TNM staging showed Stage I (48 cases), Stage II (80 cases), and Stage III (40 cases). Duration of illness ranged from 1 to 12 years, with an average of 5.33±1.42 years. Patients were allocated to a control group (n=71) and a study group (n=97) based on the intervention type. This study was approved by the Ethics Committee of Panjin Liaohe Oilfield Gem Flower Hospital.
Inclusion and exclusion criteria
Inclusion criteria: (1) Diagnosed with lung cancer per established diagnostic standards, confirmed by postoperative pathology [6]; (2) Aged 60-90 years; (3) Complete clinical data available; (4) Expected survival time > 6 months.
Exclusion criteria: (1) Prior radiotherapy or chemotherapy; (2) Other malignancies or severe dysfunction of the heart, liver, or kidneys; (3) Coexisting hematological, immunological, psychological, or psychiatric conditions; (4) Advanced lung cancer with cachexia; (5) Severe surgical complications; (6) Poor adherence or non-cooperation with nursing interventions.
Intervention methods
Control group: Patients received conventional interventions, including health education, psychological support, dietary guidance, pain management, medication instructions, monitoring, and basic rehabilitation exercises. Rehabilitation mainly comprised: (1) Breathing exercises: Patients practiced slow, deep inhalations, holding for 3-5 seconds, then exhaling while contracting the abdomen. Each session lasted 10-15 minutes, performed three times daily. (2) Cough and sputum clearance training: Patients took deep breaths, followed by forceful exhalations while crossing arms over the chest. Once sputum reached the throat, patients were encouraged to cough it out; assistance was provided with gentle back tapping if needed.
Study group: In addition to the above, patients underwent graded exercise rehabilitation according to their pulmonary function classification, based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [7]. Exercises were designed per GOLD stages:
GOLD stage 2 patients: ① Dumbbell training: Patients lifted 1-3 kg dumbbells in each hand, performing 15-20 repetitions, three times daily. ② Active cycling training: Patients lay flat, simulating pedaling for 10-15 minutes per session, three times daily. ③ Bridge training: Patients performed hip raises with bent knees, lifting hips 10-20 cm above the bed, completing 15-20 repetitions, three times daily.
GOLD stage 3 patients: ① Knee extension training: Patients flexed and extended knees while seated, completing three sets of 10-15 repetitions daily. ② Active cycling training: Patients used an air cycling device for 10-15 minutes per set, three times daily. ③ Stretch and sit-up training: Patients held the bed’s edge and elevated their upper body for 3-5 seconds, performing 10-15 repetitions per set, three times daily.
GOLD stage 4 patients: ① Elbow extension training: Patients extended elbows to 180° and flexed arms to bring hands to the shoulder, completing three sets of 15-20 repetitions daily. ② Passive cycling training: Patients selected a cycling device according to their recovery status and adjusted pedaling speed for passive cycling. Each session lasted 10-15 minutes, performed three times daily.
Regular reassessments of the patient’s GOLD stage informed adjustments to the exercise regimen based on pulmonary function evaluations. Blood oxygen saturation and heart rate were monitored closely. Exercise was discontinued if symptoms like dizziness, chest tightness, or palpitations occurred. Both groups received interventions for 12 weeks.
Outcome measures
(1) Psychological status: Psychological evaluations were conducted at baseline and after 12 weeks of intervention using the Hamilton Anxiety Rating Scale (HAMA) and the Hamilton Depression Rating Scale (HAMD) [7,8]. A HAMA score above 21 indicates significant anxiety, and scores over 29 suggest severe anxiety. For HAMD, a score exceeding 17 indicates depressive symptoms, while scores above 24 reflect severe depression.
(2) Symptom scores [9]: Symptom severity for cough, sputum production, chest pain, and shortness of breath was assessed before and after the intervention using a 0-6 severity scale, with higher scores indicating greater severity.
(3) Dyspnea: Dyspnea was measured before and after the 12-week intervention using the Modified Medical Research Council (mMRC) scale and the St. George’s Respiratory Questionnaire (SGRQ) [10,11]. The mMRC score ranges from 0 to 4, while the SGRQ score ranges from 0 to 100, with higher scores indicating more severe dyspnea.
(4) Pulmonary function [12]: Pulmonary function was assessed at baseline and after 12 weeks using a pulmonary function testing device, measuring Forced Expiratory Volume in 1 Second (FEV1), Forced Vital Capacity (FVC), Peak Expiratory Flow (PEF), Maximum Voluntary Ventilation (MVV), and Respiratory Rate (RR). Respiratory muscle strength was also evaluated.
(5) Inflammatory markers [13]: Serum levels of inflammatory markers, including Interleukin-8 (IL-8), CEA, and CYFRA21-1, were measured before and after the intervention. Blood samples were collected by venipuncture and analyzed using enzyme-linked immunosorbent assay (ELISA) kits from Shanghai Kehua Bio-engineering Co., Ltd.
(6) Exercise capacity [14]: Exercise capacity was evaluated before and after the intervention using the 6-minute walk test (6MWT), which correlates positively with exercise capacity. Additionally, a cardiopulmonary exercise testing system (COSMED, Italy, model K4b2) was used to measure VO2max, maximum workload (MWL), and anaerobic threshold (AT).
(7) Sleep quality: Sleep quality was assessed pre- and post-intervention using the Pittsburgh Sleep Quality Index (PSQI), where scores range from 0 to 21, with lower scores indicating better sleep quality [15].
(8) Quality of life: Quality of life was evaluated using the World Health Organization Quality of Life-BREF (WHOQOL-BREF) scale before and after 12 weeks of intervention. The WHOQOL-BREF includes four domains, each with a maximum score of 100, where higher scores indicate better quality of life [16].
(9) Mid-term prognosis: One-year follow-up data were collected through phone interviews and outpatient visits to assess recurrence, metastasis, and mortality rates in both groups.
Statistical methods
Statistical analysis was performed using SPSS 23.0 software. Categorical variables (e.g., gender, pathologic type) were presented as n (%), with differences tested using the chi-square (χ2) test. Continuous variables (e.g., psychological status, symptom scores, dyspnea, pulmonary function) were presented as mean ± standard deviation (x̅ ± Sd), and analyzed using the t-test. For repeated measures over multiple time points, repeated measures ANOVA was used. A p-value < 0.05 was considered significant.
Results
Comparison of baseline characteristics
Baseline characteristics, including gender, pathological type, age, TNM staging, and duration of illness, were comparable between the two groups, with no significant differences (all P > 0.05) (Table 1).
Table 1.
Comparison of relevant indicators of baseline data between the two groups n/(x̅ ± Sd)
| Group | Sex | Age (years) | Pathological type | TNM stage duration (years) | Duration of disease (years) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Male | Female | Adenocarcinoma | Squamous carcinoma | Adenosquamous carcinoma | Phase I | Phase II | Phase III | |||
| Control group (n=71) | 42 | 29 | 72.23±4.92 | 33 | 30 | 8 | 20 | 35 | 16 | 5.07±1.62 |
| Study Group (n=97) | 56 | 41 | 72.74±5.78 | 45 | 35 | 17 | 28 | 45 | 24 | 5.54±1.78 |
| χ2/t | 0.034 | 0.601 | 1.483 | 0.163 | 1.755 | |||||
| P | 0.853 | 0.549 | 0.477 | 0.922 | 0.081 | |||||
Note: TNM, tumor-node-metastasis.
Comparison of symptom scores
Before intervention, symptom scores for cough, sputum production, chest pain, and shortness of breath were similar between the groups, with no significant differences (all P > 0.05). After 12 weeks, these symptom scores decreased in both groups, with the study group showing lower scores (all P < 0.05) (Table 2).
Table 2.
Comparison of symptom scores between the two groups (x̅ ± Sd, points)
| Group | Cough | Expectoration | Thoracodynia | Short of breath | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Pre-intervention | After 12 weeks of intervention | Pre-intervention | After 12 weeks of intervention | Pre-intervention | After 12 weeks of intervention | Pre-intervention | After 12 weeks of intervention | |
| Control group (n=71) | 4.02±0.77 | 2.24±0.70* | 3.87±0.92 | 2.13±0.75* | 3.12±0.87 | 1.75±0.74* | 3.57±0.92 | 2.01±0.67* |
| Study Group (n=97) | 4.11±0.83 | 1.23±0.65* | 3.81±0.96 | 1.05±0.63* | 3.01±0.85 | 0.89±0.66* | 3.48±0.89 | 1.13±0.57* |
| t | 0.716 | 9.630 | 0.407 | 10.122 | 0.820 | 7.924 | 0.638 | 9.174 |
| P | 0.475 | < 0.001 | 0.684 | < 0.001 | 0.413 | < 0.001 | 0.524 | < 0.001 |
Note: Compared to before intervention;
P < 0.05.
Comparison of dyspnea
Dyspnea scores were comparable between groups at baseline, with no significant differences (P > 0.05). After 12 weeks, dyspnea scores significantly decreased in both groups, with a more pronounced reduction in the study group (both P < 0.05) (Table 3).
Table 3.
Comparison of dyspnea scores between the two groups (x̅ ± Sd, points)
| Group | mMRC | SGRQ | ||
|---|---|---|---|---|
|
|
|
|||
| Pre-intervention | After 12 weeks of intervention | Pre-intervention | After 12 weeks of intervention | |
| Control group (n=71) | 3.05±0.84 | 2.18±0.62* | 62.10±5.77 | 51.05±5.05* |
| Study Group (n=97) | 3.01±0.81 | 1.25±0.55* | 61.86±6.12 | 40.02±4.24* |
| t | 0.311 | 10.257 | 0.257 | 15.356 |
| P | 0.756 | < 0.001 | 0.797 | < 0.001 |
Note: Compared to before intervention;
P < 0.05.
mMRC, Modified Medical Research Council; SGRQ, St. George’s Respiratory Questionnaire.
Comparison of pulmonary function
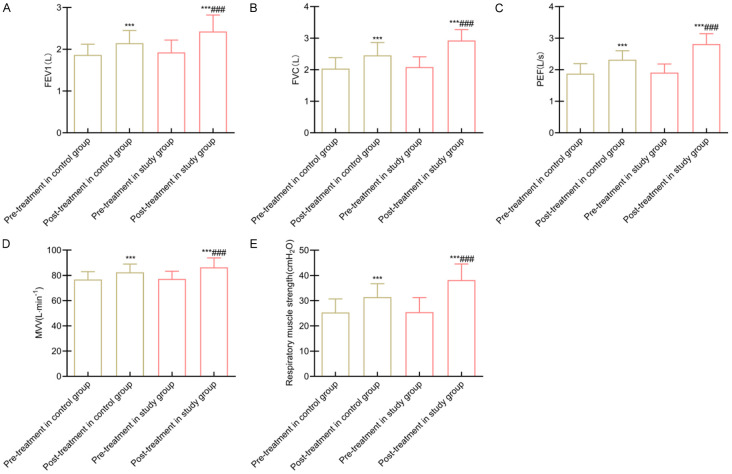
At baseline, pulmonary function parameters, including FEV1, FVC, PEF, MVV, and respiratory muscle strength, were similar between groups (all P > 0.05). Following 12 weeks of intervention, these parameters improved significantly in both groups, with the study group showing greater increases and significant differences (all P < 0.05) (Figure 1).
Figure 1.
Comparison of lung function between the two groups. Note: A: FEV1; B: FVC; C: PEF; D: MVV; E: Muscle strength of respiratory muscle. Compared to before intervention within the same group, ***P < 0.001; Compared to the control group, ###P < 0.001. FEV1, Forced Expiratory Volume in 1 Second; FVC, Forced Vital Capacity; PEF, Peak Expiratory Flow; MVV, Maximum Voluntary Ventilation.
Comparison of exercise capacity
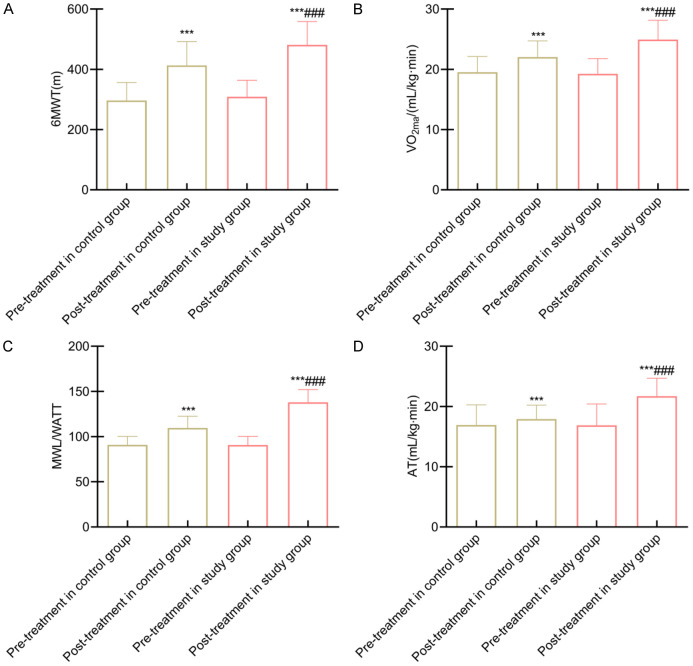
Exercise capacity, assessed by 6MWT distance, VO2max, MWL, and AT, was comparable between groups before intervention (all P > 0.05). After 12 weeks, these exercise measures improved significantly in both groups, with the study group showing greater gains (all P < 0.05) (Figure 2).
Figure 2.
Comparison of exercise capacity between groups. Note: A: 6MWT; B: VO2max; C: MWL; D: AT. Compared to pre-intervention within the same group, ***P < 0.001; Compared to the control group, ###P < 0.001. 6MWT, 6-minute walk test; VO2max, Maximum Oxygen Consumption; MWL, Maximum Workload; AT, Anaerobic Threshold.
Comparison of inflammatory and tumor markers
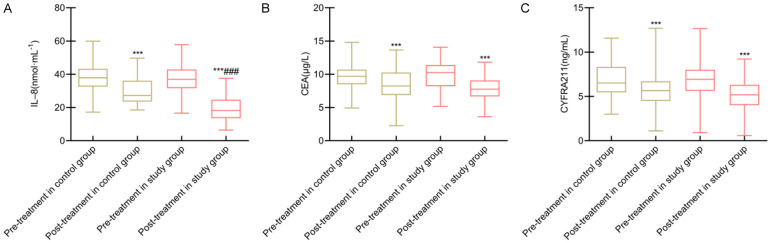
Baseline serum levels of IL-8, CEA, and CYFRA21-1 were comparable between groups (all P > 0.05). After 12 weeks, significant reductions were observed in all markers in both groups, with a more substantial decrease in IL-8 levels in the study group (all P < 0.05). However, reductions in CEA and CYFRA21-1 were not significantly different between the groups (both P > 0.05) (Figure 3).
Figure 3.
Comparison of inflammatory factors and tumor markers between the two groups. Note: A: IL-8; B: CEA; C: CYFRA21-1. Compared to before intervention within the same group, ***P < 0.001; Compared to the control group, ###P < 0.001. IL-8, Interleukin-8; CEA, Carcinoembryonic Antigen; CYFRA21-1, Cytokeratin-19 Fragment antigen 21-1.
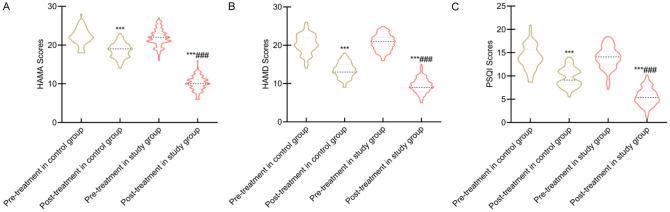
Comparison of psychological status and sleep quality
Baseline psychological status (HAMA and HAMD scores) and sleep quality (PSQI scores) were similar between groups (P > 0.05). After 12 weeks, HAMA, HAMD, and PSQI scores showed significant reductions in both groups, with the study group exhibiting a more substantial decrease (all P < 0.05) (Figure 4).
Figure 4.
Comparison of psychological status and sleep quality scores between the two groups. Note: A: HAMA; B: HAMD; C: PSQI score. Compared to before intervention within the same group, ***P < 0.001; Compared to the control group, ###P < 0.001. HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Rating Scale; PSQI, Pittsburgh Sleep Quality Index.
Comparison of quality of life
Before the intervention, quality of life scores across all domains were similar between groups (all P > 0.05). After 12 weeks, WHOQOL-BREF scores improved significantly in both groups, with higher scores in the study group (all P < 0.05) (Table 4).
Table 4.
Comparison of scores of various indexes of quality of life between the two groups (x̅ ± Sd, points)
| Group | Physiology | Psychology | Social relationships | Environment | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Pre-intervention | After 12 weeks of intervention | Pre-intervention | After 12 weeks of intervention | Pre-intervention | After 12 weeks of intervention | Pre-intervention | After 12 weeks of intervention | |
| Control group (n=71) | 48.24±4.35 | 59.37±4.72* | 53.10±4.37 | 61.57±4.87* | 55.74±4.30 | 64.05±4.93* | 52.05±4.24 | 60.55±4.87* |
| Study Group (n=97) | 48.79±4.43 | 66.87±5.12* | 52.86±5.08 | 70.33±5.34* | 56.02±4.41 | 71.27±5.29* | 52.41±4.38 | 67.58±5.67* |
| t | 0.801 | 9.691 | 0.321 | 10.897 | 0.411 | 8.991 | 0.533 | 8.418 |
| P | 0.424 | < 0.001 | 0.749 | < 0.001 | 0.682 | < 0.001 | 0.595 | < 0.001 |
Note: Compared to before intervention;
P < 0.05.
Comparison of mid-term prognosis comparison
The mid-term prognosis, including recurrence, metastasis, and mortality rates, showed no significant differences between the groups (all P > 0.05) (Table 5).
Table 5.
Comparison of mid-term prognosis between the two groups n (%)
| Group | Recurrence rate | Metastasis rate | Mortality rate |
|---|---|---|---|
| Control group (n=71) | 3 | 7 | 5 |
| Study Group (n=97) | 5 | 8 | 6 |
| χ2 | 0.008 | 0.131 | 0.005 |
| P | 0.931 | 0.717 | 0.944 |
Discussion
Lung cancer, with its high incidence and mortality rate, poses a great threat to human health. Thoracoscopic resection has become a standard treatment for lung cancer, effectively removing tumor lesions, reducing mortality, extending survival, and offering minimally invasive advantages that expedite postoperative recovery. However, lung cancer primarily affects the elderly, who often have reduced physiological function and multiple comorbidities. The decline in cardiopulmonary function in these patients intensifies dyspnea, and the invasive nature of surgery can delay pulmonary function recovery, adversely affecting prognosis [17]. Therefore, enhancing postoperative nursing interventions for elderly lung cancer patients is crucial.
While conventional rehabilitation methods, such as general exercise, are commonly employed, they often lack specificity and adaptability to individual patient needs, resulting in lower patient motivation and suboptimal outcomes [18]. Given the variability in pulmonary function among patients, standardized exercise programs may not provide adequate intensity for some, potentially diminishing training efficacy, while others may experience excessive strain, possibly worsening their condition [19]. Graded exercise rehabilitation based on pulmonary function classification offers a personalized approach by tailoring exercise intensity, frequency, and goals, thereby improving the appropriateness and efficacy of rehabilitation programs [18]. This individualized approach not only enhances patient motivation but also increases rehabilitation effectiveness.
Results from this study show that after 12 weeks of intervention, both groups exhibited significant reductions in HAMA, HAMD, cough, sputum production, chest pain, shortness of breath, mMRC, SGRQ, and PSQI scores, with the study group demonstrating greater reductions. Additionally, significant improvements were observed in FEV1, FVC, PEF, MVV, and respiratory muscle strength in both groups, with superior outcomes in the study group. These findings suggest that graded exercise rehabilitation based on pulmonary function classification can effectively alleviate clinical symptoms, improve psychological well-being, relieve dyspnea, and enhance both pulmonary function and sleep quality in elderly lung cancer surgery patients. This approach is effective likely because the GOLD grading system assesses airflow limitation, providing an accurate reflection of each patient’s pulmonary function [20].
In this study, rehabilitation training was tailored to GOLD grades: GOLD grade 2 patients performed dumbbell routines, active cycling, and bridge exercises; grade 3 patients engaged in knee extensions, active cycling, and stretching sit-ups; and grade 4 patients undertook elbow extensions and passive cycling. By aligning exercise intensity and type with each patient’s pulmonary function, the regimen meets individual needs, increases patient confidence, reduces negative emotions, and improves adherence, ultimately enhancing training outcome. While conventional breathing and cough training can improve pulmonary function, a graded rehabilitation approach based on pulmonary function classification offers a more balanced enhancement in respiratory and exercise capacities, tolerance to training, and promotes a more effective recovery of pulmonary function [21].
Clinical observations consistently reveal that lung cancer surgery patients often experience reduced exercise capacity due to compromised pulmonary function, which worsens their quality of life [22]. The 6MWT is a recognized measure of exercise capacity and is essential for evaluating the effectiveness of rehabilitation in lung disease patients. Quality of life, reflecting overall satisfaction with social, psychological, and physiological well-being, is a key goal for lung cancer patients [23]. This study showed that after 12 weeks, both groups demonstrated significant improvements in 6MWT distance, VO2max, MWL, AT, and WHOQOL-BREF scores, with the study group achieving better results. This indicates that graded exercise rehabilitation based on pulmonary function classification effectively enhances exercise capacity and quality of life in elderly lung cancer patients. The mechanism likely involves incorporating aerobic exercises targeting lower limbs, which reduces fatigue, enhances walking ability and endurance, and promotes quality of life.
Unlike conventional interventions, this customized approach tailors rehabilitation based on each patient’s pulmonary function grade, muscle strength, and exercise tolerance, fostering self-efficacy and encouraging active participation in pulmonary rehabilitation exercises [24]. Yan et al. implemented a multidisciplinary lung rehabilitation program based on protective motivation theory for elderly lung cancer patients with chemotherapy-induced weakness, significantly improving lung function, exercise self-efficacy, and physical function [25]. These findings align with our results. However, most current studies focus primarily on the mechanisms of rehabilitation training.
Previous studies have shown that postoperative lung cancer patients often experience reduced lung capacity and impaired mucociliary function, leading to sputum buildup in the airways and subsequent inflammatory responses, as indicated by elevated levels of pro-inflammatory cytokines like IL-8 [26]. Our study found that, following intervention, serum IL-8 levels were significantly lower in the study group compared to the control group, suggesting a reduction in inflammation. This may be due to the graded exercise rehabilitation program being based on pulmonary function classification, which enhances respiratory muscle strength through controlled breathing exercises, facilitating secretion clearance, reducing airway obstruction, and decreasing inflammation. Additionally, training focused on chest expansion and forced exhalation improves lung capacity and respiratory muscle endurance, enhancing coughing and expectoration ability, reducing intrapulmonary pressure, and suppressing inflammatory factor release, ultimately mitigating lung damage.
CYFRA21-1, commonly expressed in lung tissue and particularly in the cytoplasm of lung tumor epithelial cells, is released into the bloodstream during lung cancer, leading to elevated serum levels. CEA is a broad-spectrum tumor marker essential for the differential diagnosis and monitoring of lung cancer. Our study observed significant reductions in serum levels of both CEA and CYFRA21-1 in both groups post-intervention. However, no significant differences between the groups were found in these tumor markers or in recurrence, metastasis, or mortality rate. This suggests that while graded exercise rehabilitation based on pulmonary function classification effectively lowers serum CEA and CYFRA21-1 levels, it does not offer a statistically significant advantage over conventional rehabilitation in terms of these outcomes. Rehabilitation primarily benefits lung cancer patients by enhancing post-surgical function and quality of life but has limited impact on cancer recurrence and metastasis mechanisms. Although rehabilitation improves lung function, exercise capacity, and quality of life, it does not directly influence tumor cell growth and spread. Factors such as treatment duration, intervention intensity, and individual patient variability may also affect these outcomes, indicating that a comprehensive treatment approach may be necessary to manage lung cancer progression.
In summary, implementing a graded exercise rehabilitation program based on pulmonary function classification in elderly lung cancer patients effectively improves lung function, alleviates symptoms, enhances sleep and quality of life, and supports recovery, showing strong clinical applicability and potential for wider use. However, this study is retrospective, and its findings may be subject to bias. Future large-scale, prospective studies are needed to further validate these results.
Disclosure of conflict of interest
None.
References
- 1.Zhang Z, Zhang Y, Zhang J, Su P. Analysis of factors affecting intraoperative conversion from thoracoscopic radical resection of lung cancer to thoracotomy and intraoperative management experience. Pak J Med Sci. 2023;39:1389–1393. doi: 10.12669/pjms.39.5.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, An J. Effects of serratus anterior plane block and thoracic paravertebral nerve block on analgesia, immune function and serum tumor markers in patients after thoracoscopic radical resection of lung cancer. Nagoya J Med Sci. 2022;84:506–515. doi: 10.18999/nagjms.84.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, Zhang L, Gong F, Xu J. Thoracoscopic radical resection in the treatment of NSCLC patients (stage IIIA) after neoadjuvant chemotherapy. J BUON. 2021;26:313–319. [PubMed] [Google Scholar]
- 4.Huang W, Yang J, Chen H, Li P, Wei W. Preservation of the pulmonary branches of the vagus nerve during three-dimensional thoracoscopic radical resection of lung cancer: a retrospective study. BMC Surg. 2024;24:49. doi: 10.1186/s12893-024-02347-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou T, Sun C. Effect of physical manipulation pulmonary rehabilitation on lung cancer patients after thoracoscopic lobectomy. Thorac Cancer. 2022;13:308–315. doi: 10.1111/1759-7714.14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinese Medical Association; Oncology Society of Chinese Medical Association; Chinese Medical Association Publishing House. Chinese medical association guidelines for clinical diagnosis and treatment of lung cancer (2019 edition) Zhonghua Zhong Liu Za Zhi. 2020;42:257–287. doi: 10.3760/cma.j.cn112152-20200120-00049. [DOI] [PubMed] [Google Scholar]
- 7.Shen CH, Niu L, Zhang M, Yao T, Sun H, Song PF, Nie YT. Effect of staged breathing training combined with home aerobic exercise on interactive standard pulmonary rehabilitation in elderly patients with COPD. Chin J Gerontol. 2022;42:4613–4616. [Google Scholar]
- 8.Thompson E. Hamilton rating scale for anxiety (HAM-A) Occup Med (Lond) 2015;65:601. doi: 10.1093/occmed/kqv054. [DOI] [PubMed] [Google Scholar]
- 9.Leucht S, Fennema H, Engel R, Kaspers-Janssen M, Lepping P, Szegedi A. What does the HAMD mean? J Affect Disord. 2013;148:243–248. doi: 10.1016/j.jad.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Hollen PJ, Gralla RJ, Kris MG, Cox C. Quality of life during clinical trials: conceptual model for the lung cancer symptom scale (LCSS) Support Care Cancer. 1994;2:213–222. doi: 10.1007/BF00365725. [DOI] [PubMed] [Google Scholar]
- 11.Chhabra SK, Gupta AK, Khuma MZ. Evaluation of three scales of dyspnea in chronic obstructive pulmonary disease. Ann Thorac Med. 2009;4:128–132. doi: 10.4103/1817-1737.53351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 13.Farid R, Azad FJ, Atri AE, Rahimi MB, Khaledan A, Talaei-Khoei M, Ghafari J, Ghasemi R. Effect of aerobic exercise training on pulmonary function and tolerance of activity in asthmatic patients. Iran J Allergy Asthma Immunol. 2005;4:133–138. [PubMed] [Google Scholar]
- 14.Faggiano P, D’Aloia A, Gualeni A, Lavatelli A, Giordano A. Assessment of oxygen uptake during the 6-minute walking test in patients with heart failure: preliminary experience with a portable device. Am Heart J. 1997;134:203–206. doi: 10.1016/s0002-8703(97)70125-x. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28:551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 17.Xiong T, Bai X, Wei X, Wang L, Li F, Shi H, Shi Y. Exercise rehabilitation and chronic respiratory diseases: effects, mechanisms, and therapeutic benefits. Int J Chron Obstruct Pulmon Dis. 2023;18:1251–1266. doi: 10.2147/COPD.S408325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent H, Aubreton S, Galvaing G, Pereira B, Merle P, Richard R, Costes F, Filaire M. Preoperative respiratory muscle endurance training improves ventilatory capacity and prevents pulmonary postoperative complications after lung surgery. Eur J Phys Rehabil Med. 2020;56:73–81. doi: 10.23736/S1973-9087.19.05781-2. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Cai R, Liao X, Huang X, Zhao C, Chen M. The efficacy of pulmonary rehabilitation exercise training on complications and mortality after lung cancer resection: a systematic review and meta-analysis. Transl Cancer Res. 2022;11:1321–1329. doi: 10.21037/tcr-22-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng J, Li R. The effect of rehabilitation training on postoperative patients with lung cancer: a systematic review and meta-analysis. Asian J Surg. 2022;45:1968–1970. doi: 10.1016/j.asjsur.2022.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Kong M, Zheng H, Ding L, Jin K, Shen J, Ye M, Chen B. Perioperative pulmonary rehabilitation training (PPRT) can reduce the cost of medical resources in patients undergoing thoracoscopic lung cancer resection: a retrospective study. Ann Palliat Med. 2021;10:4418–4427. doi: 10.21037/apm-21-478. [DOI] [PubMed] [Google Scholar]
- 22.Ha DM, Comer A, Dollar B, Bedoy R, Ford M, Gozansky WS, Zeng C, Arch JJ, Leach HJ, Malhotra A, Prochazka AV, Keith RL, Boxer RS. Telemedicine-based inspiratory muscle training and walking promotion with lung cancer survivors following curative intent therapy: a parallel-group pilot randomized trial. Support Care Cancer. 2023;31:546. doi: 10.1007/s00520-023-07999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Q, Shen ZQ, Feng KP, Xu C, Ding C, Li C, Ju S, Chen J, Pan S, Zhao J. The efficacy of three-ball breathing apparatus exercise based on the concept of pulmonary rehabilitation in patients after lung cancer surgery. J Cardiothorac Surg. 2023;18:218. doi: 10.1186/s13019-023-02307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou H, Qin Y, Gong F, Liu J, Zhang J, Zhang L. ABCDEF pulmonary rehabilitation program can improve the mid-term lung function of lung cancer patients after thoracoscopic surgery: a randomized controlled study. Geriatr Nurs. 2022;44:76–83. doi: 10.1016/j.gerinurse.2021.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Yan WJ, Sun Y, He CY, Li Y, Ge B, Du FH. Application of multidisciplinary pulmonary rehabilitation training based on protective motivation theory in elderly patients with lung cancer complicated with debilitating chemotherapy. J Clin Pulm Med. 2023;28:1335–1340. [Google Scholar]
- 26.Tao W, Huang J, Jin Y, Peng K, Zhou J. Effect of pulmonary rehabilitation exercise on lung volume and respiratory muscle recovery in lung cancer patients undergoing lobectomy. Altern Ther Health Med. 2024;30:90–96. [PubMed] [Google Scholar]