Abstract
The respiratory microbiota significantly influence the onset and progression of asthma, as underscored by recent studies revealing discernible differences between asthma patients and healthy individuals. This review delves into the relationship between respiratory microbiota and asthma, with a particular emphasis on possible therapeutic targets and emerging treatments. Existing research is thoroughly synthesized, illuminating the association between microbial communities and the incidence of asthma. In addition to antibiotic therapy, attention is directed towards modulating the immune balance within the respiratory microbiota as a promising therapeutic approach. Specifically, the role of immunomodulators targeting key immune pathways, such as interleukins and cytokines implicated in asthma pathogenesis, is examined. Furthermore, the regulation of the gut-lung axis is explored, highlighting the significance of the gut microbiota in shaping systemic immune responses and respiratory health. Moreover, the potential of immune cell modulation as a therapeutic avenue is explored, focusing on targeting specific immune cell populations involved in asthma pathophysiology. Future research directions and challenges are also addressed, underscoring the need for a deeper understanding of the intricate interplay between respiratory microbiota and the pathogenesis of asthma.
Keywords: Respiratory microbiota, asthma, therapeutic targets, immune modulation
Introduction
Asthma is a common chronic inflammatory disease that profoundly affects the quality of life and health of millions of people worldwide. Despite decades of research, the pathogenesis of asthma remains complex and not fully understood [1]. In recent years, with advances in scientific technology, particularly in the study of respiratory microbiota, there is growing recognition of the critical role of respiratory microbiota in the onset and progression of asthma [2,3].
Respiratory microbiota refers to the microbial communities present in the respiratory tract, including bacteria, viruses, fungi, and other microorganisms [4]. The interaction between these microorganisms and the host immune system has significant implications for respiratory health and disease. Certain bacteria, such as Staphylococcus aureus [5] and Haemophilus influenzae [6-8], may trigger asthma attacks by producing toxins or stimulating the immune response. These microorganisms may further exacerbate airway inflammation and hyperreactivity by increasing the release of inflammatory cytokines, such as interleukins (IL-4, IL-5, and IL-13), leading to asthma exacerbations [9,10].
In terms of treatment, current medications mainly include corticosteroids and β2-agonists, used to alleviate asthma symptoms and control inflammation [11,12]. However, these medications do not cure asthma and may be associated with a range of side effects with long-term use [13]. Therefore, finding new therapeutic targets and approaches is imperative. Therapeutic strategies targeting respiratory microbiota have garnered significant attention in recent years. By modulating the balance of respiratory microbiota, it is possible to alter the status of the host immune system, thereby reducing the frequency of inflammatory reactions and asthma exacerbation. For example, some studies suggest that probiotics and prebiotics can modulate the gut microbiota, thereby affecting respiratory microbiota and alleviating asthma symptoms [14,15].
Considering the significant impact of respiratory microbiota on asthma pathogenesis and the urgent need for effective therapeutic interventions, this review explores the intricate relationship between respiratory microbiota and asthma. By examining recent research progress and emerging therapeutic strategies targeting respiratory microbiota, we seek to provide comprehensive insights into the mechanisms underlying asthma exacerbations and potential avenues for improved management. Through this review, we aim to facilitate a deeper understanding of the respiratory microbiota-asthma relationship and guide future research toward enhancing asthma treatment outcomes.
The relationship between respiratory microbiota and asthma pathogenesis
Respiratory microbiota disparity between healthy and asthmatic individuals
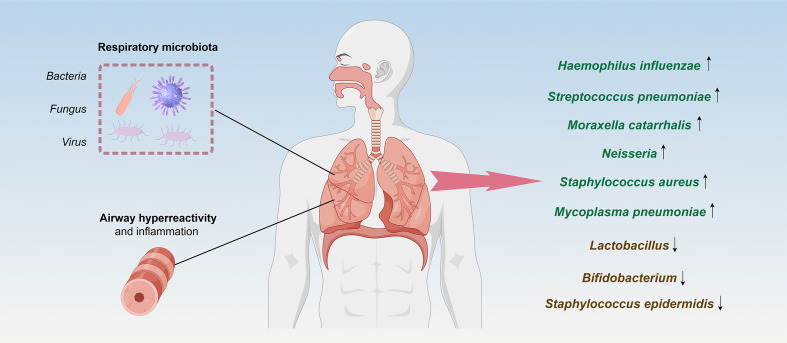
The relationship between respiratory microbiota and asthma has garnered significant attention. Recent studies indicate distinct differences in the respiratory microbiota of asthma patients compared to healthy individuals, suggesting potential implications for asthma onset and severity [16,17]. For example, Li et al analyzed the bacterial microbiota profiles in induced sputum from 31 asthma patients and 12 healthy individuals and confirmed that the airway microbiota was associated with small airway function in asthma patients [18].
Asthma patients exhibit differences in their respiratory microbiota compared to healthy individuals. Some studies have found notable disparities in the diversity and abundance of respiratory microbiota communities between asthma patients and healthy individuals [16,19,20]. Additionally, specific microbial species may inhabit the respiratory tract of asthma patients, including Haemophilus influenzae [7,21], Streptococcus pneumoniae [22], and Moraxella catarrhalis [7,23,24]. Several other respiratory microbiotas have been associated with an elevated risk of asthma. Neisseria species [25], along with Staphylococcus aureus [5,26] and Mycoplasma pneumoniae [27,28], are among the microbial populations found in higher abundance in the respiratory tract of individuals with asthma. Neisseria species have been identified as potential contributors to asthma pathogenesis, while Staphylococcus aureus has been linked to airway inflammation and exacerbations of asthma symptoms [5,25,29]. Mycoplasma pneumoniae, known primarily for causing respiratory infections, has also been associated with increased asthma severity and exacerbations [30]. The presence of these microorganisms in the respiratory tract may disrupt immune system balance and trigger airway inflammation, thereby heightening the risk of asthma development and exacerbations. In contrast, the respiratory microbiota of healthy individuals may include beneficial bacteria such as Lactobacillus, Bifidobacterium, and Staphylococcus epidermidis. The presence of these normal communities can inhibit the survival of pathogenic bacteria [31]. Furthermore, the correlation between respiratory microbiota and asthma onset has been further investigated. Some studies suggest a close association between the respiratory microbiota of asthma patients and the incidence and severity of asthma [32]. The presence of microbes may be correlated with the frequency and severity of asthma exacerbations, thus influencing the pathophysiology of asthma (Figure 1) [33].
Figure 1.
Respiratory microbiota disparities between healthy and asthmatic individuals.
Respiratory microbiota alteration and asthma induction mechanisms
Respiratory microbiota, comprising various microorganisms such as bacteria, fungi, and viruses, colonize the respiratory tract and play a crucial role in regulating the host immune system [34]. The interactions between these microorganisms and the host are pivotal for the onset and development of asthma. Common bacteria include Staphylococcus, Haemophilus, Streptococcus, and Moraxella, while fungi such as Candida and Aspergillus are also prevalent [35].
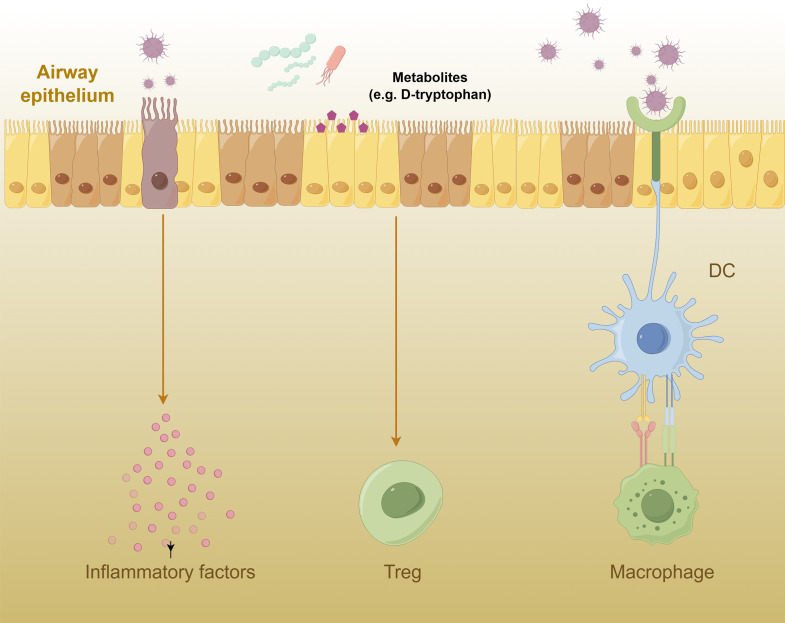
These microorganisms can influence the pathogenesis and pathologic processes of asthma through multiple mechanisms. First, they may directly stimulate the host immune system, triggering inflammatory responses and exacerbating asthma attacks [36-38]. For instance, Zheng et al investigated the role of respiratory microbiota changes in asthma progression [39]. Using an ovalbumin-induced chronic asthma mouse model, the study identified distinct microbiota profiles at various stages of the disease. Pseudomonas predominated during the acute inflammatory phase, while Staphylococcus and Cupriavidus were more prevalent during the airway remodeling stage. These findings indicated that dynamic shifts in respiratory microbiota were closely associated with the transition from acute inflammation to airway remodeling in chronic asthma. Furthermore, the concentration of IL-17 in bronchoalveolar lavage fluid from asthmatic patients was markedly elevated compared to that in healthy individuals and exhibited a significant positive correlation with Proteobacteria [40]. Infection with Haemophilus influenzae can exacerbate airway neutrophilic inflammation in a mouse model of ovalbumin-induced asthma via Th17 immune response mechanisms [41]. Additionally, Moraxella catarrhalis infection significantly enhanced neutrophil infiltration and increased levels of inflammatory cytokines such as IL-6, TNF-α, IFN-γ, and IL-17 within the airways of mice [23].
Secondly, microbial metabolites significantly contribute to the pathogenesis of asthma. Certain microorganisms may release specific molecules such as endotoxins and bacterial metabolites, which can activate immune cells to produce inflammatory mediators, worsening asthma symptoms [42-44]. Kepert et al investigated the potential of probiotics to prevent allergic airway diseases like asthma. Researchers screened probiotic supernatants for immunoactive properties and identified D-tryptophan as a bioactive metabolite. Unlike its L-tryptophan counterpart, D-tryptophan was found to be effective in increasing regulatory T cells in the lungs and gut, reducing allergic airway inflammation. These findings suggested that D-tryptophan, a product of probiotic bacteria, could be developed into a novel preventative strategy for chronic immune diseases such as asthma [45]. In addition, the microbial metabolite butyrate even directly regulates the function of type 2 innate lymphoid cell (ILC2), inhibits ILC2 production of IL-13 and IL-5, and improves ILC2-mediated airway hyperreactivity and airway inflammation [46].
Moreover, respiratory microbiota may disrupt the balance of the host immune system, leading to immune dysregulation and an increased risk of asthma onset [47]. Respiratory microbiota may indirectly affect the onset of asthma by activating specific immune cells such as alveolar macrophages and dendritic cells [48-50]. For example, Hou et al investigated the role of macrophage nuclear receptor corepressor 1 (NCOR1) in the development of asthma [51]. By using ovalbumin to induce asthma in mice deficient in macrophage NCOR1, the research demonstrated that NCOR1 deficiency significantly increased allergic airway inflammation and enhanced M2 macrophage polarization. Mechanistic studies revealed that NCOR1 regulated macrophage polarization through interaction with peroxisome proliferator-activated receptor γ (PPARγ), thereby contributing to the pathogenesis of asthma. These findings suggest that targeting macrophage NCOR1 may represent a strategy for treating asthma. These immune cells, upon sensing the presence of microorganisms, release inflammatory mediators such as cytokines and chemokines, initiating immune-inflammatory responses. Moreover, respiratory microbiota may interact with other parts of the host immune system, such as the gut microbiota, thereby influencing systemic immune status and further impacting the onset and pathologic processes of asthma (Figure 2) [15,16,52].
Figure 2.
Mechanism of respiratory microbiota in asthma.
Therapeutic targets of respiratory microbiota regulation in asthma
Asthma is a chronic airway disease characterized by airway inflammation, airway hyperresponsiveness (AHR), airflow limitation, and reversible airway obstruction. The goal of asthma treatment is to achieve long-term control by controlling symptoms, improving lung function, reducing exacerbations, and enhancing quality of life [53]. Commonly used asthma medications include inhaled short-acting β2-agonists (SABAs), inhaled corticosteroids (ICS), longacting β2-agonists (LABAs), leukotriene receptor antagonists (LTRAs), anticholinergic agents, biologics, and oral corticosteroids [54,55]. These medications exert their effects through various mechanisms, including bronchodilation, reducing airway inflammation, inhibiting the release of inflammatory mediators, and modulating immune responses. Depending on the severity of asthma and individual characteristics, healthcare providers can tailor treatment plans to achieve optimal disease control and improve quality of life.
With an improved understanding of asthma pathogenesis, researchers are exploring more targeted treatment approaches. Immunomodulators, as a novel treatment approach, may positively impact asthma treatment by targeting immune pathways associated with asthma pathology [56,57]. Furthermore, increasing evidence suggests a close relationship between the gut and lung microbiota, with the gut-lung axis playing a significant role in asthma pathogenesis [58,59]. Additionally, modulation of immune cell function, such as alveolar macrophages and dendritic cells, may also be a therapeutic approach for treating asthma [50,60]. These novel treatment approaches offer hope for asthma patients and provide new insights and strategies for improving asthma management. The following will be elaborated upon from the perspectives of traditional treatment targets and their limitations, novel immunomodulators, regulation of the gut-lung axis, and immune cell function. Table 1 presents an overview of asthma treatment modalities and novel therapeutic approaches.
Table 1.
Overview of asthma treatment modalities and novel therapeutic approaches
| Category | Common Medications | Mechanism of Action |
|---|---|---|
| Common Asthma Medications | Inhaled Short-Acting β2-Agonists (SABAs) | Dilate airway smooth muscles, rapidly relieve asthma symptoms. |
| Inhaled Corticosteroids (ICS) | Reduce airway inflammation, improve lung function. | |
| Long-Acting β2-Agonists (LABAs) | Sustain airway dilation, control asthma symptoms. | |
| Leukotriene Receptor Antagonists (LTRAs) | Block leukotriene receptors, reduce the release of inflammatory mediators. | |
| Anticholinergic Agents | Block acetylcholine receptors, reduce airway constriction. | |
| Biologics | Target specific immune pathways, reduce immune system activity. | |
| Oral Corticosteroids | Reduce systemic inflammation, control severe asthma symptoms. | |
| Novel Treatment Approaches | Omalizumab | Anti-IgE antibody, decrease allergic reactions. |
| Mepolizumab | Anti-IL-5 monoclonal antibody, reduce eosinophils, lower asthma exacerbation frequency. | |
| Reslizumab | Anti-IL-5 monoclonal antibody, improve asthma symptoms. | |
| Gut-lung axis | Improve gut microbiota, maintain immune system stability: | |
| Immune Cell Function Modulation | T Cell | Modulate Th2 cell activity, reduce secretion of IL-4, IL-5, IL-13. |
| Tregs | Enhance immune tolerance, reduce response to allergens. | |
| Macrophage | Reduce release of inflammatory mediators, suppress inflammation. | |
| Dendritic Cell | Alter antigen presentation function, influence immune response. |
Application of immunomodulators: targeting immune pathways associated with asthma pathology
Immunomodulators represent a pharmacological class of drugs that exert influence over the development and pathological processes of asthma by modulating the activity and function of the immune system. This category encompasses catecholamines, anti-IgE antibodies (e.g., Omalizumab), montelukast, among others [61]. Catecholamines, functioning as antibodies, mitigate airway inflammation and alleviate asthma symptoms by inhibiting the release of inflammatory mediators, such as leukotrienes, from immune cells [62]. Anti-IgE antibodies mitigate allergic reactions and airway inflammation by binding to and neutralizing immunoglobulin E (IgE) in the bloodstream, thus diminishing the affinity of allergens for IgE [63,64]. Montelukast contributes to the reduction of airway inflammation and asthma symptoms by impeding the binding of leukotriene receptors and diminishing the release of inflammatory mediators [65].
Immunomodulators employ diverse mechanisms to target the immune system, thereby mitigating airway inflammation and allergic reactions, ultimately enhancing asthma symptomatology, and controlling disease progression. For instance, certain immunomodulators attenuate airway inflammation and allergic responses by suppressing the activity of Th2 cells, thereby diminishing the secretion of interleukins such as IL-4, IL-5, and IL-13 [66]. Alternatively, other immunomodulators bolster the functionality of regulatory T cells (Tregs), fostering immune tolerance and consequently reducing the immune system’s reactivity to allergens [67,68]. Moreover, some immunomodulators enhance airway inflammation relief by modulating the respiratory microbiota colonization [69].
In essence, the application of immunomodulators is geared towards reinstating immune system equilibrium, alleviating airway inflammation and allergic reactions, and thereby ameliorating asthma symptoms and managing disease advancement. The use of these therapeutic agents offers a promising approach that specifically targets the pathogenesis of asthma, providing renewed optimism for patients who have inadequately responded to conventional treatment or need more efficacious therapy.
Regulation and significance of the gut-lung axis
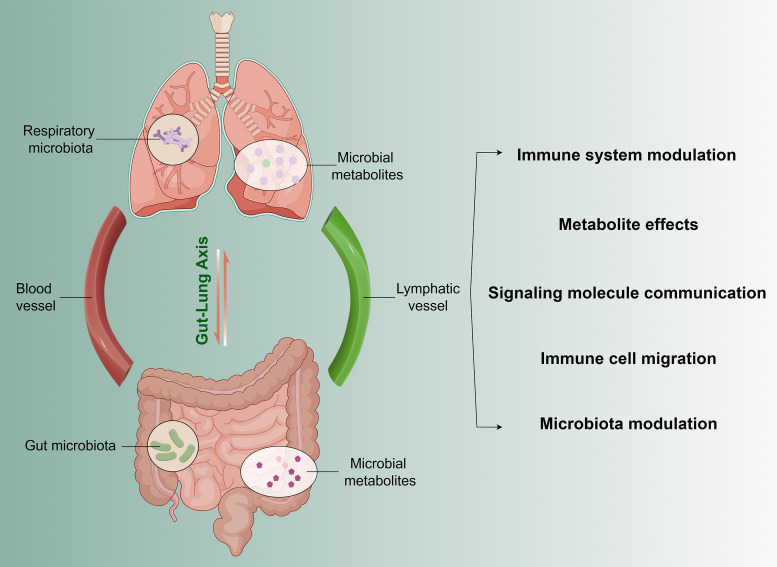
The gut-lung axis refers to the close relationship and mutual influence between the gut and the lungs. This concept highlights the interaction between the gut microbiota and the immune system of the respiratory tract, which is significant in the pathogenesis of asthma [52,70]. In essence, the gut-lung axis emphasizes the connection between gut health and respiratory health, recognizing that changes in gut microbiota may affect the immune status and inflammatory responses of the respiratory tract, thereby influencing the development and symptomatology of asthma [71].
On this axis, there exist intricate interactions between the gut and respiratory tract, where the microbiota plays a crucial role in regulating the immune system, maintaining tissue homeostasis, and influencing disease progression. Some specific aspects of gut-lung axis regulation and significance include:
Immune system modulation: Gut microbiota influence respiratory immune responses by modulating the activity and quantity of immune cells such as T cells, B cells, macrophages, and dendritic cells [72]. Certain microbiota can promote immune tolerance [73], reducing excessive reactions to allergens and thereby decreasing the frequency and severity of asthma attacks [74,75].
Metabolite effects: Changes in gut microbiota composition may lead to alterations in metabolites. These metabolites can affect the stability and immune function of the respiratory microbiota through circulation or the nervous system [71]. For instance, metabolites like short-chain fatty acids may possess anti-inflammatory and immune-modulatory effects, affecting respiratory inflammation and asthma development [76,77].
Signaling molecule communication: Various signaling molecules may facilitate communication between the gut and respiratory tract, influencing immune system activity and inflammatory responses. These molecules include cytokines (such as IL-4, IL-5, IL-13), chemokines, and intercellular signaling molecules. They can convey immune information between the two systems, modulating the activity and function of immune cells, and thus affecting asthma symptoms and severity [59,78].
Immune cell migration: Migration of immune cells between the gut and respiratory tract is possible. Immune cells like T cells, macrophages, and others may migrate to the respiratory tract through blood circulation or the lymphatic system, participating in immune responses and inflammation regulation [79,80]. This cell migration may be influenced by the gut microbiota, thereby impacting the immune status of the respiratory tract and the development of asthma.
Modulation of the gut microbiome: Recent studies show that changes in gut microbiota can impact asthma development and progression. For example, specific bacteria such as Lactobacillus and Bifidobacterium have been shown to reduce airway inflammation and enhance immune regulation in asthma models [81]. These beneficial bacteria can modulate systemic immune responses, potentially lowering the incidence and severity of respiratory conditions. Conversely, an imbalance in gut microbiota, or dysbiosis, has been associated with increased inflammatory responses and worsening asthma symptoms. For instance, a reduction in microbial diversity and an overgrowth of pathogenic bacteria in the gut can lead to heightened inflammation and immune dysregulation that exacerbates respiratory issues [82].
Interventions aimed at modulating the gut microbiome, such as probiotic supplementation and dietary adjustments, have demonstrated potential benefits. Probiotics can restore a balanced gut microbiota, enhance regulatory T cells, and decrease inflammatory cytokines, thereby improving lung health. These findings suggest that targeting the gut microbiome through such interventions may offer novel therapeutic strategies for managing asthma and other respiratory diseases (Figure 3).
Figure 3.
Role of the Gut-lung axis regulation in asthma.
Immune cell modulation as a therapeutic approach
Asthma is characterized by chronic inflammation of the airways, which involves various immune cells such as T cells, B cells, eosinophils, mast cells, and macrophages [83]. The rationale behind immune cell modulation in asthma therapy lies in the understanding that these cells play crucial roles in the initiation and perpetuation of airway inflammation and hyperreactivity. Murphy et al investigated the mechanisms underlying indirect AHR in asthma, with a particular focus on exercise-induced bronchoconstriction (EIB). RNA sequencing of epithelial brushings from individuals with and without EIB identified 120 differentially expressed genes. Notable signaling pathways involving IL-33, IL-18, and IFN-γ were highlighted. The expressions of IL1RL1, IL18R1, and IFNG were found to correlate with the densities of mast cells and eosinophils. Ex vivo studies demonstrated that airway epithelial cells foster type 2 inflammation and enhance T2 gene expression in response to IL-33 [84]. These findings underscore the critical role of epithelial interactions with mast cells and eosinophils in indirect AHR. By modulating the activity, function, or numbers of specific immune cells, it is possible to intervene in the inflammatory cascade and mitigate asthma symptoms. Several approaches are being explored in immune cell modulation for asthma treatment.
Biological therapies are an emerging frontier in asthma treatment, focusing on targeting specific immune cell types or cytokines involved in the disease process. Monoclonal antibodies against interleukins such as IL-4, IL-5, and IL-13 have been developed to counter eosinophilic inflammation, a hallmark of allergic asthma [85,86]. Examples include omalizumab, which targets IgE [87], and mepolizumab [88,89], targeting IL-5 [90], both demonstrating efficacy in reducing exacerbations and improving asthma control. Additionally, cellular therapies complement these approaches, involving the manipulation of immune cells ex vivo and their reintroduction to modulate immune responses [91]. Regulatory T cells (Tregs) have emerged as a promising candidate for suppressing airway inflammation and promoting immune tolerance [92]. Clinical trials exploring Treg adoptive transfer have shown promise in reducing AHR and inflammation in asthma [93]. Meanwhile, small molecule modulators offer a unique avenue by selectively targeting immune cell signaling pathways [94]. For instance, Janus kinase (JAK) inhibitors like tofacitinib aim to modulate inflammatory responses [95]. Although still under investigation, they hold potential for regulating immune cell activation and cytokine production to alleviate inflammation and improve asthma control [96]. Immunotherapy, particularly allergen-specific immunotherapy, remains a cornerstone in allergic asthma management [97]. By gradually exposing patients to specific allergens, it induces immune tolerance and reduces allergic inflammation. Subcutaneous and sublingual immunotherapy have shown effectiveness in reducing asthma symptoms and medication use, especially in patients with identifiable allergic triggers [98,99].
In essence, immune cell modulation represents a promising direction for asthma therapy, offering targeted interventions tailored to the underlying immune dysregulation. With ongoing advancements in biological agents, cellular therapies, small molecule modulators, and immunotherapy, the future of asthma treatment holds the promise of more personalized and effective approaches, addressing the diverse immune profiles and disease phenotypes of individual patients [100].
Discussion
The intricate interplay between respiratory microbiota and asthma represents a multifaceted relationship, influenced by a myriad of factors spanning genetic predisposition, environmental exposures, and lifestyle influences. Genetic variations within individuals can significantly affect the composition and function of the respiratory microbiota, thereby influencing susceptibility to asthma and the severity of its manifestations. Environmental factors, including allergen exposure, air pollution, and dietary habits, further shape the respiratory microbiota composition and immune responses, contributing to the complex pathogenesis of asthma.
Despite advancements in our understanding, the precise mechanisms underlying the microbiota-asthma relationship remain incompletely understood, necessitating comprehensive research endeavors to elucidate these intricate interactions. Studies exploring the dynamics of microbial communities in asthmatic individuals have revealed alterations in microbial diversity, abundance, and composition compared to healthy controls [101]. However, the causal relationships between specific microbial taxa and asthma development or exacerbation require further investigation to decipher the underlying mechanisms driving these associations.
Microbiota modulation holds considerable promise as a novel therapeutic approach for asthma management. Recent studies have demonstrated the effects of probiotics, including intratracheal and intranasal injections of Lactobacillus rhamnosus and Lactobacillus fermentum, as well as Lactobacillus paracasei, Lactobacillus salivarius, and Lactobacillus brevis, on various lung diseases such as infections and cancer. These findings suggest that these probiotics can directly colonize the lungs. However, the application of this therapy in the treatment of asthma has yet to be investigated [102-104]. By targeting dysbiotic microbial communities and promoting the growth of beneficial microorganisms, microbiota-based interventions aim to restore microbial balance within the respiratory tract and alleviate asthma symptoms. Probiotics, prebiotics, and dietary interventions represent potential strategies for modulating the respiratory microbiota and mitigating asthma-related inflammation and AHR. Additionally, interventions targeting the gut-lung axis, such as fecal microbiota transplantation and respiratory microbial colonization through intratracheal and intranasal injection, offer innovative avenues for manipulating microbial communities to improve asthma outcomes. However, the translation of microbiota-based interventions into clinical practice faces several challenges. Interindividual variability in microbiota composition and treatment response poses a significant obstacle, necessitating the development of personalized treatment strategies tailored to individual patient profiles. Furthermore, the safety, efficacy, and long-term effects of microbiota-based therapies require rigorous evaluation through well-designed clinical trials to establish their utility and inform evidence-based clinical practice guidelines [105,106].
Looking ahead, advancements in technology and our evolving understanding of disease pathophysiology offer opportunities for innovative asthma treatment. Gene editing techniques such as CRISPR/Cas9 offer the potential for precise interventions by targeting specific genetic mutations associated with asthma. Immunomodulatory therapies and stem cell-based approaches provide additional avenues for achieving sustained disease control and improving patient outcomes. By integrating these cutting-edge technologies with a deeper understanding of respiratory microbiota, we can envision a future where personalized, targeted therapies transform asthma management, enhancing patients’ quality of life and long-term health [107]. Continued research efforts and collaboration across disciplines will be essential to realize this vision and address the unmet needs of asthma patients worldwide.
In conclusion, the relationship between respiratory microbiota and asthma represents a complex and dynamic interplay influenced by genetic, environmental, and lifestyle factors. While microbiota-based interventions hold promise for revolutionizing asthma management, several challenges must be addressed before their use in clinical practice.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82074365, 82104761 and 82274420).
Disclosure of conflict of interest
None.
References
- 1.Miller RL, Grayson MH, Strothman K. Advances in asthma: new understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J Allergy Clin Immunol. 2021;148:1430–1441. doi: 10.1016/j.jaci.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P, Armstrong-James DPH, Adcock IM, Chotirmall SH, Chung KF, Hansbro PM. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med. 2019;7:907–920. doi: 10.1016/S2213-2600(18)30510-1. [DOI] [PubMed] [Google Scholar]
- 3.Yagi K, Huffnagle GB, Lukacs NW, Asai N. The lung microbiome during health and disease. Int J Mol Sci. 2021;22:10872. doi: 10.3390/ijms221910872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emonet S, Lazarevic V, Leemann Refondini C, Gaia N, Leo S, Girard M, Nocquet Boyer V, Wozniak H, Despres L, Renzi G, Mostaguir K, Dupuis Lozeron E, Schrenzel J, Pugin J. Identification of respiratory microbiota markers in ventilator-associated pneumonia. Intensive Care Med. 2019;45:1082–1092. doi: 10.1007/s00134-019-05660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachert C, Humbert M, Hanania NA, Zhang N, Holgate S, Buhl R, Broker BM. Staphylococcus aureus and its IgE-inducing enterotoxins in asthma: current knowledge. Eur Respir J. 2020;55:1901592. doi: 10.1183/13993003.01592-2019. [DOI] [PubMed] [Google Scholar]
- 6.Ackland J, Barber C, Heinson A, Azim A, Cleary DW, Christodoulides M, Kurukulaaratchy RJ, Howarth P, Wilkinson TMA, Staples KJ WATCH study investigators. Nontypeable Haemophilus influenzae infection of pulmonary macrophages drives neutrophilic inflammation in severe asthma. Allergy. 2022;77:2961–2973. doi: 10.1111/all.15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MA, Jabeen M, Bharj G, Hinks TSC. Non-typeable Haemophilus influenzae airways infection: the next treatable trait in asthma? Eur Respir Rev. 2022;31:220008. doi: 10.1183/16000617.0008-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versi A, Ivan FX, Abdel-Aziz MI, Bates S, Riley J, Baribaud F, Kermani NZ, Montuschi P, Dahlen SE, Djukanovic R, Sterk P, Maitland-Van Der Zee AH, Chotirmall SH, Howarth P, Adcock IM, Chung KF U-BIOPRED consortium. Haemophilus influenzae and Moraxella catarrhalis in sputum of severe asthma with inflammasome and neutrophil activation. Allergy. 2023;78:2906–2920. doi: 10.1111/all.15776. [DOI] [PubMed] [Google Scholar]
- 9.Dunn RM, Wechsler ME. Anti-interleukin therapy in asthma. Clin Pharmacol Ther. 2015;97:55–65. doi: 10.1002/cpt.11. [DOI] [PubMed] [Google Scholar]
- 10.Gibeon D, Menzies-Gow AN. Targeting interleukins to treat severe asthma. Expert Rev Respir Med. 2012;6:423–439. doi: 10.1586/ers.12.38. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PJ. Efficacy of inhaled corticosteroids in asthma. J Allergy Clin Immunol. 1998;102:531–538. doi: 10.1016/s0091-6749(98)70268-4. [DOI] [PubMed] [Google Scholar]
- 12.McCracken JL, Veeranki SP, Ameredes BT, Calhoun WJ. Diagnosis and management of asthma in adults: a review. JAMA. 2017;318:279–290. doi: 10.1001/jama.2017.8372. [DOI] [PubMed] [Google Scholar]
- 13.Heffler E, Madeira LNG, Ferrando M, Puggioni F, Racca F, Malvezzi L, Passalacqua G, Canonica GW. Inhaled Corticosteroids safety and adverse effects in patients with asthma. J Allergy Clin Immunol Pract. 2018;6:776–781. doi: 10.1016/j.jaip.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Alcazar CG, Paes VM, Shao Y, Oesser C, Miltz A, Lawley TD, Brocklehurst P, Rodger A, Field N. The association between early-life gut microbiota and childhood respiratory diseases: a systematic review. Lancet Microbe. 2022;3:e867–e880. doi: 10.1016/S2666-5247(22)00184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018;20:e12966. doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 16.Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52:241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hufnagl K, Pali-Scholl I, Roth-Walter F, Jensen-Jarolim E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol. 2020;42:75–93. doi: 10.1007/s00281-019-00775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Zou C, Li J, Wang W, Guo Y, Zhao L, Jiang C, Zhao P, An X. Upper respiratory tract microbiota is associated with small airway function and asthma severity. BMC Microbiol. 2023;23:13. doi: 10.1186/s12866-023-02757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson DJ, Gern JE. Rhinovirus infections and their roles in asthma: etiology and exacerbations. J Allergy Clin Immunol Pract. 2022;10:673–681. doi: 10.1016/j.jaip.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi H, Zhao T, Geng R, Sun L, Fan H. The associations between gut microbiota and chronic respiratory diseases: a Mendelian randomization study. Front Microbiol. 2023;14:1200937. doi: 10.3389/fmicb.2023.1200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor SL, Leong LEX, Mobegi FM, Choo JM, Wesselingh S, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, Baraket M, Marks GB, Gibson PG, Rogers GB, Simpson JL. Long-term azithromycin reduces haemophilus influenzae and increases antibiotic resistance in severe asthma. Am J Respir Crit Care Med. 2019;200:309–317. doi: 10.1164/rccm.201809-1739OC. [DOI] [PubMed] [Google Scholar]
- 22.Ahluwalia TS, Eliasen AU, Sevelsted A, Pedersen CT, Stokholm J, Chawes B, Bork-Jensen J, Grarup N, Pedersen O, Hansen T, Linneberg A, Sharma A, Weiss ST, Evans MD, Jackson DJ, Morin A, Krogfelt KA, Schjorring S, Mortensen PB, Hougaard DM, Bybjerg-Grauholm J, Baekvad-Hansen M, Mors O, Nordentoft M, Borglum AD, Werge T, Agerbo E, Gern JE, Lemanske RF Jr, Ober C, Pedersen AG, Bisgaard H, Bonnelykke K. FUT2-ABO epistasis increases the risk of early childhood asthma and Streptococcus pneumoniae respiratory illnesses. Nat Commun. 2020;11:6398. doi: 10.1038/s41467-020-19814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alnahas S, Hagner S, Raifer H, Kilic A, Gasteiger G, Mutters R, Hellhund A, Prinz I, Pinkenburg O, Visekruna A, Garn H, Steinhoff U. IL-17 and TNF-alpha are key mediators of moraxella catarrhalis triggered exacerbation of allergic airway inflammation. Front Immunol. 2017;8:1562. doi: 10.3389/fimmu.2017.01562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dissanayake E, Brockman-Schneider RA, Stubbendieck RM, Helling BA, Zhang Z, Bochkov YA, Kirkham C, Murphy TF, Ober C, Currie CR, Gern JE. Rhinovirus increases Moraxella catarrhalis adhesion to the respiratory epithelium. Front Cell Infect Microbiol. 2023;12:1060748. doi: 10.3389/fcimb.2022.1060748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Yu Y, Du W, Liu Y, Dai R, Tang W, Wang P, Zhang C, Shi G. Fungal and bacterial microbiome dysbiosis and imbalance of trans-kingdom network in asthma. Clin Transl Allergy. 2020;10:42. doi: 10.1186/s13601-020-00345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorde I, Schreiber J, Stegemann-Koniszewski S. The role of staphylococcus aureus and its toxins in the pathogenesis of allergic asthma. Int J Mol Sci. 2022;24:654. doi: 10.3390/ijms24010654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giavina-Bianchi P, Kalil J. Mycoplasma pneumoniae infection induces asthma onset. J Allergy Clin Immunol. 2016;137:1024–1025. doi: 10.1016/j.jaci.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Wang Y, Chen C, Liu K. Mycoplasma pneumoniae infection and risk of childhood asthma: a systematic review and meta-analysis. Microb Pathog. 2021;155:104893. doi: 10.1016/j.micpath.2021.104893. [DOI] [PubMed] [Google Scholar]
- 29.Losol P, Choi JP, Kim SH, Chang YS. The role of upper airway microbiome in the development of adult asthma. Immune Netw. 2021;21:e19. doi: 10.4110/in.2021.21.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao S, Wang L, Zhu W, Jiang J. Mycoplasma pneumonia infection and asthma: a clinical study. Pak J Med Sci. 2015;31:548–551. doi: 10.12669/pjms.313.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paulo AC, Lanca J, Almeida ST, Hilty M, Sa-Leao R. The upper respiratory tract microbiota of healthy adults is affected by Streptococcus pneumoniae carriage, smoking habits, and contact with children. Microbiome. 2023;11:199. doi: 10.1186/s40168-023-01640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valverde-Molina J, Garcia-Marcos L. Microbiome and asthma: microbial dysbiosis and the origins, phenotypes, persistence, and severity of asthma. Nutrients. 2023;15:486. doi: 10.3390/nu15030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards MR, Bartlett NW, Hussell T, Openshaw P, Johnston SL. The microbiology of asthma. Nat Rev Microbiol. 2012;10:459–471. doi: 10.1038/nrmicro2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuksel N, Gelmez B, Yildiz-Pekoz A. Lung microbiota: its relationship to respiratory system diseases and approaches for lung-targeted probiotic bacteria delivery. Mol Pharm. 2023;20:3320–3337. doi: 10.1021/acs.molpharmaceut.3c00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenck LP, Surette MG, Bowdish DM. Composition and immunological significance of the upper respiratory tract microbiota. FEBS Lett. 2016;590:3705–3720. doi: 10.1002/1873-3468.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun. 2017;8:1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elgamal Z, Singh P, Geraghty P. The upper airway microbiota, environmental exposures, inflammation, and disease. Medicina (Kaunas) 2021;57:823. doi: 10.3390/medicina57080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martens K, Pugin B, De Boeck I, Spacova I, Steelant B, Seys SF, Lebeer S, Hellings PW. Probiotics for the airways: potential to improve epithelial and immune homeostasis. Allergy. 2018;73:1954–1963. doi: 10.1111/all.13495. [DOI] [PubMed] [Google Scholar]
- 39.Zheng J, Wu Q, Zou Y, Wang M, He L, Guo S. Respiratory microbiota profiles associated with the progression from airway inflammation to remodeling in mice with OVA-induced asthma. Front Microbiol. 2021;12:723152. doi: 10.3389/fmicb.2021.723152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136:874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Essilfie AT, Simpson JL, Horvat JC, Preston JA, Dunkley ML, Foster PS, Gibson PG, Hansbro PM. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7:e1002244. doi: 10.1371/journal.ppat.1002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Tang W, Dai R, Wang P, Shi G, Du W, Ni Y. Disentangling the potential roles of the human gut mycobiome and metabolites in asthma. Clin Transl Med. 2022;12:e1012. doi: 10.1002/ctm2.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pivniouk V, Gimenes-Junior JA, Ezeh P, Michael A, Pivniouk O, Hahn S, VanLinden SR, Malone SP, Abidov A, Anderson D, Gozdz J, DeVries A, Martinez FD, Pasquali C, Vercelli D. Airway administration of OM-85, a bacterial lysate, blocks experimental asthma by targeting dendritic cells and the epithelium/IL-33/ILC2 axis. J Allergy Clin Immunol. 2022;149:943–956. doi: 10.1016/j.jaci.2021.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wypych TP, Pattaroni C, Perdijk O, Yap C, Trompette A, Anderson D, Creek DJ, Harris NL, Marsland BJ. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat Immunol. 2021;22:279–286. doi: 10.1038/s41590-020-00856-3. [DOI] [PubMed] [Google Scholar]
- 45.Kepert I, Fonseca J, Muller C, Milger K, Hochwind K, Kostric M, Fedoseeva M, Ohnmacht C, Dehmel S, Nathan P, Bartel S, Eickelberg O, Schloter M, Hartmann A, Schmitt-Kopplin P, Krauss-Etschmann S. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol. 2017;139:1525–1535. doi: 10.1016/j.jaci.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Thio CL, Chi PY, Lai AC, Chang YJ. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J Allergy Clin Immunol. 2018;142:1867–1883. e1812. doi: 10.1016/j.jaci.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boonpiyathad T, Sozener ZC, Satitsuksanoa P, Akdis CA. Immunologic mechanisms in asthma. Semin Immunol. 2019;46:101333. doi: 10.1016/j.smim.2019.101333. [DOI] [PubMed] [Google Scholar]
- 49.Obieglo K, van Wijck Y, de Kleijn S, Smits HH, Taube C. Microorganism-induced suppression of allergic airway disease: novel therapies on the horizon? Expert Rev Respir Med. 2014;8:717–730. doi: 10.1586/17476348.2014.949244. [DOI] [PubMed] [Google Scholar]
- 50.Paplinska-Goryca M, Misiukiewicz-Stepien P, Proboszcz M, Nejman-Gryz P, Gorska K, Zajusz-Zubek E, Krenke R. Interactions of nasal epithelium with macrophages and dendritic cells variously alter urban PM-induced inflammation in healthy, asthma and COPD. Sci Rep. 2021;11:13259. doi: 10.1038/s41598-021-92626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou C, Yan L, Sun K, Zhou T, Zou Y, Xiong W, Duan SZ. Nuclear receptor corepressor 1 deficiency exacerbates asthma by modulating macrophage polarization. Cell Death Discov. 2023;9:429. doi: 10.1038/s41420-023-01724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 53.Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF Jr, Pace W, Schatz M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the national asthma education and prevention program. JAMA. 2020;324:2301–2317. doi: 10.1001/jama.2020.21974. [DOI] [PubMed] [Google Scholar]
- 54.Castillo JR, Peters SP, Busse WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. 2017;5:918–927. doi: 10.1016/j.jaip.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hinks TSC, Levine SJ, Brusselle GG. Treatment options in type-2 low asthma. Eur Respir J. 2021;57:2000528. doi: 10.1183/13993003.00528-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dimov VV, Stokes JR, Casale TB. Immunomodulators in asthma therapy. Curr Allergy Asthma Rep. 2009;9:475–483. doi: 10.1007/s11882-009-0070-x. [DOI] [PubMed] [Google Scholar]
- 57.Edwards MR, Walton RP, Jackson DJ, Feleszko W, Skevaki C, Jartti T, Makrinoti H, Nikonova A, Shilovskiy IP, Schwarze J, Johnston SL, Khaitov MR EAACI Anti-infectives in Asthma and Asthma Exacerbations Task Force. The potential of anti-infectives and immunomodulators as therapies for asthma and asthma exacerbations. Allergy. 2018;73:50–63. doi: 10.1111/all.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alharris E, Mohammed A, Alghetaa H, Zhou J, Nagarkatti M, Nagarkatti P. The ability of resveratrol to attenuate ovalbumin-mediated allergic asthma is associated with changes in microbiota involving the gut-lung axis, enhanced barrier function and decreased inflammation in the lungs. Front Immunol. 2022;13:805770. doi: 10.3389/fimmu.2022.805770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahhaleh FG, Barrientos G, Conrad ML. The gut-lung axis and asthma susceptibility in early life. Acta Physiol (Oxf) 2024;240:e14092. doi: 10.1111/apha.14092. [DOI] [PubMed] [Google Scholar]
- 60.Biggs SE, Ownby DR, May KR. Use of over-the-counter inhaled epinephrine in asthma. Ann Allergy Asthma Immunol. 2022;129:637–638. doi: 10.1016/j.anai.2022.08.992. [DOI] [PubMed] [Google Scholar]
- 61.Eremija J, Carr TF. Immunotherapy for asthma. Semin Respir Crit Care Med. 2022;43:709–719. doi: 10.1055/s-0042-1749454. [DOI] [PubMed] [Google Scholar]
- 62.KleinJan A. Airway inflammation in asthma: key players beyond the Th2 pathway. Curr Opin Pulm Med. 2016;22:46–52. doi: 10.1097/MCP.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 63.Guntern P, Eggel A. Past, present, and future of anti-IgE biologics. Allergy. 2020;75:2491–2502. doi: 10.1111/all.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfeffer PE, Ali N, Murray R, Ulrik C, Tran TN, Maspero J, Peters M, Christoff GC, Sadatsafavi M, Torres-Duque CA, Altraja A, Lehtimaki L, Papadopoulos NG, Salvi S, Costello RW, Cushen B, Heffler E, Iwanaga T, Al-Ahmad M, Larenas-Linnemann D, Kuna P, Fonseca JA, Al-Lehebi R, Rhee CK, Perez-de-Llano L, Perng Steve DW, Mahboub B, Wang E, Goh C, Lyu J, Newell A, Alacqua M, Belevskiy AS, Bhutani M, Bjermer L, Bjornsdottir U, Bourdin A, Bulow AV, Busby J, Canonica GW, Cosio BG, Dorscheid DR, Munoz-Esquerre M, FitzGerald JM, Gil EG, Gibson PG, Heaney LG, Hew M, Hilberg O, Hoyte F, Jackson DJ, Koh MS, Ko HB, Lee JH, Lehmann S, Chaves Loureiro C, Luethviksdottir D, Menzies-Gow AN, Mitchell P, Papaioannou AI, Popov TA, Porsbjerg CM, Salameh L, Sirena C, Taille C, Taube C, Tohda Y, Wechsler ME, Price DB. Comparative effectiveness of anti-IL5 and anti-IgE biologic classes in patients with severe asthma eligible for both. Allergy. 2023;78:1934–1948. doi: 10.1111/all.15711. [DOI] [PubMed] [Google Scholar]
- 65.Zuberi FF, Haroon MA, Haseeb A, Khuhawar SM. Role of montelukast in asthma and allergic rhinitis patients. Pak J Med Sci. 2020;36:1517–1522. doi: 10.12669/pjms.36.7.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo W, Hu J, Xu W, Dong J. Distinct spatial and temporal roles for Th1, Th2, and Th17 cells in asthma. Front Immunol. 2022;13:974066. doi: 10.3389/fimmu.2022.974066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dembele M, Tao S, Massoud AH, Miah SMS, Lelias S, De Groot AS, Mazer BD. Tregitopes improve asthma by promoting highly suppressive and antigen-specific tregs. Front Immunol. 2021;12:634509. doi: 10.3389/fimmu.2021.634509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas R, Qiao S, Yang X. Th17/Treg imbalance: implications in lung inflammatory diseases. Int J Mol Sci. 2023;24:4865. doi: 10.3390/ijms24054865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. Intraalveolar catecholamines and the human lung microbiome. Am J Respir Crit Care Med. 2015;192:257–259. doi: 10.1164/rccm.201502-0326LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019;12:843–850. doi: 10.1038/s41385-019-0160-6. [DOI] [PubMed] [Google Scholar]
- 71.Liu Q, Tian X, Maruyama D, Arjomandi M, Prakash A. Lung immune tone via gut-lung axis: gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1beta, FFAR2, and FFAR3 expression. Am J Physiol Lung Cell Mol Physiol. 2021;321:L65–L78. doi: 10.1152/ajplung.00421.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, Delhaes L. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bingula R, Filaire M, Radosevic-Robin N, Bey M, Berthon JY, Bernalier-Donadille A, Vasson MP, Filaire E. Desired turbulence? Gut-lung axis, immunity, and lung cancer. J Oncol. 2017;2017:5035371. doi: 10.1155/2017/5035371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheffold A, Bacher P. Anti-fungal T cell responses in the lung and modulation by the gut-lung axis. Curr Opin Microbiol. 2020;56:67–73. doi: 10.1016/j.mib.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Stricker S, Hain T, Chao CM, Rudloff S. Respiratory and intestinal microbiota in pediatric lung diseases-current evidence of the gut-lung axis. Int J Mol Sci. 2022;23:6791. doi: 10.3390/ijms23126791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang YH, Yan ZZ, Luo SD, Hu JJ, Wu M, Zhao J, Liu WF, Li C, Liu KX. Gut microbiota-derived succinate aggravates acute lung injury after intestinal ischaemia/reperfusion in mice. Eur Respir J. 2023;61:2200840. doi: 10.1183/13993003.00840-2022. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Liu J, Li F, Luo Y, Ge P, Zhang Y, Wen H, Yang Q, Ma S, Chen H. The gut-lung axis in severe acute Pancreatitis-associated lung injury: the protection by the gut microbiota through short-chain fatty acids. Pharmacol Res. 2022;182:106321. doi: 10.1016/j.phrs.2022.106321. [DOI] [PubMed] [Google Scholar]
- 78.Jia Y, He T, Wu D, Tong J, Zhu J, Li Z, Dong J. The treatment of Qibai Pingfei Capsule on chronic obstructive pulmonary disease may be mediated by Th17/Treg balance and gut-lung axis microbiota. J Transl Med. 2022;20:281. doi: 10.1186/s12967-022-03481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fidelle M, Rauber C, Alves Costa Silva C, Tian AL, Lahmar I, de La Varende AM, Zhao L, Thelemaque C, Lebhar I, Messaoudene M, Pizzato E, Birebent R, Mbogning Fonkou MD, Zoppi S, Reni A, Dalban C, Leduc M, Ferrere G, Durand S, Ly P, Silvin A, Mulder K, Dutertre CA, Ginhoux F, Yonekura S, Roberti MP, Tidjani-Alou M, Terrisse S, Chen J, Kepp O, Schippers A, Wagner N, Suarez-Gosalvez J, Kobold S, Fahrner JE, Richard C, Bosq J, Lordello L, Vitali G, Galleron N, Quinquis B, Le Chatelier E, Blanchard L, Girard JP, Jarry A, Gervois N, Godefroy E, Labarriere N, Koschny R, Daillere R, Besse B, Truntzer C, Ghiringhelli F, Coatnoan N, Mhanna V, Klatzmann D, Drubay D, Albiges L, Thomas AM, Segata N, Danlos FX, Marabelle A, Routy B, Derosa L, Kroemer G, Zitvogel L. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science. 2023;380:eabo2296. doi: 10.1126/science.abo2296. [DOI] [PubMed] [Google Scholar]
- 80.Ni S, Yuan X, Cao Q, Chen Y, Peng X, Lin J, Li Y, Ma W, Gao S, Chen D. Gut microbiota regulate migration of lymphocytes from gut to lung. Microb Pathog. 2023;183:106311. doi: 10.1016/j.micpath.2023.106311. [DOI] [PubMed] [Google Scholar]
- 81.Lee DH, Park HK, Lee HR, Sohn H, Sim S, Park HJ, Shin YS, Kim YK, Choi Y, Park HS. Immunoregulatory effects of Lactococcus lactis-derived extracellular vesicles in allergic asthma. Clin Transl Allergy. 2022;12:e12138. doi: 10.1002/clt2.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukherjee S, Joardar N, Sengupta S, Sinha Babu SP. Gut microbes as future therapeutics in treating inflammatory and infectious diseases: lessons from recent findings. J Nutr Biochem. 2018;61:111–128. doi: 10.1016/j.jnutbio.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184:1469–1485. doi: 10.1016/j.cell.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 84.Murphy RC, Lai Y, Liu M, Al-Shaikhly T, Altman MC, Altemeier WA, Frevert CW, Debley JS, Piliponsky AM, Ziegler SF, Gharib SA, Hallstrand TS. Distinct epithelial-innate immune cell transcriptional circuits underlie airway hyperresponsiveness in asthma. Am J Respir Crit Care Med. 2023;207:1565–1575. doi: 10.1164/rccm.202209-1707OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi: 10.3389/fimmu.2020.603312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scioscia G, Nolasco S, Campisi R, Quarato CMI, Caruso C, Pelaia C, Portacci A, Crimi C. Switching biological therapies in severe asthma. Int J Mol Sci. 2023;24:9563. doi: 10.3390/ijms24119563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan YL, Zhang X, Liu L, Wang G, Chen-Yu Hsu A, Huang D, Wang G, Oliver BG. Total IgE variability is associated with future asthma exacerbations: a 1-year prospective cohort study. J Allergy Clin Immunol Pract. 2021;9:2812–2824. doi: 10.1016/j.jaip.2021.04.065. [DOI] [PubMed] [Google Scholar]
- 88.Charles D, Shanley J, Temple SN, Rattu A, Khaleva E, Roberts G. Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: a systematic review and meta-analysis. Clin Exp Allergy. 2022;52:616–627. doi: 10.1111/cea.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, Humbert M, Katz LE, Keene ON, Yancey SW, Chanez P MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 90.Singh D, Fuhr R, Bird NP, Mole S, Hardes K, Man YL, Cahn A, Yancey SW, Pouliquen IJ. A Phase 1 study of the long-acting anti-IL-5 monoclonal antibody GSK3511294 in patients with asthma. Br J Clin Pharmacol. 2022;88:702–712. doi: 10.1111/bcp.15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brennan LC, O’Sullivan A, MacLoughlin R. Cellular therapy for the treatment of paediatric respiratory disease. Int J Mol Sci. 2021;22:8906. doi: 10.3390/ijms22168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benamar M, Chen Q, Martinez-Blanco M, Chatila TA. Regulatory T cells in allergic inflammation. Semin Immunol. 2023;70:101847. doi: 10.1016/j.smim.2023.101847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abbasi-Dokht T, Sadrifar S, Forouzandeh S, Malek F, Hemmati M, Kokhaei P, Salek Farrokhi A, Baharlou R. Multistrain probiotics supplement alleviates asthma symptoms via increasing treg cells population: a randomized, double-blind, placebo-controlled trial. Int Arch Allergy Immunol. 2023;184:291–301. doi: 10.1159/000526739. [DOI] [PubMed] [Google Scholar]
- 94.Thomson NC. New and developing non-adrenoreceptor small molecule drugs for the treatment of asthma. Expert Opin Pharmacother. 2017;18:283–293. doi: 10.1080/14656566.2017.1284794. [DOI] [PubMed] [Google Scholar]
- 95.Zak M, Dengler HS, Rajapaksa NS. Inhaled Janus Kinase (JAK) inhibitors for the treatment of asthma. Bioorg Med Chem Lett. 2019;29:126658. doi: 10.1016/j.bmcl.2019.126658. [DOI] [PubMed] [Google Scholar]
- 96.Malaviya R, Laskin DL, Malaviya R. Janus kinase-3 dependent inflammatory responses in allergic asthma. Int Immunopharmacol. 2010;10:829–836. doi: 10.1016/j.intimp.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, Muraro A, Roberts G, Akdis CA, Bonini M, Cavkaytar O, Flood B, Gajdanowicz P, Izuhara K, Kalayci O, Mosges R, Palomares O, Pfaar O, Smolinska S, Sokolowska M, Asaria M, Netuveli G, Zaman H, Akhlaq A, Sheikh A. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy. 2017;72:1825–1848. doi: 10.1111/all.13208. [DOI] [PubMed] [Google Scholar]
- 98.Rice JL, Diette GB, Suarez-Cuervo C, Brigham EP, Lin SY, Ramanathan M Jr, Robinson KA, Azar A. Allergen-specific immunotherapy in the treatment of pediatric asthma: a systematic review. Pediatrics. 2018;141:e20173833. doi: 10.1542/peds.2017-3833. [DOI] [PubMed] [Google Scholar]
- 99.Virchow JC. Allergen immunotherapy (AIT) in asthma. Semin Immunol. 2019;46:101334. doi: 10.1016/j.smim.2019.101334. [DOI] [PubMed] [Google Scholar]
- 100.Lommatzsch M, Buhl R, Canonica GW, Ribas CD, Nagase H, Brusselle GG, Jackson DJ, Pavord ID, Korn S, Milger K, Taube C, Virchow JC. Pioneering a paradigm shift in asthma management: remission as a treatment goal. Lancet Respir Med. 2024;12:96–99. doi: 10.1016/S2213-2600(23)00415-0. [DOI] [PubMed] [Google Scholar]
- 101.Alamri A. Diversity of microbial signatures in asthmatic airways. Int J Gen Med. 2021;14:1367–1378. doi: 10.2147/IJGM.S304339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fangous MS, Gosset P, Galakhoff N, Gouriou S, Guilloux CA, Payan C, Vallet S, Hery-Arnaud G, Le Berre R. Priming with intranasal lactobacilli prevents Pseudomonas aeruginosa acute pneumonia in mice. BMC Microbiol. 2021;21:195. doi: 10.1186/s12866-021-02254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Glieca S, Quarta E, Bottari B, Lal VC, Sonvico F, Buttini F. The role of airways microbiota on local and systemic diseases: a rationale for probiotics delivery to the respiratory tract. Expert Opin Drug Deliv. 2024;21:991–1005. doi: 10.1080/17425247.2024.2380334. [DOI] [PubMed] [Google Scholar]
- 104.Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M, Storti C, Triulzi T, Castelli C, Balsari A, Tagliabue E, Sfondrini L. Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep. 2018;24:3528–3538. doi: 10.1016/j.celrep.2018.08.090. [DOI] [PubMed] [Google Scholar]
- 105.Guo S, Shi Y, Xu A, Wang Y, Xu P. Liubao tea extract ameliorates ovalbumin-induced allergic asthma by regulating gut microbiota in mice. Food Funct. 2023;14:10605–10616. doi: 10.1039/d3fo03470d. [DOI] [PubMed] [Google Scholar]
- 106.Wu Y, Chen Y, Li Q, Ye X, Guo X, Sun L, Zou J, Shen Y, Mao Y, Li C, Yang Y. Tetrahydrocurcumin alleviates allergic airway inflammation in asthmatic mice by modulating the gut microbiota. Food Funct. 2021;12:6830–6840. doi: 10.1039/d1fo00194a. [DOI] [PubMed] [Google Scholar]
- 107.Sudre CH, Antonelli M, Cheetham NJ, Molteni E, Canas LS, Bowyer V, Murray B, Rjoob K, Modat M, Capdevila Pujol J, Hu C, Wolf J, Spector TD, Hammers A, Steves CJ, Ourselin S, Duncan EL. Symptoms before and after COVID-19: a population and case-control study using prospective data. Eur Respir J. 2024;64:2301853. doi: 10.1183/13993003.01853-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]