Abstract
Objective: To investigate the effectiveness of combining transurethral resection of bladder tumor (TURBT) with Bacille Calmette-Guerin (BCG) intravesical instillation in treating high-risk non-muscle invasive bladder cancer (HRNMIBC). Methods: We retrospectively reviewed the clinical data from 118 HRNMIBC patients treated at Tongren Hospital between March 2020 and June 2022. The patients were categorized into two groups based on their treatment regimen: the control group (n=60) which received regular pirarubicin intravesical instillation, and the observation group (n=58) which received additional BCG intravesical instillation alongside pirarubicin. All patients underwent TURBT. Postoperative follow-up included monitoring recurrence and adverse reactions. Quality of life was assessed for both groups before and after treatment. Peripheral blood T lymphocyte subsets and immunoglobulin levels were measured pre- and post-instillation. Patients were further classified into recurrence and non-recurrence groups to analyze factors influencing HRNMIBC recurrence. Results: The observation group exhibited a recurrence rate of 22.41% (13/58), notably lower than the 41.66% (25/60) in the control group (P<0.05). Both groups showed improved postoperative scores in somatic, emotional, social, cognitive, and role functions compared to preoperative scores, with the observation group demonstrating significantly higher scores across all domains (all P<0.05). Post-instillation, CD4+, CD4+/CD8+, Immunoglobulin M (IgM), Immunoglobulin G (IgG), and Immunoglobulin A (IgA) levels were elevated in both groups relative to pre-instillation levels, whereas CD8+ levels were reduced. The observation group showed significantly higher levels of CD4+, CD4+/CD8+, IgM, IgG, and IgA, and lower levels of CD8+ compared to the control group following instillation (all P<0.05). Univariate analysis revealed that tumor number, stage, grade, and primary tumor status were all significantly associated with HRNMIBC recurrence (all P<0.05). Multivariate logistic regression further identified tumor number, stage, grade, and primary status as independent predictors of HRNMIBC recurrence (all P<0.05). Conclusion: The combination of TURBT with pirarubicin and BCG intravesical instillation significantly lowers the recurrence rate in HRNMIBC patients and enhances both quality of life and immune function recovery. Independent factors affecting HRNMIBC recurrence include tumor number, stage, grade, and primary tumor status.
Keywords: High-risk non-muscle invasive bladder cancer, transurethral resection of bladder tumor, Bacille Calmette-Guerin vaccine, efficacy, relapse, influencing factor
Introduction
Bladder cancer (BC) is characterized with a superficial growth, and presents symptoms such as urinary irritation and obstruction. As the condition advances, it can cause complications including lower abdominal masses, urinary retention, involvement of pelvic lymph nodes, and metastasis to other organs, significantly affecting the patient’s overall health [1,2]. BC is categorized into non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC), depending on whether the muscle layer of the bladder wall is affected. NMIBC constitutes 75% of the cases, while MIBC makes up 25% [3,4]. Studies have shown that nearly 3/4 high-risk NMIBC (HRNMIBC) patients experience relapse or disease progression within 10 years after diagnosis [5]. While NMIBC itself rarely causes death directly, recurrence and progression present ongoing challenges for most patients [6].
Radical cystectomy is often used in the early-stage BC, yet its clinical application is limited due to severe trauma, long recovery time, high complication rates, and many contraindications [7,8]. Recently, minimally invasive techniques have developed rapidly in the field of urology. Minimally invasive surgery, represented by transurethral resection of bladder tumor (TURBT), has gradually become the standard treatment for bladder cancer due to minimal trauma, wide adaptability and bladder-preserving benefits. However, the recurrence rate still exceeds 70% within 5 years after lesion resection, with some recurrent tumors exhibiting increased malignancy. TURBT alone cannot effectively prevent the recurrence and progress of tumors [9,10].
To reduce the recurrence rate and eliminate residual tumor cells, adjuvant bladder perfusion chemotherapy is recommended post-TURBT. Various drugs are available for bladder perfusion treatment [11,12]. A commonly used drug for bladder perfusion immunotherapy is Bacille Calmette-Guerin vaccine (BCG), with chemotherapeutic agents like epirubicin, pirarubicin, gemcitabine, doxorubicin, hydroxycamptothecin, and mitomycin also widely used for NMIBC after surgery. BCG remains the first-line drug for HRNMIBC [13,14]. Therefore, it is particularly important to explore more effective adjuvant treatment schemes. Intravesical instillation of BCG has potential advantages in improving patient prognosis and reducing recurrence rate, though existing literature is limited.
This study retrospectively analyzed clinical data from HRNMIBC patients to explore the efficacy of TURBT combined with BCG intravesical instillation in HRNMIBC treatment and identify factors influencing recurrence. Specifically, this research examines the efficacy of TURBT combined with BCG intravesical instillation in high-risk patients. Unlike previous studies, it evaluates both postoperative recurrence rate and influencing factors, aiming to address research gaps in this area and provide a basis for developing individualized follow-up and treatment strategies for high-risk patients by analyzing recurrence-associated factors.
Patients and methods
Patients
A retrospective review was performed on the clinical data from 118 HRNMIBC patients treated at Tongren Hospital between March 2020 and June 2022 after being approved by the Ethics Committee of Tongren Hospital (Figure 1).
Figure 1.
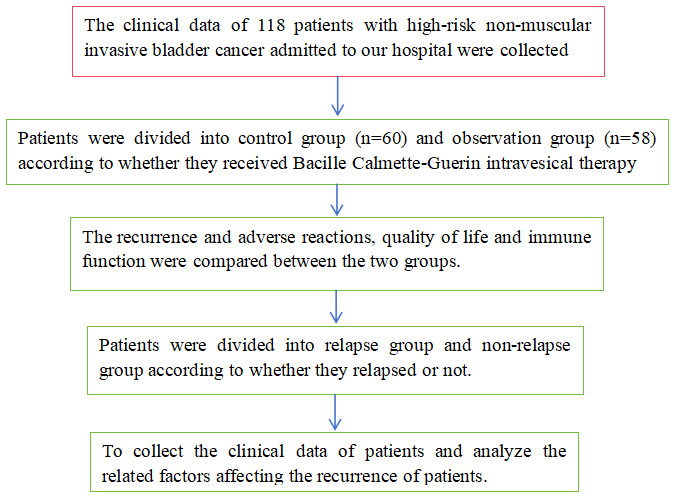
Flowchart of the study process.
Inclusion criteria: patients met the diagnostic criteria for HRNMIBC [15]; Patients eligible for TURBT; and patients with complete clinical records. Exclusion criteria: invasive bladder cancer; presence of malignant tumors in other organs; coagulation disorders; prior radiotherapy or chemotherapy for other systemic cancers; or loss to follow-up. Patients were categorized into two groups based on whether they received BCG intravesical instillation: the control group (n=60) and the observation group (n=58). Clinical data such as age, sex, Body Mass Index (BMI), tumor number, tumor stage, tumor diameter, tumor grade, tumor location, hypertension and diabetes, and primary tumor were collected. There were no significant differences in baseline characteristics between the two groups (P>0.05), as shown in Table 1.
Table 1.
Comparison of general information between the two groups
| Group | Age (years) | Gender | Number of tumors | Tumor staging | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Male | Female | Single | Multiple | Ta | T1 | ||
| Control group (n=60) | 63.65±9.24 | 51 | 9 | 36 | 24 | 22 | 38 |
| Observation group (n=58) | 64.06±9.78 | 44 | 14 | 31 | 27 | 30 | 28 |
| t/χ2 | 0.234 | 1.569 | 0.516 | 2.713 | |||
| P | 0.815 | 0.210 | 0.473 | 0.100 | |||
Treatment methods
Patients in both groups underwent TURBT in the lithotomy position under general anesthesia. After anesthesia, an electrosurgical cystoscope was inserted through the urethra to observe the lesion characteristics. Electrosurgical power was set at 160-180 W and the electrocoagulation power was set at 60-80 W. Smaller lesions were removed via anterograde resection from the base, while larger lesions were resected layer-by-layer from one side of the lesion. Additionally, a 1.5 cm margin of bladder wall surrounding the tumor was excised. Wounds were cauterized, and lesions were rinsed with normal saline. Normal-temperature perfusion chemotherapy drugs were given immediately after operation. Postoperatively, control group patients received weekly bladder instillations of 40 mg pirarubicin for 4-8 weeks, followed by monthly instillations up to one year after surgery. In addition to this regimen, observation group patients received a 60 mg BCG vaccine + 40 ml of 0.9% sodium chloride solution weekly from the second to seventh weeks post-surgery, followed by three BCG injections every two weeks, each containing 60 mg BCG + 40 ml sodium chloride. Subsequently, they received monthly instillations, alternating between pirarubicin and BCG: one pirarubicin dose and two BCG doses per month, for a total of 10 instillations.
Observation indicators
Primary outcome measures included recurrence and adverse reactions. All patients were followed up after operation through telephone or WeChat every 3 months until June 2024, with a minimum follow-up time of 2 years. Postoperative recurrence rates were recorded, with recurrence defined as the reappearance of a tumor confirmed by pathological biopsy. Adverse reactions during treatment were also recorded. Patients were divided into a recurrence group and a non-recurrence group based on their recurrence status. Clinical data for all patients were analyzed retrospectively to identify factors affecting HRNMIBC recurrence, utilizing both univariate and multivariate analyses.
Secondary outcome measures included quality of life and immune function. Quality of life was evaluated for both groups before and one year after surgery using the European Organization for Research and Treatment of Cancer Quality of Life Scale [16]. This scale assesses five dimensions: somatic function, emotional function, social function, cognitive function, and role function. Fasting venous blood samples were collected from patients before and six months post-perfusion for serum biochemical analysis. T lymphocyte subsets (CD4+, CD8+, CD4+/CD8+) in peripheral blood were measured using an Epics XL flow cytometer (Beckman Kurt gmbh, USA), while immunoglobulin levels (Immunoglobulin M (IgM), IgG, IgA) were measured by nephelometry with immune scattering. Changes in these markers were compared to evaluate immune function variations.
Statistical methods
Data analysis was conducted using SPSS 22.0 statistical software, while GraphPad Prism 8 was used for creating graphical representations of the data. Count data were presented as rates, and comparisons between groups were performed using the chi-square test. For pairwise comparisons of continuous variables between groups, the t-test was utilized. Univariate and multivariate logistic regression analyses were employed to identify factors influencing the HRNMIBC recurrence. Statistical significance was set at P<0.05.
Results
Comparison of recurrence rates between groups
The recurrence rate in the observation group was 22.41% (13/58), significantly lower than the 41.66% (25/60) observed in the control group (P<0.05), as shown in Figure 2.
Figure 2.
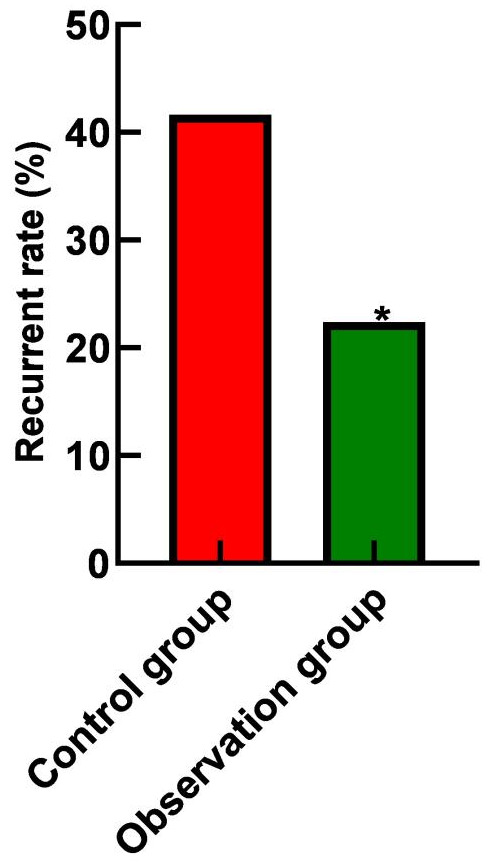
Comparison of recurrence rate between the two groups. Note: Compared with the control group, *P<0.05.
Comparison of quality-of-life scores between groups
Postoperative evaluations revealed that scores for somatic, emotional, social, cognitive, and role functions were higher in both groups compared to preoperative levels. The observation group consistently had higher scores across all dimensions compared to the control group (all P<0.05), as shown in Figure 3.
Figure 3.
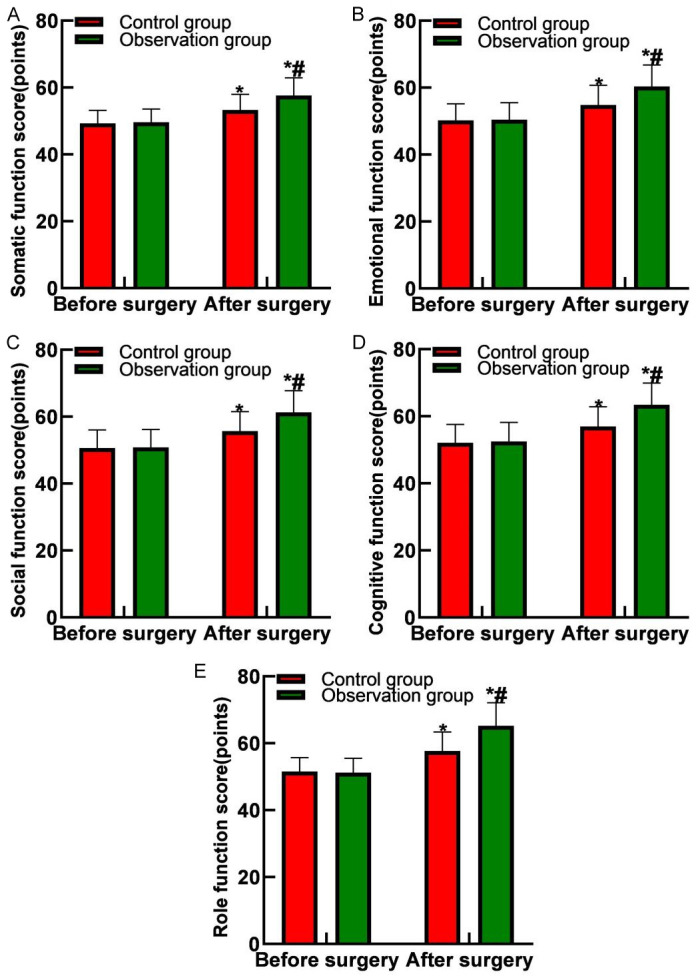
Comparison of quality-of-life scores between the two groups. A. Physical function scores; B. Emotional function scores; C. Social function scores; D. Cognitive function scores; E. Role function scores. Note: Compared with before surgery, *P<0.05; compared with the control group, #P<0.05.
Comparison of immune function between groups
Following instillation, both groups exhibited increased proportions of CD4+, CD4+/CD8+ T lymphocytes, IgM, IgG, and IgA levels, with a decrease in CD8+ T lymphocyte proportion. Moreover, the observation group had significantly higher proportions of CD4+, CD4+/CD8+ T lymphocytes, IgM, IgG, and IgA levels, and lower levels of CD8+ T lymphocytes compared to the control group (all P<0.05), as shown in Figures 4 and 5.
Figure 4.
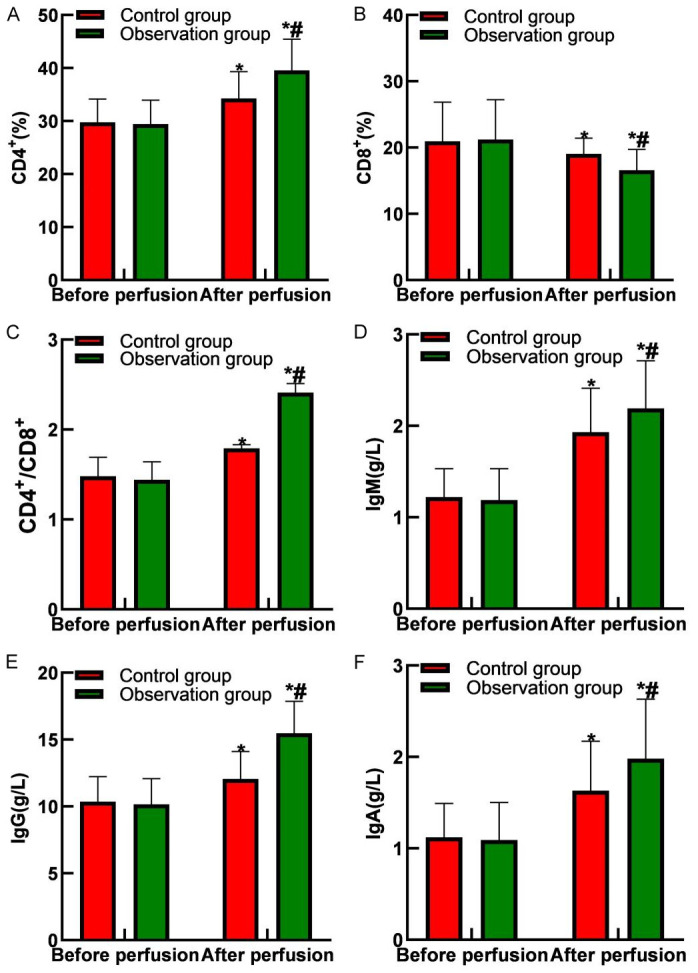
Comparison of immune function between the two groups. A. CD4+ levels; B. CD8+ levels; C. CD4+/CD8+ levels; D. IgM levels; E. IgG levels; F. IgA levels. Note: Compared with before perfusion, *P<0.05; compared with the control group, #P<0.05. IgM: Immunoglobulin M, IgG: Immunoglobulin G, IgA: Immunoglobulin A.
Figure 5.
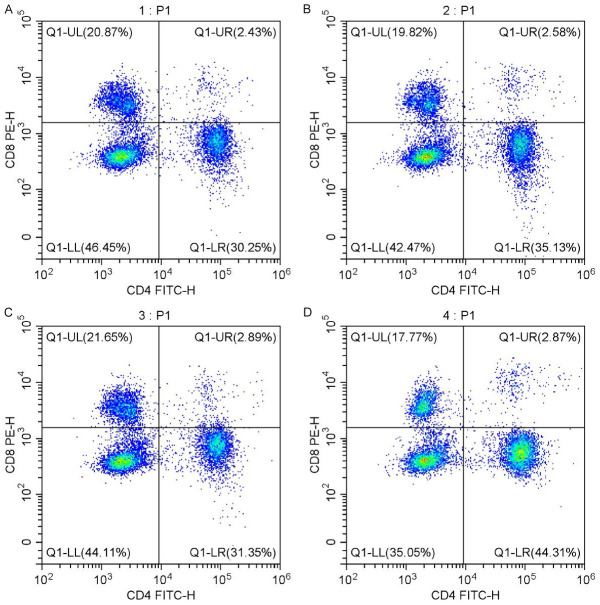
Flow cytometry of peripheral blood T lymphocyte subsets in two groups. A. T lymphocyte subsets before perfusion in the control group; B. T lymphocyte subsets after perfusion in the control group; C. T lymphocyte subsets before perfusion in the observation group; D. T lymphocyte subsets after perfusion in the observation group.
Comparison of adverse reactions between groups
The overall incidence of adverse reactions did not differ significantly between the two groups (P>0.05), as shown in Table 2.
Table 2.
Comparison of adverse reactions between the two groups
| Group | Nausea and vomiting | Thrombocytopenia | Anorexia | Total incidence rate |
|---|---|---|---|---|
| Control group (n=60) | 5 (8.33%) | 4 (6.67%) | 6 (10.00%) | 15 (25.00%) |
| Observation group (n=58) | 3 (5.17%) | 2 (3.45%) | 5 (8.62%) | 10 (17.24%) |
| χ2 | 1.063 | |||
| P | 0.302 |
Univariate analysis of HRNMIBC recurrence
Univariate analysis indicated that factors such as the number of tumors, tumor stage, tumor grade, and primary tumor status were associated with HRNMIBC recurrence (all P<0.05), as shown in Table 3.
Table 3.
Univariate analysis of factors associated with recurrence in HRNMIBC patients
| Factors | Recurrence group (n=38) | Non-recurrence group (n=80) | χ2 | P | |
|---|---|---|---|---|---|
| Age (years) | <60 | 18 (47.37%) | 26 (32.50%) | 2.436 | 0.119 |
| ≥60 | 20 (52.63%) | 55 (67.50%) | |||
| Gender | Male | 29 (76.32%) | 66 (82.50%) | 0.628 | 0.428 |
| Female | 9 (23.68%) | 14 (17.50%) | |||
| BMI (kg/m2) | <23 | 19 (50.00%) | 33 (41.25%) | 0.800 | 0.371 |
| ≥23 | 19 (50.00%) | 47 (58.75%) | |||
| Number of tumors | Single | 15 (39.47%) | 52 (65.00%) | 6.841 | 0.009 |
| Multiple | 23 (60.53%) | 28 (35.00%) | |||
| Tumor staging | Ta | 10 (26.32%) | 42 (52.50%) | 7.166 | 0.007 |
| T1 | 28 (73.68%) | 38 (47.50%) | |||
| Tumor diameter (cm) | <5 | 16 (42.11%) | 45 (56.25%) | 2.064 | 0.151 |
| ≥5 | 22 (57.89%) | 35 (43.75%) | |||
| Tumor grading | Poorly differentiated | 19 (50.00%) | 15 (18.75%) | 12.267 | 0.002 |
| Intermediately differentiated | 11 (28.95%) | 38 (47.50%) | |||
| Highly differentiated | 8 (21.05%) | 27 (33.75%) | |||
| Tumor site | Bladder wall | 14 (36.84%) | 28 (35.00%) | 0.307 | 0.858 |
| Trigone/bladder neck | 14 (36.84%) | 27 (33.75%) | |||
| Both | 10 (26.32%) | 25 (31.25%) | |||
| Combined hypertension | Yes | 18 (47.37%) | 42 (52.50%) | 0.271 | 0.602 |
| No | 20 (52.63%) | 38 (47.50%) | |||
| Combined diabetes | Yes | 14 (36.84%) | 38 (47.50%) | 1.187 | 0.276 |
| No | 24 (63.16%) | 42 (52.50%) | |||
| Whether primary tumor | Primary | 16 (42.11%) | 56 (70.00%) | 8.428 | 0.004 |
| Recurrent | 22 (57.89%) | 24 (30.00%) | |||
Note: BMI: Body Mass Index.
Multivariate analysis of HRNMIBC recurrence
Further multivariate logistic regression analysis identified the number of tumors, tumor stage, tumor grade, and primary tumor status as independent factors influencing HRNMIBC recurrence (all P<0.05), as shown in Tables 4 and 5.
Table 4.
Multi-factor analysis assignment table
| Factors | Variable | Evaluation |
|---|---|---|
| Number of tumors | X1 | Single =1; Multiple =2 |
| Tumor staging | X2 | Ta =1; T1 =2 |
| Tumor grading | X3 | Poorly differentiated =1; Intermediately differentiated =2; Highly differentiated =3 |
| Whether primary tumor | X4 | Primary =1; Recurrent =2 |
Table 5.
Multivariate analysis of factors affecting recurrence in HRNMIBC patients
| Factors | B | SE | Wald | Sig. | Exp (B) |
|---|---|---|---|---|---|
| Number of tumors | 1.191 | 0.472 | 6.358 | 0.012 | 3.292 |
| Tumor staging | 1.393 | 0.499 | 7.806 | 0.005 | 4.027 |
| Tumor grading | -1.600 | 0.599 | 7.127 | 0.008 | 0.202 |
| Whether primary tumor | 1.090 | 0.473 | 5.308 | 0.021 | 2.973 |
Discussion
Bladder cancer (BC) significantly affects patients’ health and quality of life, with non-muscle invasive bladder cancer (NMIBC) accounting for over 70% of cases, primarily involving Ta and T1 stage tumors. Studies have shown that more than 40% of NMIBC patients experience relapse within 2 years of treatment, and approximately 10% progress to muscle invasive bladder cancer (MIBC) [17,18]. At present, NMIBC treatment predominantly involves TURBT followed by regular intravesical infusion of chemotherapy or immunotherapy drugs, yielding positive clinical results [19,20]. NMIBC is a highly heterogeneous disease, with recurrence and progression risks varying significantly across different risk grades. High-risk NMIBC (HRNMIBC) patients have the highest likelihood of recurrence and progression, necessitating tailored treatment strategies [21,22]. To mitigate postoperative tumor recurrence and progression, bladder perfusion immunotherapy and chemotherapy are often used, as they effectively reduce recurrence by targeting tumor cells disseminated during surgery [23,24]. Bacille Calmette-Guerin (BCG) therapy remains the “gold standard” for HRNMIBC treatment. While the precise mechanisms by which BCG exerts its antitumor effects is not entirely understood, it is known to involve direct targeting of bladder tumor cells, leading to cell uptake and activation of adaptive immune responses [25,26]. Preserving bladder function and maintaining urinary health are key priorities for most patients under current treatment protocols. However, HRNMIBC patients still face significant challenges, such as high rates of recurrence and disease progression. Therefore, improving the prognosis of HRNMIBC and enhancing patient quality of life under current treatment paradigms remains a critical focus of clinical investigation. Our study investigated the efficacy of combining standard intravesical pirarubicin instillations post-TURBT with adjunctive BCG instillation therapy, yielding promising clinical outcomes.
The study findings revealed that the recurrence rate in the observation group was 20.67% (12/58), significantly lower than the 38.33% (23/60) observed in the control group. Postoperative assessments showed improvements in physical, emotional, social, cognitive, and role functions for both groups compared to their preoperative scores, with the observation group performing better in all areas. Additionally, after perfusion, levels of CD4+, CD4+/CD8+ T lymphocytes, IgM, IgG, and IgA were all elevated in both groups, while CD8+ levels were reduced. The observation group exhibited higher levels of CD4+, CD4+/CD8+, IgM, IgG, and IgA, and lower levels of CD8+ compared to the control group. These results suggest that combining TURBT with pirarubicin and BCG vaccine for HRNMIBC treatment can significantly decrease recurrence rates and improve both quality of life and immune function of patients. The mechanism behind BCG’s efficacy lies in its ability to induce a local immune response in the bladder wall, recruiting a large number of immune cells, such as macrophages, neutrophils and T lymphocytes. The activation and accumulation of these immune cells can directly attack and kill residual cancer cells, thus reducing tumor recurrence [27,28]. This immune response is predominantly cell-mediated, with T cell activation playing a crucial role, especially cytotoxic T cells, which recognize and kill tumor cells expressing specific antigens [29,30]. Additionally, BCG vaccine fosters a long-lasting immune memory and promotes the release of cytokines, enhancing both immune response and the anti-tumor activity of immune cells, providing sustained protection against tumors reoccurrence [31,32].
While bladder perfusion therapy effectively reduces tumor recurrence, it can also cause local or systemic adverse reactions. For patients undergoing continuous perfusion, these reactions may impact compliance and, in severe cases, lead to treatment discontinuation [33,34]. Therefore, it is essential to monitor adverse reactions during bladder perfusion while evaluating the efficacy of treatment regimens. The study findings revealed no significant difference in overall adverse reaction rates between the two groups, and no severe adverse reactions necessitating treatment cessation. Furthermore, symptomatic treatment or rest successfully alleviated most adverse reactions without significantly affecting treatment compliance. These results indicate the high safety profile of both drugs in bladder perfusion therapy.
Both univariate and multivariate analyses conducted in this study identified the number of tumors, tumor stage, tumor grade, and presence of a primary tumor independently influenced HRNMIBC recurrence. The number of tumors will have a certain impact on postoperative recurrence. When multiple tumors are present, it may be challenging to identify all lesions during TURBT, and smaller tumors may go undetected, potentially leading to regrowth and recurrence after surgery. Tumor stage is an important predictor of recurrence in patients with HRNMIBC. Higher-stage tumors are associated with a greater likelihood of recurrence. For example, in T1-stage tumors, deeper infiltration may make complete resection challenging, increasing the likelihood of residual cancer cells. Additionally, therapeutic agents may not reach effective concentrations at deeper infiltration sites, reducing their efficacy and increasing the risk of recurrence. Tumor differentiation is closely related to survival. Poorly differentiated tumors tend to have higher malignancy, rapid growth potential, and a greater capacity for tissue invasion, leading to a higher risk of lymph node metastasis and making surgical removal more challenging. In the study of Lin N et al. [35], tumor stage and tumor grade were identified as the main influencing factors of postoperative recurrence, aligning with our study findings.
This study highlights the importance of differentiating between primary and recurrent HRNMIBC in assessing recurrence risk. Recurrent HRNMIBC patients often exhibit some tolerance to therapeutic agents, such as postoperative instillation therapies. This acquired tolerance, along with the body’s decreased ability to recognize tumor cells, further increases the likelihood of recurrence [36,37]. The ongoing advancements in HRNMIBC diagnosis and treatment, including innovative diagnostic techniques and more comprehensive methodologies, continue to improve diagnostic accuracy and theoretical understanding. However, further research, particularly through large prospective studies, is essential to predict recurrence risk factors in bladder cancer. Future work should focus on strategies to prevent initial onset, optimize surgical methods, and improve postoperative follow-up care for recurrent cases to further reduce recurrence rates.
Numerous factors influence postoperative recurrence in HRNMIBC patients. This study is a retrospective analysis conducted at a single center, focusing on HRNMIBC patients treated in our department within a specific timeframe. A key limitation is the limited sample size, which inevitably introduces potential biases and influences from the study design, limiting the generalizability of our findings. Future multicenter prospective studies are necessary to validate these findings comprehensively and derive conclusions that better guide clinical diagnosis and treatment strategies.
In conclusion, combining TURBT with bladder perfusion using pirarubicin and Bacille Calmette-Guerin vaccine effectively lowers recurrence rates in HRNMIBC patients, supporting enhanced quality of life and immune function recovery. The number of tumors, tumor stage, tumor grade, and primary tumor status can independently influence recurrence in HRNMIBC patients.
Disclosure of conflict of interest
None.
References
- 1.Huang L, Jia K, Yao K, Liu D, Xu Y, Liu Q. Effect of neoadjuvant chemotherapy on survival in patients with T1 high-grade non-muscle-invasive bladder cancer who underwent radical cystectomy. Medicine (Baltimore) 2023;102:e34501. doi: 10.1097/MD.0000000000034501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Zutphen M, Hof JP, Aben KK, Kampman E, Witjes JA, Kiemeney LA, Vrieling A. Adherence to lifestyle recommendations after non-muscle invasive bladder cancer diagnosis and risk of recurrence. Am J Clin Nutr. 2023;117:681–690. doi: 10.1016/j.ajcnut.2022.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Black PC, Tangen CM, Singh P, McConkey DJ, Lucia MS, Lowrance WT, Koshkin VS, Stratton KL, Bivalacqua TJ, Kassouf W, Porten SP, Bangs R, Plets M, Thompson IM Jr, Lerner SP. Phase 2 trial of atezolizumab in Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer: SWOG S1605. Eur Urol. 2023;84:536–544. doi: 10.1016/j.eururo.2023.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afferi L, Moschini M, Cumberbatch MG, Catto JW, Scarpa RM, Porpiglia F, Mattei A, Sanchez-Salas R, Esperto F European Association of Urology - European Society of Resident Urologists (EAU-ESRU) Biomarkers predicting oncological outcomes of high-risk non-muscle-invasive bladder cancer. Minerva Urol Nefrol. 2020;72:265–278. doi: 10.23736/S0393-2249.20.03786-8. [DOI] [PubMed] [Google Scholar]
- 5.García-Perdomo HA, Sánchez AL, Spiess PE. Immune checkpoints inhibitors in the management of high-risk non-muscle-invasive bladder cancer. A scoping review. Urol Oncol. 2022;40:409.e1–409.e8. doi: 10.1016/j.urolonc.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Bedke J, Black PC, Szabados B, Guerrero-Ramos F, Shariat SF, Xylinas E, Brinkmann J, Blake-Haskins JA, Cesari R, Redorta JP. Optimizing outcomes for high-risk, non-muscle-invasive bladder cancer: the evolving role of PD-(L)1 inhibition. Urol Oncol. 2023;41:461–475. doi: 10.1016/j.urolonc.2023.10.004. [DOI] [PubMed] [Google Scholar]
- 7.de Jong FC, Laajala TD, Hoedemaeker RF, Jordan KR, van der Made ACJ, Boevé ER, van der Schoot DKE, Nieuwkamer B, Janssen EAM, Mahmoudi T, Boormans JL, Theodorescu D, Costello JC, Zuiverloon TCM. Non-muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette-Guérin. Sci Transl Med. 2023;15:eabn4118. doi: 10.1126/scitranslmed.abn4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veeratterapillay R, Heer R, Johnson MI, Persad R, Bach C. High-risk non-muscle-invasive bladder cancer-therapy options during intravesical BCG shortage. Curr Urol Rep. 2016;17:68. doi: 10.1007/s11934-016-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang WL, Huang CY, Huang KH, Pu YS, Chang HC, Chow PM. Outcomes of stratified transurethral resection of bladder tumor: a propensity score-matched analysis. J Formos Med Assoc. 2022;121:73–80. doi: 10.1016/j.jfma.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Taskovska M, Kreft ME, Smrkolj T. Current and innovative approaches in the treatment of non-muscle invasive bladder cancer: the role of transurethral resection of bladder tumor and organoids. Radiol Oncol. 2020;54:135–143. doi: 10.2478/raon-2020-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood EL, Djaladat H. Radical transurethral resection of bladder tumor in organ-confined muscle-invasive bladder cancer: yes! Eur Urol Focus. 2023;9:225–226. doi: 10.1016/j.euf.2022.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Bree KK, Kokorovic A, Westerman ME, Hensley PJ, Brooks NA, Qiao W, Shen Y, Kamat AM, Dinney CP, Navai N. Repeat transurethral resection of muscle-invasive bladder cancer prior to radical cystectomy is prognostic but not therapeutic. J Urol. 2023;209:140–149. doi: 10.1097/JU.0000000000003015. [DOI] [PubMed] [Google Scholar]
- 13.Choi SY, Ha MS, Kim JH, Chi BH, Kim JW, Chang IH, Kim TH, Myung SC. Low-dose versus standard-dose bacille Calmette-Guérin for non-muscle-invasive bladder cancer: systematic review and meta-analysis of randomized controlled trials. Investig Clin Urol. 2022;63:140–150. doi: 10.4111/icu.20210340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durant AM, Lee YS, Mi L, Faraj K, Lyon TD, Singh P, Tyson Ii MD. Effect of Bacille Calmette-Guérin for non-muscle-invasive bladder cancer after prostate radiotherapy. Bladder Cancer. 2024;10:35–45. doi: 10.3233/BLC-230073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF, Seisen T, Soukup V, Sylvester RJ. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and Carcinoma in Situ) Eur Urol. 2022;81:75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Park J, Shin DW, Kim TH, Jung SI, Nam JK, Park SC, Hong S, Jung JH, Kim H, Kim WT. Development and validation of the Korean version of the European organization for research and treatment of cancer quality of life questionnaire for patients with non-muscle invasive bladder cancer: EORTC QLQ-NMIBC24. Cancer Res Treat. 2018;50:40–49. doi: 10.4143/crt.2016.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caño Velasco J, Polanco Pujol L, Moreno Cortés JC, Lafuente Puentedura A, Hernández Fernández C. Bladder epicheck for surveillance in high-risk non-muscle-invasive bladder cancer: Initial experience and follow-up proposal. Actas Urol Esp (Engl Ed) 2023;47:471–473. doi: 10.1016/j.acuroe.2023.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Hornak J, Brisuda A, Babjuk M. Transurethral resection of bladder cancer with or without fluorescence. Curr Opin Urol. 2023;33:152–156. doi: 10.1097/MOU.0000000000001071. [DOI] [PubMed] [Google Scholar]
- 19.Lin L, Guo X, Ma Y, Zhu J, Li X. Does repeat transurethral resection of bladder tumor influence the diagnosis and prognosis of T1 bladder cancer? A systematic review and meta-analysis. Eur J Surg Oncol. 2023;49:29–38. doi: 10.1016/j.ejso.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Bolat D, Baltaci S, Akgul M, Karabay E, Izol V, Aslan G, Eskicorapci S, Sahin H, Turkeri L Members of Bladder Cancer Study Group of Turkish Urooncology Association. Predictive role of the systemic immune inflammation index for intravesical BCG response in intermediate- and high-risk non-muscle-invasive bladder cancer. Urol Int. 2023;107:617–623. doi: 10.1159/000528740. [DOI] [PubMed] [Google Scholar]
- 21.Kondo Y, Nagamine Y, Yoshikawa N, Echigo N, Kida T, Sumitomo M, Yoshida M, Inagawa G, Goto T. Incidence of perioperative hypotension in patients undergoing transurethral resection of bladder tumor after oral 5-aminolevulinic acid administration: a retrospective multicenter cohort study. J Anesth. 2023;37:703–713. doi: 10.1007/s00540-023-03222-3. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi S, Watanabe M, Tambo M, Machida H, Yokoyama K, Fukuhara H. Proposal for a new vesical imaging-reporting and data system (VI-RADS)-based algorithm for the management of bladder cancer: a paradigm shift from the current transurethral resection of bladder tumor (TURBT)-dependent practice. Clin Genitourin Cancer. 2022;20:e291–e295. doi: 10.1016/j.clgc.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Packiam VT, Johnson SC, Steinberg GD. Non-muscle-invasive bladder cancer: intravesical treatments beyond Bacille Calmette-Guérin. Cancer. 2017;123:390–400. doi: 10.1002/cncr.30392. [DOI] [PubMed] [Google Scholar]
- 24.Jeong SH, Han JH, Jeong CW, Kim HH, Kwak C, Yuk HD, Ku JH. Clinical determinants of recurrence in pTa bladder cancer following transurethral resection of bladder tumor. BMC Cancer. 2022;22:631. doi: 10.1186/s12885-022-09733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hensley PJ, Bree KK, Brooks N, Matulay J, Li R, Nogueras González GM, Navai N, Grossman HB, Dinney CP, Kamat AM. Time interval from transurethral resection of bladder tumour to bacille Calmette-Guérin induction does not impact therapeutic response. BJU Int. 2021;128:634–641. doi: 10.1111/bju.15413. [DOI] [PubMed] [Google Scholar]
- 26.Davalos L, Kushlaf H. New onset of seropositive generalized myasthenia gravis following intravesical bacille Calmette-Guerin treatment for bladder cancer: a case study. Muscle Nerve. 2019;59:E1–E2. doi: 10.1002/mus.26328. [DOI] [PubMed] [Google Scholar]
- 27.Guerrero-Ramos F, Alvarez-Maestro M, Pinto Marín A, Domínguez Escrig JL, Rodríguez Faba Ó. Multidisciplinary consensus document on the current treatment of bacille Calmette-Guérin-unresponsive non-muscle invasive bladder tumor. Actas Urol Esp (Engl Ed) 2024;48:262–272. doi: 10.1016/j.acuroe.2024.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Nowak L, Krajewski W, Poterek A, Sliwa A, Zdrojowy R. The prognostic value of programmed cell death protein ligand 1 in patients with non-muscle-invasive bladder cancer treated with bacille Calmette-Guérin immunotherapy: current status. Arab J Urol. 2020;19:67–70. doi: 10.1080/2090598X.2020.1791562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nurminen P, Ettala O, Seppänen M, Taimen P, Boström PJ, Kaipia A. Urine cytology is a feasible tool for assessing erythematous bladder lesions after bacille Calmette-Guérin (BCG) treatment. BJU Int. 2019;123:246–251. doi: 10.1111/bju.14470. [DOI] [PubMed] [Google Scholar]
- 30.Lu JL, Xia QD, Liu CQ, Sun JX, Yang YY, Hu HL, Wang SG. Efficacy and toxicity in scheduled intravesical gemcitabine versus Bacille Calmette-Guérin for Ta and T1 bladder cancer: a systematic review and meta-analysis. Transl Cancer Res. 2021;10:2849–2858. doi: 10.21037/tcr-21-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurminen P, Ettala O, Uusitalo-Seppala R, Nummi A, Jarvinen R, Antti K, Bostrom PJ. Incidence of and mortality from Bacille Calmette-Guerin (BCG) infections after BCG instillation therapy. BJU Int. 2022;129:737–743. doi: 10.1111/bju.15608. [DOI] [PubMed] [Google Scholar]
- 32.Gellings P, Galeas-Pena M, Morici LA. Mycobacterium bovis bacille Calmette-Guerin-derived extracellular vesicles as an alternative to live BCG immunotherapy. Clin Exp Med. 2023;23:519–527. doi: 10.1007/s10238-022-00794-4. [DOI] [PubMed] [Google Scholar]
- 33.Yergin CG, Pafford R, Pirris J, Rao D, Rahmathulla G. Spinal tuberculosis secondary to intravesical bacille Calmette-Guerin treatment for bladder cancer. Cureus. 2021;13:e17446. doi: 10.7759/cureus.17446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marx AH, Nowicki DN, Carlson RB, Schultz KM, Sickbert-Bennett E, Weber DJ. Bacille Calmette-Guérin preparation and intravesical administration to patients with bladder cancer: risks to healthcare personnel and patients, and mitigation strategies. Infect Control Hosp Epidemiol. 2024;45:520–525. doi: 10.1017/ice.2023.259. [DOI] [PubMed] [Google Scholar]
- 35.Lin N, Wu YP, Lin YZ, Tao X, Chen SH, Ke ZB, Wei Y, Zheng QS, Xue XY, Xu N. Risk factors for upper tract urothelial recurrence following local excision of bladder cancer. Cancer Med. 2018;7:4098–4103. doi: 10.1002/cam4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Benedetto I, Barco A, Rossi M, Lapadula G, Lupia T, Bonfanti P, Bonora S, Di Perri G, Calcagno A. BCGitis after Bacille Calmette-Guerin intravesical administration from two referral centers: clinical characteristics and risk factors. Infez Med. 2022;30:242–246. doi: 10.53854/liim-3002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Wang L, Li H, Zhang P, Li Z, Xue L, Wang Z, Fu D, Chen Q, Luo Q, Chong T, Wang Z. Analysis of risk factors for recurrence after transurethral resection of bladder tumor in patients with non-muscle invasive bladder cancer: 2-year follow-up outcomes. Oncology. 2024;102:337–342. doi: 10.1159/000533410. [DOI] [PMC free article] [PubMed] [Google Scholar]