Abstract
Objective: To evaluate the diagnostic value of the expression of the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in distinguishing endometrial cancer from benign uterine lesions. Methods: In this retrospective analysis, clinical data were collected from 112 patients treated at Hengshui People’s Hospital (Harrison International Peace Hospital) between January 2022 and December 2023. The cohort comprised 56 patients diagnosed with endometrial cancer and 56 patients with benign uterine lesions, matched 1:1. Demographic details, comorbidities, and serological parameters - including WBC, RBC, Hb, MPV, neutrophil, lymphocyte, monocyte, and platelet counts - were recorded. NLR, LMR, and PLR values were subsequently calculated. Results: Significant serological differences were observed between the endometrial cancer and benign lesion groups, including NLR (4.25 ± 1.23 vs. 2.18 ± 0.95, P < 0.001), LMR (3.12 ± 0.98 vs. 5.08 ± 1.75, P < 0.001), and PLR (201.23 ± 45.66 vs. 150.27 ± 30.45, P < 0.001). Correlation analysis indicated a strong association between endometrial cancer and NLR (r = 0.689, P < 0.001), LMR (r = -0.572, P < 0.001), and PLR (r = 0.552, P < 0.001). ROC analysis demonstrated that NLR (AUC = 0.91) offered superior diagnostic value relative to LMR (AUC = 0.841) and PLR (AUC = 0.83). Logistic regression identified significant associations for NLR ≥ 3.4 (OR = 69.173, P < 0.001), LMR ≥ 4.055 (OR = 0.048, P < 0.001), and PLR ≥ 150.445 (OR = 18.134, P = 0.002). DeLong’s test revealed no significant differences in diagnostic performance among the ratios (NLR vs. LMR, P = 0.149; NLR vs. PLR, P = 0.08; LMR vs. PLR, P = 0.842). Conclusion: NLR, LMR, and PLR are valuable hematological markers for diagnosing endometrial cancer, with NLR demonstrating the highest sensitivity and specificity. These findings support the inclusion of these serological parameters in routine diagnostic protocols to enhance the accurate identification of endometrial cancer.
Keywords: Neutrophil-lymphocyte ratio, lymphocyte-monocyte ratio, platelet-lymphocyte ratio, endometrial cancer
Introduction
Endometrial cancer (EC) is one of the most prevalent gynecological malignancies worldwide, with rising numbers in both developed and developing regions. According to the International Agency for Research on Cancer (IARC), approximately 417,000 new cases of endometrial cancer were recorded globally in 2020, making it the sixth most common cancer among women [1,2]. Notably, around 97,000 deaths were attributed to the disease, underscoring its considerable clinical and public health impact [3,4].
Despite advances in diagnostic and therapeutic methods, early detection and accurate prognostication of endometrial cancer remain challenging [5]. Traditional diagnostic approaches, such as imaging and histopathology, although effective, are invasive, costly, and often inaccessible, particularly in resource-limited settings [6-8]. Identifying reliable, non-invasive biomarkers for early diagnosis, prognosis, and monitoring treatment response is therefore crucial.
Recent studies indicate a significant role for systemic inflammatory responses in cancer development and progression [9,10]. Chronic inflammation, mediated through diverse cellular and molecular mechanisms, is increasingly recognized as a cancer hallmark [11-13]. In this context, inflammatory biomarkers from routine blood tests are promising due to their cost-effectiveness, accessibility, and ease of measurement in clinical settings. Among these, the neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) have shown prognostic and predictive value in various cancers, including colorectal, lung, breast, and ovarian cancers [14-16].
NLR, LMR, and PLR represent composite markers of systemic inflammation and immune response, reflecting the complex interaction between host immunity and the tumor microenvironment [17,18]. Elevated NLR, indicative of heightened inflammation and immunosuppression, has been associated with poor outcomes in cancer patients [19,20]. Conversely, high LMR, suggesting an effective anti-tumor immune response, has been linked to better prognosis [21,22]. Similarly, increased PLR, reflecting platelet activation and release of pro-tumorigenic factors, is implicated in cancer progression and metastasis [23]. However, the diagnostic and prognostic utility of these hematological ratios in endometrial cancer is relatively underexplored [24-26], with existing studies presenting inconsistent findings that highlight the need for further research [27-29].
Several studies emphasize the association between inflammatory markers and cancer prognosis. For example, Muangto et al. [30] reported that elevated NLR is linked to poor overall survival in endometrial cancer, supporting its role as a prognostic marker. However, the roles of LMR and PLR remain debated. Research by Eo et al. [31] showed a significant correlation between low LMR and disease progression in endometrial cancer, whereas Dong et al. [32] reported no significant prognostic value for LMR, indicating variability in its predictive utility. Similarly, while some studies link elevated PLR with aggressive tumor behavior and poor prognosis, such as findings by Ural et al. [33], opposing results have been reported by Xiong et al. [34], who found no significant correlation in their cohort.
This study aims to elucidate the significance of NLR, LMR, and PLR in endometrial cancer by assessing their expression in peripheral blood and examining their diagnostic and prognostic value. To our knowledge, this is the first comprehensive study conducted in a Chinese population to systematically investigate these inflammatory biomarkers in endometrial cancer, addressing a crucial gap in the literature.
Materials and methods
Patients
This study retrospectively analyzed clinical data from 112 patients who received initial treatment at the Department of Gynecology, Hengshui People’s Hospital (Harrison International Peace Hospital), between January 2022 and December 2023. Among these, 56 patients were diagnosed with endometrial cancer through pathological examination, and 56 patients were diagnosed with benign uterine lesions, confirmed by imaging examinations and consistent with diagnostic criteria outlined in “Obstetrics and Gynecology”. This study received approval from the Ethics Committee of Hengshui People’s Hospital (Harrison International Peace Hospital).
Inclusion criteria: Patients in the endometrial cancer group met the International Federation of Gynecology and Obstetrics (FIGO) diagnostic criteria for endometrial cancer and were confirmed through surgical pathology. Patients in the benign uterine lesion group met the diagnostic criteria for benign uterine lesions as specified in “Obstetrics and Gynecology” and were confirmed via imaging examinations. Additional criteria included complete clinical data and no history of hormone treatment within the past 6 months.
Exclusion criteria: Patients with a history of tumors, organ dysfunction, severe fluid imbalance or metabolic disturbances, prior treatment before hospitalization, mental or consciousness disorders affecting normal communication, severe infectious diseases, or autoimmune diseases were excluded.
Sample size estimation
Sample size was estimated using G*Power version 3.1.9.7 with the “Means: Difference between two independent means (two groups)” option, based on t-tests for post hoc analysis. The analysis was two-tailed with an effect size of d = 0.6 and an alpha error probability (α) of 0.05. Given the sample sizes of each group, the calculated power (1 - β error probability) was 0.882. Typically, a minimum power of 0.80 (80%) is acceptable, indicating an 80% probability of correctly rejecting a false null hypothesis. With a power of 0.882, this study exceeds this benchmark, suggesting a strong likelihood of detecting a true effect if present.
Data collection
Patient medical records were accessed via the hospital information system. General information included age, smoking history, alcohol intake, comorbidities (e.g., hypertension, diabetes), family history of cancer, weight status, number of deliveries, and marital status.
Serological parameters included white blood cell (WBC) count, red blood cell (RBC) count, hemoglobin (Hb) level, mean platelet volume (MPV), neutrophil count, lymphocyte count, monocyte count, platelet count, and calculated values for NLR, LMR, and PLR. Four milliliters of fasting venous blood were drawn from each patient in the morning, allowed to stand for 2 hours, then centrifuged at 3000 rpm for 10 minutes. The serum supernatant was stored at -20°C. NLR, LMR, and PLR values were measured with an automated blood cell analyzer (Sysmex Corporation, XT-4000i).
Statistical analysis
Data were analyzed using SPSS 25.0 statistical software. Categorical data were expressed as n (%), while normally distributed continuous data (Table S1) were presented as mean ± standard deviation (mean ± standard deviation) and analyzed with t-tests. Spearman’s correlation analysis was used to assess correlations. Variables showing statistically significant differences between groups were included in a binary logistic regression analysis, with endometrial cancer as the outcome variable and the identified factors as predictor variables.
Results
Comparison of general information
Comparison of general and demographic characteristics between the Endometrial Cancer and Benign Uterine Lesions groups revealed no statistically significant differences in age (58.52 ± 5.24 vs. 59.81 ± 4.75, P = 0.176), smoking history (5.92 ± 1.24 vs. 6.24 ± 1.55, P = 0.225), or alcohol intake (5.95 ± 1.53 vs. 6.03 ± 1.85, P = 0.812) (Table 1). There were also no significant differences in comorbidities such as hypertension (P = 1.000) and diabetes (P = 1.000), family history of cancer (P = 1.000), weight status (P = 0.515), number of deliveries (P = 0.398), or marital status (P = 0.994), indicating comparable baseline characteristics between the two groups.
Table 1.
General information and demographic characteristics of patients
| Parameter | Benign Uterine Lesions (n = 56) | Endometrial Cancer (n = 56) | t/x2 | P |
|---|---|---|---|---|
| Age (years) | 59.81 ± 4.75 | 58.52 ± 5.24 | 1.361 | 0.176 |
| Smoking history (pack-years) | 6.24 ± 1.55 | 5.92 ± 1.24 | 1.221 | 0.225 |
| Alcohol intake (g/week) | 6.03 ± 1.85 | 5.95 ± 1.53 | 0.239 | 0.812 |
| Comorbidities (%) | ||||
| -Hypertension | 12 (21.43%) | 11 (19.64%) | 0.000 | 1.000 |
| -Diabetes | 10 (17.86%) | 9 (16.07%) | 0.000 | 1.000 |
| Family history of cancer | 11 (19.64%) | 12 (21.43%) | 0.000 | 1.000 |
| Weight status [n (%)] | 1.329 | 0.515 | ||
| -Normal | 45 (80.36%) | 41 (73.21%) | ||
| -Overweight | 6 (10.71%) | 6 (10.71%) | ||
| -Underweight | 5 (8.93%) | 9 (16.07%) | ||
| Deliveries Number | 0.714 | 0.398 | ||
| < 2 | 38 (67.86%) | 43 (76.79%) | ||
| ≥ 2 | 18 (32.14%) | 13 (23.21%) | ||
| Marital Status | 0.234 | 0.994 | ||
| -Unmarried | 16 (28.57%) | 17 (30.36%) | ||
| -Married | 23 (41.07%) | 22 (39.29%) | ||
| -Divorced | 5 (8.93%) | 6 (10.71%) | ||
| -Bereave | 6 (10.71%) | 5 (8.93%) | ||
| -Other | 6 (10.71%) | 6 (10.71%) |
Comparison of serological detection
Serological comparisons between the Endometrial Cancer and Benign Uterine Lesions groups showed no statistically significant differences in WBC count (8.21 ± 2.81 vs. 7.65 ± 2.61, P = 0.278), RBC count (4.25 ± 0.45 vs. 4.35 ± 0.41, P = 0.225), Hb (12.59 ± 1.86 vs. 13.21 ± 2.04, P = 0.096), MPV (9.77 ± 1.22 vs. 9.33 ± 1.53, P = 0.099), neutrophil count (4.38 ± 1.15 vs. 3.98 ± 1.02, P = 0.057), lymphocyte count (1.97 ± 0.88 vs. 2.25 ± 0.69, P = 0.071), monocyte count (0.6 ± 0.2 vs. 0.54 ± 0.14, P = 0.1), and platelet count (280.04 ± 35.87 vs. 269.33 ± 31.42, P = 0.096) (Table 2).
Table 2.
Comparison of serological detection between the two groups
| Parameter | Benign Uterine Lesions (n = 56) | Endometrial Cancer (n = 56) | t | P |
|---|---|---|---|---|
| WBC count | 7.65 ± 2.61 | 8.21 ± 2.81 | 1.091 | 0.278 |
| RBC count | 4.35 ± 0.41 | 4.25 ± 0.45 | 1.221 | 0.225 |
| Hb | 13.21 ± 2.04 | 12.59 ± 1.86 | 1.677 | 0.096 |
| MPV | 9.33 ± 1.53 | 9.77 ± 1.22 | 1.665 | 0.099 |
| NLR | 2.18 ± 0.95 | 4.25 ± 1.23 | 9.982 | P < 0.001 |
| LMR | 5.08 ± 1.75 | 3.12 ± 0.98 | 7.323 | P < 0.001 |
| PLR | 150.27 ± 30.45 | 201.23 ± 45.66 | 6.948 | P < 0.001 |
| Neutrophil | 3.98 ± 1.02 | 4.38 ± 1.15 | 1.927 | 0.057 |
| Lymphocyte | 2.25 ± 0.69 | 1.97 ± 0.88 | 1.822 | 0.071 |
| Monocyte | 0.54 ± 0.14 | 0.60 ± 0.20 | 1.659 | 0.100 |
| Platelet | 269.33 ± 31.42 | 280.04 ± 35.87 | 1.680 | 0.096 |
Note: WBC: white blood cell count; RBC: red blood cell; Hb: hemoglobin; MPV: mean platelet volume; NLR: neutrophil-lymphocyte ratio; LMR: lymphocyte-monocyte ratio; PLR: platelet-lymphocyte ratio.
However, significant differences were observed in NLR (4.25 ± 1.23 vs. 2.18 ± 0.95, P < 0.001), LMR (3.12 ± 0.98 vs. 5.08 ± 1.75, P < 0.001), and PLR (201.23 ± 45.66 vs. 150.27 ± 30.45, P < 0.001), indicating notable differences in these inflammatory markers between the groups.
Comparison of correlation analysis
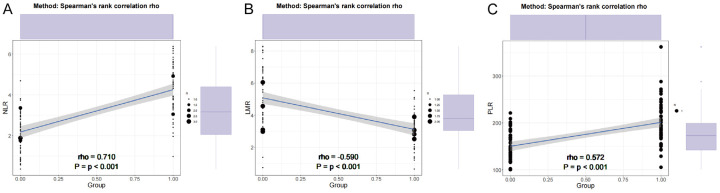
Correlation analysis between serum biomarkers and endometrial cancer revealed significant associations (Figure 1). NLR showed a strong positive correlation with endometrial cancer (r = 0.689, P < 0.001), while the LMR exhibited a moderate negative correlation (r = -0.572, P < 0.001). Additionally, PLR demonstrated a moderate positive correlation (r = 0.552, P < 0.001), suggesting a potential relationship between these biomarkers and the risk of endometrial cancer. These findings underscore the potential of these serum biomarkers as indicators for endometrial cancer, warranting further investigation.
Figure 1.
Correlation analysis between serum biomarkers and endometrial cancer. A: Neutrophil-lymphocyte ratio; B: Lymphocyte-monocyte ratio; C: Platelet-lymphocyte ratio.
ROC and DCA analysis
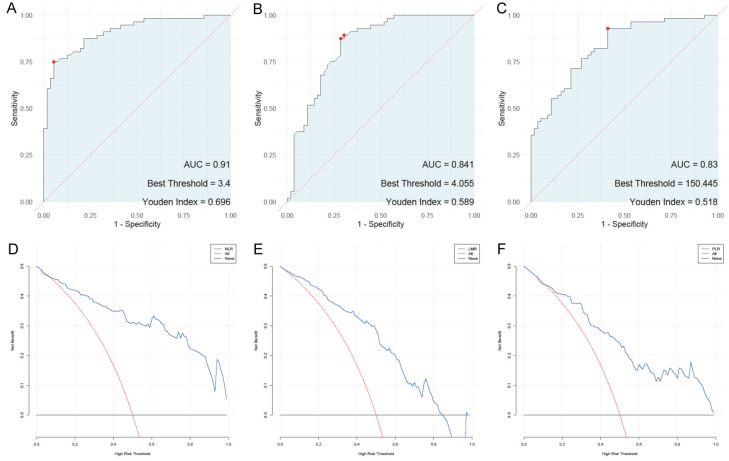
Among the hematological parameters, the NLR, LMR, and PLR demonstrated varying diagnostic performances for endometrial cancer (Figure 2). The optimal threshold for NLR was 3.4, with a sensitivity of 0.75 and specificity of 0.946. NLR had an area under the curve (AUC) of 0.91 and a Youden index of 0.696, indicating it as a highly reliable marker. LMR, with a threshold of 4.055, showed higher sensitivity (0.893) but lower specificity (0.696), yielding an AUC of 0.841 and a Youden index of 0.589. The PLR, with a threshold of 150.445, achieved the highest sensitivity at 0.929 but had the lowest specificity among the ratios at 0.589, resulting in an AUC of 0.83 and a Youden index of 0.518. The DCA plot further confirmed these findings. These results suggest that while all three ratios are useful, NLR offers the greatest combined sensitivity and specificity for diagnosing endometrial cancer.
Figure 2.
ROC and DCA analysis. A: ROC analysis of neutrophil-lymphocyte ratio; B: ROC analysis of lymphocyte-monocyte ratio; C: ROC analysis of platelet-lymphocyte ratio; D: DCA analysis of neutrophil-lymphocyte ratio; E: DCA analysis of lymphocyte-monocyte ratio; F: DCA analysis of platelet-lymphocyte ratio.
Delong analysis of influencing factors
The DeLong analysis (Table 3) compared the diagnostic accuracy of the NLR, LMR, and PLR for endometrial cancer. Results indicated no significant difference between these parameters: the comparison between NLR and LMR yielded a P-value of 0.149, while the comparison between NLR and PLR returned a P-value of 0.08. Additionally, the comparison between LMR and PLR produced a P-value of 0.842, reinforcing the lack of significant difference in their diagnostic performance. These findings suggest that, although each hematological parameter offers diagnostic value, their comparative effectiveness is not significantly different.
Table 3.
Delong analysis of influencing factors
| NLR | LMR | PLR | |
|---|---|---|---|
| NLR | / | 0.149 | 0.080 |
| LMR | 0.149 | / | 0.842 |
| PLR | 0.080 | 0.842 | / |
Note: NLR: neutrophil-lymphocyte ratio; LMR: lymphocyte-monocyte ratio; PLR: platelet-lymphocyte ratio.
Regression analysis of influencing factors
Regression analysis (Table 4) revealed significant associations between the NLR, LMR, and PLR and endometrial cancer. Univariate analysis showed that NLR ≥ 3.4 (OR, 53.000; 95% CI, 16.342-242.624; P < 0.001), LMR ≥ 4.055 (OR, 0.052; 95% CI, 0.017-0.137; P < 0.001), and PLR ≥ 150.445 (OR, 18.652; 95% CI, 6.519-68.115; P < 0.001) were all statistically significant. Multivariate analysis confirmed these associations, with NLR ≥ 3.4 (OR, 69.173; 95% CI, 9.866-484.999; P < 0.001), LMR ≥ 4.055 (OR, 0.048; 95% CI, 0.009-0.261; P < 0.001), and PLR ≥ 150.445 (OR, 18.134; 95% CI, 2.898-113.483; P = 0.002) all remaining significant. These results highlight the clinical relevance of these hematological markers in the assessment and management of endometrial cancer.
Table 4.
Regression analysis of influencing factors
| Coefficient | Std. Error | Wald | P Value | OR | |
|---|---|---|---|---|---|
| Univariate | |||||
| NLR ≥ 3.400 | 3.970 | 0.669 | 5.935 | < 0.001 | 53.000 (16.342-242.624) |
| LMR ≥ 4.055 | -2.951 | 0.521 | 5.667 | < 0.001 | 0.052 (0.017-0.137) |
| PLR ≥ 150.445 | 2.926 | 0.586 | 4.996 | < 0.001 | 18.652 (6.519-68.115) |
| Multivariate | |||||
| NLR ≥ 3.400 | 4.237 | 0.994 | 4.264 | < 0.001 | 69.173 (9.866-484.999) |
| LMR ≥ 4.055 | -3.028 | 0.859 | -3.525 | < 0.001 | 0.048 (0.009-0.261) |
| PLR ≥ 150.445 | 2.898 | 0.936 | 3.097 | 0.002 | 18.134 (2.898-113.483) |
Note: NLR: neutrophil-lymphocyte ratio; LMR: lymphocyte-monocyte ratio; PLR: platelet-lymphocyte ratio.
Discussion
This study investigated NLR, LMR, and PLR in peripheral blood as potential biomarkers to differentiate endometrial cancer from benign uterine lesions. The significant findings not only underscore their diagnostic value but also offer insights into the biological mechanisms underlying these associations.
The elevated NLR observed in endometrial cancer patients points to an enhanced inflammatory response [35,36]. Neutrophils release a range of cytokines, chemokines, and enzymes, establishing a microenvironment conducive to tumor growth [37]. They generate reactive oxygen species (ROS) and nitrogen intermediates, which can induce DNA damage in both tumor and surrounding stromal cells, thereby promoting tumor progression through mutagenic processes [38,39].
Neutrophils also interact with circulating tumor cells (CTCs), supporting metastatic development [40]. This interaction is facilitated by neutrophil extracellular traps (NETs), which capture CTCs and shield them from immune surveillance [41]. Furthermore, neutrophils produce arginase and ROS, which can suppress lymphocyte function, thus contributing to an immunosuppressive tumor microenvironment [42]. This may partly explain the elevated NLR in cancer patients, reflecting an imbalance that favors a pro-tumorigenic state over an effective anti-tumor immune response [43].
Conversely, lymphopenia observed in cancer patients can be attributed to the immunosuppressive nature of the tumor microenvironment [44]. Tumors secrete various suppressive factors, such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), which inhibit lymphocyte proliferation and function [45]. A reduction in lymphocyte count weakens the adaptive immune system’s capacity to recognize and attack tumor cells, further tipping the balance toward tumor progression [46].
These findings align with those of Dong et al. [32], who reported elevated NLR levels in endometrial cancer patients associated with poorer overall survival outcomes. This evidence highlights the role of systemic inflammatory responses in cancer biology, supporting the hypothesis that NLR may function as a universal biomarker across various cancer types.
The elevated PLR observed in endometrial cancer patients indicates increased platelet activity, which plays a critical role in tumor biology [47]. Platelets facilitate tumor cell survival and dissemination [36], forming aggregates with circulating tumor cells (CTCs) that protect them from immune-mediated destruction and aid their survival in the bloodstream [48]. This interaction is crucial for metastasis, as it helps tumor cells anchor in distant tissues, establishing secondary growth sites [49].
Platelets are also reservoirs of growth factors, such as vascular endothelial growth factor and platelet-derived growth factor [50]. These factors are released into the tumor microenvironment, promoting angiogenesis and supporting the tumor’s blood supply, fueling growth and metastatic potential [51]. Furthermore, platelet interactions with the coagulation system contribute to a hypercoagulable state often observed in cancer patients, linked to poorer prognosis and increased metastasis [52].
The prognostic implications of PLR have been validated in numerous studies. Song et al. [53] reported that elevated PLR levels are associated with advanced disease stages and predict a more aggressive cancer phenotype. Similarly, a study by Ni et al. [54] established a strong link between high PLR and poor clinical outcomes, suggesting that platelets not only facilitate cancer progression but also serve as key modulators within the tumor microenvironment, corroborating our findings in endometrial cancer.
The significant reduction in LMR in the endometrial cancer group deserves attention [55]. Monocytes serve as precursors to tumor-associated macrophages (TAMs), a prominent component of the tumor microenvironment that promotes tumor growth, invasion, and metastasis [55]. Influenced by tumor-derived signals, TAMs often polarize towards an M2 phenotype associated with immunosuppression and tissue remodeling that supports tumor progression [36].
Monocytes and TAMs release factors such as matrix metalloproteinases (MMPs) and cytokines (e.g., IL-10, TGF-β), which promote extracellular matrix degradation and facilitate metastasis [55]. Elevated monocyte counts in peripheral blood reflect increased recruitment of these cells to the tumor site, where they enhance tumor-supportive processes, resulting in a lower LMR [56]. This decreased LMR signifies a higher tumor burden and a more aggressive disease course characterized by substantial monocyte involvement [52].
The interrelationship among hematological ratios - NLR, PLR, and LMR - and cancer underscores the intricate interplay of inflammation, immunity, and tumor biology [52]. The systemic inflammation indicated by elevated NLR and PLR, along with the immunosuppressive environment reflected by a reduced LMR, highlights the multifaceted nature of cancer progression [52]. Elevated neutrophils and platelets characterize a chronic inflammatory response, while reduced lymphocytes and increased monocytes indicate a weakened adaptive and innate immune response, providing a snapshot of the body’s reaction to the tumor presence [56].
From a clinical perspective, these findings are significant. Elevated NLR and PLR and lower LMR can serve as accessible, cost-effective biomarkers for the early detection, risk stratification, and prognostic evaluation of endometrial cancer. Given their simplicity, these markers could be easily integrated into routine clinical practice, supplementing traditional diagnostic tools with valuable additional information.
However, further investigation is needed to elucidate the pathophysiological mechanisms underlying these associations. Future research should aim to clarify the molecular pathways through which neutrophils, platelets, and monocytes contribute to tumorigenesis and explore how modulating these ratios might offer therapeutic benefits. Additionally, larger prospective studies are essential to validate these findings across diverse populations and clinical settings.
This study has several limitations. As a retrospective analysis, it is inherently subject to selection bias, particularly regarding patient record selection and the availability of comprehensive clinical data. Relying on pre-existing hospital records may also introduce inconsistencies and potential inaccuracies. Furthermore, the sample size, although meaningful, is relatively small and may not fully represent the broader population, limiting the generalizability of the findings. The lack of longitudinal follow-up also restricts conclusions about the temporal relationship between hematological markers and disease progression.
In conclusion, the diagnostic value of NLR, PLR, and LMR in distinguishing endometrial cancer from benign uterine lesions reflects their roles in tumor biology. Elevated NLR and PLR indicate a heightened inflammatory and immunosuppressive state, while decreased LMR suggests monocyte activity involvement in the tumor microenvironment. These markers offer insights into tumor-associated immune and inflammatory responses and hold promise as practical tools for improving endometrial cancer management.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399:1412–1428. doi: 10.1016/S0140-6736(22)00323-3. [DOI] [PubMed] [Google Scholar]
- 2.Lu KH, Broaddus RR. Endometrial cancer. N Engl J Med. 2020;383:2053–2064. doi: 10.1056/NEJMra1514010. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton CA, Pothuri B, Arend RC, Backes FJ, Gehrig PA, Soliman PT, Thompson JS, Urban RR, Burke WM. Endometrial cancer: a society of gynecologic oncology evidence-based review and recommendations. Gynecol Oncol. 2021;160:817–826. doi: 10.1016/j.ygyno.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Trojano G, Olivieri C, Tinelli R, Damiani GR, Pellegrino A, Cicinelli E. Conservative treatment in early stage endometrial cancer: a review. Acta Biomed. 2019;90:405–410. doi: 10.23750/abm.v90i4.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passarello K, Kurian S, Villanueva V. Endometrial cancer: an overview of pathophysiology, management, and care. Semin Oncol Nurs. 2019;35:157–165. doi: 10.1016/j.soncn.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, Wang B, Xu W, Liu K, Gao Y, Guo C, Chen J, Kamal MA, Yuan C. Endometrial cancer: genetic, metabolic characteristics, therapeutic strategies and nanomedicine. Curr Med Chem. 2021;28:8755–8781. doi: 10.2174/0929867328666210705144456. [DOI] [PubMed] [Google Scholar]
- 7.Karpel H, Slomovitz B, Coleman RL, Pothuri B. Biomarker-driven therapy in endometrial cancer. Int J Gynecol Cancer. 2023;33:343–350. doi: 10.1136/ijgc-2022-003676. [DOI] [PubMed] [Google Scholar]
- 8.Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19:510–521. doi: 10.1038/s41568-019-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, Tatebe K, Veneris JL. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin. 2019;69:258–279. doi: 10.3322/caac.21561. [DOI] [PubMed] [Google Scholar]
- 10.Karpel HC, Slomovitz B, Coleman RL, Pothuri B. Treatment options for molecular subtypes of endometrial cancer in 2023. Curr Opin Obstet Gynecol. 2023;35:270–278. doi: 10.1097/GCO.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 11.Marín-Jiménez JA, García-Mulero S, Matías-Guiu X, Piulats JM. Facts and hopes in immunotherapy of endometrial cancer. Clin Cancer Res. 2022;28:4849–4860. doi: 10.1158/1078-0432.CCR-21-1564. [DOI] [PubMed] [Google Scholar]
- 12.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 13.Vermij L, Smit V, Nout R, Bosse T. Incorporation of molecular characteristics into endometrial cancer management. Histopathology. 2020;76:52–63. doi: 10.1111/his.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahdi H, Chelariu-Raicu A, Slomovitz BM. Immunotherapy in endometrial cancer. Int J Gynecol Cancer. 2023;33:351–357. doi: 10.1136/ijgc-2022-003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitric C, Bernardini MQ. Endometrial cancer: transitioning from histology to genomics. Curr Oncol. 2022;29:741–757. doi: 10.3390/curroncol29020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nees LK, Heublein S, Steinmacher S, Juhasz-Böss I, Brucker S, Tempfer CB, Wallwiener M. Endometrial hyperplasia as a risk factor of endometrial cancer. Arch Gynecol Obstet. 2022;306:407–421. doi: 10.1007/s00404-021-06380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal S. Neutrophil-lymphocyte ratio predicting case severity in SARS-CoV-2 infection: a review. Cureus. 2022;14:e29760. doi: 10.7759/cureus.29760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atum M, Alagöz G. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with retinal artery occlusion. J Ophthalmic Vis Res. 2020;15:195–200. doi: 10.18502/jovr.v15i2.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtul BE, Ozer PA. Neutrophil-to-lymphocyte ratio in ocular diseases: a systematic review. Int J Ophthalmol. 2019;12:1951–1958. doi: 10.18240/ijo.2019.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zawiah M, Hayat Khan A, Abu Farha R, Usman A, Bitar AN. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio, and platelet-lymphocyte ratio in stroke-associated pneumonia: a systematic review and meta-analysis. Curr Med Res Opin. 2023;39:475–482. doi: 10.1080/03007995.2023.2174327. [DOI] [PubMed] [Google Scholar]
- 21.Chidambaram AC, Ramamoorthy JG, Anantharaj A. Neutrophil-lymphocyte ratio for predicting coronary artery lesions in children with Kawasaki disease. Indian Pediatr. 2023;60:207–211. [PubMed] [Google Scholar]
- 22.Sharma D, Spring KJ, Bhaskar SMM. Neutrophil-lymphocyte ratio in acute ischemic stroke: immunopathology, management, and prognosis. Acta Neurol Scand. 2021;144:486–499. doi: 10.1111/ane.13493. [DOI] [PubMed] [Google Scholar]
- 23.Farias JS, Villarreal EG, Savorgnan F, Acosta S, Flores S, Loomba RS. The use of neutrophil-lymphocyte ratio for the prediction of refractory disease and coronary artery lesions in patients with Kawasaki disease. Cardiol Young. 2023;33:1409–1417. doi: 10.1017/S1047951123000653. [DOI] [PubMed] [Google Scholar]
- 24.Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23:3636. doi: 10.3390/ijms23073636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159–2167. doi: 10.1182/blood-2018-11-844548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shadman M. Diagnosis and treatment of chronic lymphocytic leukemia: a review. JAMA. 2023;329:918–932. doi: 10.1001/jama.2023.1946. [DOI] [PubMed] [Google Scholar]
- 27.Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends Immunol. 2019;40:228–242. doi: 10.1016/j.it.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Figueroa E, Álvarez-Carrasco P, Ortega E, Maldonado-Bernal C. Neutrophils: many ways to die. Front Immunol. 2021;12:631821. doi: 10.3389/fimmu.2021.631821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed J, Reichelt M, Wetzel SA. Lymphocytes and trogocytosis-mediated signaling. Cells. 2021;10:1478. doi: 10.3390/cells10061478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muangto T, Maireang K, Poomtavorn Y, Thaweekul Y, Punyashthira A, Chantawong N, Wisarnsirirak P, Pattaraarchachai J, Suwannarurk K. Study on preoperative neutrophil/lymphocyte (NLR) and platelet/lymphocyte ratio (PLR) as a predictive factor in endometrial cancer. Asian Pac J Cancer Prev. 2022;23:3317–3322. doi: 10.31557/APJCP.2022.23.10.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eo WK, Kwon S, Koh SB, Kim MJ, Ji YI, Lee JY, Suh DS, Kim KH, Kim HY. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of endometrial cancer. J Cancer. 2016;7:538–545. doi: 10.7150/jca.14206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y, Cheng Y, Wang J. The ratio of neutrophil to lymphocyte is a predictor in endometrial cancer. Open Life Sci. 2019;14:110–118. doi: 10.1515/biol-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ural ÜM, Şehitoğlu İ, Tekin YB, Şahin FK. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in patients with endometrial hyperplasia and endometrial cancer. J Obstet Gynaecol Res. 2015;41:445–448. doi: 10.1111/jog.12536. [DOI] [PubMed] [Google Scholar]
- 34.Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14:173. doi: 10.1186/s13045-021-01187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet-to-lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39:345–357. doi: 10.3343/alm.2019.39.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22:8002. doi: 10.3390/ijms22158002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song S, Bai M, Li X, Gong S, Yang W, Lei C, Tian H, Si M, Hao X, Guo T. Early predictive value of circulating biomarkers for sorafenib in advanced hepatocellular carcinoma. Expert Rev Mol Diagn. 2022;22:361–378. doi: 10.1080/14737159.2022.2049248. [DOI] [PubMed] [Google Scholar]
- 38.Manuel V, Miana LA, Jatene MB. Neutrophil-lymphocyte ratio in congenital heart surgery: what is known and what is new? World J Pediatr Congenit Heart Surg. 2022;13:208–216. doi: 10.1177/21501351211064143. [DOI] [PubMed] [Google Scholar]
- 39.Mishra RK, Galwankar S, Gerber J, Jain A, Yunus M, Cincu R, Moscote-Salazar LR, Quiñones-Ossa GA, Agrawal A. Neutrophil-lymphocyte ratio as a predictor of outcome following traumatic brain injury: systematic review and meta-analysis. J Neurosci Rural Pract. 2022;13:618–635. doi: 10.25259/JNRP-2022-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aroca-Crevillén A, Vicanolo T, Ovadia S, Hidalgo A. Neutrophils in physiology and pathology. Annu Rev Pathol. 2024;19:227–259. doi: 10.1146/annurev-pathmechdis-051222-015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Souza FW, Miao EA. Neutrophils only die twice. Sci Adv. 2023;9:eadm8715. doi: 10.1126/sciadv.adm8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikora JP, Karawani J, Sobczak J. Neutrophils and the systemic inflammatory response syndrome (SIRS) Int J Mol Sci. 2023;24:13469. doi: 10.3390/ijms241713469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu S, Wu W, Du Y, Yin H, Chen Q, Yu W, Wang W, Yu J, Liu L, Lou W, Pu N. The evolution and heterogeneity of neutrophils in cancers: origins, subsets, functions, orchestrations and clinical applications. Mol Cancer. 2023;22:148. doi: 10.1186/s12943-023-01843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonaka T, Kawashiro S, Ishikawa H, Ito Y, Nemoto K, Ishihara R, Oyama T, Oyama T, Kato K, Kato H, Kawakubo H, Kawachi H, Kuribayashi S, Kono K, Kojima T, Takeuchi H, Tsushima T, Toh Y, Booka E, Makino T, Matsuda S, Matsubara H, Mano M, Minashi K, Miyazaki T, Muto M, Yamaji T, Yamatsuji T, Yoshida M, Kitagawa Y Esophageal Cancer Practice Guidelines Preparation Committee. Concurrent chemoradiotherapy using proton beams can reduce cardiopulmonary morbidity in esophageal cancer patients: a systematic review. Esophagus. 2023;20:605–616. doi: 10.1007/s10388-023-01015-x. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Choi H, Jeun SS, Ahn S. From lymphopenia to restoration: IL-7 immunotherapy for lymphocyte recovery in glioblastoma. Cancer Lett. 2024;588:216714. doi: 10.1016/j.canlet.2024.216714. [DOI] [PubMed] [Google Scholar]
- 46.Kosianova А, Pak O, Bryukhovetskiy I. Regulation of cancer stem cells and immunotherapy of glioblastoma (review) Biomed Rep. 2023;20:24. doi: 10.3892/br.2023.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An S, Shim H, Kim K, Kim B, Bang HJ, Do H, Lee HR, Kim Y. Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer. Ann Coloproctol. 2022;38:97–108. doi: 10.3393/ac.2021.01004.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misiewicz A, Dymicka-Piekarska V. Fashionable, but what is their real clinical usefulness? NLR, LMR, and PLR as a promising indicator in colorectal cancer prognosis: a systematic review. J Inflamm Res. 2023;16:69–81. doi: 10.2147/JIR.S391932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang CL, Jiang XC, Li Y, Pan X, Gao MQ, Chen Y, Pang B. Independent predictive value of blood inflammatory composite markers in ovarian cancer: recent clinical evidence and perspective focusing on NLR and PLR. J Ovarian Res. 2023;16:36. doi: 10.1186/s13048-023-01116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faria AVS, Andrade SS, Peppelenbosch MP, Ferreira-Halder CV, Fuhler GM. Platelets in aging and cancer-“double-edged sword”. Cancer Metastasis Rev. 2020;39:1205–1221. doi: 10.1007/s10555-020-09926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabrkhany S, Kuijpers MJE, Oude Egbrink MGA, Griffioen AW. Platelets as messengers of early-stage cancer. Cancer Metastasis Rev. 2021;40:563–573. doi: 10.1007/s10555-021-09956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki-Inoue K. Platelets and cancer-associated thrombosis: focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood. 2019;134:1912–1918. doi: 10.1182/blood.2019001388. [DOI] [PubMed] [Google Scholar]
- 53.Song H, Jeong MJ, Cha J, Lee JS, Yoo JG, Song MJ, Kim JH, Lee SJ, Lee HN, Yoon JH, Park DC, Kim SI. Preoperative neutrophil-to-lymphocyte, platelet-to-lymphocyte and monocyte-to-lymphocyte ratio as a prognostic factor in non-endometrioid endometrial cancer. Int J Med Sci. 2021;18:3712–3717. doi: 10.7150/ijms.64658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni L, Tao J, Xu J, Yuan X, Long Y, Yu N, Wu R, Zhang Y. Prognostic values of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:251–261. doi: 10.1007/s00404-019-05372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiang Q, Liu Y, Xiao J, Ou L, Du J. Prognostic value of lymphocyte-to-monocyte ratio (LMR) in patients with prostate cancer: a systematic review and meta-analysis. Am J Mens Health. 2024;18:15579883241234747. doi: 10.1177/15579883241234747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazar S, Goldfinger LE. Platelets and extracellular vesicles and their cross talk with cancer. Blood. 2021;137:3192–3200. doi: 10.1182/blood.2019004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.