Abstract
Objective: To assess and compare the clinical efficacy of scar pregnancy debridement by a combination of reversible bilateral uterine and internal iliac artery blockade with hysterolaparoscopy, as a management strategy for cesarean scar pregnancy. Methods: This retrospective study included patients diagnosed with cesarean scar pregnancy who underwent combined surgical intervention involving hysteroscopy and laparoscopy between May 2020 and February 2024. The study population was divided into two groups based on the type of arterial blockade used: a uterine artery blockade group and an internal iliac artery blockade group. Data were retrospectively collected from patient medical records, including baseline characteristics, surgery-related details, and postoperative outcome. Result: The initial data revealed no discernible differences between the two groups in terms of age, number of caesarean sections, menstrual period, menstrual flow (light/medium/heavy), presence of dysmenorrhea, days of menopause, maximum human chorionic gonadotropin (hCG) levels, gestational tissue size on ultrasound imaging, reproductive hormones levels (E2/FSH/LH/progesterone), or anti-Müllerian hormone (AMH) (all P > 0.05). There were no apparent associations between the two groups in terms of the occurrence of adverse pregnancy outcome or ultrasound findings. However, the uterine artery group demonstrated shorter operative time, less bleeding, fewer postoperative hospital days, and lower overall hospital costs compared to the internal iliac artery group. Furthermore, the uterine artery group exhibited greater improvements in hCG and progesterone levels, menstrual periods, menstrual flow and dysmenorrhea than the internal iliac artery group. Additionally, the quality-of-life scores and a cumulative pregnancy rate were both significantly higher than in the control group. Conclusion: The uterine artery group exhibited superior efficacy in the removal of scar pregnancies compared to the internal iliac artery group, under reversible uterine artery blockade by uterolaparoscopy in conjunction with bilateral uterine artery ligation. This procedure should be considered the preferred surgical approach for the aforementioned indication.
Keywords: Uterolaparoscopy, uterine artery, internal iliac artery, scarred pregnancy
Introduction
A uterine scar pregnancy following Caesarean section represents a distinct form of ectopic pregnancy, one of the complications following Caesarean section that with long-term side effects [1,2]. This specific form of ectopic pregnancy is characterized by the implantation of the gestational tissue within the uterine scar tissue left from a previous Caesarean section. Moreover, the pregnancy is entirely situated outside the uterine cavity, encompassed by myometrium and fibrous scar tissue. Uterine scar pregnancies are associated with significant medical complications [3]. At present, patients with Cesarean Scar Pregnancy (CSP) diagnosed in our hospital mainly undergo Uterine Artery Embolization (UAE) as an important auxiliary means prior to curettage or lesion resection to reduce the risk of major bleeding. However, there are drawbacks such as high treatment cost, complications related to uterine embolization, inability to complete subsequent surgeries simultaneously with the embolization procedure, and prolonged hospital stays. If not addressed properly, they may result in uterine rupture, hemorrhage and even life-threatening complications.
The aim of this study is to evaluate the clinical efficacy of two types of reversible arterial blockades for the surgical repair of uterine scars through a minimally invasive laparoscopic approach. The goal is to identify a treatment method that is minimally invasive, with advantages of less damage, faster healing, high cure rate, and safety.
Materials and methods
General information
This retrospective study analyzed clinical data from 142 patients diagnosed with cesarean scar pregnancy who were treated at Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, between May 2020 and February 2024. The patient cohort was segregated into two groups based on the vascular conduit utilized during the surgical operation, a uterine artery blockade group and an internal iliac artery blockade group. Selection for uterine artery intervention or internal iliac artery intervention was based on the size and depth of the scar pregnancy, as determined by ultrasound or MRI imaging. Larger or more deeply embedded pregnancies with extensive vascularization were typically treated with uterine artery intervention, while smaller, more localized pregnancies were deemed suitable for internal iliac artery intervention. The patient’s reproductive history and any contraindications were also considered in the decision-making process.
The uterine artery intervention group comprised 78 patients, while the internal iliac artery intervention group comprised 64 patients. In the uterine artery group, the scar pregnancies were removed under reversible blockade of the uterine artery ligation using uterolaparoscopy. In the internal iliac artery group, the scar pregnancies were removed under the reversible blockade of the bilateral internal iliac artery ligation in conjugation with uterolaparoscopy. Key baseline variables, such as age, number of caesarean sections, and menstrual period, were selected for analysis. A direct matching method was employed to ensure comparability between the two groups. Finally, a total of 50 patients were included in each group for the purpose of the study.
Subjects were included if they met the diagnostic criteria outlined in the Expert Consensus on the Diagnosis and Treatment of Uterine Scar Pregnancy after Cesarean Section, had a documented history of prior caesarean sections, and exhibited serum-β human chorionic gonadotropin (β-hCG) levels greater than 5000 mIU/mL. Cesarean scar pregnancy was diagnosed by anterior ultrasound or MRI, with some patients presenting with symptoms such as mild vaginal bleeding and abdominal pain. In other cases, the diagnosis was made by ultrasound or MRI following a medicated abortion or negative-pressure suction surgery. The patients’ data were complete. Patients were excluded if they exhibited unstable vital signs, significant cardiac, hepatic, or renal impairment, coagulation disorders, a history of uterine hemorrhage, or contrast allergy, as these conditions posed an elevated risk of complications.
This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University.
Surgical method
All patients were admitted to the hospital following a confirmed diagnosis. During the operation, laparoscopy was used to access the abdomen, followed by the opening of the bladder and a peritoneal push to expose the scar pregnancy mass. For patients with scar pregnancies, the uterine artery group underwent pregnancy removal under reversible uterine artery blockade, performed using a combination of laparoscopy and bilateral uterine artery ligation. In comparison, the internal iliac artery group underwent a similar procedure, but with reversible bilateral internal iliac artery blockade. Hysteroscopy was utilized to inspect the uterine cavity for residual pregnancy tissue and to examine the scar site. This procedure also assessed the presence or absence of a diverticulum and evaluated the degree of scar alignment. To prevent infection, patients who had undergone surgical treatment received symptomatic care postoperatively.
Collection of indicators
Baseline data were collected from all included cases, including age, number of prior caesarean sections, length of menstrual period, volume and character of menstrual blood, and menopausal characteristics such as dysmenorrhea and the duration of the menopausal phase. Additionally, serum level of HCG and the maximum diameter of the gestational tissue observed under ultrasound imaging were recorded. Reproductive hormone levels, including estradiol (E2), follicle-stimulating hormone (FSH), luteinizing hormone (LH), progesterone, and antimullerian hormone (AMH), were also evaluated. A comprehensive dataset related to surgical procedures was compiled for both groups, encompassing variables such as surgical duration, postoperative hemorrhage, length of hospital stay, and total hospital expenditure. After resumption of menstruation, hCG, reproductive hormone levels, AMH levels, menstrual cycle characteristics (period length, flow volume), and the presence or absence of dysmenorrhea were collected for both groups over a three-month follow-up period post-surgery. Ultrasound data, including uterine position, depths of uterine cavity penetration, and the thicknesses of residual uterine muscle, were also extracted from both groups of patients.
The patient quality of life for post-intervention was assessed using the SF-36 scale, a comprehensive tool that evaluates four domains: psychological, physiological, social, and environmental. Each domain is scored on a 100-point scale, with higher scores indicating better quality of life.
Endpoint
Data from the medical records of inpatients, outpatients, and patients followed up by telephone were extracted. The endpoints of the study were defined as pregnancy, delivery, and the end of follow-up, which was 31 January 2024. Information regarding pregnancy outcome, including miscarriage, ectopic pregnancy, and fetal survival, was gathered.
Data extraction principles
To guarantee the reliability and integrity of the extracted data, the following principles were employed: First, comprehensive data sets were collected, encompassing all relevant baseline data, surgical and postoperative follow-up data. Second, standardized diagnostic and examination techniques were used throughout the study. Third, a rigorous screening procedure was conducted in line with the established inclusion and exclusion criteria; and finally, robust data protection measures were implemented to guarantee data confidentiality.
Statistical methods
The collected data were analyzed using SPSS 22.0 statistical software. The Shapiro-Wilk method was employed to determine the data distribution. The variables with normal distribution were expressed as the mean and standard deviation (x̅±s). Independent samples t-test was applied to compare between the two groups. The variables not conforming to normal distribution were compared using the Mann-Whitney U test between the two groups. Counted data were expressed as a rate (%) and compared between groups using the chi-square test, the continuity-corrected test, or Fisher’s exact probability test as appropriate. A significant difference was set as P value < 0.05.
Results
Comparison of baseline data between the two groups of patients
No significant differences were observed between the two groups in terms of age, prior Caesarean sections, menstrual period and characters, reproductive hormone levels (E2, FSH, LH, progesterone, AMH), and HCG levels, etc. (P > 0.05, Table 1), indicating the two groups were comparable.
Table 1.
Comparison of baseline information between the two groups
| Index | Uterine Artery Group (n=50) | Internal Iliac Artery Group (n=50) | t/χ2/Z | P |
|---|---|---|---|---|
| Age (years) | 29.34 ± 4.26 | 30.16 ± 5.18 | -0.865 | 0.389 |
| Cesarean sections (times) | 1 (1, 2) | 1 (1, 2) | -1.210 | 0.226 |
| Menstrual period (days) | 12.26 ± 3.19 | 12.86 ± 3.91 | -0.841 | 0.403 |
| Menstrual flow [n (%)] | 0.204 | 0.903 | ||
| Less | 23 (46.00) | 21 (42.00) | ||
| Medium | 5 (10.00) | 6 (12.00) | ||
| Most | 22 (44.00) | 23 (46.00) | ||
| Dysmenorrhea [n (%)] | 0.396 | 0.529 | ||
| Yes | 34 (68.00) | 31 (62.00) | ||
| No | 16 (32.00) | 19 (38.00) | ||
| Days to menopause (days) | 44.82 ± 5.26 | 45.00 ± 5.69 | -0.164 | 0.870 |
| Maximum hCG value (U/L) | 54989.68 ± 512.00 | 54893.90 ± 787.90 | 0.721 | 0.473 |
| Maximum diameter of pregnancy (cm) | 3.12 ± 1.69 | 3.20 ± 1.41 | -0.257 | 0.798 |
| E2 (pmol/L) | 216.25 ± 11.52 | 218.52 ± 15.36 | -0.836 | 0.405 |
| FSH (U/L) | 6.78 ± 1.59 | 6.89 ± 1.45 | -0.360 | 0.719 |
| LH (U/L) | 7.52 ± 1.25 | 7.45 ± 1.63 | 0.240 | 0.811 |
| Progesterone (nmol/L) | 27.52 ± 6.26 | 27.85 ± 6.87 | -0.251 | 0.803 |
| AMH (ng/mL) | 4.50 ± 2.12 | 4.58 ± 2.15 | -0.187 | 0.852 |
HCG: human chorionic gonadotropin; E2: estradiol/estradiol-sulfate; FSH: follicle-stimulating hormone; LH: luteinizing hormone; AMH: antimüllerian hormone.
Comparison of surgical data between the two groups
Table 2 provides analysis of the surgical data, including the duration of surgery, postoperative hospital days, and total hospital costs for both groups. A comparison between the two groups demonstrated that the patients undergoing uterine artery intervention exhibited shorter operating time, less bleeding, fewer postoperative hospital days, and lower total hospital costs compared to those in the internal iliac artery group (P < 0.05).
Table 2.
Comparison of surgery-related data between the two groups of patients
| Item | Uterine artery group (n=50) | Internal iliac artery group (n=50) | t | P |
|---|---|---|---|---|
| Surgery duration (min) | 55.58 ± 18.57 | 86.52 ± 12.47 | -9.782 | < 0.001 |
| Hemorrhage (mL) | 99.30 ± 15.17 | 150.28 ± 25.67 | -12.089 | < 0.001 |
| Hospital stay (d) | 7.26 ± 2.65 | 13.56 ± 5.23 | -7.595 | < 0.001 |
| Total hospitalization costs (RMB) | 820.48 ± 102.00 | 1025.54 ± 100.55 | -10.126 | < 0.001 |
Comparison of post-operative menstruation indices between the two groups
After the treatment, the concentrations of hCG and progesterone decreased greatly in both groups. Notably, the uterine artery group exhibited significantly lower levels of HCG and progesterone levels compared to those in the internal iliac artery group (all P < 0.05). However, there was no statistically significant difference in the post-operative levels of E2, FSH, LH, and AMH observed between the two groups (all P > 0.05) (Table 3).
Table 3.
Comparison of postoperative hormone levels between the two groups of patients
| Index | Uterine artery group (n=50) | Internal iliac artery group (n=50) | t | P |
|---|---|---|---|---|
| LH (U/L) | 8.22 ± 2.85 | 8.54 ± 2.96 | -0.552 | 0.582 |
| hCG (U/L) | 21210.00 ± 510.00 | 38474.50 ± 625.00 | -151.335 | < 0.001 |
| E2 (pmol/L) | 200.22 ± 15.20 | 205.58 ± 12.05 | -1.954 | 0.054 |
| FSH (U/L) | 7.25 ± 2.10 | 7.85 ± 2.35 | -1.343 | 0.182 |
| Progesterone (nmol/L) | 5.54 ± 1.52 | 7.44 ± 1.68 | -5.932 | < 0.001 |
| AMH (ng/mL) | 2.18 ± 0.52 | 2.31 ± 0.76 | -0.998 | 0.321 |
hCG: human chorionic gonadotropin; E2: estradiol/estradiol-sulfate; FSH: follicle-stimulating hormone; LH: luteinizing hormone; AMH: antimüllerian hormone.
A comparison of postoperative indicators at three months between the two groups
A comparison of the mean menstruation days, the proportion of regular menstruation, and dysmenorrhea was conducted between the two groups. The uterine artery group demonstrated better outcomes in all three indices (all P < 0.05). Definitions for menstruation periods were as follows: ‘shorter’ refers to a menstruation period lasting fewer than 4 days with total blood loss of less than 30 mL, ‘regular’ refers to a period lasting 4 to 6 days with blood loss between 30 and 60 mL, and ‘lengthy’ refers to periods lasting more than 6 days or with blood loss exceeding 60 mL. These results are detailed in Table 4.
Table 4.
Comparison of menstruation relevant indices at 3 months postoperatively between the two groups of patients
| Index | Uterine artery group (n=50) | Internal iliac artery group (n=50) | χ2/Z | P |
|---|---|---|---|---|
| Mean menstruation days | 6 (5, 7) | 9 (7, 9.25) | -5.555 | < 0.001 |
| Menstruation duration [n (%)] | 8.136 | 0.017 | ||
| Shorter | 12 (24.00) | 19 (38.00) | ||
| Regular | 18 (36.00) | 6 (12.00) | ||
| Lengthy | 20 (40.00) | 25 (50.00) | ||
| Dysmenorrhea [n (%)] | 4.857 | 0.028 | ||
| Yes | 19 (38.00) | 28 (56.00) | ||
| No | 31 (62.00) | 22 (44.00) |
Comparison of ultrasound findings between the two groups
The statistical comparison of ultrasound outcomes between the two patient groups revealed no significant difference (all P > 0.05, Table 5), except the median uterine cavity depth showed a P value of 0.043.
Table 5.
Comparison of ultrasound findings between the two groups of patients
| Index | Uterine artery group (n=50) | Internal iliac artery group (n=50) | t/χ2 | P |
|---|---|---|---|---|
| Uterine Position [n, %] | 2.219 | 0.528 | ||
| AVF | 12 (24.00) | 17 (34.00) | ||
| Stretched | 10 (20.00) | 6 (12.00) | ||
| RVF | 21 (42.00) | 22 (44.00) | ||
| Extreme RVF | 7 (14.00) | 5 (10.00) | ||
| Median Uterine Cavity Depth (mm) | 10.35 ± 4.50 | 8.50 ± 4.20 | 2.057 | 0.043 |
| Median Residual Fiber Thickness (mm) | 0.92 ± 0.40 | 1.00 ± 0.48 | 1.537 | 0.128 |
AVF: Anteverted Flexed; RVF: Retroverted Flexed.
Comparison of quality of life between the two groups
A comparison of the quality-of-life scores between the two groups revealed that patients in uterine artery group had significantly higher quality-of-life scores across all domains than the internal iliac artery group (all P < 0.05, Table 6).
Table 6.
Comparison of quality-of-life indicators between the two groups of patients (x̅±s, Points)
| Index | Uterine artery group (n=50) | Internal iliac artery group (n=50) | t | P |
|---|---|---|---|---|
| Mental function | 90.28 ± 5.43 | 80.28 ± 6.33 | 8.479 | < 0.001 |
| Physiologic function | 90.76 ± 5.37 | 80.52 ± 5.45 | 9.462 | < 0.001 |
| Environment | 91.40 ± 5.64 | 81.24 ± 5.37 | 9.229 | < 0.001 |
| Social function | 90.44 ± 5.95 | 79.58 ± 5.54 | 9.452 | < 0.001 |
Comparison of adverse pregnancy outcomes between the two groups
The statistical analysis demonstrated that there was no significant difference in the incidence of adverse pregnancy outcomes between the two groups (P > 0.05), as detailed in Table 7.
Table 7.
Comparison of adverse pregnancy outcomes between the two groups of patients
| Index | Uterine artery group (n=50) | Internal iliac artery group (n=50) | χ2 | P |
|---|---|---|---|---|
| Abortion [n (%)] | 3 (6.00) | 4 (8.00) | 1.667 | 0.435 |
| Ectopic Pregnancy [n (%)] | 0 (0.00) | 1 (2.00) | ||
| Neonatal Asphyxia [n (%)] | 1 (2.00) | 1 (2.00) |
Comparison of successful pregnancy rates between the two groups
The mean duration of the observation period was 13.10 ± 6.57 months, with 43 pregnancies in the uterine artery group and 34 pregnancies in the internal iliac artery group. As demonstrated in Figure 1, the cumulative pregnancy rate was significantly higher in the uterine artery group compared to the internal iliac artery group.
Figure 1.
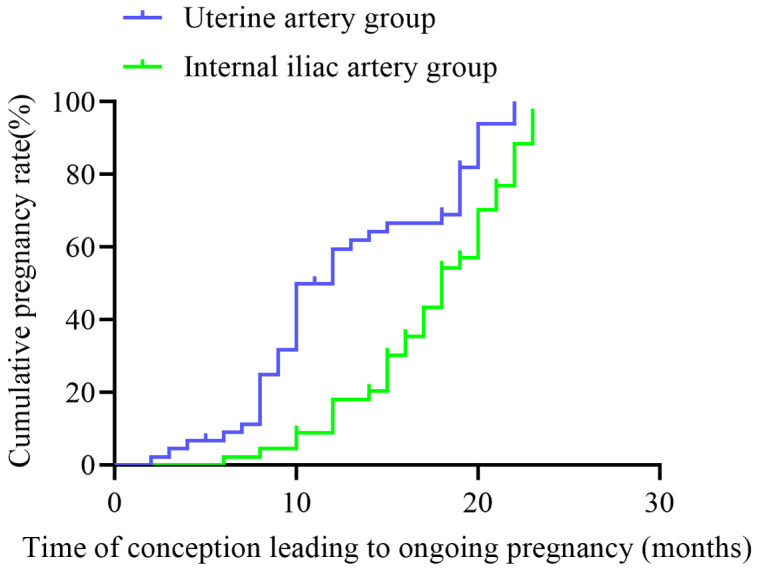
Comparison of distant pregnancy rates between two distinct groups of patients.
Discussion
Despite a relatively low incidence, scar pregnancy has been on the rise, particularly in recent years [4]. This increase is attributed, in part, to the growing rate of caesarean sections, coupled with repeated abortions and damage to the endometrium caused by the procedure itself. The most prominent clinical symptoms are recurrent or abrupt heavy bleeding during early pregnancy or hemorrhage during abortion [5]. Additionally, positive blood and urine chorionic gonadotropin tests are indicative of this condition. Furthermore, an ultrasound examination reveals a gestational sac at the uterine scar, accompanied by abundant periphery blood flow signals and the gestational sac reaching the uterine plasma membrane, with no discernible boundary between the sac and the bladder [6,7]. Misdiagnosis of uterine scar pregnancy can occur easily, and without timely diagnosis and termination of pregnancy, the consequences can be severe, including uncontrollable hemorrhage, potentially leading to complications such as uterine removal or life-threatening injury [8,9]. However, there is no uniform treatment principle. Consequently, when a patient is diagnosed with uterine scar pregnancy [10], a comprehensive assessment of the individual’s circumstances is essential, particularly regarding the desire for future childbearing. This enables the selection of the most appropriate treatment plan following a comprehensive assessment. With the advancement of medical technology, hysteroscopy has become an invaluable tool in clinical practice. For patients presenting with an endogenous, stable CSP, hysteroscopic surgery is considered the optimal primary treatment modality, offering significant benefits including enhanced safety, effectiveness, rapid postoperative recovery, and a shorter hospital stay [11,12]. Hysteroscopy allows for direct visualization of the gestational sac, enabling the avoidance of blood vessels and precise removal of the pregnancy lesion. Simultaneously, electrocoagulation of the bleeding site helps to significantly reduce intraoperative blood loss [13].
Nevertheless, studies have demonstrated that for patients with a manifest proclivity towards bleeding and substantial gestational sacs, direct hysteroscopic electrodesiccation may induce significant hemorrhage, complicating the surgical procedures and extending operative time. Furthermore, it may result in inadequate electrodesiccation, whereby residual lesions persist within the uterine cavity, giving rise to long-term complications. The technique of uterine artery embolization (UAE) has become more established, and patients with CSP can be treated with UAE combined with hysteroscopic electrosurgery [14]. Prior to hysteroscopic electrosurgery, UAE is carried out to reduce blood flow around the gestational sac and lower B-hCG level, leading to the deformation and necrosis of chorionic villi and reducing the size of the mass. Then, electrosurgical treatment can be carried out, minimizing the incidence of residual lesions and promoting a complete cure. The combination of UAE with hysteroscopic electrosurgery represents a promising approach to the treatment of pregnancy at the site of a previous caesarean section, offering several key advantages such as reduced operative time, decreased intraoperative blood loss, complete excision of the target lesion, and a lower incidence of postoperative complications [15,16].
Laparoscopic surgery represents a minimally invasive approach to the treatment of CSP [17]. The primary risks associated with laparoscopic removal of keloidal pregnancy lesion and uterine scar repair include intraoperative hemorrhage and incomplete scar repair. The success of laparoscopic reversible uterine artery block combined with hysteroscopic suction hinges on the isolation of the uterine artery. This is achieved using the ureter as a reference point to identify the uterine artery in its external and superior position, similar to techniques employed in conventional gynecological surgery. Additionally, it is essential to ascertain the presence of peristalsis and uterine arterial pulsation before tying a live knot to temporarily block the uterine artery. Conversely, laparoscopic reversible internal iliac artery block is an effective method for preventing intraoperative hemorrhage [18,19]. Additionally, the procedure does not affect menstruation, as blood supply is quickly restored postoperatively. Positive dissection of the internal iliac artery has been proposed as an effective method for reducing intraoperative bleeding compared to uterine artery ligation, which has proven successful in preventing xenograft vascularization in CSP. Consequently, it may be a valuable technique for reducing intraoperative bleeding. The laparoscopic approach offers several advantages for the removal of scarred pregnancy lesions, including the ability to completely and directly remove the pregnancy tissue, eliminating any remaining trophoblastic cells. Additionally, the microtubular structures surrounding the scar can be repaired, and the incision site fully restored. The magnification provided by laparoscopy enables more precise identification of the lesion [20,21]. In comparison, transabdominal surgery has the advantage of reduced intraoperative bleeding due to minimal invasiveness and the ability to perform the procedure through a small incision. Postoperative recovery is also faster, and hospital stay is shorter. Furthermore, the intervention costs can be reduced by nearly ten thousand yuan.
This study compares the effectiveness of uterine artery ligation and internal iliac ligation in the surgical treatment for scar pregnancies. The comparison revealed that those in the uterine artery group exhibited superior outcomes, including enhanced hCG and progesterone levels, regular menstrual cycles, reduced menstrual flow and dysmenorrhea, shorter operative time and hospital stay, lower total hospital cost, and higher quality of life scores. Furthermore, the uterine artery group exhibited a higher pregnancy rate compared to the internal iliac artery group. Although some patients conceived within 5 to 10 months postoperatively, clinical guidelines generally recommend waiting at least 6 months before attempting conception. This allows sufficient time for uterine healing and minimizes the risk of complications such as uterine rupture or poor pregnancy outcome.
Conclusion
This study demonstrates that the removal of scar pregnancies under the reversible blockade of bilateral uterine arteries, in conjunction with hysterolaparoscopy, offers significant benefits to patients compared to the internal iliac artery approach.
Acknowledgements
This work was supported by Hangzhou Health & Scientific Technology Plan (A20240298) and Zhejiang Provincial Medical and Health Scientifical Technology Plan (2020KY161).
Disclosure of conflict of interest
None.
References
- 1.Park SE, Ryu JE, Jang TK. Laparoscopic excision and repair of a cesarean scar pregnancy in a woman with uterine didelphys: a case report. J Yeungnam Med Sci. 2023;40:202–206. doi: 10.12701/jyms.2022.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye M, Zhang Q, Li Z, Gu C, Meng Y. Robotic CSP resection and hysterotomy repair. J Minim Invasive Gynecol. 2021;28:945–946. doi: 10.1016/j.jmig.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Gkegkes ID, Psomiadou V, Minis E, Iavazzo C. Robot-assisted laparoscopic repair of cesarean scar defect: a systematic review of clinical evidence. J Robot Surg. 2023;17:745–751. doi: 10.1007/s11701-022-01502-w. [DOI] [PubMed] [Google Scholar]
- 4.Silva B, Viana Pinto P, Costa MA. Cesarean scar pregnancy: a systematic review on expectant management. Eur J Obstet Gynecol Reprod Biol. 2023;288:36–43. doi: 10.1016/j.ejogrb.2023.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Timor-Tritsch IE, Monteagudo A. Unforeseen consequences of the increasing rate of cesarean deliveries: early placenta accreta and cesarean scar pregnancy. a review. Am J Obstet Gynecol. 2012;207:14–29. doi: 10.1016/j.ajog.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Bacila IF, Balulescu L, Dabica A, Brasoveanu S, Pirtea M, Ratiu A, Pirtea L. Laparoscopic management of cesarean scar pregnancy with temporary clipping of anterior trunk of hypogastric arteries: a case report. J Pers Med. 2024;14:469. doi: 10.3390/jpm14050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho-Almeida J, Cardoso A, Cruz-Correia R, Pereira-Rodrigues P. Fast healthcare interoperability resources-based support system for predicting delivery type: model development and evaluation study. JMIR Form Res. 2024;8:e54109. doi: 10.2196/54109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murji A, Sanders AP, Monteiro I, Haiderbhai S, Matelski J, Walsh C, Abbott JA, Munro MG, Maheux-Lacroix S International Federation of Gynecology and Obstetrics (FIGO) Committee on Menstrual Disorders and Related Health Impacts. Cesarean scar defects and abnormal uterine bleeding: a systematic review and meta-analysis. Fertil Steril. 2022;118:758–766. doi: 10.1016/j.fertnstert.2022.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Lin C, Wu J, Xu D, Zhu S, Jiang B. Value and influencing factors of preoperative MRI evaluation for previous cesarean scar defect associated abnormal uterine bleeding in patients undergoing laparoscopic surgery. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2023;48:1316–1324. doi: 10.11817/j.issn.1672-7347.2023.230123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HF, Chen HH, Ting WH, Lu HF, Lin HH, Hsiao SM. Robotic or laparoscopic treatment of cesarean scar defects or cesarean scar pregnancies with a uterine sound guidance. Taiwan J Obstet Gynecol. 2021;60:821–826. doi: 10.1016/j.tjog.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Smith SN, Workman M, Alrowaily N, Rattray D. Cesarean scar ectopic pregnancy at laparoscopy. J Obstet Gynaecol Can. 2023;45:101912. doi: 10.1016/j.jogc.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Zhang G, Hua K, Ding J. Single-port laparoscopy versus conventional laparoscopy of benign adnexal masses during pregnancy: a retrospective case-control study. J Int Med Res. 2022;50:3000605221128153. doi: 10.1177/03000605221128153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legris ML, Gabriele V, Host A, Akladios C, Garbin O, Lecointre L. Cesarean scar pregnancy: two case report and therapeutic management algorithm. J Gynecol Obstet Hum Reprod. 2021;50:102056. doi: 10.1016/j.jogoh.2020.102056. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Jiang ZB, Huang MS, Guan SH, Zhu KS, Qian JS, Zhou B, Li MA, Shan H. Uterine artery embolization combined with methotrexate in the treatment of cesarean scar pregnancy: results of a case series and review of the literature. J Vasc Interv Radiol. 2012;23:1582–1588. doi: 10.1016/j.jvir.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Di Spiezio Sardo A, Zizolfi B, Saccone G, Ferrara C, Sglavo G, De Angelis MC, Mastantuoni E, Bifulco G. Hysteroscopic resection vs ultrasound-guided dilation and evacuation for treatment of cesarean scar ectopic pregnancy: a randomized clinical trial. Am J Obstet Gynecol. 2023;229:437.e431–437.e437. doi: 10.1016/j.ajog.2023.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Li H, Jiang J, Zhang X, Shan S, Zhao X, Shi B. Dilatation and curettage versus lesion resection in the treatment of cesarean-scar-pregnancy: a systematic review and meta-analysis. Taiwan J Obstet Gynecol. 2021;60:412–421. doi: 10.1016/j.tjog.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Tipiani-Rodríguez O, Elías-Estrada JC, Bocanegra-Becerra YL, Ponciano-Biaggi MA. Treatment of ectopic pregnancy implanted on cesarea scar: cohort study 2018-2022, Lima, Peru. Rev Colomb Obstet Ginecol. 2023;74:15–30. doi: 10.18597/rcog.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sziller I, Hupuczi P, Papp Z. Hypogastric artery ligation for severe hemorrhage in obstetric patients. J Perinat Med. 2007;35:187–192. doi: 10.1515/JPM.2007.049. [DOI] [PubMed] [Google Scholar]
- 19.Kostov S, Kornovski Y, Watrowski R, Slavchev S, Ivanova Y, Yordanov A. Internal iliac artery ligation in obstetrics and gynecology: surgical anatomy and surgical considerations. Clin Pract. 2023;14:32–51. doi: 10.3390/clinpract14010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su X, Yang M, Na Z, Wen C, Liu M, Cai C, Zhong Z, Zhou B, Tang X. Application of laparoscopic internal iliac artery temporary occlusion and uterine repair combined with hysteroscopic aspiration in type III cesarean scar pregnancy. Am J Transl Res. 2022;14:1737–1741. [PMC free article] [PubMed] [Google Scholar]
- 21.Fu P, Zhang L, Zhou T, Wang S, Liu R. Clinical application of a new cesarean scar pregnancy classification and evaluation system and a risk scoring system. Int J Gen Med. 2024;17:115–126. doi: 10.2147/IJGM.S445327. [DOI] [PMC free article] [PubMed] [Google Scholar]


