Abstract
Objective: To compare the safety and effectiveness between long-term GnRH agonist plus HCG (dual trigger) and HCG trigger alone in high ovarian responders. Methods: A retrospective study was conducted on clinical data from 314 cases of high ovarian response who underwent in-vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment at Hunan Provincial Maternal and Child Healthcare Hospital from July 2018 to January 2023. Participants were divided into two groups based on their triggering regimen: the Combined treatment group (GnRH agonist + HCG) and the HCG group (HCG alone). Blood routine, ovary ultrasound parameters, baseline hormone levels, clinical outcomes of controlled ovarian stimulation, clinical outcomes of the first transplantation, and incidence of ovarian hyperstimulation syndrome (OHSS) were compared between the two groups. Results: There were no significant differences in patient characteristics, blood routines, ovary ultrasound parameters, clinical pregnancy rate, implantation rate and abortion rate between the two groups (all P > 0.05). However, the incidence of Ovarian Hyperstimulation Syndrome (OHSS) in combined treatment group was significantly lower than that in HCG group (mild OHSS: 31% vs. 46.26%, P=0.015; moderate/severe OHSS: 3.00% vs. 11.68%, P=0.021). Conclusion: Long-term GnRH agonist plus HCG (dual trigger) does not affect the number of metaphase II (MII) oocytes, high-quality embryos, or clinical pregnancy rate in patients with high ovarian response. Furthermore, the incidence of OHSS is significantly lower with the dual trigger compared to the HCG trigger alone.
Keywords: High ovarian response, dual trigger, OHSS, GnRH agonist protocol
Introduction
Infertility is diagnosed when a couple has failed to conceive after one year of regular, unprotected sexual intercourse [1]. The challenge of infertility, often referred to as the “infertility predicament”, imposes significant physical, social, and economic pressures, profoundly affecting individuals’ lives [2]. Globally, infertility is a widespread medical issue, impacting approximately 10-15% of couples of reproductive age, with the incidence in China estimated to be between 12.5% and 15% [3].
Assisted reproductive techniques, such as in-vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI), are essential interventions for couples facing infertility [4]. The variability in response to ovulation-inducing drugs can significantly affect their success rates. Although ovarian stimulation can yield multiple eggs, it also poses risks, including reduced egg quality, decreased endometrial receptivity, and ovarian hyperstimulation syndrome (OHSS). As a result, there is growing interest in optimizing the type and dosage of medications used to prevent OHSS in patients undergoing ovarian stimulation [5].
Controlled ovarian stimulation (COS) is a fundamental component of assisted reproductive technology (ART) cycles and involves administering gonadotropins to stimulate the development of multiple follicles [6]. Two extensive retrospective analyses have demonstrated a direct correlation between the number of oocytes retrieved and pregnancy/live birth rates in cycles with immediate fresh embryo transfers. This relationship is nearly linear up to a point, beyond which live birth rates plateau or decline with excessively high oocyte counts [7,8]. However, COS can lead to complications such as ovarian hyperstimulation syndrome (OHSS), occurring in approximately 20-30% of cycles and potentially resulting in severe consequences [9]. To reduce the risks of OHSS, various strategies have been investigated, including the use of gonadotropin-releasing hormone (GnRH) agonists and human chorionic gonadotropin (HCG) triggers [10].
GnRH agonists are commonly used in IVF cycles for pituitary suppression, facilitating the synchronization of follicular development and preventing spontaneous luteinizing hormone (LH) surges [11]. The long GnRH agonist protocol is a traditional strategy for controlled ovarian stimulation (COS) due to its efficiency in recruiting multiple follicles for synchronous development and suppressing spontaneous LH surges, which enhances egg quality [12]. However, prolonged use of GnRH agonists can elevate the risk of ovarian hyperstimulation syndrome (OHSS) because of excessive follicle development, imposing significant psychological and physical burdens on patients [13]. HCG is typically used to trigger final oocyte maturation in IVF cycles [14]. Despite its effectiveness, HCG triggers can heighten the risk of OHSS, particularly in patients with high ovarian response, as it mimics the physiological LH surge but may intensify OHSS symptoms. To mitigate the risk of OHSS while preserving the advantages of both GnRH agonists and HCG, a dual trigger approach has been proposed. This involves combining a GnRH agonist with a lower dose of HCG, aiming to replicate the natural hormonal milieu during oocyte maturation [15]. The GnRH agonist initiates a positive feedback mechanism on the hypothalamic-pituitary-ovarian (HPO) axis, releasing follicle-stimulating hormone (FSH), which is crucial for oocyte development, while the HCG component provides the LH-like effect necessary for final oocyte maturation. Research indicates that this dual trigger method can significantly reduce the incidence of moderate to severe OHSS (< 2%) and increase the number of mature oocytes [16]. Therefore, combining a GnRH agonist with a low-dose HCG trigger retains the benefits of the classic protocol while reducing OHSS occurrences.
Currently, there is no research examining the long-term safety and efficacy of GnRH agonists and HCG triggers in patients with high ovarian response. This study aims to explore the safety and effectiveness of HCG triggering compared to a combined GnRH agonist and HCG trigger in patients undergoing ovarian stimulation.
Materials and methods
Patients
A retrospective analysis was conducted on the clinical data from 314 cases of IVF/ICSI that performed at Hunan Provincial Maternal and Child Healthcare Hospital from July 2018 to January 2023. Patients were divided into a combined treatment group (GnRH-a + HCG) (n=100) and an HCG group (n=214) based on different trigger methods. The study was approved by the ethics committee of Hunan Provincial Maternal and Child Healthcare Hospital. Since this study solely utilized de-identified patient data, informed consent on this study was exempted.
Inclusion and exclusion criteria
Inclusion criteria: (1) age ≤ 35 years; (2) patients underwent long protocol of GnRH-a and IVF/ICSI treatment; (3) first treatment with IVF-ET or IntraCytoplasmic Sperm Injection with Embryo Transfer (ICSI-ET); (4) patients with ovarian stimulation showing serum estradiol (E2) ≥ 15000 nmol/ml, anti mullerian hormone (AMH) level ≥ 4.5 ng/ml, or number of oocytes retrieved ≥ 15 [17].
Exclusion criteria: (1) patients with intrauterine adhesions or endometriosis; (2) body mass index (BMI) ≥ 28 kg/m2; (3) patients with poor ovarian reserve.
Treatment method
Ovarian stimulation
Ovarian stimulation was performed using GnRH-a for all patients. Patients were injected with 1.0-1.875 mg of Leuprorelin (Takeda Pharmaceutical Company Ltd., Japan) at the mid-luteal stage of their last menstrual period. Serum concentrations of FSH, LH and progesterone (P) were tested, the number and size of ovarian effect follicles were evaluated by ultrasound after two weeks. A starting dose of 112.5-225 IU of recombinant FSH (Lizhu Pharmaceutical Trading Co., Zhuhai, China) was administered for all patients, with gonadotropins (Gn) dosage fine-tuned based on the patient’s reaction.
Oocyte retrieval
Final maturation of oocytes was initiated immediately upon the presence of a dominant follicle reaching or exceeding 18 mm in diameter, which was achieved through the administration of 0.2 mg of the GnRH agonist triptorelin-acetate (GeneScience Pharmaceuticals Co., Ltd., Changchun, China) plus 2000 IU of HCG (Chorionic Gonadotrophin, Lizhu Pharmaceutical Trading Co., Zhuhai, China) in combined treatment group or 250 μg of rHCG (Merck Serono, Geneva, Switzerland) alone in HCG Group. Transvaginal ultrasound-guided oocyte retrieval was performed 36 hours later, and the collected oocytes were inseminated through IVF/ICSI.
Embryo freezing
Embryos of grades I and II, with 6-9 cells on day 3, were defined as “top-quality embryos”. In the combined treatment group, all fresh embryos were cryopreserved (freeze-all approach). In the HCG Group, embryo transfer was canceled, and all embryos were cryopreserved if the E2 level was ≥ 20000 nmol/L, the number of oocytes retrieved was ≥ 20, the mean diameter of unilateral ovaries on the day of transfer was ≥ 7 cm, significant pelvic effusion was present, or if poor endometrial morphology or hydrosalpinx was observed.
Embryo transfer
Daily intramuscular progesterone 60 mg (Zhejiang Xianju Pharmaceutical Co., Ltd., Taizhou, China) was started on the day of fresh embryo transfer. Serum β-HCG levels were tested 14 days post-transfer, and pregnancy was confirmed if serum β-HCG levels exceeded 10 U/L. Progesterone was continued until 8-10 weeks of gestation.
Patients with frozen embryo transplantation and without bilateral ovarian cysts were given oral administration of estradiol valerate (Bayer, Germany), at a dosage of 2 mg administered bi-daily, increasing to 3 mg twice per day after 3 days. Drug dosages were adjusted based on endometrial thickness as determined by ultrasonography. Intramuscular injection of progesterone, 40-60 mg per day, was administered for endometrial transformation when estradiol valerate administration exceeded 12 days, and the endometrial thickness was ≥ 8 mm. The timing of embryo thawing and transfer was determined based on the duration of estradiol valerate administration. Serum β-HCG levels were measured 14 days post-transfer, and pregnancy was confirmed if serum β-HCG level > 10 U/L. Progesterone was continued until 8-10 weeks of gestation.
A summary of the treatment methods for the patients are shown in Figure 1.
Figure 1.
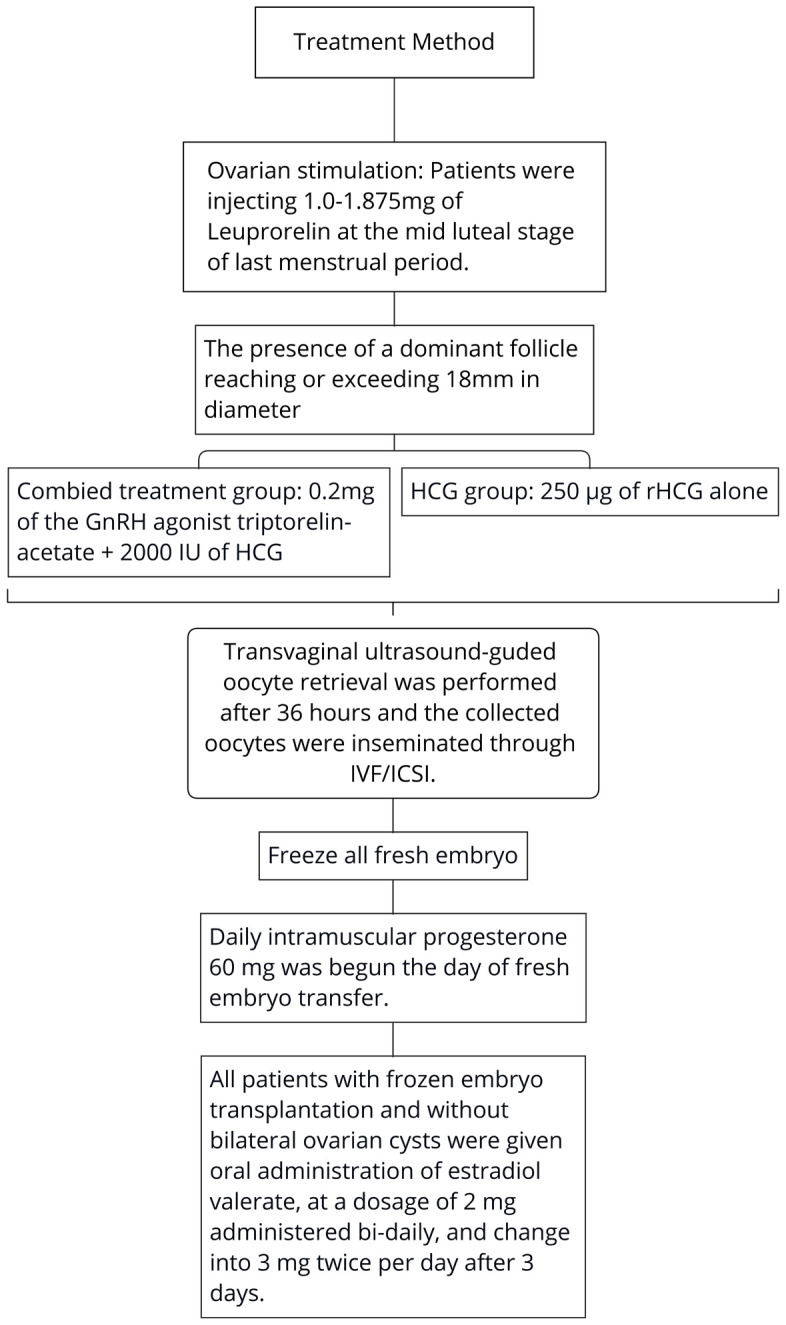
Flowchart of treatment procedures. Note: HCG: human chorionic gonadotropin; IVF/ICSI: in-vitro fertilization/intracytoplasmic sperm injection.
Baseline information
Demographic information and disease-related indicators of patients were extracted and documented from the medical records system, including age, BMI, smoking history, alcohol consumption, hypertension, diabetes, infertility duration, primary infertility and polycystic ovary syndrome (PCOS).
Blood test
A complete blood count and hormone level assessment was conducted on the day of medication administration. Fasting venous blood samples of 4 mL were collected from all patients in the morning, allowed to stand for 2 hours, and then centrifuged at 3000 rpm for 10 minutes. The upper serum was stored at -20°C for further analysis. Hemoglobin, red blood cell count, white blood cell count, neutrophil count, and platelet count were measured using an automated blood cell analyzer (Sysmex Corporation, XT-4000i). Serum levels of anti-Mullerian hormone (AMH) (Ashlab, USA) were assessed using commercially procured kits. E2 and P were analyzed utilizing the Immulite 1000 assay based on chemiluminescence (DPC, Poway, CA).
Ultrasound examinations
Before treatment, patient underwent an ultrasound examination of the ovaries. Ultrasounds were performed using an Aloka SSD-650 or SSD-620 (Aloka Co., Tokyo, Japan), equipped with a 5 MHz probe to evaluate endometrial thickness. The assessment included measuring endometrial thickness and ovarian diameter, as well as checking for ascites, ovarian tissue damage, premature ovarian failure, and invasion of other organs.
OHSS occurrence rate
The incidence of OHSS was the primary outcome measure. The occurrence of OHSS was recorded throughout the treatment cycle. Mild OHSS: abdominal distension, mild abdominal pain, ovarian size < 8 cm3; Moderate OHSS: increased abdominal distension, abdominal pain, nausea, and/or vomiting, ascites confirmed by ultrasound, ovarian size of 8-12 cm3; Severe OHSS: ascites (or pleural effusion), oliguria (< 300 ml/day or < 30 ml/hour), hematocrit > 0.45, hyponatremia (serum sodium < 135 mmol/L), hyperkalemia (serum potassium > 5 mmol/L), hypoproteinemia (serum albumin < 35 g/L), and ovarian size > 12 cm3.
Clinical outcomes
Post-treatment, various clinical outcomes were recorded, including duration of Gn administration, total Gn dosage, follicle diameter, total number of oocytes obtained, fertilization rate, metaphase II (MII) oocytes, 2 pronuclei (2PN) embryos, available embryos, and high-quality embryos. High-quality embryos were defined as those reaching the 6-8 cell stage, with cytoplasmic particles covering no more than 10% of the embryo’s outer surface, and uniform size. The fertilization rate was calculated by dividing the count of 2PN embryos by the total number of fertilized oocytes. Clinical parameters recorded during the treatment cycle included clinical pregnancy rate, implantation rate, and abortion rate.
Statistical analysis
Statistical analysis was performed using SPSS 29.0 software (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was employed to ascertain the normality of continuous variables. For continuous variables that conformed to a normal distribution, results were expressed as mean ± standard deviation and analyzed using Student’s t-test with corrected variance. Categorical variables, displayed as frequencies, were compared using the chi-square test. Statistical significance was established at P < 0.05.
Results
Baseline characteristics
Table 1 summarizes the fundamental patient characteristics. Comparisons of mean age, BMI, smoking history, alcohol consumption history, hypertension, and diabetes status revealed no significant differences between the two groups (all P > 0.05). Additionally, comparison of disease-related indicators, including infertility duration, primary infertility, and PCOS also revealed no significant differences between the two groups (all P > 0.05).
Table 1.
Comparison of baseline characteristics between the two groups
| Parameters | Combined treatment group (n=100) | HCG Group (n=214) | t/χ2 | P-value |
|---|---|---|---|---|
| Age (years) | 28.85 ± 3.29 | 29.12 ± 3.23 | 0.691 | 0.491 |
| BMI (kg/m2) | 23.27 ± 3.67 | 22.95 ± 2.62 | 0.780 | 0.436 |
| Smoking history | 12 (12.00%) | 21 (9.81%) | 0.153 | 0.696 |
| Alcohol consumption | 23 (23.00%) | 37 (17.29%) | 1.092 | 0.296 |
| Hypertension | 7 (7.00%) | 12 (5.61%) | 0.052 | 0.820 |
| Diabetes | 5 (5.00%) | 9 (4.21%) | 0.001 | 0.981 |
| Infertility duration (years) | 3.45 ± 0.83 | 3.62 ± 0.95 | 1.623 | 0.106 |
| Primary infertility | 44 (44.00%) | 83 (38.79%) | 0.568 | 0.451 |
| PCOS | 56 (56.00%) | 118 (55.14%) | 0.000 | 0.983 |
HCG: human chorionic gonadotropin; BMI: body mass index; PCOS: polycystic ovary syndrome.
Routine blood tests
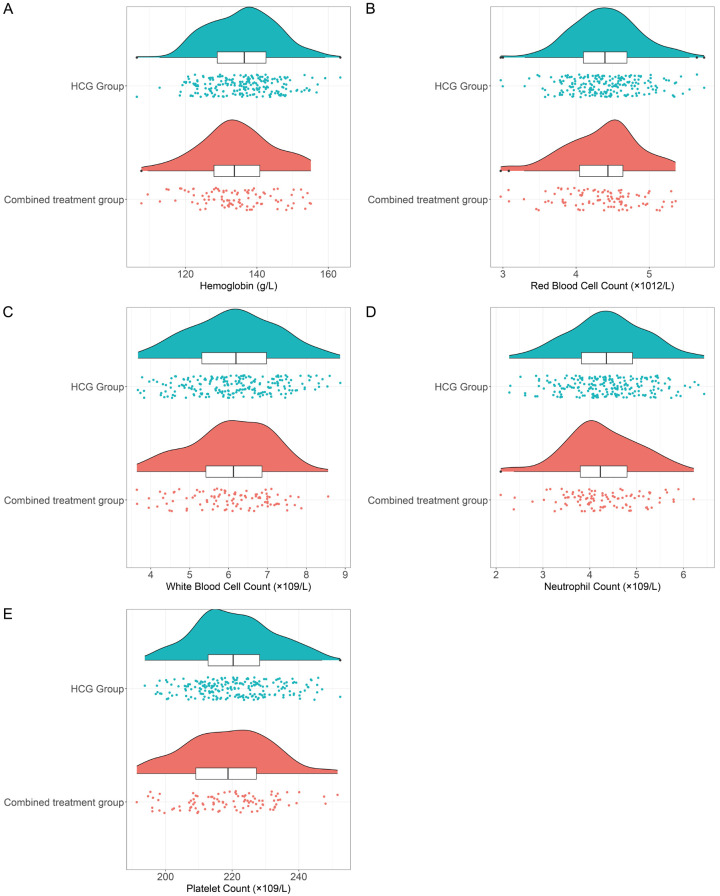
Examination of routine blood indices showed no notable disparities between the two cohorts concerning hemoglobin levels, red blood cell counts, white blood cell counts, neutrophil counts, and platelet counts (all P > 0.05, Figure 2A-E).
Figure 2.
Comparison of blood routine between the two groups. A. Hemoglobin level; B. Red blood cell count; C. White blood cell count; D. Neutrophil count; E. Platelet count. HCG: human chorionic gonadotropin.
Ultrasound examination
Comparison of clinical outcomes between the two groups showed no significant differences in endometrial thickness (14.27 ± 1.96 mm vs. 14.52 ± 2.08 mm), ovarian diameter (6.35 ± 1.51 cm vs. 6.41 ± 1.48 cm), ascites (9.00% vs. 11.21%), ovarian tissue damage (2.00% vs. 3.27%), premature ovarian failure (1.00% vs. 1.87%), and organ invasion (0% vs. 0.47%) (all P > 0.05, Table 2).
Table 2.
Comparison of ultrasonographic parameters of the ovary between the two groups
| Characteristic | Combined treatment group (n=100) | HCG Group (n=214) | t/χ2 | P-value |
|---|---|---|---|---|
| Average endometrial thickness (mm) | 14.27 ± 1.96 | 14.52 ± 2.08 | 1.007 | 0.315 |
| Ovarian diameter | 6.35 ± 1.51 | 6.41 ± 1.48 | 0.315 | 0.753 |
| Ascites | 9 (9.00%) | 24 (11.21%) | 0.356 | 0.551 |
| Ovarian tissue damage | 2 (2.00%) | 7 (3.27%) | 0.071 | 0.790 |
| Premature ovarian failure | 1 (1.00%) | 4 (1.87%) | 0.008 | 0.929 |
| Invasion of other organs | 0 (0.00%) | 1 (0.47%) | None | 1.000 |
HCG: human chorionic gonadotropin.
Baseline hormone level
Before treatment initiation, serum hormone levels in both groups were tested and compared. There were no significant differences observed in the baseline levels of AMH, E2, and P between the two groups (all P > 0.05) (Table 3).
Table 3.
Comparison of basal hormone levels between the two groups
| Parameters | Combined treatment group (n=100) | HCG Group (n=214) | t | P-value |
|---|---|---|---|---|
| Basal AMH level (ng/ml) | 7.11 ± 3.92 | 6.46 ± 2.82 | 1.482 | 0.141 |
| Basal serum E2 level (pg/mL) | 2645.00 ± 1101.00 | 2658.00 ± 1122.00 | 0.097 | 0.923 |
| Basal serum progesterone level (ng/mL) | 109.95 ± 14.75 | 111.57 ± 21.07 | 0.784 | 0.433 |
HCG: human chorionic gonadotropin; AMH: anti-Müllerian hormone; E2: estradial.
Clinical outcomes of the controlled ovarian stimulation
As shown in Table 4, no significant differences were noted between the two groups regarding the duration of Gn administration, total Gn dosage, number of oocytes retrieved, MII stage count, and the number of available and high-quality embryos (all P > 0.05). However, the follicle diameter (18.78 ± 5.32 vs. 17.47 ± 4.42, t=2.145, P=0.033) and the number of 2PN embryos (11.09 ± 3.37 vs. 8.64 ± 4.60, t=5.316, P < 0.001) were significantly higher in the combined treatment group compared to the HCG group.
Table 4.
Comparison of clinical outcomes of the controlled ovarian stimulation between the two groups
| Parameters | Combined treatment group (n=100) | HCG Group (n=214) | t/χ2 | P-value |
|---|---|---|---|---|
| Duration of Gn administration | 15.27 ± 3.68 | 15.18 ± 3.05 | 0.223 | 0.824 |
| Total Gn dosage | 1896.25 ± 411.83 | 1963.41 ± 510.42 | 1.244 | 0.215 |
| Follicle diameter | 18.78 ± 5.32 | 17.47 ± 4.42 | 2.145 | 0.033 |
| Oocytes retrieved | 13.11 ± 4.40 | 14.1 ± 5.60 | 1.702 | 0.090 |
| IVF rate | 82 (82.00%) | 181 (84.58%) | 0.171 | 0.680 |
| MII oocytes | 12.85 ± 3.99 | 12.71 ± 3.2 | 0.310 | 0.757 |
| 2PN embryos | 11.09 ± 3.37 | 8.64 ± 4.60 | 5.316 | < 0.001 |
| Available embryo | 5.24 ± 1.57 | 4.93 ± 1.36 | 1.704 | 0.090 |
| High-quality embryo | 11.2 ± 1.68 | 11.18 ± 1.46 | 0.107 | 0.915 |
HCG: human chorionic gonadotropin; Gn: gonadotropins; IVF: in vitro fertilization; MII: metaphase II; 2PN: 2 pronuclei.
Clinical outcomes of the first transplantation in the two groups
The clinical outcomes of the first transplantation in the two groups are shown in Table 5. The clinical pregnancy rate in combined treatment group was 58%, compared to 49.53% in the HCG group (P=0.078). Additionally, there were no significant differences in the embryo implantation rate (P=0.265) and abortion rate (P=1.000) between the two groups.
Table 5.
Comparison of clinical outcomes of the first transplantation between the two groups
| Parameters | Combined treatment group (n=100) | HCG Group (n=214) | χ2 | P-value |
|---|---|---|---|---|
| Clinical pregnancy rate | 58 (58.00%) | 106 (49.53%) | 1.634 | 0.201 |
| Implantation rate | 76 (76.00%) | 148 (69.16%) | 1.243 | 0.265 |
| Abortion rate | 2 (2.00%) | 5 (2.34%) | 0.000 | 1.000 |
HCG: human chorionic gonadotropin.
Incidence of ovarian hyperstimulation syndrome (OHSS)
In the combined treatment group, 31 cases developed mild OHSS and 3 cases developed moderate OHSS. There were 99 cases of mild OHSS and 25 cases of moderate or severe OHSS in HCG group (Table 6). The proportions of mild (χ2=5.929, P=0.015) and moderate/severe OHSS (χ2=5.302, P=0.021) were significantly higher in the HCG group compared to the combined treatment group.
Table 6.
Comparison of OHSS rate between the two groups
| Parameter | Combined treatment group (n=100) | HCG Group (n=214) | χ2 | P-value |
|---|---|---|---|---|
| Mild OHSS | 31 (31.00%) | 99 (46.26%) | 5.929 | 0.015 |
| Moderate/Severe OHSS | 3 (3.00%) | 25 (11.68%) | 5.302 | 0.021 |
HCG: human chorionic gonadotropin; OHSS: Ovarian Hyperstimulation Syndrome.
Discussion
Infertility has emerged as a significant health issue affecting reproductive capabilities, with intracytoplasmic sperm injection (ICSI) and in vitro fertilization (IVF) serving as important treatment options [18,19]. Ovarian hyperstimulation syndrome (OHSS) is a common complication in assisted reproduction and ovarian hyperstimulation, occurring in approximately 20% to 30% of cases [20,21]. Severe OHSS can adversely impact liver and kidney function, lead to thrombosis, and even become life-threatening, imposing substantial economic and psychological burdens on patients [22,23]. Currently, various strategies are employed to prevent OHSS in the context of IVF and embryo transfer (IVF-ET), including pre-treatment with controlled ovarian hyperstimulation (COH), individualized COH protocols, and Gn dosage reduction. Research focuses on drug selection and dosage adjustment on the trigger day as key measures to mitigate OHSS risk.
Since the introduction of GnRH-a in IVF controlled ovarian hyperstimulation in 1984, the GnRH-a long protocol has remained a classical strategy for controlled ovarian hyperstimulation (COH) for over 30 years. After pituitary downregulation by GnRH-a, multiple follicles are effectively recruited for synchronized development while spontaneous LH surges are suppressed, enhancing egg quality [24,25]. This results in the retrieval of more high-quality oocytes and reduces treatment cycle cancellation rates. Research indicates that GnRH-a not only improves oocyte quality and endometrial receptivity but also enhances the pelvic microenvironment, thereby increasing the success rate of assisted reproduction. However, prolonged GnRH-a therapy often heightens the risk of OHSS due to excessive follicle development, imposing significant psychological and physical burdens on patients [26,27].
In this study, patients with high ovarian response were treated with GnRH-a combined with 2000 IU HCG trigger. Our study showed that this regimen achieved optimal egg retrieval levels. In addition, the quantity of MII oocytes, 2PN embryos, and the rate of implantation and clinical pregnancy were slightly higher in the combined treatment group than those in the HCG group, while the incidence of OHSS was significantly lower. Several studies have compared the outcomes of dual trigger with HCG alone in patients undergoing IVF/ICSI. For instance, a study by Yan et al. found that the dual trigger approach led to a similar number of MII oocytes and high-quality embryos as the HCG trigger alone, with a significantly lower incidence of OHSS [28]. Similarly, another study found that the dual trigger group had a lower incidence of moderate/severe OHSS compared to the HCG group, with no significant difference in the clinical pregnancy rate [29]. These findings are consistent with our results, supporting the use of the dual trigger approach as a safe and effective option for high ovarian responders.
Studies have found that in a normal menstrual cycle, the final maturation of the egg is completed under the trigger of the FSH and the LH [30]. In the COH process of IVF-ET, HCG is used to mimic the physiological effect of LH peak in the human menstrual cycle. HCG combined with GnRH-a not only simulates the LH peak, but also provides positive feedback to the HPO axis, promoting the release of FSH, thereby more closely resembling natural hormonal changes during egg maturation. FSH plays an indispensable role in the development and maturation of oocytes. Research has confirmed that, compared with the HCG trigger alone, the additional application of the GnRH-a can greatly reduce the incidence of moderate to severe OHSS (< 2%), and can increase the number of mature eggs [31]. However, the luteinizing effect of GnRH-a can induce the inadequate luteal function and reduced endometrial receptivity, potentially lowering pregnancy rate and increasing miscarriage rate, which limits its clinical application [32,33]. In this study, the fresh embryo transfer was canceled for the patients receiving the double-trigger treatment, replaced by frozen embryo transfer after cryopreservation, which mitigates the impact high estrogen environment on the endometrial receptivity and reduces the occurrence of moderate to severe OHSS after pregnancy. Long-term GnRH-a treatment effectively inhibits the premature ovulation and synchronizes follicular development during ovulation induction [34,35]. The combination of long-term GnRH-a with a low-dose HCG trigger not only retains the advantages of the classic long-term treatment, but also reduces the occurrence of OHSS. Therefore, for patients with high ovarian response, GnRH-a combined with a low-dose HCG trigger can significantly reduce the incidence of OHSS, which is worthy of promotion and in-depth study.
The mechanism behind the reduced incidence of OHSS with the dual trigger approach is likely related to the combined effects of GnRH agonists and HCG. The GnRH agonist triggers a positive feedback loop that releases FSH, which is crucial for oocyte development. The HCG provides the necessary LH-like effect for final oocyte maturation, while the lower dose helps minimize the risk of OHSS. This synergistic effect ensures optimal oocyte maturity without significantly affecting the rates of MII oocytes, high-quality embryos, or clinical pregnancy in single embryo transfers. This approach offers a promising strategy for mitigating OHSS risks while maintaining high-quality embryo production.
Our study has several limitations. First, it is a retrospective analysis, which introduces potential biases and confounding factors. Second, the inclusion criteria have certain limitations, and the sample size is relatively small. Consequently, larger, multi-center randomized controlled studies are required to validate our findings. Future research should focus on optimizing the dual trigger regimen, particularly the timing and dosing of GnRH agonists and HCG, to further enhance its safety and effectiveness.
Conclusion
In patients with high ovarian response, the long-term use of GnRH-a combined with a low-dose HCG trigger does not significantly affect the rates of MII oocytes, high-quality embryos, or clinical pregnancy in single embryo transfers. Importantly, this approach significantly reduces the incidence of OHSS. With the serum E2 level ≥ 15000 pmol/L on the trigger day, a dual trigger treatment appears to effectively lower the incidence of OHSS.
Acknowledgements
This study was supported by Hunan Provincial National Science Foundation of China (2019JJ80038); the Key Grant of Prevention and Treatment of Birth Defect from Hunan Province (2019SK1012); and Hunan Provincial Health Commission National Science Foundation (20190795 and B2019032).
Disclosure of conflict of interest
None.
References
- 1.Dougherty MP, Poch AM, Chorich LP, Hawkins ZA, Xu H, Roman RA, Liu H, Brakta S, Taylor HS, Knight J, Kim HG, Diamond MP, Layman LC. Unexplained female infertility associated with genetic disease variants. N Engl J Med. 2023;388:1055–1056. doi: 10.1056/NEJMc2211539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontbonne A. Infertility in Queens: clinical approach, experiences and challenges. J Feline Med Surg. 2022;24:825–836. doi: 10.1177/1098612X221118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, Cao Y, Chen X, Zhu Y, Xu S, Chen ZJ, Mol BW, Qiao J. Epidemiology of infertility in China: a population-based study. BJOG. 2018;125:432–441. doi: 10.1111/1471-0528.14966. [DOI] [PubMed] [Google Scholar]
- 4.Koatz JG, Souza MDCB. Obese women and in vitro fertilization: results. JBRA Assist Reprod. 2022;17:353–356. [PubMed] [Google Scholar]
- 5.Haas J, Bassil R, Samara N, Zilberberg E, Mehta C, Orvieto R, Casper RF. GnRH agonist and hCG (dual trigger) versus hCG trigger for final follicular maturation: a double-blinded, randomized controlled study. Hum Reprod. 2020;35:1648–1654. doi: 10.1093/humrep/deaa107. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Li L, Lin N, Ma R, Li Y, Wu Z. Low endogenous LH on the COS initiation day of a GnRH-agonist regimen increases the risk of early pregnancy loss and adverse ART outcomes. Front Endocrinol (Lausanne) 2022;13:830567. doi: 10.3389/fendo.2022.830567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE, Fauser BC, Macklon NS. Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online. 2006;13:476–480. doi: 10.1016/s1472-6483(10)60633-5. [DOI] [PubMed] [Google Scholar]
- 8.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. 2011;26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 9.Tshzmachyan R, Hambartsoumian E. The role of Letrozole (LE) in controlled ovarian stimulation (COS) in patients at high risk to develop ovarian hyper stimulation syndrome (OHSS). A prospective randomized controlled pilot study. J Gynecol Obstet Hum Reprod. 2020;49:101643. doi: 10.1016/j.jogoh.2019.101643. [DOI] [PubMed] [Google Scholar]
- 10.Yılmaz N, Ceran MU, Ugurlu EN, Gülerman HC, Engin Ustun Y. GnRH agonist versus HCG triggering in different IVF/ICSI cycles of same patients: a retrospective study. J Obstet Gynaecol. 2020;40:837–842. doi: 10.1080/01443615.2019.1674262. [DOI] [PubMed] [Google Scholar]
- 11.Kadoura S, Alhalabi M, Nattouf AH. Conventional GnRH antagonist protocols versus long GnRH agonist protocol in IVF/ICSI cycles of polycystic ovary syndrome women: a systematic review and meta-analysis. Sci Rep. 2022;12:4456. doi: 10.1038/s41598-022-08400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuan KKW, Omoseni S, Tello JA. Comparing ART outcomes in women with endometriosis after GnRH agonist versus GnRH antagonist ovarian stimulation: a systematic review. Ther Adv Endocrinol Metab. 2023;14:20420188231173325. doi: 10.1177/20420188231173325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo JC, Haahr T, Martínez-Moya M, Humaidan P. Gonadotropin-releasing hormone agonist ovulation trigger-beyond OHSS prevention. Ups J Med Sci. 2020;125:138–143. doi: 10.1080/03009734.2020.1737599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryniec J, Esfandiari N. Early serum hCG in IVF: are we trending in the right direction? Reprod Sci. 2021;28:1827–1838. doi: 10.1007/s43032-020-00347-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Yang X, Wang Y, Xi J, Pan H, Wang M, Zhou Y, Xiao Y. Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles. Hum Reprod. 2022;37:1795–1805. doi: 10.1093/humrep/deac114. [DOI] [PubMed] [Google Scholar]
- 16.Hsia LH, Lee TH, Lin YH, Huang YY, Chang HJ, Liu YL. Dual trigger improves the pregnancy rate in fresh in vitro fertilization (IVF) cycles compared with the human chorionic gonadotropin (hCG) trigger: a systematic review and meta-analysis of randomized trials. J Assist Reprod Genet. 2023;40:2063–2077. doi: 10.1007/s10815-023-02888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Eshre Guideline Group On Ovarian Stimulation. Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, Kolibianakis E, Kunicki M, La Marca A, Lainas G, Le Clef N, Massin N, Mastenbroek S, Polyzos N, Sunkara SK, Timeva T, Töyli M, Urbancsek J, Vermeulen N, Broekmans F. ESHRE guideline: ovarian stimulation for IVF/ICSI(†) Hum Reprod Open. 2020;2020:hoaa009. doi: 10.1093/hropen/hoaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng D, Zeng L, Yang R, Lian Y, Zhu YM, Liang X, Tang L, Wang H, Cao Y, Hao G, Liu J, Zhao J, Wang R, Mol BW, Li R, Huang HF, Qiao J. Intracytoplasmic sperm injection (ICSI) versus conventional in vitro fertilisation (IVF) in couples with non-severe male infertility (NSMI-ICSI): protocol for a multicentre randomised controlled trial. BMJ Open. 2019;9:e030366. doi: 10.1136/bmjopen-2019-030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asplund K. Use of in vitro fertilization-ethical issues. Ups J Med Sci. 2020;125:192–199. doi: 10.1080/03009734.2019.1684405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams A, Adams C. Ovarian hyperstimulation syndrome: a case report. J Emerg Nurs. 2023;49:8–11. doi: 10.1016/j.jen.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Timmons D, Montrief T, Koyfman A, Long B. Ovarian hyperstimulation syndrome: a review for emergency clinicians. Am J Emerg Med. 2019;37:1577–1584. doi: 10.1016/j.ajem.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Buca D, D’Antonio F, Liberati M, Tinari S, Pagani G, Greco P, Nappi L. Ovarian hyperstimulation syndrome and adverse pregnancy outcome. Minerva Obstet Gynecol. 2022;74:178–185. doi: 10.23736/S2724-606X.21.04806-5. [DOI] [PubMed] [Google Scholar]
- 23.Sun B, Ma Y, Li L, Hu L, Wang F, Zhang Y, Dai S, Sun Y. Factors associated with ovarian hyperstimulation syndrome (OHSS) severity in women with polycystic ovary syndrome undergoing IVF/ICSI. Front Endocrinol (Lausanne) 2021;11:615957. doi: 10.3389/fendo.2020.615957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pang LL, Mei J, Fan LX, Zhao TT, Li RN, Wen Y. Efficacy of high-intensity focused ultrasound combined with GnRH-a for adenomyosis: a systematic review and meta-analysis. Front Public Health. 2021;9:688264. doi: 10.3389/fpubh.2021.688264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian L, Xia L, Wu Q. Retrospective analysis of GnRH-a prolonged protocol for in vitro fertilization in 18,272 cycles in China. J Ovarian Res. 2022;15:110. doi: 10.1186/s13048-022-01044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan Y, Sun YF, Xu YM, Cao ZY, Luo ZY, Sun Y, Zhao ZM, Hao GM, Gao BL. Factors affecting clinical pregnancy in controlled ovarian hyperstimulation with GnRH-a long protocol: a retrospective cross-sectional study. J Obstet Gynaecol. 2022;42:2486–2491. doi: 10.1080/01443615.2022.2081490. [DOI] [PubMed] [Google Scholar]
- 27.Iorio GG, Carbone L, Conforti A, Rovetto MY, Picarelli S, Cariati F, Strina I, Papanikolaou E, Alviggi C. Ovarian hyperstimulation syndrome after GnRH agonist triggering and Freeze-all protocol? Never not, hardly ever: a systematic review of case reports. Gynecol Obstet Invest. 2022;87:259–265. doi: 10.1159/000524904. [DOI] [PubMed] [Google Scholar]
- 28.Yan MH, Cao JX, Hou JW, Jiang WJ, Wang DD, Sun ZG, Song JY. GnRH agonist and hCG (dual trigger) versus hCG trigger for final oocyte maturation in expected normal Responders with a high immature oocyte rate: study protocol for a randomized, superiority, parallel group, controlled trial. Front Endocrinol (Lausanne) 2022;13:831859. doi: 10.3389/fendo.2022.831859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu K, Wang J, Yang S, Wang Z, Hou N, Sun M. Comparison of HCG trigger versus dual trigger in improving pregnancy outcomes in patients with different ovarian responses: a retrospective study. Int J Endocrinol. 2024;2024:2507026. doi: 10.1155/2024/2507026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cadenas J, Poulsen LC, Nikiforov D, Grøndahl ML, Kumar A, Bahnu K, Englund ALM, Malm J, Marko-Varga G, Pla I, Sanchez A, Pors SE, Andersen CY. Regulation of human oocyte maturation in vivo during the final maturation of follicles. Hum Reprod. 2023;38:686–700. doi: 10.1093/humrep/dead024. [DOI] [PubMed] [Google Scholar]
- 31.Shen X, Yang Q, Li L, Lu W. Clinical pregnancy and incidence of ovarian hyperstimulation syndrome in high ovarian responders receiving different doses of hCG supplementation in a GnRH-agonist trigger protocol. Evid Based Complement Alternat Med. 2021;2021:2180933. doi: 10.1155/2021/2180933. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Wang Y, Hu WH, Wan Q, Li T, Qian Y, Chen MX, Tang XJ, Feng Q, Meng XQ, Adu-Gyamfi EA, Ding YB, Geng LH, Lv XY, Zhong ZH. Effect of artificial cycle with or without GnRH-a pretreatment on pregnancy and neonatal outcomes in women with PCOS after frozen embryo transfer: a propensity score matching study. Reprod Biol Endocrinol. 2022;20:56. doi: 10.1186/s12958-022-00929-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu S, Wang X, Zhang Y, Han Y, Zhang C. Comparison the effects of progestin-primed ovarian stimulation (PPOS) protocol and GnRH-a long protocol in patients with normal ovarian reserve function. Gynecol Endocrinol. 2023;39:2217263. doi: 10.1080/09513590.2023.2217263. [DOI] [PubMed] [Google Scholar]
- 34.Filindris T, Papakonstantinou E, Keramida M, Panteris E, Kalogeropoulos S, Georgopoulos N, Taniguchi F, Adonakis G, Harada T, Kaponis A. The effect of GnRH-a on the angiogenesis of endometriosis. Hormones (Athens) 2024;23:509–515. doi: 10.1007/s42000-024-00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Xing W, Li T, Lin H, Ou J. GnRH antagonist protocol versus GnRH agonist long protocol: a retrospective cohort study on clinical outcomes and maternal-neonatal safety. Front Endocrinol (Lausanne) 2022;13:875779. doi: 10.3389/fendo.2022.875779. [DOI] [PMC free article] [PubMed] [Google Scholar]