Abstract
Objectives: This retrospective study mainly analyzed the relationship between prostate tumor overexpressed 1 (PTOV1) and cytokeratin 19 fragment (Cyfra21-1) and neoadjuvant chemosensitivity in patients with lung adenocarcinoma (LUAC). Methods: A total of 110 LUAC patients who received neoadjuvant chemotherapy (cisplatin 75 mg/m2 combined with pemetrexed 500 mg/m2) from February 2022 to February 2024 were selected. They were divided into a chemoresistant group (n=32) and a chemosensitive group (n=78) based on treatment response, and their clinical data were analyzed. Serum levels of PTOV1 and Cyfra21-1 in both groups were measured using enzyme-linked immunosorbent assays for comparison. The association between PTOV1 and Cyfra21-1 and neoadjuvant chemosensitivity was evaluated, along with their predictive value for chemosensitivity in LUAC patients. Logistic multivariate regression analysis was conducted to further explore the factors influencing neoadjuvant chemosensitivity. Results: Significant differences were observed between the chemoresistant and chemosensitive groups in age, distant metastasis, and tumor differentiation. The chemoresistant group showed significantly higher levels of PTOV1 and Cyfra21-1 compared to the chemosensitive group. Both PTOV1 and Cyfra21-1 were significantly correlated with neoadjuvant chemosensitivity, with an area under the curve of no less than 0.700 for predicting chemosensitivity in LUAC patients. Logistic regression analysis indicated that distant metastasis, differentiation status, PTOV1, and Cyfra21-1 were independent factors influencing neoadjuvant chemosensitivity in LUAC patients. Conclusions: PTOV1 and Cyfra21-1 are upregulated in LUAC patients who are resistant to neoadjuvant chemotherapy. Both markers not only have predictive value for chemosensitivity in these patients, but are also independent factors affecting neoadjuvant chemosensitivity.
Keywords: Prostate tumor overexpressed 1, cytokeratin 19 fragment, lung adenocarcinoma, neoadjuvant chemotherapy, chemosensitivity
Introduction
Lung cancer ranks second in incidence and first in mortality, accounting for nearly 12% of all cancers, with a mortality rate of 18% [1]. Non-small-cell lung cancer (NSCLC), particularly lung adenocarcinoma (LUAC), is the most common form of lung cancer [2]. Risk factors for LUAC include environmental tobacco smoke, air pollution, radon and arsenic exposure, and occupational hazards like asbestos [3]. Although early surgery offers favorable outcomes and prognosis for LUAC patients, many miss the window for optimal intervention, leading to poor overall outcomes [4,5]. Standard chemotherapy for LUAC includes cisplatin and pemetrexed, but resistance to chemotherapy remains a significant challenge, resulting in suboptimal efficacy [6,7]. Neoadjuvant chemotherapy (NACT) has proven effective in treating advanced LUAC, but tumor heterogeneity often reduces chemosensitivity, limiting the therapeutic benefits [8]. Therefore, identifying new biomarkers to predict NACT sensitivity in LUAC patients is crucial for improving treatment and prognosis.
Prostate tumor overexpressed 1 (PTOV1) consists of 12 exons and is located on chromosome 19 (19q13) [9]. Initially identified in prostate cancer, PTOV1 is closely linked to tumor proliferation, invasion, and metastasis. It also promotes cell proliferation in cervical cancer as an oncogene [10-12]. Overexpression of PTOV1 has been associated with tumor development and progression in esophageal squamous cell carcinoma, suggesting it could serve as a prognostic marker and therapeutic target [13]. In NSCLC, reducing PTOV1 expression enhances chemosensitivity by weakening cancer stem cell properties [14]. Cytokeratin 19 fragment (Cyfra21-1), a fragment of cytokeratin 19, is a useful biomarker in lung cancer for diagnosis, pathological classification, and staging [15]. According to Bi et al. [16], Cyfra21-1 levels are significantly elevated in lung cancer patients compared to healthy individuals, making it valuable for early diagnosis, differential diagnosis, and monitoring disease progression. Additionally, it can complement contrast-enhanced computerized tomography (CT) in diagnosing NSCLC and evaluating chemotherapy response [17].
This study aims to explore the correlation between PTOV1 and Cyfra21-1 and neoadjuvant chemosensitivity in LUAC patients. The association will be validated through clinical data analysis, correlation studies, and an evaluation of their predictive value for NACT sensitivity.
Materials and methodology
Study population
Inclusion criteria: ① Pathologically confirmed stage III or IV LUAC [18]; ② a Karnovsky Performance Scale (KPS) score ≥60 [19]; ③ an estimated survival >3 months; ④ one or more measurable lesions; ⑤ complete clinical data; ⑥ no contraindications to NACT; ⑦ no prior local radiotherapy or chemotherapy; ⑧ no brain metastasis; ⑨ no deliberate concealment of clinical history; ⑩ at least two cycles of chemotherapy required.
Exclusion criteria: ① Pregnant or lactating women; ② other malignancies or contraindications to concomitant treatments; ③ symptoms of comorbid dyspnea; ④ use of anti-tumor treatments, such as traditional Chinese medicine, during chemotherapy; ⑤ history of anti-tumor therapy or blood transfusion within one month before admission for chemotherapy; ⑥ severe heart, liver, brain, kidney, or hematopoietic dysfunction; ⑦ mental disorders or poor compliance.
The Ethics Committee of Shanxi Medical University approved this retrospective study. A total of 110 LUAC cases admitted to the School of Basic Medical Sciences, Shanxi Medical University, were selected after rigorous screening based on the above inclusion and exclusion criteria from February 2022 to February 2024. All patients received NACT and were categorized into a chemoresistant group (n=32) and a chemosensitive group (n=78) based on treatment response.
NACT regimen
All patients received NACT, with 500 mg/m2 of pemetrexed dissolved in 100 mL of normal saline administered intravenously over 10-30 minutes, and 75 mg/m2 of cisplatin intravenously infused on days 1 to 3, with a 21-day treatment cycle. Patients were assessed by computed tomography (CT), magnetic resonance imaging (MRI), or electrochemotherapy (ECT) before treatment and received at least two cycles of chemotherapy.
Efficacy evaluation
Efficacy was assessed every two cycles of chemotherapy according to the Response Evaluation Criteria for Solid Tumors (RECIST) [20]: Complete remission (CR) is defined as the disappearance of all lesions with no new lesions, lasting for 4 weeks. Partial remission (PR) is a ≥30% reduction in the sum of the longest diameters of baseline target lesions, sustained for 4 weeks. Stable disease (SD) refers to a reduction in the sum of the longest diameters of baseline lesions without reaching PR, or an increase without reaching progressive disease (PD). PD is defined as a ≥20% increase in the sum of the longest diameters of target lesions from the smallest recorded measurement, or the emergence of one or more new lesions. Patients with CR and PR were included in the chemosensitive group, while those with SD and PD were placed in the chemoresistant group.
Detection methods
Cubital venous blood was collected from all patients after fasting, prior to chemotherapy. The serum was separated by centrifugation and stored at low temperatures. Serum PTOV1 and Cyfra21-1 levels were measured using enzyme-linked immunosorbent assays.
Statistical methods
Statistical analyses were performed using SPSS 23.0. Quantitative data were expressed as mean ± standard deviation (±sd), and comparisons between groups were made using t-tests. Categorical data were presented as n (%), and group comparisons were analyzed using χ2 tests. Receiver operating characteristic (ROC) curves were used to determine the thresholds of PTOV1 and Cyfra21-1 for predicting chemoresistance. Independent risk factors influencing neoadjuvant chemosensitivity in LUAC patients were identified through multivariate logistic regression analysis. Statistical significance was set at P<0.05.
The sample size was rigorously selected based on inclusion and exclusion criteria, ensuring that the minimum required sample size (approximately 25 per group) was met, as calculated by the sample size estimation formula. The formula is
where the expected effective rate for the chemoresistant group is p1=30%, the expected effective rate for the chemosensitive group is p2=70%, the significance level (α) is 0.05, the test power (1-β) is 0.80, the combined expected incidence rate is p = (p1 + p2)/2 = 0.50, Z1-α/2 corresponding to α=0.05 is 1.96, and Z1-β corresponding to β=0.20 is 0.84. Using this formula, the estimated sample size for each group was n=24.5, rounded up to 25.
Results
Clinical data analysis of the chemoresistant and chemosensitive groups
The analysis of clinical data (Table 1) revealed no significant differences between the chemoresistant and chemosensitive groups in terms of gender, tumor location, clinical stage, smoking history, or alcohol consumption (all P>0.05). However, significant differences were found in age, distant metastasis, and degree of differentiation (all P<0.05).
Table 1.
Clinical data analysis of the chemoresistant and chemosensitive groups
| Clinical data | Chemoresistant group (n=32) | Chemosensitive group (n=78) | χ2/t | P |
|---|---|---|---|---|
| Sex | 0.019 | 0.890 | ||
| Male | 18 (56.25) | 45 (57.69) | ||
| Female | 14 (43.75) | 33 (42.31) | ||
| Age (years old) | 63.69±5.88 | 60.23±8.59 | 2.084 | 0.040 |
| Tumor site | 0.598 | 0.440 | ||
| Left | 13 (40.63) | 38 (48.72) | ||
| Right | 19 (59.38) | 40 (51.28) | ||
| Clinical staging | 0.186 | 0.666 | ||
| III | 15 (46.88) | 32 (41.03) | ||
| IV | 17 (53.13) | 46 (58.97) | ||
| Distant metastasis | 6.529 | 0.011 | ||
| Without | 12 (37.50) | 50 (64.10) | ||
| With | 20 (62.50) | 28 (35.90) | ||
| Differentiation degree | 10.147 | 0.001 | ||
| Moderately or well differentiated | 9 (28.13) | 48 (61.54) | ||
| Poorly differentiated | 23 (71.88) | 30 (38.46) | ||
| History of smoking | 0.135 | 0.714 | ||
| Without | 16 (50.00) | 36 (46.15) | ||
| With | 16 (50.00) | 42 (53.85) | ||
| History of alcoholism | 1.064 | 0.302 | ||
| Without | 14 (43.75) | 26 (33.33) | ||
| With | 18 (56.25) | 52 (66.67) |
Comparison of PTOV1 and Cyfra21-1 levels between the chemoresistant and chemosensitive groups
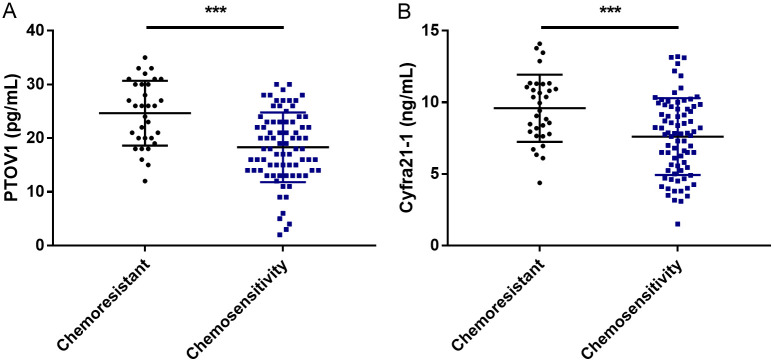
As shown in Figure 1, the PTOV1 levels in the chemoresistant and chemosensitive groups were 24.66±6.04 pg/mL and 18.31±6.49 pg/mL, respectively. The Cyfra21-1 levels were 9.59±2.34 ng/mL in the chemoresistant group and 7.61±2.68 ng/mL in the chemosensitive group. These results indicate significantly higher PTOV1 and Cyfra21-1 levels in the chemoresistant group compared to the chemosensitive group (P<0.001).
Figure 1.
Comparison of PTOV1 and Cyfra21-1 levels between the chemoresistant and chemosensitive groups. A. PTOV1 in the chemoresistant and chemosensitive groups. B. Cyfra21-1 in the chemoresistant and chemosensitive groups. Note: ***P<0.001. PTOV1, prostate tumor overexpressed 1; Cyfra21-1, cytokeratin 19 fragment.
Correlation analysis between PTOV1 and Cyfra21-1 levels and neoadjuvant chemosensitivity
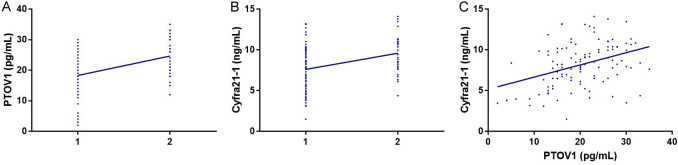
Chemosensitivity was coded as 1 and chemoresistance as 2, and the correlation between PTOV1 and Cyfra21-1 levels and neoadjuvant chemosensitivity was analyzed using Spearman correlation coefficients. As shown in Figure 2, PTOV1 and Cyfra21-1 were both positively correlated with neoadjuvant chemosensitivity (r=0.402, P<0.001; r=0.329, P<0.001). Pearson correlation coefficient analysis also indicated a positive correlation between PTOV1 and Cyfra21-1 (r=0.381, P<0.001).
Figure 2.
Correlation analysis between levels of PTOV1 and Cyfra21-1 and neoadjuvant chemosensitivity. A. Correlation of PTOV1 levels with neoadjuvant chemosensitivity. B. Correlation of Cyfra21-1 levels with neoadjuvant chemosensitivity. C. Correlation between PTOV1 and Cyfra21-1. PTOV1, prostate tumor overexpressed 1; Cyfra21-1, cytokeratin 19 fragment.
Predictive efficacy of PTOV1 and Cyfra21-1 levels for NACT sensitivity in LUAC patients
ROC analysis showed that the area under the curve (AUC) of PTOV1 for predicting NACT sensitivity in LUAC patients was 0.755, with an optimal cutoff value of 17.50, a specificity of 90.63%, and a sensitivity of 46.15%. For Cyfra21-1, the AUC was 0.709, with an optimal cutoff of 10.36, a specificity of 46.88%, and a sensitivity of 88.46%. See Figure 3 and Table 2.
Figure 3.
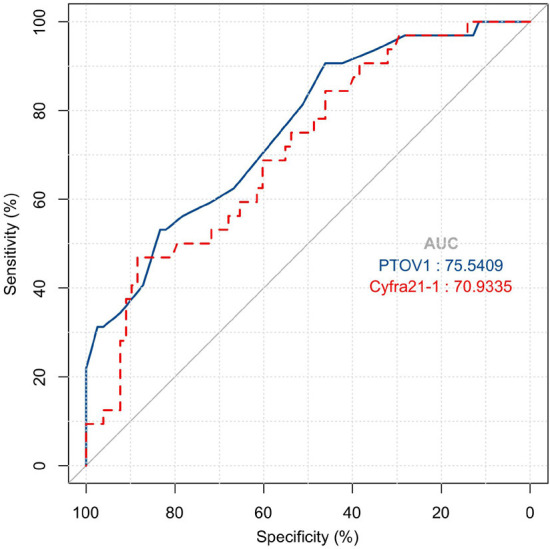
Efficacy of PTOV1 and Cyfra21-1 levels in predicting the sensitivity of LUAC patients to neoadjuvant chemotherapy. Note: PTOV1, prostate tumor overexpressed 1; Cyfra21-1, cytokeratin 19 fragment; LUAC, lung adenocarcinoma; AUC, area under the curve.
Table 2.
Efficacy of PTOV1 and Cyfra21-1 levels in predicting neoadjuvant chemosensitivity in lung adenocarcinoma patients
| Indicators | AUC | Standard error | 95% CI | Cut-off value | Specificity (%) | Sensitivity (%) |
|---|---|---|---|---|---|---|
| PTOV1 | 0.755 | 0.050 | 0.658-0.853 | 17.50 | 90.63 | 46.15 |
| Cyfra21-1 | 0.709 | 0.053 | 0.606-0.813 | 10.36 | 46.88 | 88.46 |
Note: PTOV1, prostate tumor overexpressed 1; Cyfra21-1, cytokeratin 19 fragment; AUC, area under the curve.
Multivariate logistic regression analysis of factors affecting NACT sensitivity in LUAC patients
Factors showing significant differences, such as age, distant metastasis, differentiation degree, PTOV1, and Cyfra21-1, were included and assigned (Table 3), followed by Logistic multiple regression analysis (Table 4). The results showed that distant metastasis (OR: 4.947, 95% CI: 1.600-15.300), differentiation degree (OR: 5.522, 95% CI: 1.685-18.091), PTOV1 (OR: 16.133, 95% CI: 3.263-79.758), and Cyfra21-1 (OR: 5.683, 95% CI: 1.532-21.088) were independent factors influencing NACT in LUAC patients (all P<0.05).
Table 3.
Assignment table
| Variable | Assignment |
|---|---|
| Age (years old) | <60 = 0 (n=66), ≥60 = 1 (n=44) |
| Distant metastasis | Without = 0 (n=62), with = 1 (n=48) |
| Differentiation degree | Medium-high differentiation = 0 (n=57), low differentiation = 1 (n=53) |
| PTOV1 (pg/mL) | <17.50 = 0 (n=40), ≥17.50 = 1 (n=70) |
| Cyfra21-1 (ng/mL) | <10.36 = 0 (n=86), ≥10.36 = 1 (n=24) |
Note: PTOV1, prostate tumor overexpressed 1; Cyfra21-1, cytokeratin 19 fragment.
Table 4.
Multivariate logistic regression analysis of related factors affecting neoadjuvant chemosensitivity in lung adenocarcinoma patients
| Variable | β | SE | Wald | P | Exp (β) | 95% CI |
|---|---|---|---|---|---|---|
| Age (years old) | 0.895 | 0.607 | 2.176 | 0.140 | 2.448 | 0.745-8.041 |
| Distant metastasis | 1.599 | 0.576 | 7.705 | 0.006 | 4.947 | 1.600-15.300 |
| Differentiation degree | 1.709 | 0.605 | 7.964 | 0.005 | 5.522 | 1.685-18.091 |
| PTOV1 (pg/mL) | 2.781 | 0.815 | 11.631 | 0.001 | 16.133 | 3.263-79.758 |
| Cyfra21-1 (ng/mL) | 1.738 | 0.669 | 6.746 | 0.009 | 5.683 | 1.532-21.088 |
Note: PTOV1, prostate tumor overexpressed 1; Cyfra21-1, cytokeratin 19 fragment.
Discussion
The efficacy of conventional therapies for LUAC patients remains limited, with a five-year survival rate of only 15% [21]. Even with NACT, the improvement in the five-year survival rate is modest, at just 5% [22]. To enhance chemosensitivity and improve long-term disease control, it is essential to identify novel biomarkers that can provide clinical guidance and predictive value.
Exploring predictors of chemotherapy efficacy in NSCLC has long been a challenging research area. Currently, some markers have been validated, including excision repair cross-complementing gene 1 (ERCC1) and ribonucleotide reductase catalytic subunit M1 (RRM1) for platinum-based drugs, gemcitabine, and pemetrexed; human equilibrative nucleoside transporter 1 (hENT1) for gemcitabine; and centromeric region of chromosome 17 (CEP17; 17p11.1-q11.1) for anthracyclines [23]. However, few studies have investigated the correlation between PTOV1, Cyfra21-1, and neoadjuvant chemotherapy sensitivity in LUAC patients. This study aims to further explore biomarkers with both specificity and sensitivity for such patients.
First, we found that PTOV1 and Cyfra21-1 levels were significantly higher in the chemoresistant group compared to the chemosensitive group, suggesting that these markers may contribute to NACT (cisplatin+pemetrexed) resistance in LUAC patients. Elevated levels of these markers may inhibit sensitivity to NACT. Previous studies have shown that PTOV1 promotes cisplatin resistance, possibly by increasing the activity of the nuclear factor kappa-B (NF-κB) pathway [24]. Moreover, downregulating PTOV1 has been shown to enhance chemosensitivity to cisplatin and docetaxel, potentially by inhibiting Dickkopf-related protein 1 (DKK1)/β-catenin signaling [14]. Wang et al. [25] also reported that high Cyfra21-1 levels are associated with lower chemoradiotherapy efficacy in NSCLC patients, consistent with our findings.
Second, we observed a strong positive correlation between PTOV1 and Cyfra21-1 levels and neoadjuvant chemosensitivity, as well as a significant correlation between the two markers themselves, suggesting they may play a synergistic role in influencing chemosensitivity in LUAC patients. Cánovas et al. [26] found a correlation between PTOV1 and docetaxel resistance in prostate tumors, similar to our results.
According to ROC analysis, the AUC for PTOV1 and Cyfra21-1 in predicting NACT sensitivity in LUAC patients was 0.755 and 0.709, respectively. PTOV1 had a specificity of 90.63%, while Cyfra21-1 demonstrated a sensitivity of 88.46%. These findings indicate that PTOV1 and Cyfra21-1 can effectively distinguish between NACT-sensitive and NACT-resistant patients. In line with our observations, Wu Z et al. [14] reported that high PTOV1 levels can predict shorter survival and a poorer chemotherapy response in NSCLC patients. Pang et al. [27] highlighted that Cyfra21-1, as the most sensitive serum tumor marker for predicting chemotherapy response, can be used to forecast the response of NSCLC patients to various chemotherapy regimens, which supports our findings. Fu et al. [28] also reported that Cyfra21-1 aids in distinguishing benign lung disease from lung cancer, as well as separating both benign lung disease and lung cancer from healthy individuals. According to multivariate logistic regression analysis, distant metastasis, differentiation degree, PTOV1, and Cyfra21-1 were identified as independent factors influencing neoadjuvant chemosensitivity in LUAC patients.
In the study by Lu et al. [29], age and platinum-based chemotherapy were identified as factors affecting first-line chemotherapy sensitivity in advanced non-squamous NSCLC through univariate analysis. However, multivariate analysis revealed that neither age nor platinum was independently correlated with chemotherapy sensitivity, consistent with our findings. In the multivariate analysis by Yang et al. [30], a ≥60% reduction in Cyfra21-1 was found to be a prognostic factor for improved survival in advanced NSCLC patients, and this marker was helpful in predicting chemotherapy response, further supporting our results. Similarly, Cox regression conducted by Reinmuth et al. [31] identified Cyfra21-1 as an independent prognostic factor for overall survival and disease-free interval in patients with completely resected NSCLC. Patients with distant metastasis and poor differentiation tend to have worse treatment responses due to the more advanced nature of their disease.
Several limitations of this study warrant further consideration. First, the imbalance in sample size between the chemoresistant group (n=32) and the chemosensitive group (n=78) could have influenced the results. Second, this study is not a multicenter prospective study, and future multicenter prospective research will enhance the accuracy and generalizability of the findings. Third, no basic mechanistic research was performed. Supplementary analyses exploring the underlying mechanisms of chemotherapy sensitivity and the target pathways of PTOV1 and Cyfra21-1 would provide a deeper understanding of their roles. Future research will focus on these aspects to improve and expand upon the current study.
In conclusion, PTOV1 and Cyfra21-1 are upregulated in LUAC patients with chemoresistance, and both are closely associated with neoadjuvant chemosensitivity, possibly working synergistically. Additionally, distant metastasis and differentiation degree, alongside PTOV1 and Cyfra21-1, are independent factors affecting neoadjuvant chemosensitivity in LUAC patients. For patients with high PTOV1, high Cyfra21-1, distant metastasis, and poor differentiation, alternative therapies should be considered to avoid the low efficacy or ineffectiveness of NACT (cisplatin combined with pemetrexed).
Disclosure of conflict of interest
None.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Liu H, Li G. What’s the difference between lung adenocarcinoma and lung squamous cell carcinoma? Evidence from a retrospective analysis in a cohort of Chinese patients. Front Endocrinol (Lausanne) 2022;13:947443. doi: 10.3389/fendo.2022.947443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu HI, Chiang CJ, Su SY, Jhuang JR, Tsai DR, Yang YW, Lin LJ, Wang YC, Lee WC. Incidence trends and spatial distributions of lung adenocarcinoma and squamous cell carcinoma in Taiwan. Sci Rep. 2023;13:1655. doi: 10.1038/s41598-023-28253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montagne F, Guisier F, Venissac N, Baste JM. The role of surgery in lung cancer treatment: present indications and future perspectives-state of the art. Cancers (Basel) 2021;13:3711. doi: 10.3390/cancers13153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Ma X, Shen X, Wang S, Li Y, Hu H, Chen H. Surgery for pre- and minimally invasive lung adenocarcinoma. J Thorac Cardiovasc Surg. 2022;163:456–464. doi: 10.1016/j.jtcvs.2020.11.151. [DOI] [PubMed] [Google Scholar]
- 6.Zheng F, Xu R. CircPVT1 contributes to chemotherapy resistance of lung adenocarcinoma through miR-145-5p/ABCC1 axis. Biomed Pharmacother. 2020;124:109828. doi: 10.1016/j.biopha.2020.109828. [DOI] [PubMed] [Google Scholar]
- 7.Breathnach OS, Freidlin B, Conley B, Green MR, Johnson DH, Gandara DR, O’Connell M, Shepherd FA, Johnson BE. Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J. Clin. Oncol. 2001;19:1734–1742. doi: 10.1200/JCO.2001.19.6.1734. [DOI] [PubMed] [Google Scholar]
- 8.Seguin L, Durandy M, Feral CC. Lung adenocarcinoma tumor origin: a guide for personalized medicine. Cancers (Basel) 2022;14:1759. doi: 10.3390/cancers14071759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Li N, Tian G. Prognostic significance of PTOV1 expression in cancers: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e28149. doi: 10.1097/MD.0000000000028149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Li Z, Wan S, Li Y, Ye Z, Li D. Pan-cancer analysis confirms the prognostic and immunological effects of prostate tumor overexpressed-1 in human cancers. Curr Cancer Drug Targets. 2023 doi: 10.2174/1568009623666230316153813. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Pennington KL, McEwan CM, Woods J, Muir CM, Pramoda Sahankumari AG, Eastmond R, Balasooriya ER, Egbert CM, Kaur S, Heaton T, McCormack KK, Piccolo SR, Kurokawa M, Andersen JL. SGK2, 14-3-3, and HUWE1 cooperate to control the localization, stability, and function of the oncoprotein PTOV1. Mol Cancer Res. 2022;20:231–243. doi: 10.1158/1541-7786.MCR-20-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Hu Z, Chai D. High expression of prostate tumor overexpressed 1 (PTOV1) is a potential prognostic biomarker for cervical cancer. Int J Clin Exp Pathol. 2017;10:11044–11050. [PMC free article] [PubMed] [Google Scholar]
- 13.Li R, Leng AM, Liu XM, Hu TZ, Zhang LF, Li M, Jiang XX, Zhou YW, Xu CX. Overexpressed PTOV1 associates with tumorigenesis and progression of esophageal squamous cell carcinoma. Tumour Biol. 2017;39:1010428317705013. doi: 10.1177/1010428317705013. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Liu Z, Jiang X, Mi Z, Meng M, Wang H, Zhao J, Zheng B, Yuan Z. Depleting PTOV1 sensitizes non-small cell lung cancer cells to chemotherapy through attenuating cancer stem cell traits. J Exp Clin Cancer Res. 2019;38:341. doi: 10.1186/s13046-019-1349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Chen Y, Wang X, Wang C, Xiao M. The value of combined detection of CEA, CYFRA21-1, SCC-Ag, and pro-GRP in the differential diagnosis of lung cancer. Transl Cancer Res. 2021;10:1900–1906. doi: 10.21037/tcr-21-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi H, Yin L, Fang W, Song S, Wu S, Shen J. Association of CEA, NSE, CYFRA 21-1, SCC-Ag, and ProGRP with clinicopathological characteristics and chemotherapeutic outcomes of lung cancer. Lab Med. 2023;54:372–379. doi: 10.1093/labmed/lmac122. [DOI] [PubMed] [Google Scholar]
- 17.He X, Wang M. Application value of serum TK1 and PCDGF, CYFRA21-1, NSE, and CEA plus enhanced CT scan in the diagnosis of nonsmall cell lung cancer and chemotherapy monitoring. J Oncol. 2022;2022:8800787. doi: 10.1155/2022/8800787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, Veronesi G, Reck M ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:358–376. doi: 10.1016/j.annonc.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Park H, Chung HT, Kim JW, Dho YS, Lee EJ. A 3-month survival model after Gamma Knife surgery in patients with brain metastasis from lung cancer with Karnofsky performance status ≤ 70. Sci Rep. 2023;13:13159. doi: 10.1038/s41598-023-40356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart LL, Ferrarotto R, Andric ZG, Beck JT, Subramanian J, Radosavljevic DZ, Zaric B, Hanna WT, Aljumaily R, Owonikoko TK, Verhoeven D, Xiao J, Morris SR, Antal JM, Hussein MA. Myelopreservation with trilaciclib in patients receiving topotecan for small cell lung cancer: results from a randomized, double-blind, placebo-controlled phase II study. Adv Ther. 2021;38:350–365. doi: 10.1007/s12325-020-01538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spella M, Stathopoulos GT. Immune resistance in lung adenocarcinoma. Cancers (Basel) 2021;13:384. doi: 10.3390/cancers13030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai F, Wu X, Wang X, Li K, Wang Y, Shen C, Zhou J, Niu H, Deng B, Tan Q, Wang R, Guo W. Neoadjuvant immunotherapy combined with chemotherapy significantly improved patients’ overall survival when compared with neoadjuvant chemotherapy in non-small cell lung cancer: a cohort study. Front Oncol. 2022;12:1022123. doi: 10.3389/fonc.2022.1022123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olaussen KA, Postel-Vinay S. Predictors of chemotherapy efficacy in non-small-cell lung cancer: a challenging landscape. Ann Oncol. 2016;27:2004–2016. doi: 10.1093/annonc/mdw321. [DOI] [PubMed] [Google Scholar]
- 24.Shen H, Liao B, Wan Z, Zhao Y, You Z, Liu J, Lan J, He S. PTOV1 promotes cisplatin-induced chemotherapy resistance by activating the nuclear factor kappa B pathway in ovarian cancer. Mol Ther Oncolytics. 2021;20:499–507. doi: 10.1016/j.omto.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Yi Y, Li B, Wang Z, Sun H, Zhang P, Huang W. CYFRA21-1 can predict the sensitivity to chemoradiotherapy of non-small-cell lung carcinoma. Biomarkers. 2010;15:594–601. doi: 10.3109/1354750X.2010.504308. [DOI] [PubMed] [Google Scholar]
- 26.Canovas V, Punal Y, Maggio V, Redondo E, Marin M, Mellado B, Olivan M, Lleonart M, Planas J, Morote J, Paciucci R. Prostate tumor overexpressed-1 (PTOV1) promotes docetaxel-resistance and survival of castration resistant prostate cancer cells. Oncotarget. 2017;8:59165–59180. doi: 10.18632/oncotarget.19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang L, Wang J, Jiang Y, Chen L. Decreased levels of serum cytokeratin 19 fragment CYFRA 21-1 predict objective response to chemotherapy in patients with non-small cell lung cancer. Exp Ther Med. 2013;6:355–360. doi: 10.3892/etm.2013.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Li D, Zhu Y, Yan S, Wang X, Lian Z, Xie Q, He Z. Application value of CYFRA21-1 combined with NSE, CEA, and SCC-Ag in lung cancer. Clin Lab. 2024;70 doi: 10.7754/Clin.Lab.2023.230662. [DOI] [PubMed] [Google Scholar]
- 29.Lu YY, Huang XE, Xu L, Liu DG, Cao J, Wu XY, Liu J, Xiang J. Potential predictors of sensitivity to pemetrexed as first-line chemotherapy for patients with advanced non-squamous NSCLCs. Asian Pac J Cancer Prev. 2013;14:2005–2008. doi: 10.7314/apjcp.2013.14.3.2005. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Chen X, Li Y, Yang J, Tang L. Declines in serum CYFRA21-1 and carcinoembryonic antigen as predictors of chemotherapy response and survival in patients with advanced non-small cell lung cancer. Exp Ther Med. 2012;4:243–248. doi: 10.3892/etm.2012.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinmuth N, Brandt B, Semik M, Kunze WP, Achatzy R, Scheld HH, Broermann P, Berdel WE, Macha HN, Thomas M. Prognostic impact of Cyfra21-1 and other serum markers in completely resected non-small cell lung cancer. Lung Cancer. 2002;36:265–270. doi: 10.1016/s0169-5002(02)00009-0. [DOI] [PubMed] [Google Scholar]