Abstract
Objective: Rheumatoid arthritis-associated pneumonia (RAP) is a common complication of rheumatoid arthritis (RA) and is related to poor prognosis. Inflammation plays an important role in the development of RAP. This study aims to analyze and explore the predictive value of the neutrophil/lymphocyte ratio (NLR) combined with the albumin to globulin ratio (AGR) for assessing RAP. Methods: Data for this study were collected retrospectively from the database of Xuancheng People’s Hospital between February 2021 and November 2023. Patients with RAP were assigned to the observation group (n=78), while patients with rheumatoid arthritis (RA) alone were assigned to the control group (n=75). The differences in general clinical data, NLR, and AGR were compared between the two groups. Risk factors for RAP were analyzed using univariate and multivariate Logistic regression. Results: The observation group had significantly lower AGR levels and higher NLR levels compared to the control group (all P<0.05). Univariate and multivariate logistic regression analyses identified age (95% CI 1.265-3.468; P=0.007), glucocorticoid use (95% CI 1.187-3.187; P=0.009), usage of disease-modifying anti-rheumatic drugs (DMARDs) (95% CI 1.257-2.997; P=0.006), AGR (95% CI 1.147-3.578; P=0.012), NLR (95% CI 1.198-2.978; P=0.008) and course of disease (95% CI 11.178-2.971; P=0.005) as independent prognostic factors for RAP. In addition, the ROC curve analysis showed that joint detection of NLR and AGR had a sensitivity of 98.8% and specificity of 81.8% for predicting RAP. Conclusion: NLR and AGR play significant roles in the occurrence and progression of RAP and can serve as predictive factors for early detection of RAP.
Keywords: Neutrophil-to-lymphocyte ratio (NLR), albumin to globulin ratio (AGR), predicting value, rheumatoid arthritis, pneumonia
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease mainly manifested by symmetrical, chronic and progressive polyarthritis. Chronic inflammation and hyperplasia of the joint synovium lead to the formation of pannus, which invades joint cartilage, subchondral bone, ligaments and tendons, etc. This invasion causes the destruction of joint cartilage, bone and joint capsule, eventually leading to joint deformity and loss of function [1]. As RA progresses, various complications may arise, with rheumatoid arthritis-associated pneumonia (RAP) being one of the most common and closely related to poor prognosis of RA [2]. RAP greatly increases mortality and poor outcomes [3], leading to prolonged hospital stay, higher medical expenses, and a greater economic burden on patients. These factors contribute to poor functional recovery at discharge and heightened mortality among RA patients [4]. Therefore, research on RAP is of crucial importance. In the early stage of RA, the common diagnostic features of pneumonia, such as fever, cough and purulent sputum, may not always be present. Clinically, the relevant scoring is rather cumbersome, necessitating the identification of specific biomarkers to predict RAP risk and facilitate early intervention [5]. Although numerous laboratory indicators have been suggested to predict the occurrence of RAP [6], their practical application in clinical settings still need further research.
Neutrophils are the front-line immune cells activated by inflammation following the onset of RA [7]. The number of neutrophils significantly increases after RA. Catecholamine-mediated lymphocytes play an important role in RAP, leading to a reduction in lymphocyte count and inactivation of T cells [8]. The neutrophil/lymphocyte ratio (NLR) was recognized as an inflammatory marker as early as 2001 [9]. It reflects systemic inflammation and has been extensively studied as a predictor of bacterial infection, showing a higher predictive value than traditional inflammatory markers [10]. In addition, NLR can also predict the occurrence of pneumonia and provide dose-response information related to the burden of community-acquired pneumonia [11]. Studies have shown that elevated NLR in the early stages can be an important indicator for predicting the occurrence of RA [12,13]. The albumin to globulin ratio (AGR) is often used as a prognostic biomarker and has been studied in many diseases, mainly including small cell lung cancer [14], liver cancer [15], metastatic gastric cancer [16], nasopharyngeal carcinoma [17], chronic heart failure [18], non-ST segment elevation myocardial infarction [19], and myasthenia gravis [20]. Lv GY et al. conducted a meta-analysis and suggested that a lower AGR is associated with a poor cancer prognosis; they also emphasized that AGR should be considered as a prognostic indicator during cancer treatment [21], highlighting AGR as a valuable clinical indicator for evaluating cancer patient prognosis. However, there is currently only a few studies on the predictive value and clinical application of AGR in RAP.
In this study, we explore a novel combined approach by integrating the NLR and AGR. This approach provides a more comprehensive and potentially more accurate assessment tool compared to using a single biomarker alone. Additionally, we focus specifically on the prediction of RAP, a critical complication of RA, addressing a significant gap in the current research. It offers a potential early warning system for clinicians to identify patients at higher risk of developing RAP, enabling earlier detection and intervention, which is crucial for improving patient outcomes. The combined biomarker approach may also aid in personalized treatment decisions, guiding the selection of appropriate therapeutic strategies based on an individual’s risk profile.
There are numerous biomarkers available for predicting RAP, and the combined use of biomarkers has shown greater efficacy than individual predictors. While NLR is a well-recognized biomarker for predicting inflammation, AGR, as a newly proposed predictive biomarker, still needs further verification. Therefore, this study employs a retrospective analysis to identify risk factors for RAP and to assess the predictive and diagnostic value of NLR and AGR for RAP. Moreover, it evaluates whether NLR combined with AGR provides a higher predictive value for RAP than either biomarker alone, contributing to the early diagnosis and treatment of RAP.
Methods
Study design
The data for this study were retrospectively collected from the database of Xuancheng People’s Hospital between February 2021 and November 2023. The study was conducted under the principles of the Declaration of Helsinki. Ethics committee of Xuancheng People’s Hospital approved the study protocol. RAP cases were assigned to the observation group (n=78), while RA cases were assigned to the control group (n=75).
Inclusion and exclusion criteria
Inclusion criteria: 1) Both groups met the diagnostic criteria of RA (2010 ACR/EULAR) [22]; 2) The observation group met the “Diagnostic Criteria for Nosocomial Infection (trial)” and the “Diagnostic Criteria for Pneumonia” [23]; 3) Age ≥ 18 years.
Exclusion criteria: 1) Presence with other autoimmune diseases; 2) Presence with malignant tumors, hematological diseases, or cardio-cerebrovascular stroke diseases; 3) Presence of systemic infectious diseases; 4) Previous anti-infection treatment before the diagnosis of pulmonary infection at the time of hospitalization.
Diagnosis of RAP
(1) Confirmed history of RA: Patients exhibited typical clinical manifestations of RA along with relevant examination evidence. (2) Chest imaging manifestations: High-resolution CT scans showed characteristics indicative of interstitial lung disease, such as reticular shadow, honeycombing, ground-glass opacity, etc. (3) Exclusion of other causes of pneumonia: A detailed medical history, physical examination, and laboratory tests were used to exclude pneumonia caused by environmental factors, infection, medications, or other autoimmune diseases. (4) Pulmonary function test: The test results showed abnormalities such as restrictive ventilation dysfunction.
Data collection
Primary measurements
Laboratory data collected upon admission included albumin, globulin, NLR, white blood cell (WBC) count, C-reactive protein (CRP), Immunoglobulin A (IgA), IgG, urea, creatinine, urea/creatinine ratio, and uric acid. NLR was calculated based on neutrophil and lymphocyte counts. AGR was calculated based on albumin and globulin levels.
Secondary measurements
General patient information was collected through the hospital’s case query system, including gender, age, height, weight, diabetes mellitus, history of smoking and hypertension.
Statistical analysis
SPSS 23.0 statistical software was used for statistical analysis. Quantitative data following a normal distribution were expressed as mean ± standard deviation (X ± SD), and compared using independent t-test between groups. Categorical data were expressed as percentage (%). The Chi-square test was used for the comparison between groups of qualitative data. Single-factor logistic regression was applied to screen for confounding factors among independent variables. For multivariate logistic regression, the forward stepwise method was employed to identify independent risk factors affecting RAP. The area under the receiver operating characteristic (ROC) curve (AUC) was compared by the DeLong test. A P-value of <0.05 was considered statistically significant for all tests.
Results
Comparison of the general data between the two groups
There were no significant differences between the two groups in terms of general clinical data, including gender, age, body mass index (BMI), smoking history, alcohol consumption, hypertension, diabetes, coronary heart disease, and history of atrial fibrillation (all P>0.05). However, significant differences were observed between the two groups in terms of length of stay and use of disease-modifying anti-rheumatic drugs (DMAROs) for RA (all P<0.05) (Table 1).
Table 1.
Comparison of general data between the two groups
| Clinical features | Control group (n=75) | Observation group (n=78) | t/χ2 | p |
|---|---|---|---|---|
| Age | 71.56±8.62 | 74.84±7.89 | -1.987 | 0.056 |
| Gender | 3.373 | 0.066 | ||
| Male | 52 (69.33%) | 64 (82.05%) | ||
| Female | 23 (30.67%) | 14 (17.95%) | ||
| BMI | 23.84±4.12 | 22.89±4.03 | 1.364 | 0.186 |
| Smoking history | 37 (49.33%) | 42 (53.85%) | 1.123 | 0.289 |
| History of alcohol consumption | 16 (21.33%) | 14 (17.95%) | 0.165 | 0.685 |
| Length of stay | 11.69±3.23 | 12.84±3.12 | -3.041 | 0.004 |
| Hypertension | 25 (33.33%) | 35 (44.87%) | 3.426 | 0.064 |
| Diabetes | 32 (42.67%) | 42 (53.85%) | 3.433 | 0.064 |
| Coronary heart disease | 32 (42.67%) | 37 (47.44%) | 0.671 | 0.413 |
| History of atrial fibrillation | 27 (36.00%) | 24 (32.00%) | 0.262 | 0.609 |
| Treatment for RA | ||||
| Non-steroidal diseases | 46 (58.97%) | 45 (60.00%) | 0.017 | 0.897 |
| Glucocorticoid | 40 (51.28%) | 19 (25.33%) | 10.867 | 0.001 |
| Biopharma | 1 (1.33%) | 1 (1.28%) | 0.001 | 0.978 |
| Intermediate drug | 23 (29.49%) | 24 (32.00%) | 0.001 | 0.989 |
| DMAROs | 29 (37.18%) | 52 (69.33%) | 15.867 | 0.000 |
Notes: DMARO, Disease-modifying anti-rheumatic drugs; RA, Rheumatoid arthritis; BMI, Body mass index.
Comparison of biochemical indicators between the two groups
Compared to the control group, the observation group had lower levels of albumin, globulin, and AGR, and these differences were statistically significant (all P<0.05) (Table 2).
Table 2.
Comparison of biochemical indicators between the two groups
| Index | Control group (n=75) | Observation group (n=78) | t/χ2 | p |
|---|---|---|---|---|
| Total protein | 68.12±7.51 | 65.48±6.94 | 1.567 | 0.123 |
| Albumin | 39.64±4.81 | 36.45±4.23 | 4.621 | 0.001 |
| Globulin | 27.84±3.87 | 29.87±3.41 | -2.987 | 0.005 |
| AGR | 1.46±0.23 | 1.27±0.23 | 5.867 | 0.001 |
Note: AGR, Albumin/Globulin ratio.
Comparison of inflammatory indexes between the two groups
Compared to the control group, the observation group showed significantly higher levels of neutrophils, NLR, WBC, and CRP (all P<0.05). However, no significant differences were observed between the two groups in terms of IgA and IgC (both P>0.05) (Table 3).
Table 3.
Comparison of inflammatory indices between the two groups
| Index | Observation group (n=78) | Control group (n=75) | t/χ2 | P |
|---|---|---|---|---|
| Neutrophil | 7.23±2.22 | 5.67±1.74 | -5.52 | <0.05 |
| Lymphocyte | 1.51±0.84 | 2.04±1.37 | -4.03 | <0.05 |
| NLR | 6.27±2.07 | 2.67±1.13 | -5.89 | <0.05 |
| WBC | 8.32±2.13 | 5.74±1.26 | 8.272 | 0.001 |
| CRP | 39.56±12.56 | 5.56±1.17 | 26.874 | <0.001 |
| IgA | 3.06±0.52 | 2.98±0.72 | 0.468 | 0.665 |
| IgG | 14.68±2.23 | 14.98±2.37 | 0.587 | 0.574 |
Notes: NLR, Neutrophil/Lymphocyte ratio; WBC, White blood cell; CRP, C-reactive protein; IgA, Immunoglobulin A; IgG, Immunoglobulin G.
Comparison of renal function between the two groups
The two groups did not differ significantly in terms of renal function parameters, including urea, creatinine, urea/creatinine, and uric acid (all P>0.05) (Table 4).
Table 4.
Comparison of renal function between the two groups
| Observation group (n=78) | Control group (n=75) | T | p | |
|---|---|---|---|---|
| Urea | 4.46±3.61 | 4.89±3.54 | 0.257 | 0.365 |
| Creatinine | 55.65±8.65 | 56.96±8.56 | 0.257 | 0.147 |
| Urea/creatinine | 0.16±0.05 | 0.08±0.07 | 0.178 | 0.147 |
| Uric acid | 268.78±75.98 | 259.58±59.87 | 0.487 | 0.178 |
Univariate and multivariate analysis
The univariate and multivariate analyses showed that age (95% CI 1.265-3.468; P=0.007), glucocorticoid use (95% CI 1.187-3.187; P=0.009), DMARO use (95% CI 1.257-2.997; P=0.006), AGR (95% CI 1.147-3.578; P=0.012), NLR (95% CI 1.198-2.978; P=0.008) and course of disease (95% CI 11.178-2.971; P=0.005) were independent prognostic factors for RAP (Tables 5, 6).
Table 5.
Univariate analysis of risk factors for rheumatoid arthritis-associated pneumonia
| Item | Observation group (n=78) | Control group (n=75) | T | p |
|---|---|---|---|---|
| Age | 8.904 | 0.0034 | ||
| <60 | 50 (64.10%) | 30 (40.00%) | ||
| ≥60 | 28 (35.90%) | 45 (60.00%) | ||
| Gender | 3.373 | 0.0667 | ||
| Male | 52 (69.33%) | 64 (82.05%) | ||
| Female | 23 (30.67%) | 14 (17.95%) | ||
| Course of disease | 9.889 | 0.0028 | ||
| <5 | 51 (65.38%) | 30 (40.00%) | ||
| ≥5 | 27 (34.62%) | 45 (60.00%) | ||
| Smoking | 37 (49.33%) | 42 (53.85%) | 1.123 | 0.2894 |
| Diabetes | 32 (42.67%) | 42 (53.85%) | 3.433 | 0.064 |
| Glucocorticoid use | 40 (51.28%) | 19 (25.33%) | 10.867 | 0.001 |
| DMARO use | 29 (37.18%) | 52 (69.33%) | 15.867 | 0.000 |
| Hypertension | 25 (33.33%) | 35 (44.87%) | 0.05 | 0.89 |
| Albumin | 36.45±4.23 | 39.64±4.81 | 4.621 | 0.001 |
| Globulin | 29.87±3.41 | 27.84±3.87 | -2.987 | 0.005 |
| AGR | 1.27±0.23 | 1.46±0.23 | 5.867 | 0.001 |
| Neutrophil | 7.23±2.22 | 5.67±1.74 | -5.52 | <0.05 |
| Lymphocyte | 1.51±0.84 | 2.04±1.37 | -4.03 | <0.05 |
| NLR | 6.27±2.07 | 2.67±1.13 | -5.89 | <0.05 |
| WBC | 8.32±2.13 | 5.74±1.26 | 8.272 | 0.001 |
| CRP | 39.56±12.56 | 5.56±1.17 | 26.874 | <0.001 |
| Urea | 4.46±3.61 | 4.89±3.54 | 0.257 | 0.365 |
| Creatinine | 55.65±8.65 | 56.96±8.56 | 0.257 | 0.147 |
| Urea/creatinine | 0.16±0.05 | 0.08±0.07 | 0.178 | 0.147 |
| Uric acid | 268.78±75.98 | 259.58±59.87 | 0.487 | 0.178 |
Notes: NLR, Neutrophil/Lymphocyte ratio; WBC, White blood cell; CRP, C-reactive protein; DMARO, Disease-modifying anti-rheumatic drugs; AGR, Albumin/Globulin ratio.
Table 6.
Multivariate analysis of risk factors for rheumatoid arthritis-associated pneumonia
| Variable | β | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.742 | 0.268 | 7.456 | 0.007 | 2.037 | 1.265-3.468 |
| Glucocorticoid use | 0.668 | 0.247 | 7.146 | 0.009 | 1.987 | 1.187-3.187 |
| DMARO use | 0.647 | 0.227 | 7.044 | 0.006 | 1.987 | 1.257-2.997 |
| AGR | 0.749 | 0.287 | 6.664 | 0.012 | 2.064 | 1.147-3.578 |
| NLR | 0.598 | 0.247 | 7.852 | 0.008 | 1.874 | 1.198-2.978 |
| Course of disease | 0.657 | 0.237 | 7.469 | 0.005 | 1.885 | 1.178-2.971 |
Notes: NLR, Neutrophil/Lymphocyte Ratio; AGR, Albumin/Globulin Ratio; DMARO, Disease disease-modifying anti-rheumatic drugs.
ROC curve analysis
The ROC analysis showed that the sensitivity and specificity of NLR in predicting RAP were 92.4% and 84.9% respectively. The sensitivity and specificity of AGR in predicting RAP were 83.4% and 80.1%, respectively. The sensitivity and specificity of their combination in predicting RAP were 98.8% and 81.8%, respectively. DeLong analysis revealed that their combined prediction outperformed either of their individual application (P=0.019) (Table 7 and Figure 1).
Table 7.
ROC curve analysis for NLR and AGR in early diagnosis of rheumatoid arthritis-associated pneumonia
| Factor | AUC | Sensitivity | Specificity | Cut-off | P |
|---|---|---|---|---|---|
| NLR | 0.675 | 92.4 | 84.9 | 5.27 | 0.002 |
| AGR | 0.778 | 83.4 | 80.1 | 1.33 | 0.004 |
| Joint detection | 0.986 | 98.8 | 81.8 | 4.35 | 0.001 |
Notes: NLR, Neutrophil/Lymphocyte Ratio; AGR, Albumin/Globulin Ratio.
Figure 1.
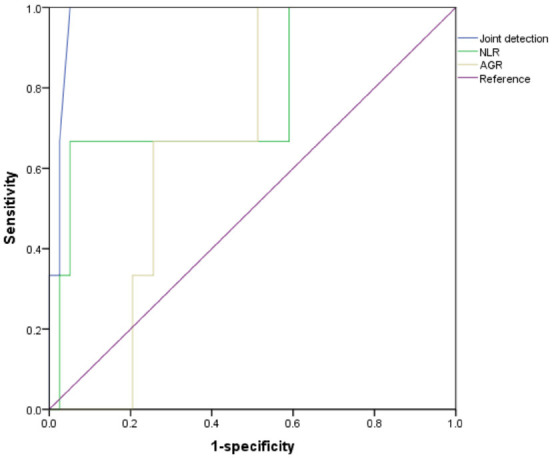
ROC curve analysis for NLR and AGR in early diagnosis of rheumatoid arthritis-associated pneumonia. Notes: NLR, Neutrophil/Lymphocyte ratio; AGR, Albumin/Globulin ratio.
Discussion
In this study, we found that the observation group had significantly lower levels of AGR and higher NLR levels compared to the control group. Univariate and multivariate logistic regression analyses identified age, glucocorticoid use, DMARO use, AGR, NLR and course of disease as independent prognostic factors for rheumatoid arthritis-associated pneumonia (RAP). In addition, the ROC curve results showed that joint detection of NLR and AGR exhibited robust performance in predicting RAP, with sensitivity and specificity of 98.8% and 81.8%, respectively.
Neutrophil-to-lymphocyte Ratio (NLR) is a simple, cost-effective laboratory marker derived from routine blood analysis that reflects leukocyte characteristics. It combines the pro-inflammatory effects of neutrophils, which can cause endothelial damage, with the protective anti-atherosclerotic effects of lymphocytes. NLR is widely recognized a reliable indicator of systemic inflammation [24,25]. Activated neutrophils release various inflammatory mediators and proteases, which can directly damage lung tissue and promote fibroblast activation and collagen deposition, contributing to lung fibrosis [26]. At the same time, a reduction in lymphocytes may compromise the regulatory function of the immune system, further exacerbating the inflammatory response and tissue damage in the lungs [27]. Our findings showed that NLR levels were significantly higher in the observation group than in the control group, indicating that patients with RAP are in an inflammatory state, which is consistent current understanding. The ROC curve analysis demonstrated that NLR predicted RAP with AUC of 0.675 (P=0.002), suggesting that NLR is a more effective tool for diagnosing early-stage RAP.
The albumin-to-globulin ratio (AGR) is an important index in medical and biological fields. Albumin reflects a patient’s nutritional status and systemic inflammatory response, while globulin, as the main cortisol-binding protein, plays an important role in immune function and inflammatory response [28]. An imbalanced AGR reflects disrupted immune and metabolic homeostasis in RA patients. This imbalance can compromise the integrity and defense mechanisms of the lungs, making them more vulnerable to infectious insults, especially from pneumonia-causing pathogens. Additionally, the chronic inflammation and immune abnormalities associated with RA may directly or indirectly alter the pulmonary microenvironment, increasing the risk of pneumonia [29,30].
This study does have a few limitations. Firstly, it is a single-center retrospective study with a limited patient population. The small sample size may affect the generalizability of the research findings. Secondly, the pathogenesis of RAP is multifaceted, and due to the limitations of the available clinical data, further research is still needed to incorporate more factors that may contribute to RAP development for a more comprehensive analysis. In conclusion, we found that NLR and AGR hold promise in monitoring the occurrence and progression of RAP, serving as valuable predictive factors for early detection of RAP.
Disclosure of conflict of interest
None.
References
- 1.Gravallese EM, Firestein GS, Koscal N, Ling E, Longo DL, Messenger LA, Schubach Al. What is rheumatoid arthritis? N Engl J Med. 2024;390:e32. doi: 10.1056/NEJMp2310178. [DOI] [PubMed] [Google Scholar]
- 2.Fukada A, Toyoshima M, Nozue T, Suda T. Bucillamine-induced pneumonitis in a patient with rheumatoid arthritis-associated interstitial pneumonia: a case report and review of the literature. Intern Med. 2019;58:2207–2211. doi: 10.2169/internalmedicine.2515-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie M, Zhu C, Ye Y. Incidence, risk factors, and prognosis of acute exacerbation of rheumatoid arthritis-associated interstitial lung disease: a systematic review and meta-analysis. BMC Pulm Med. 2023;23:255. doi: 10.1186/s12890-023-02532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmadipour M, Lashkari M, Ahmadinejad M. Comparison of morbidity, mortality, and costs of VAP patients with Non-VAP patients in the tertiary referral hospital of Kerman, Iran. Tanaffos. 2023;22:61–69. [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu Y, Liu C, Shi Y, Hao N, Tan W, Wang F. Integrating bioinformatic resources to identify characteristics of rheumatoid arthritis-related usual interstitial pneumonia. BMC Genomics. 2023;24:450. doi: 10.1186/s12864-023-09548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia J, Gao H, Tang J, Jiang R, Xiao L, Sheng H, Lin J. A novel diagnostic model based on lncRNA PTPRE expression, neutrophil count and red blood cell distribution width for diagnosis of seronegative rheumatoid arthritis. Clin Exp Med. 2024;24:86. doi: 10.1007/s10238-024-01343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orange DE, Blachere NE, DiCarlo EF, Mirza S, Pannellini T, Jiang CS, Frank MO, Parveen S, Figgie MP, Gravallese EM, Bykerk VP, Orbai AM, Mackie SL, Goodman SM. Rheumatoid arthritis morning stiffness is associated with synovial fibrin and neutrophils. Arthritis Rheumatol. 2020;72:557–564. doi: 10.1002/art.41141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storrer KM, Müller CS, Pessoa MCA, Pereira CAC. Connective tissue disease-associated interstitial lung disease. J Bras Pneumol. 2024;50:e20230132. doi: 10.36416/1806-3756/e20230132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alakuş H, Göksu M. Does parathyroidectomy affect the neutrophil/lymphocyte ratio, a systemic inflammatory marker? Cureus. 2021;13:e13708. doi: 10.7759/cureus.13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Verde M, Luciano M, Fordellone M, Sampogna G, Lettieri D, Palma M, Torella D, Marrapodi MM, Di Vincenzo M, Torella M. Postpartum depression and inflammatory biomarkers of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, and monocyte-lymphocyte ratio: a prospective observational study. Gynecol Obstet Invest. 2024;89:140–149. doi: 10.1159/000536559. [DOI] [PubMed] [Google Scholar]
- 11.Wang RH, Wen WX, Jiang ZP, Du ZP, Ma ZH, Lu AL, Li HP, Yuan F, Wu SB, Guo JW, Cai YF, Huang Y, Wang LX, Lu HJ. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. 2023;14:1115031. doi: 10.3389/fimmu.2023.1115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Li J, Sun L, Wang T, Liang W. The association between neutrophil-to-lymphocyte ratio and disease activity in rheumatoid arthritis. Inflammopharmacology. 2023;31:2237–2244. doi: 10.1007/s10787-023-01273-2. [DOI] [PubMed] [Google Scholar]
- 13.Khan T, Nawal CL, Meena PD, Singh A. Study neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patient with rheumatoid arthritis. J Assoc Physicians India. 2022;70:11–12. [PubMed] [Google Scholar]
- 14.Lu P, Ma Y, Wei S, Liang X. A low albumin-to-globulin ratio predicts a poor prognosis in patients with metastatic non-small-cell lung cancer. Front Med (Lausanne) 2021;8:621592. doi: 10.3389/fmed.2021.621592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts WS, Delladio W, Price S, Murawski A, Nguyen H. The efficacy of albumin-globulin ratio to predict prognosis in cancer patients. Int J Clin Oncol. 2023;28:1101–1111. doi: 10.1007/s10147-023-02380-4. [DOI] [PubMed] [Google Scholar]
- 16.Bozkaya Y, Erdem GU, Demirci NS, Yazıcı O, Özdemir NY, Köstek O, Zengin N. Prognostic importance of the albumin to globulin ratio in metastatic gastric cancer patients. Curr Med Res Opin. 2019;35:275–282. doi: 10.1080/03007995.2018.1479683. [DOI] [PubMed] [Google Scholar]
- 17.Mao H, Yang F. Prognostic significance of albumin-to-globulin ratio in patients with renal cell carcinoma: a meta-analysis. Front Oncol. 2023;13:1210451. doi: 10.3389/fonc.2023.1210451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niedziela JT, Hudzik B, Szygula-Jurkiewicz B, Nowak JU, Polonski L, Gasior M, Rozentryt P. Albumin-to-globulin ratio as an independent predictor of mortality in chronic heart failure. Biomark Med. 2018;12:749–757. doi: 10.2217/bmm-2017-0378. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Yang L, Wu X, Zheng X, Zhao Yl. Urea nitrogen-to-albumin ratio predicts ventricular aneurysm formation in ST-segment elevation myocardial infarction. ESC Heart Fail. 2024;11:974–985. doi: 10.1002/ehf2.14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang A, Zhang Y, Xia G, Tian X, Zuo Y, Chen P, Wang Y, Meng X, Han X. Association of serum albumin to globulin ratio with outcomes in acute ischemic stroke. CNS Neurosci Ther. 2023;29:1357–1367. doi: 10.1111/cns.14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv GY, An L, Sun XD, Hu YL, Sun DW. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta. 2018;476:81–91. doi: 10.1016/j.cca.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann GH, Norli ES, Bøyesen P, van der Heijde D, Grøvle L, Haugen AJ, Nygaard H, Bjørneboe O, Thunem C, Kvien TK, Mjaavatten MD, Lie E. Role of erosions typical of rheumatoid arthritis in the 2010 ACR/EULAR rheumatoid arthritis classification criteria: results from a very early arthritis cohort. Ann Rheum Dis. 2017;76:1911–1914. doi: 10.1136/annrheumdis-2017-211350. [DOI] [PubMed] [Google Scholar]
- 23.Campos CGP, Pacheco A, Gaspar MDDR, Arcaro G, Reche PM, Nadal JM, Farago PV. Analysis of diagnostic criteria for ventilator-associated pneumonia: a cohort study. Rev Bras Enferm. 2021;74:e20190653. doi: 10.1590/0034-7167-2019-0653. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Wang X, Jia W, Wang K, Wang W, Diao W, Ou F, Ma J, Yang Y. Association of the systemic immuno-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio with diabetic microvascular complications. Front Endocrinol (Lausanne) 2024;15:1367376. doi: 10.3389/fendo.2024.1367376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albayrak H. Neutrophil-to-lymphocyte ratio, neutrophil-to-monocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index in psoriasis patients: response to treatment with biological drugs. J Clin Med. 2023;12:5452. doi: 10.3390/jcm12175452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal BH, Giridharan T, Suzuki S, Khan ANH, Zsiros E, Emmons TR, Yaffe MB, Gankema AAF, Hoogeboom M, Goetschalckx I, Matlung HL, Kuijpers TW. Neutrophil interactions with T cells, platelets, endothelial cells, and of course tumor cells. Immunol Rev. 2023;314:13–35. doi: 10.1111/imr.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danne C, Skerniskyte J, Marteyn B, Sokol H. Neutrophils: from IBD to the gut microbiota. Nat Rev Gastroenterol Hepatol. 2024;21:184–197. doi: 10.1038/s41575-023-00871-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Song C, Yao Z, Sun J, Liu W. Associations between albumin, globulin, albumin to globulin ratio and muscle mass in adults: results from the national health and nutrition examination survey 2011-2014. BMC Geriatr. 2022;22:383. doi: 10.1186/s12877-022-03094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köse Çobanoglu R, Şentürk T. The role of albumin-to-globulin ratio in undifferentiated arthritis: rheumatoid arthritis versus primary Sjögren syndrome. Arch Rheumatol. 2022;37:245–251. doi: 10.46497/ArchRheumatol.2022.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurindo LF, de Maio MC, Barbalho SM, Guiguer EL, Araújo AC, de Alvares Goulart R, Flato UAP, Júnior EB, Detregiachi CRP, Dos Santos Haber JF, Bueno PCS, Girio RSJ, Eleutério RG, Bechara MD. Organokines in rheumatoid arthritis: a critical review. Int J Mol Sci. 2022;23:6193. doi: 10.3390/ijms23116193. [DOI] [PMC free article] [PubMed] [Google Scholar]


