Abstract
Objectives: To explore the risk factors associated with immune-related recurrent spontaneous abortion (IRSA) and to develop a predictive model to identify high-risk individuals, aiming to provide theoretical guidance for RSA prevention. Methods: This retrospective study included 120 patients with RSA who were treated at Jiangxi University of Traditional Chinese Medicine Hospital between January 2022 and January 2024. Patients were divided into two groups based on the nature of their miscarriages: the IRSA group (observation group, n=58) and the non-IRSA group (control group, n=62). Univariate and multivariate analyses were conducted to identify independent risk factors for IRSA. Using these factors, a nomogram prediction model was constructed and validated with R software (version 4.1.2). Results: Independent prognostic factors for immune-related RSA included average platelet aggregation rate (95% CI 1.005-4.387; P=0.027), hemoglobin (95% CI 0.856-6.205; P=0.014), antiphospholipid antibodies (95% CI 0.954-5.465; P=0.024), triglycerides (TG) (95% CI 1.009-8.369; P=0.016), and free triiodothyronine (FT3) (95% CI 1.236-7.069; P=0.026). Using these factors, a nomogram was developed for predicting immune-related RSA incidence. The model achieved an AUC of 0.7975882 (95% CI 0.635-0.947), indicating good predictive accuracy, and decision curve analysis demonstrated positive net benefits. Conclusion: FT3, TG, antiphospholipid antibodies, hemoglobin, and average platelet aggregation rate are independent risk factors for IRSA onset.
Keywords: Immune, recurrent spontaneous abortion, risk factors, predictive models
Introduction
Recurrent spontaneous abortion (RSA) refers to the consecutive loss of two or more fetuses before 28 weeks of pregnancy, including consecutive biochemical pregnancies. Its incidence rate ranges from 1-5% [1,2]. The etiology of RSA is multifactorial and includes immunological factors (autoimmunity, alloimmunity), anatomical abnormalities (e.g., intrauterine adhesions, uterine fibroids), endocrine abnormalities (e.g., insulin resistance, polycystic ovary syndrome, thyroid dysfunction), and chromosomal abnormalities in either the embryo or the parents (e.g., chromosomal translocations, aneuploidies) [3-6]. Recent research indicates that approximately 50-60% of RSA cases are related to immune dysfunction, leading to the classification of such cases as immune-related recurrent spontaneous abortion (IRSA) [7,8].
The etiology of IRSA remains largely unknown, imposing considerable psychological stress on affected individuals and presenting a significant clinical challenge. Factors contributing to IRSA are complex and may include genetic predispositions, reproductive anatomy, endocrine imbalances, infections, immune dysregulation, thrombotic disorders, and other influences (e.g., environmental exposures, lifestyle factors, physical and psychological stress, and medication use) [9-11]. Dinda et al. suggest that genetic polymorphisms might predispose certain individuals to immune abnormalities associated with RSA, potentially affecting immune responses and increasing susceptibility [12]. Consequently, patients with IRSA are highly susceptible to psychological issues, with about 40% experiencing depression or anxiety to varying degrees [13].
Identifying patients at risk for IRSA based on personal and medical history prior to conception could enable proactive diagnostic and therapeutic interventions, reducing the incidence of IRSA. However, to our knowledge, no prediction model currently exists to assess IRSA risk.
Given the complexity of IRSA and recent advancements in related research, establishing a comprehensive, standardized etiological screening process for IRSA patients is essential. This approach involves identifying IRSA-related risk factors, predicting its occurrence, and implementing early interventions to prevent further miscarriages and improve pregnancy outcomes. This study aims to identify IRSA risk factors and develop a predictive model to support more effective clinical management of IRSA.
Methods
Study design and patient selection
The medical records of 120 patients with recurrent spontaneous abortion treated at Jiangxi University of Traditional Chinese Medicine Hospital between January 2022 and January 2024 were retrospectively reviewed. Patients were categorized into two groups based on the etiology of miscarriage: the IRSA group (observation group, n=58) and the non-IRSA group (control group, n=62). The protocol was approved by the Ethics Committee of Jiangxi University of Traditional Chinese Medicine Hospital.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients with two or more instances of abortion, biochemical pregnancy, or embryo implantation failure. (2) Patients with a normal chromosomal karyotype. (3) Patients aged ≥18 years without significant hearing or vision impairment, or dementia. (4) Patients with a normal uterine cavity, as confirmed by ultrasound or hysteroscopy.
Exclusion criteria: (1) Patients diagnosed with polycystic ovary syndrome or uterine fibroids. (2) Patients with genital tract infections, including positive findings in cervical/vaginal secretions and viral infection screenings (e.g., TORCH). (3) Patients with prior immunotherapy. (4) Patients with infectious, endocrine, genetic diseases, or hypercoagulable conditions.
Diagnostic criteria
(1) Abortion: Defined as the termination of pregnancy before 28 weeks of gestation, with fetal weight less than 1000 grams [14]. (2) Biochemical Pregnancy: A condition where human chorionic gonadotropin (β-hCG) levels in blood or urine exceed normal values, but no gestational sac is visible on ultrasound [15]. (3) IRSA: Characterized by a history of two or more consecutive miscarriages without a live birth or stillbirth, with no chromosomal or anatomical abnormalities detected in routine etiological screening, and without concurrent infectious or endocrine diseases [16].
Outcome measures
Detailed patient information was retrieved from the hospital information system (HIS), including clinical characteristics, medical history, and both clinical and biochemical indicators. Then the primary outcomes covered the concordance index (c-index), a calibration curve, DCA, and area under the receiver operating characteristic (ROC) curve (AUC). The secondary outcomes included the clinical data and laboratory indicators. Clinical characteristics encompassed age, body mass index (BMI), smoking and alcohol history, previous gestational weeks of miscarriage, antiphospholipid antibody, and abortion frequency. Laboratory indicators included routine blood tests, thyroid function, coagulation-related indicators (DIC, thromboelastogram), 25-hydroxyvitamin D, erythrocyte sedimentation rate, glucose metabolism, blood lipids, renal function, and antiphospholipid syndrome (APS) markers (quantitative assays of anticardiolipin, anti-β2 glycoprotein 1 antibody, and lupus anticoagulant).
Sample size estimation
Sample size was calculated using power analysis, following the formula: corrected sample size = sample size/(1 - [% attrition/100]) [17]. A final sample size of approximately 100 was determined.
Statistical analysis
Data analysis and statistical plotting were performed using IBM SPSS 25.0 (IBM, New York, USA) and R software (Version 4.2.0, https://www.r-project.org/). For normally distributed data, values were expressed as mean ± standard deviation (mean ± SD) and analyzed using the two-sample t-test. Non-normally distributed data were presented as median and quartiles, with the Mann-Whitney U test used for comparisons. Categorical data were expressed as frequency and compared using the chi-square or Fisher’s exact test.
Variables with statistically significant differences were included in a binary multivariate logistic regression analysis to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for independent predictors of immune-related RSA. Statistical significance was set at P<0.05.
A nomogram for predicting immune-related RSA was constructed using R software. The model’s discriminatory ability was assessed using the ROC curve and AUC. Calibration was evaluated with a calibration plot, and clinical applicability was determined through a Decision Curve Analysis (DCA) plot.
Results
Comparison of general characteristics between the two groups
No significant differences were observed between the two groups in general clinical data such as age, antinuclear antibody presence, smoking, drinking, and previous gestational weeks of miscarriage (all P>0.05). However, significant differences were found in abortion frequency, antiphospholipid antibodies, 25(OH)VD3, maximum platelet aggregation rate, and average platelet aggregation rate (all P<0.05) (Table 1).
Table 1.
Comparison of general characteristics between the two groups
| Control group (n=62) | Observation group (n=58) | χ2/t | P | |
|---|---|---|---|---|
| Age | 32.95±4.65 | 32.64±5.21 | 0.543 | 0.587 |
| ≥35 | 16 (25.81%) | 19 (32.76%) | ||
| 30-35 | 20 (32.26%) | 21 (36.21%) | ||
| ≤30 | 26 (41.93%) | 18 (31.03%) | ||
| Abortion frequency | 9.087 | 0.006 | ||
| ≥3 | 14 (22.58%) | 28 (48.28%) | ||
| <3 | 48 (77.42%) | 30 (50.72%) | ||
| Antiphospholipid antibody | 4.784 | 0.027 | ||
| Single Yang | 14 (22.58%) | 22 (37.93%) | ||
| Shuangyang | 16 (25.81%) | 14 (24.14%) | ||
| San Yang | 2 (3.23%) | 0 (0.00%) | ||
| Antinuclear antibody | 42 (67.74%) | 37 (63.79%) | 0.897 | 0.357 |
| 25(OH)VD3 | 28.65±9.64 | 23.41±8.64 | -3.078 | 0.001 |
| Maximum platelet aggregation rate | 56.78±17.48 | 6.08±1.57 | 1.987 | 0.047 |
| Average platelet aggregation rate | 52.47±18.27 | 68.57±20.85 | 6.459 | 0.012 |
| BMI | 20.11±1.27 | 20.89±2.27 | 0.398 | 0.078 |
| Smoking | 14 (22.58%) | 13 (22.41%) | 0.108 | 0.754 |
| Drinking | 13 (20.97%) | 13 (22.41%) | 1.497 | 0.475 |
| Previous gestational weeks of miscarriage | 13.43±3.45 | 16.38±2.89 | 1.574 | 0.207 |
Note: BMI: body mass index, 25(OH)VD3: 25-hydroxyvitamin D3.
Comparison of endocrine-related index levels between the two groups
Significant differences were identified between the groups in levels of 2h insulin, FT3, TSH, and TG (all P<0.05). However, there were no significant differences in T3, T4, FT4, thyroglobulin, thyroid globulin antibody, peroxidase antibody, fasting blood glucose, fasting insulin, C-peptide at fasting, 2h blood glucose, 2h C-peptide, CHO, HDL, and LDL levels between the two groups (all P>0.05) (Table 2).
Table 2.
Comparison of endocrine-related index levels between the two groups
| Control group (n=62) | Observation group (n=58) | t | p | |
|---|---|---|---|---|
| T3 | 1.78±0.25 | 1.87±0.21 | 1.009 | 0.165 |
| T4 | 112.23±24.01 | 112.54±17.04 | 0.278 | 0.112 |
| FT3 | 5.65±1.65 | 10.18±0.57 | 5.498 | 0.024 |
| FT4 | 19.87±12.65 | 16.54±2.87 | 0.487 | 0.123 |
| TSH | 12.68±8.54 | 15.98±8.51 | 6.125 | 0.012 |
| Thyroglobulin | 15.65±6.24 | 12.34±9.54 | 1.665 | 0.154 |
| Thyroid globulin antibody | 50.24±7.45 | 49.58±7.41 | 1.024 | 0.124 |
| Peroxidase antibody | 56.24±7.68 | 56.41±7.12 | 0.098 | 0.147 |
| Fasting blood glucose | 4.98±0.64 | 4.82±0.41 | 0.287 | 0.087 |
| Fasting insulin | 11.65±6.34 | 10.87±3.54 | 0.065 | 0.090 |
| Empty dispersed C-peptide | 1.69±0.54 | 1.28±0.54 | 0.125 | 0.128 |
| 2h blood glucose | 5.64±1.12 | 5.89±1.32 | 0.089 | 0.336 |
| 2h insulin | 46.57±32.45 | 42.74±28.65 | 0.274 | 0.047 |
| 2h C-peptide | 4.56±1.98 | 4.69±1.81 | 0.015 | 0.189 |
| CHO | 3.98±1.89 | 3.97±0.79 | 0.256 | 0.178 |
| TG | 1.28±1.01 | 6.87±0.37 | 3.897 | 0.048 |
| HDL | 1.56±0.54 | 1.98±0.24 | 0.087 | 0.124 |
| LDL | 2.29±0.72 | 2.18±0.87 | 0.474 | 0.074 |
Note: T3: triiodothyronine, T4: thyroxine, FT3: Free Triiodothyronine, FT4: Free Thyroxine, FSH: Thyroid Stimulating Hormone, CHO: cholesterol, TG: triglyceride, HDL: high-density lipoprotein, LDL: low-density lipoprotein.
Comparison of routine blood parameters between the two groups
The two groups showed no significant differences in routine blood parameters, including white blood cell (WBC), neutrophil ratio, lymphocyte ratio, monocyte ratio, eosinophil ratio, basophil ratio, neutrophil count, lymphocyte count, monocyte count, eosinophil count, basophil count, and red blood cell (RBC) count (all P>0.05). However, there was a significant difference in hemoglobin levels between the two groups (P<0.05) (Table 3).
Table 3.
Comparison of routine blood parameters between the two groups
| Observation group (n=58) | Control group (n=62) | t | p | |
|---|---|---|---|---|
| WBC | 6.87±4.65 | 6.48±1.78 | 0.398 | 0.178 |
| Neutrophil ratio | 0.78±0.31 | 1.03±0.56 | 0.278 | 0.165 |
| Lymphocyte ratio | 0.36±0.16 | 0.36±0.18 | 0.871 | 0.187 |
| Monocyte ratio | 0.16±0.07 | 0.21±0.14 | 0.487 | 0.154 |
| Eosinophil ratio | 0.49±0.28 | 0.59±0.27 | 0.874 | 0.289 |
| Basophil ratio | 0.59±0.47 | 0.64±0.52 | 1.264 | 0.254 |
| Neutrophil count | 3.98±1.65 | 3.64±1.56 | 1.007 | 0.165 |
| Lymphocyte count | 1.89±0.56 | 1.59±0.54 | 0.458 | 0.154 |
| Number of monocytes | 0.36±0.14 | 0.64±0.24 | 1.364 | 0.178 |
| Number of eosinophils | 0.13±0.05 | 0.45±0.04 | 0.174 | 0.297 |
| Number of basophils | 0.46±0.16 | 0.65±0.11 | 0.184 | 0.123 |
| RBC | 4.69±0.37 | 4.49±0.78 | 0.174 | 0.378 |
| Hemoglobin | 96.87±12.87 | 139.25±20.87 | -7.156 | 0.034 |
Note: WBC: White Blood Cell, RBC: Red Blood Cell.
Univariate and multivariate analysis
Variables such as abortion frequency, 25(OH)VD3, maximum platelet aggregation rate, average platelet aggregation rate, 2h insulin, hemoglobin, antiphospholipid antibody, TG, FT3, and TSH were included in the univariate analysis. The univariate analysis showed significant associations for average platelet aggregation rate (95% CI 0.656-1.595; P=0.040), hemoglobin (95% CI 0.504-0.987; P=0.037), antiphospholipid antibody (95% CI 1.039-1.513; P=0.018), TG (95% CI 1.306-3.132; P=0.002), and FT3 (95% CI 1.574-8.235; P=0.003) (Table 4). These factors were then incorporated into the multivariate analysis, which confirmed that average platelet aggregation rate (95% CI 1.005-4.387; P=0.027), hemoglobin (95% CI 0.856-6.205; P=0.014), antiphospholipid antibody (95% CI 0.954-5.465; P=0.024), TG (95% CI 1.009-8.369; P=0.016), and FT3 (95% CI 1.236-7.069; P=0.026) are independent prognostic factors for immune-related RSA (Table 5).
Table 4.
Univariate analysis of influencing factors of IRSA
| Index | β | SE | OR (95% CI) | Wald | P |
|---|---|---|---|---|---|
| Abortion frequency | -0.049 | 0.143 | 0.952 (0.719-1.261) | 0.116 | 0.733 |
| 25(OH)VD3 | -0.078 | 0.099 | 0.925 (0.761-1.123) | 0.623 | 0.430 |
| Maximum platelet aggregation rate | -1.069 | 0.743 | 0.343 (0.080-1.473) | 2.070 | 0.150 |
| Average platelet aggregation rate | 0.069 | 0.743 | 0.343 (0.656-1.595) | 7.070 | 0.040 |
| 2h insulin | 0.004 | 0.002 | 1.004 (0.999-1.009) | 2.534 | 0.111 |
| Hemoglobin | 0.001 | 0.007 | 0.714 (0.504, 0.987) | 7.798 | 0.037 |
| Antiphospholipid antibody | 0.226 | 0.096 | 1.254 (1.039-1.513) | 5.580 | 0.018 |
| TG | 0.704 | 0.223 | 2.023 (1.306-3.132) | 9.970 | 0.002 |
| FT3 | 0.609 | 0.333 | 3.589 (1.574, 8.235) | 10.378 | 0.003 |
| TSH | 0.007 | 0.009 | 1.007 (0.990-1.025) | 0.628 | 0.428 |
Note: 25(OH)VD3: 25-hydroxyvitamin D3, TG: triglyceride, FT3: Free Triiodothyronine, TSH: Thyroid Stimulating Hormone, IRSA: immune-related recurrent spontaneous abortion.
Table 5.
Multivariate analysis of influencing factors of IRSA
| β | SE | p | OR (95% CI) | |
|---|---|---|---|---|
| Average platelet aggregation rate | 4.569 | 1 | 0.027 | 1.333 (1.005-4.387) |
| Hemoglobin | -3.387 | 1 | 0.014 | 1.287 (0.856-6.205) |
| Antiphospholipid antibody | -3.987 | 1 | 0.024 | 3.498 (0.954-5.465) |
| TG | 4.897 | 1 | 0.016 | 4.398 (1.009-8.369) |
| FT3 | 5.687 | 1 | 0.026 | 2.398 (1.236-7.069) |
Note: β: regression coefficient, SE: standard error, OR: odds ratio, TG: triglyceride, FT3: Free Triiodothyronine, IRSA: immune-related recurrent spontaneous abortion.
Development and validation of the nomogram
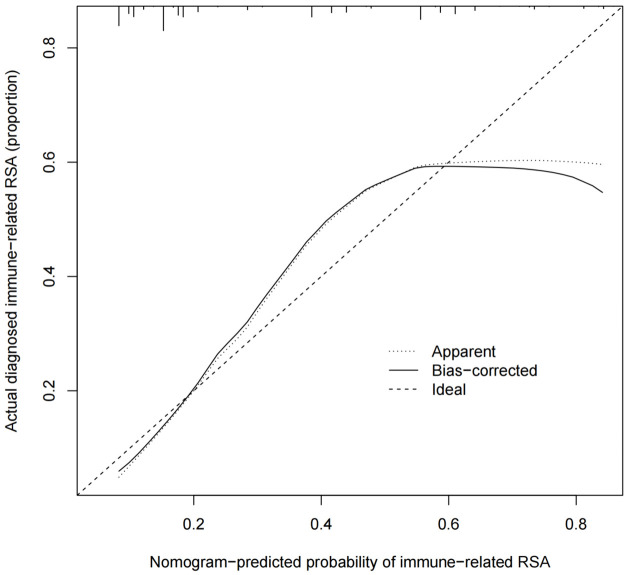
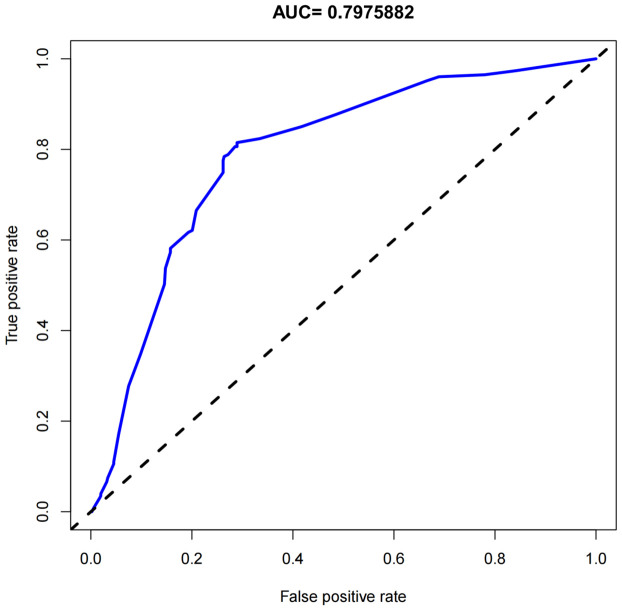
Using results from the multivariate logistic regression analysis, a nomogram was developed incorporating the independent risk factors FT3, TG, antiphospholipid antibody, hemoglobin, and average platelet aggregation rate (Figure 1). The calibration curve (Figure 2) in the training set demonstrated close alignment with the ideal curve, indicating that the model’s predictions for immune-related RSA risk are consistent with actual risk, confirming high model accuracy. Additionally, the area under the ROC curve (AUC) was 0.798, suggesting good predictive performance for immune-related RSA (Figure 3).
Figure 1.
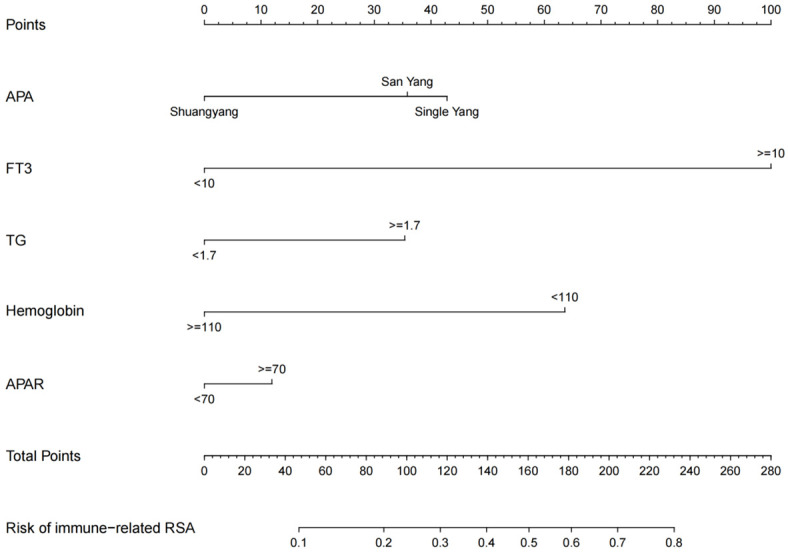
Nomogram for predicting IRSA. IRSA: immune-related recurrent spontaneous abortion, TG: triglyceride, FT3: Free Triiodothyronine, APA: Antiphospholipid antibody, APAR: Average platelet aggregation rate.
Figure 2.
Calibration curve of the nomogram. RSA: Related recurrent spontaneous abortion.
Figure 3.
ROC curve area. The value of the area under ROC curve (AUC) was 0.7975882 (95% confidence interval of 0.635-0.947). ROC: Receiver Operating Characteristic.
Clinical utility evaluation and validation
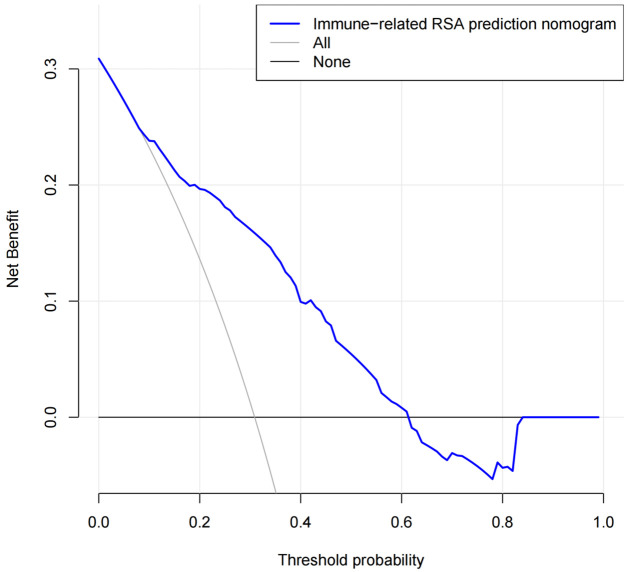
DCA indicated that the nomogram provides a high level of clinical utility for predicting immune-related RSA risk (Figure 4). DCA findings suggest that, within a predicted probability range of 20%-60% for immune-related RSA, application of the nomogram model is beneficial in clinical settings.
Figure 4.
Decision curve analysis of the nomogram model. RSA: Related recurrent spontaneous abortion.
Discussion
This study retrospectively analyzed 120 patients with RSA, focusing on general clinical data and various clinical indicators to identify independent risk factors for IRSA. The findings revealed that FT3, TG, antiphospholipid antibody, hemoglobin, and average platelet aggregation rate are independent risk factors for IRSA onset. Using these five identified risk factors, a predictive model for IRSA risk was successfully developed, evaluated, and validated.
FT3 emerged as an independent risk factor for immune-related RSA. Studies indicate that thyroid hormones play a crucial role in regulating physiological processes, including reproduction. Specifically, imbalances in thyroid function, particularly alterations in FT3 levels, can significantly affect the immune system and pregnancy outcomes [17-19]. Thyroid hormone receptors are widely expressed in immune cells, and changes in FT3 levels can directly impact immune cell function and behavior. Abnormal FT3 levels may lead immune cells to produce excessive pro-inflammatory cytokines or alter immune regulation, thereby exacerbating immune-mediated pregnancy damage. Elevated FT3 levels can disrupt immune system function, leading to imbalances in immune regulation that affect homeostasis. This imbalance may result in excessive immune cell activation and dysregulated cytokine production [20]. Moreover, abnormal thyroid hormone levels, such as elevated FT3, can adversely affect the endometrium, which is critical for embryo implantation and maintenance, leading to inadequate endometrial receptivity and impairing proper embryo development [21]. Thus, monitoring and maintaining appropriate thyroid function, including FT3 levels, may be crucial in managing at-risk pregnancies and improving outcomes.
Additionally, TG was identified as an independent risk factor for immune-related RSA. Elevated triglycerides can contribute to endothelial dysfunction and inflammation, potentially causing abnormal placental blood vessel function. This affects the supply of nutrients and oxygen to the fetus, raising the risk of miscarriage [22]. Dyslipidemia, particularly elevated triglycerides, can disrupt immune balance by promoting immune cell activation and pro-inflammatory cytokine release, which negatively impacts the pregnancy environment and fetal development, contributing to RSA [23]. Lipid accumulation and related metabolic disturbances may also influence reproductive hormone function and signaling pathways, further impacting pregnancy stability and progression [24].
The results of this study indicate that antiphospholipid antibodies are independent risk factors for IRSA. Antiphospholipid antibodies can interact with cell membranes and proteins, activating platelets and the coagulation cascade, ultimately resulting in a hypercoagulable state [25]. This hypercoagulability can lead to placental thrombosis, infarction, and reduced blood supply to the fetus, which may result in miscarriage. Additionally, antiphospholipid antibodies can directly target trophoblast cells, impairing their functions in implantation, invasion, and development [26]. This interference can compromise placental integrity and functionality, affecting nutrient and gas exchange between mother and fetus. Furthermore, antiphospholipid antibodies can induce an inflammatory response, damaging placental tissues and endothelial cells, thereby compromising pregnancy viability [27]. The presence of these antibodies disrupts multiple aspects of placental function and immune regulation, significantly increasing the risk of IRSA.
This study also suggests that hemoglobin levels are independent risk factors for immune-related RSA. Low hemoglobin levels may result in insufficient oxygen supply to tissues, including the reproductive system [28], potentially disrupting the physiological functions of the uterus and placenta. This can interfere with embryo implantation and development, thereby increasing RSA risk. Moreover, low hemoglobin levels may signal underlying health issues or nutritional deficiencies that adversely impact the immune system [29]. Such deficiencies may contribute to immune imbalance, disrupting the tolerance required for successful pregnancy and promoting RSA. Additionally, anemia from low hemoglobin can alter metabolism and microenvironmental conditions, potentially destabilizing pregnancy and increasing susceptibility to immune-related complications [30].
The study also found that average platelet aggregation rate is an independent risk factor for IRSA. Platelets play a pivotal role in immune response. An elevated platelet aggregation rate may signal abnormal platelet activation, which can interact with immune cells and mediators, potentially leading to immune dysregulation in the reproductive system [31]. Abnormal platelet aggregation may impair microcirculation in reproductive organs, reducing nutrient and oxygen delivery, which could damage the endometrium and other tissues, hindering embryo implantation and development [32].
This study does have a few limitations. Firstly, due to the small sample size, single-center design, and retrospective nature of the analysis, there may be inherent biases. Although we made efforts to ensure sample representativeness, the findings may differ in broader populations. Secondly, some relevant factors may not have been fully considered in the analysis. Despite conducting a comprehensive literature review and data analysis, potential factors may have been overlooked. Lastly, while the prediction model underwent internal validation, it lacks external validation, which may affect its accuracy and applicability across different clinical settings and patient groups. Nevertheless, a series of measures were implemented to minimize these limitations. We adhered strictly to scientific protocols in study design and data analysis, carefully interpreting and discussing the results. These limitations are also clearly noted to advise readers to exercise caution when referencing and applying the findings. Future research will aim to expand the sample size, conduct further in-depth studies, and refine the model to improve reliability and applicability.
In conclusion, this study developed a nomogram prediction model based on risk factors for IRSA, demonstrating good predictive performance, high accuracy, and clinical applicability. The model is simple, easy to implement in clinical practice, safe and non-invasive, and widely accepted by both doctors and patients, aiding in the early identification of high-risk populations for IRSA. This model not only enhances the detection rate of IRSA but also reduces complications associated with excessive invasive examinations, providing a cost-effective means for IRSA screening in clinical practice with substantial medical and social significance.
Disclosure of conflict of interest
None.
References
- 1.Yao Y, Ye Y, Chen J, Zhang M, Cai X, Zheng C. Maternal-fetal immunity and recurrent spontaneous abortion. Am J Reprod Immunol. 2024;91:e13859. doi: 10.1111/aji.13859. [DOI] [PubMed] [Google Scholar]
- 2.Deng T, Liao X, Zhu S. Recent advances in treatment of recurrent spontaneous abortion. Obstet Gynecol Surv. 2022;77:355–366. doi: 10.1097/OGX.0000000000001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li MM, Lin J, Wu HF, Zheng GJ, Cai RN. Analysis of the risk factors in patients with unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2023;90:e13774. doi: 10.1111/aji.13774. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Zheng L, Zhao D, Xu Y, Wang Y. The role of immune cells in recurrent spontaneous abortion. Reprod Sci. 2021;28:3303–3315. doi: 10.1007/s43032-021-00599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu A, Zhao Y, Yu R, Zhou J, Tuo Y. Untargeted metabolomics analysis reveals the metabolic disturbances and exacerbation of oxidative stress in recurrent spontaneous abortion. PLoS One. 2023;18:e0296122. doi: 10.1371/journal.pone.0296122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafiee M, Sereshki N, Alipour R, Ahmadipanah V, Pashoutan Sarvar D, Wilkinson D. The effect of probiotics on immunogenicity of spermatozoa in couples suffering from recurrent spontaneous abortion. BMC Immunol. 2022;23:32. doi: 10.1186/s12865-022-00506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang C, Hu W. Non-coding RNA regulates the immune microenvironment in recurrent spontaneous abortion (RSA): new insights into immune mechanisms† . Biol Reprod. 2024;110:220–229. doi: 10.1093/biolre/ioad157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu H, Hu X, Huang X, Yin T, Liu L, Yue C, Du M. Causal relationship between circulating immune cells and recurrent spontaneous abortion: a bidirectional mendelian randomization study. Am J Reprod Immunol. 2024;91:e13888. doi: 10.1111/aji.13888. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Song QL, Ji R, Wang JY, Cao ML, Guo DY, Zhang Y, Yang Jl. JPT2 affects trophoblast functions and macrophage polarization and metabolism, and acts as a potential therapeutic target for recurrent spontaneous abortion. Adv Sci (Weinh) 2024;11:e2306359. doi: 10.1002/advs.202306359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Tan YQ, Wang FY. Tim-3: an inhibitory immune checkpoint is associated with maternal-fetal tolerance and recurrent spontaneous abortion. Clin Immunol. 2022;245:109185. doi: 10.1016/j.clim.2022.109185. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Lu Y, Qi Q, Li M, Zhou J, Wang J, Lin J, Cao L, Du Y, Li L, Wang L. Advanced age - a critical risk factor for recurrent miscarriage. Glob Health Med. 2023;5:316–318. doi: 10.35772/ghm.2023.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinda RN, Iman R, Mariana M. Risk factors of spontaneous abortion. Sriw J Med. 2022;5:137–141. [Google Scholar]
- 13.Ning L, Wang X, Xuan B, Ma Y, Yan Y, Gao Z, Tong T, Cui Z, Chen H, Li X, Hong J, Wang Z. Identification and investigation of depression-related molecular subtypes in inflammatory bowel disease and the anti-inflammatory mechanisms of paroxetine. Front Immunol. 2023;14:1145070. doi: 10.3389/fimmu.2023.1145070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall LL, Yemane A. Infectious complications of abortion. Open Forum Infect Dis. 2022;9:ofac553. doi: 10.1093/ofid/ofac553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neill S. Management of early pregnancy loss. JAMA. 2023;329:1399–1400. doi: 10.1001/jama.2023.0933. [DOI] [PubMed] [Google Scholar]
- 16.Mu F, Wang M, Zeng X, Wang F. Predicting risk of subsequent pregnancy loss among women with recurrent pregnancy loss: an immunological factor-based multivariable model. Am J Reprod Immunol. 2024;91:e13837. doi: 10.1111/aji.13837. [DOI] [PubMed] [Google Scholar]
- 17.Vomstein K, Egerup P, Kolte AM, Behrendt-Møller I, Boje AD, Bertelsen ML, Eiken CS, Reiersen MR, Toth B, la Cour Freiesleben N, Nielsen HS. Biopsy-free profiling of the uterine immune system in patients with recurrent pregnancy loss and unexplained infertility. Reprod Biomed Online. 2023;47:103207. doi: 10.1016/j.rbmo.2023.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Wieringa JW, Driessen GJ, Van Der Woude CJ. Pregnant women with inflammatory bowel disease: the effects of biologicals on pregnancy, outcome of infants, and the developing immune system. Expert Rev Gastroenterol Hepatol. 2018;12:811–818. doi: 10.1080/17474124.2018.1496820. [DOI] [PubMed] [Google Scholar]
- 19.Andersen SL, Christensen PA, Knøsgaard L, Andersen S, Handberg A, Hansen AB, Vestergaard P. Classification of thyroid dysfunction in pregnant women differs by analytical method and type of thyroid function test. J Clin Endocrinol Metab. 2020;105:dgaa567. doi: 10.1210/clinem/dgaa567. [DOI] [PubMed] [Google Scholar]
- 20.Kravchenko V, Zakharchenko T. Thyroid hormones and minerals in immunocorrection of disorders in autoimmune thyroid diseases. Front Endocrinol (Lausanne) 2023;14:1225494. doi: 10.3389/fendo.2023.1225494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum Reprod Update. 2014;20:884–904. doi: 10.1093/humupd/dmu028. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Sun J, Jin L. The N6-methyladenosine regulator ALKBH5 mediated stromal cell-macrophage interaction via VEGF signaling to promote recurrent spontaneous abortion: a bioinformatic and in vitro study. Int J Mol Sci. 2022;23:15819. doi: 10.3390/ijms232415819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Tan D, Hao B, Zhang X, Geng W, Wang Y, Sun J, Zhao Y. Efficacy of intravenous immunoglobulin in the treatment of recurrent spontaneous abortion: a systematic review and meta-analysis. Am J Reprod Immunol. 2022;88:e13615. doi: 10.1111/aji.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai M, Hong L, Yin T, Liu S. Disturbed follicular microenvironment in polycystic ovary syndrome: relationship to oocyte quality and infertility. Endocrinology. 2024;165:bqae023. doi: 10.1210/endocr/bqae023. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Sun S, Xu H, Zhang X, Chen C, Fu R, Li C, Guo F, Zhao A. Prognostic analysis of antibody typing and treatment for antiphospholipid syndrome-related recurrent spontaneous abortion. Int J Gynaecol Obstet. 2022;156:107–111. doi: 10.1002/ijgo.13621. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, He L. Aspirin and heparin in the treatment of recurrent spontaneous abortion associated with antiphospholipid antibody syndrome: a systematic review and meta-analysis. Exp Ther Med. 2021;21:57. doi: 10.3892/etm.2020.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu C, Liu Y, Jiang HL. Aspirin or heparin or both in the treatment of recurrent spontaneous abortion in women with antiphospholipid antibody syndrome: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2019;32:1299–1311. doi: 10.1080/14767058.2017.1404979. [DOI] [PubMed] [Google Scholar]
- 28.Zhao QY, Li QH, Fu YY, Ren CE, Jiang AF, Meng YH. Decidual macrophages in recurrent spontaneous abortion. Front Immunol. 2022;13:994888. doi: 10.3389/fimmu.2022.994888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naaz A, Muneshwar KN. How maternal nutritional and mental health affects child health during pregnancy: a narrative review. Cureus. 2023;15:e48763. doi: 10.7759/cureus.48763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerra A, Parhiz H, Rivella S. Novel potential therapeutics to modify iron metabolism and red cell synthesis in diseases associated with defective erythropoiesis. Haematologica. 2023;108:2582–2593. doi: 10.3324/haematol.2023.283057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La X, Wang W, Zhang M, Liang L. Definition and multiple factors of recurrent spontaneous abortion. Adv Exp Med Biol. 2021;1300:231–257. doi: 10.1007/978-981-33-4187-6_11. [DOI] [PubMed] [Google Scholar]
- 32.Wang XH, Xu S, Zhou XY, Zhao R, Lin Y, Cao J, Zang WD, Tao H, Xu W, Li MQ, Zhao SM, Jin LP, Zhao JY. Low chorionic villous succinate accumulation associates with recurrent spontaneous abortion risk. Nat Commun. 2021;12:3428. doi: 10.1038/s41467-021-23827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]