Abstract
Objective: To evaluate the efficacy and safety of combining GLP-1 receptor agonists (GLP-1RA) with SGLT-2 inhibitors (SGLT-2i) in elderly patients with type 2 diabetes mellitus (T2DM). Methods: A comprehensive search of Web of Science, Cochrane Library, Embase, and PubMed was conducted from inception to May 2024. Eligible randomized controlled trials (RCTs) assessing the efficacy of GLP-1RA combined with SGLT-2i for managing T2DM in elderly patients were included. Data extraction and quality evaluation were performed on eligible studies, and meta-analysis was conducted using RevMan 5.3 software. Results: This meta-analysis included 15 studies with a total of 11,679 elderly T2DM patients. The combined GLP-1RA and SGLT-2i therapy significantly improved several clinical outcomes. Hemoglobin A1c (HbA1c) levels were significantly reduced (mean difference [MD] -0.26; 95% confidence interval [CI] -0.32, -0.20; P < 0.0001), indicating effective glycemic control. Renal function, as measured by estimated glomerular filtration rate (eGFR), improved significantly (MD 2.18; 95% CI 1.56, 2.80; P < 0.0001), suggesting renoprotective effects. Lipid profiles also showed improvement, with reductions in LDL cholesterol (MD -0.56; 95% CI -0.64, -0.48; P < 0.0001) and increases in HDL cholesterol (MD 0.18; 95% CI 0.14, 0.22; P < 0.0001). Additionally, systolic blood pressure decreased significantly (MD -3.15 mmHg; 95% CI -4.72, -1.58; P < 0.0001). The combination therapy did not increase the risk of hypoglycemia or other adverse events compared to monotherapy, demonstrating a favorable safety profile. These results support the potential of combined SGLT-2i and GLP-1RA therapy as a comprehensive treatment approach for improving metabolic and cardiovascular health outcomes in elderly T2DM patients. Conclusion: Combining GLP-1RA with SGLT-2i provides superior benefits over monotherapy in elderly T2DM patients, particularly for glycemic control, weight management, and cardiovascular protection. This combination also demonstrates a positive safety profile, making it a viable option for clinical use. Ongoing research into the long-term effects and mechanisms of this therapy is necessary to optimize and personalize treatment for elderly patients with type 2 diabetes.
Keywords: GLP-1 receptor agonist, SGLT-2 inhibitor, geriatric type 2 diabetes mellitus, meta-analysis
Introduction
Type 2 diabetes mellitus (T2DM) presents a major global health challenge, significantly raising the risk of cardiovascular and renal complications, thus imposing a considerable disease burden [1-3]. Older adults are particularly vulnerable to T2DM and frequently present with multiple components of metabolic syndrome, including dyslipidemia, hypertension, and obesity. Effective management of T2DM in the elderly is further complicated by age-related metabolic and physiological changes, necessitating a comprehensive and tailored treatment strategy. Recent clinical studies have highlighted the potential of GLP-1 receptor agonists (GLP-1RA) and SGLT-2 inhibitors (SGLT-2i) to enhance metabolic control, assist in weight management, and address cardiovascular risk factors [4-6]. Acting through distinct pathways, these medications may work synergistically to reduce diabetes-associated cardiovascular complications [7]. GLP-1RA enhances insulin secretion, inhibits glucagon release, slows gastric emptying, and reduces appetite, thus aiding glycemic control and supporting weight management [8,9]. Conversely, SGLT-2i promotes urinary glucose excretion, effectively lowering blood glucose, blood pressure, and body weight [10]. Because these two classes of drugs have been used in the clinic for a relatively short time, and older people with type 2 diabetes may differ from other age groups in terms of drug reactions and adverse events, this study aims to systematically evaluate the efficacy and safety of combining GLP-1RA and SGLT-2i therapy through meta-analysis. The objective is to provide comprehensive evidence and clinical recommendations for optimizing T2DM management in older adults.
Materials and methods
Literature search
This study was registered with PROSPERO (CRD42024585939). To rigorously assess the therapeutic effectiveness of combined GLP-1RA and SGLT-2i therapy in elderly T2DM patients, a comprehensive literature search was conducted using strict criteria. Databases searched included Web of Science, Cochrane Library, PubMed, and Embase, encompassing studies published up to May 2024. Keywords were carefully selected, including terms like “Sodium-Glucose Co-transporter 2 Inhibitors”, “Glucagon-Like Peptide 1 Receptor Agonists”, and “Type 2 Diabetes Mellitus”, to capture all relevant randomized controlled trials (RCTs) while minimizing redundancy.
Inclusion criteria
Strict inclusion and exclusion criteria were applied during literature selection. Eligible studies included published RCTs examining the combined use of SGLT-2i and GLP-1RA in elderly patients with T2DM. Both blinded and unblinded studies, with or without follow-up, were accepted. Participants had to be 60 years or older and diagnosed with T2DM following the diagnostic standards of the American Diabetes Association or the World Health Organization. The control group received monotherapy with either an SGLT-2i (e.g., dapagliflozin, empagliflozin, canagliflozin, ertugliflozin) or a GLP-1RA (e.g., liraglutide, lixisenatide, dulaglutide, semaglutide, albiglutide, exenatide, oral semaglutide), while the experimental group was treated with the combination of GLP-1RA and SGLT-2i. Both groups were required to have an intervention period of at least six months.
Exclusion criteria
Exclusion criteria included duplicate publications, studies involving non-T2DM patients (e.g., type 1 diabetes), study protocols, conference abstracts, reviews, animal studies, non-English literature, and publications with design flaws, such as mismatched interventions or incomplete data.
Quality assessment of included literature
A double-blind approach was used for literature screening, where two researchers independently assessed the studies based on the predefined inclusion and exclusion criteria, ensuring objectivity and accuracy. For each eligible study, comprehensive data extraction was performed, capturing details like study design, sample size, primary author, publication year, baseline characteristics, interventions, patient demographics, outcome measures, and other relevant data. A standardized data extraction form was used to ensure consistency and completeness. In cases of disagreement, a third researcher was consulted for resolution. Quality assessment followed internationally accepted criteria, examining factors such as randomization, blinding, attrition rates, sample size adequacy, baseline comparability, and outcome measure precision. Scores were assigned based on these criteria, providing an overall quality rating for each study to ensure the reliability of the included literature.
Statistical analysis
The extracted data were analyzed through meta-analysis using STATA 12.0 and RevMan 5.3 software. For categorical variables, relative risk (RR) and its 95% confidence interval (CI) were used as effect measures to assess the association between the combination therapy and disease incidence in T2DM. For continuous variables, standardized mean difference (SMD) with a 95% CI was applied to account for variations in measurement timing and follow-up across studies, quantifying mean differences and evaluating reliability through confidence intervals, with significance set at P < 0.05. Heterogeneity among studies, indicating result inconsistencies, was assessed using the χ2 test (α = 0.1) and the I2 statistic to measure heterogeneity magnitude. Model selection between random and fixed effects was guided by the I2 statistic and P value: if I2 > 50% and P < 0.1, significant heterogeneity was indicated, and a random-effects model was chosen, accommodating inter-study variability for a conservative treatment effect estimate. Conversely, if I2 < 50% and P > 0.1, suggesting low heterogeneity, a fixed-effects model was applied, assuming consistent treatment effects across studies for more precise estimation. Thus, a random-effects model was applied if P < 0.1 and I2 > 50%; otherwise, a fixed-effects model was used.
Results
Literature screening outcomes
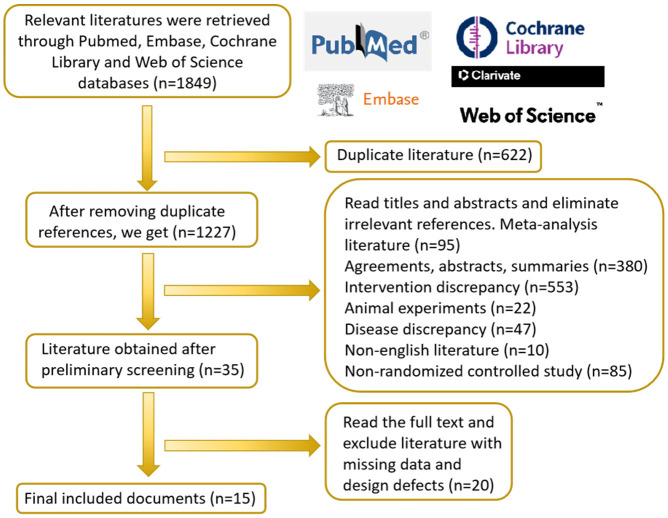
An extensive literature search following the established strategy initially identified 1,845 articles for preliminary screening. To ensure the study’s precision and reliability, duplicates, protocols, conference abstracts, reviews, and animal studies were excluded according to predefined inclusion and exclusion criteria. Additionally, articles with incomplete data or significant design flaws were omitted to maintain the credibility and representativeness of included studies. This rigorous review process ultimately yielded 15 articles, with the literature screening process illustrated in Figure 1.
Figure 1.
Flow chart of literature screening.
Literature quality evaluation
The 15 selected studies [11-25] included a total of 11,679 participants, with 10,199 in the experimental group and 1,480 in the control group. Study characteristics are detailed in Tables 1A and 1B. The quality of each study was evaluated using Cochrane Collaboration’s risk of bias tool. Three studies [12,15,18] employed double-blind randomized controlled designs, though none specified the methods of randomization. Additionally, four studies [13-16] reported patient attrition, introducing potential bias. Through a comprehensive quality evaluation using the Cochrane risk of bias tool, the safety and efficacy of combined SGLT-2i and GLP-1RA therapy in elderly T2DM patients were assessed with greater accuracy (Figure 2).
Table 1A.
Basic information of each document
| Number | Author | years | Country of publication | Sample size | Gender (Male/female) | Age (years) | Intervention program | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||
| A | B | A | B | A | B | A | B | ||||
| 1 | J.J. Gorgojo-Martínez | 2016 | Spain | 104 | 109 | 65/59 | 49/55 | 59.1±10.7 | 59.7±10.8 | SGLT-2 | SGLT-2+GLP-1 |
| 2 | João Sérgio Neves | 2023 | Portugal | 8887 | 575 | 6133/2754 | 435/140 | 64.2±8.7 | 62.9±8.2 | GLP-1 | SGLT-2+GLP-1 |
| 3 | Rafaele Marfella | 2024 | Italy | 99 | 214 | 64/35 | 131/83 | 68 (63-73) | 70 (64-70) | SGLT-2 | SGLT-2+GLP-1 |
| 4 | Jingpu Wang | 2023 | China | 63 | 62 | 40/23 | 30/32 | 57.5±9.9 | 56.2±10.2 | DAPA 10 mg/day + placebo | SGLT-2 Dagliprazin (DAPA) 10 mg/day +GLP-1 Exenatide (ExQW) 2 mg/week |
| 5 | Yan Pi | 2022 | China | 40 | 40 | 21/19 | 23/17 | 52.12±10.2 | 51.23±10.4 | Liraglutide (GLP-1 receptor agonist, initial dose 0.6 mg/day, increased to 1.8 mg/day after one week of treatment) | Liraglutide (GLP-1 receptor agonist, initial dose 0.6 mg/day, increased to 1.8 mg/day after one week of treatment) + Dagliprazin (SGLT-2 inhibitor, 10 mg/day orally) |
| 6 | Lin, Yu-Hao | 2024 | China | 35 | 35 | / | / | / | / | SGLT-2 | SGLT-2+GLP-1 |
| 7 | Toru Ishikawa | 2024 | Japan | 15 | 15 | 5/10 | 5/10 | 67.3 (61.9-74.5) | 67.3 (61.9-74.5) | SGLT-2 | SGLT-2+GLP-1 |
| 8 | Liv Vernstrøm MD | 2024 | Denmark | 30 | 30 | 22/8 | 25/5 | 70±7 | 70±7 | SGLT-2 (Engalizin) | SGLT-2 (Englaglizin) +GLP-1 (Semaglutide) |
| 9 | Konstantinos Katogiannis | 2024 | Greece | 50 | 50 | 37/13 | 38/12 | 58.1±8.8 | 59.3±8.9 | SGLT-2 (Engalizin) | SGLT-2+GLP-1 |
| 10 | Francesco Giorgino | 2024 | Italy | 746 | 219 | 379/267 | 136/83 | 62.0±9.9 | 60.5±10.7 | GLP-1 | SGLT-2+GLP-1 |
| 11 | Suvanjaa Sivalingam | 2024 | Denmark | 30 | 30 | 23/7 | 24/6 | 69.4±9.1 | 70.5±6.8 | placebo | SGLT-2+GLP-1 (Semaglutide) |
| 12 | Annemarie B | 2023 | Netherlands | 20 | 20 | 4/16 | 16/4 | 70.5±5.1 | 70.5±5.1 | SGLT-2 (Dagliazine 10 mg/day) | SGLT-2 (Dagaglizin 10 mg/day) +GLP-1 (exenatide 2 mg/week) |
| 13 | Søren Gullaksen | 2023 | Denmark | 30 | 30 | 22/8 | 25/5 | 70.4±6.6 | 69.5±6.8 | SGLT-2 (Engalizin) | SGLT-2 (Englaglizin) +GLP-1 (Semaglutide) |
| 14 | Søren Gullaksen | 2024 | Denmark | 20 | 20 | 14/6 | 17/3 | 69.6±6.0 | 67.9±6.2 | SGLT-2 (10 mg Empaglizin) | SGLT-2 (10 mg Empaglizin) +GLP-1 (1.0 mg Semaglutide) |
| 15 | Juan P Frias | 2023 | America | 30 | 31 | 23/17 | 18/13 | 57±10 | 62±7 | SGLT-2 (once a week subcutaneous injection of caglutide 2.4 mg) | SGLT-2 (once a week subcutaneous injection of caglutide 2.4 mg) +GLP-1 (Semaglutide 2.4 mg) |
Table 1B.
Basic information of the literature
| Number | Author | Years | Course of treatment | Observation index |
|---|---|---|---|---|
| 1 | J.J. Gorgojo-Martínez | 2016 | 12 months | Glycosylated hemoglobin (HbA1c), systolic blood pressure (SBP), fasting blood glucose (FBG), diastolic blood pressure (DBP), triglycerides (TC), estimated glomerular filtration rate (eGFR), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) |
| 2 | João Sérgio Neves | 2023 | 19 months | Glycosylated hemoglobin, estimated glomerular filter rate angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, mineral corticosteroid receptor antagonists, HBA1C, SBP, DBP |
| 3 | Rafaele Marfella | 2024 | 24 months | Myocardial salvage index (MSI), blood glucose control, body weight, LDL-C, BG, HbA1c, total cholesterol, HDL-C, TC, creatinine, troponin, lesion length, minimum lumen diameter (MLD), reference diameter, minimum lumen diameter after stent, STEMI incidence, hypertension, dyslipidemia, smoking status, drug use (including metformin, DPP-IV inhibitors, thiazolidinedione, beta blockers, ARBs ACE inhibitors, calcium channel blockers, statins, diuretics, insulin, antiplatelet drugs, anticoagulants), single vessel disease, double vessel disease, three vessel disease |
| 4 | Jingpu Wang | 2023 | 52 weeks | HbA1c, fasting plasma glucose (FPG), SBP |
| 5 | Yan Pi | 2022 | 12 months | TG, TC, HDL-C and LDL-C levels, FBG, DBP and SBP, adverse reactions, changes in body weight, combined BMI and HBA1c, insulin dose, kidney function, and blood pressure change |
| 6 | Lin, Yu-Hao | 2024 | 13 months | LSM, HbA1c, 25[OH]D3, PTH, cytokine, hsCRP, BMI, NFS, HOMA-IR, LSM |
| 7 | Toru Ishikawa | 2024 | 12 months | ALT, ALP, GGT; BMI, visceral fat index (VATI) (based on CT scan), subcutaneous fat index (SATI) (based on CT scan), skeletal muscle index (SMI), waist circumference and other body weight and body fat distribution; Hemoglobin A1c (HbA1c) and other diabetes control indicators; TG, total cholesterol, LDL-C, HDL-C, eGFR, Alb, UA |
| 8 | Liv Vernstrøm MD | 2024 | 8 months | Arterial stiffness, cervical and femoral pulse velocity (cf-PWV), kidney oxygenation, 24-hour blood pressure, office-measured brachial artery blood pressure, central blood pressure, urinary albumin-to-creatinine ratio (UACR), blood glucose control, continuous glucose monitoring (CGM), glycated hemoglobin (HbA1c), weight change, biochemical markers, low-density lipoprotein cholesterol, glucose Oil triester, drug use |
| 9 | Konstantinos Katogiannis | 2024 | 6 months | Left Atrial Strain (Left Atrial Strain), PWV, Central Systolic Blood Pressure, GLS, HbA1c |
| 10 | Francesco Giorgino | 2024 | 26 weeks | HbA1c, FPG, AEs, incidence and rate of hypoglycemic events |
| 11 | Suvanjaa Sivalingam | 2024 | 26 weeks | Changes in UACR, GFR, 24-hour systolic blood pressure, HbA1c levels, body weight, and vasoactive hormone (plasma renin and aldosterone) levels |
| 12 | Annemarie B | 2023 | 26 weeks | UACR, blood pressure, HbA1c level, body weight, extracellular volume, lithium ion fraction excretion, and renal hemodynamic variables determined by magnetic resonance imaging, including RBF, ERPF and FF, eGFR |
| 13 | Søren Gullaksen | 2023 | 32 weeks | Central retinal thickness, central retinal thickness |
| 14 | Søren Gullaksen | 2024 | 32 weeks | Renal oxygenation (R2 value), renal perfusion, erythropoietin and hematocrit, GFR, UACR, body weight, BP |
| 15 | Juan P Frias | 2023 | 32 weeks | HbA1c, body weight, FBG, fasting serum insulin, fasting glucagon,hsCRP, leptin, soluble leptin receptor and lipid profile |
Note: A: Control group, B: Experimental group.
Figure 2.
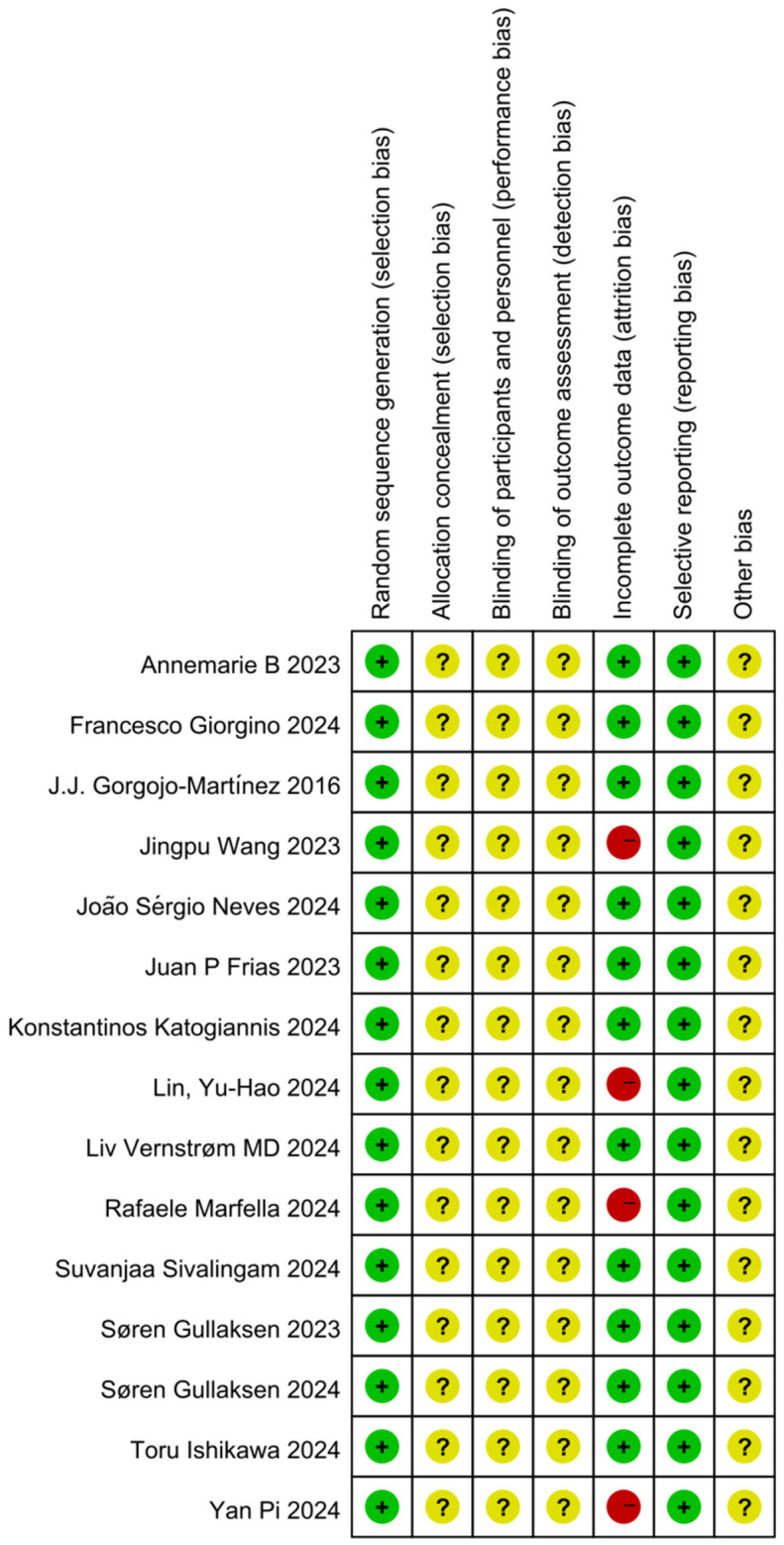
Literature risk of bias summary chart.
Analysis of outcome indicators
HbA1c analysis
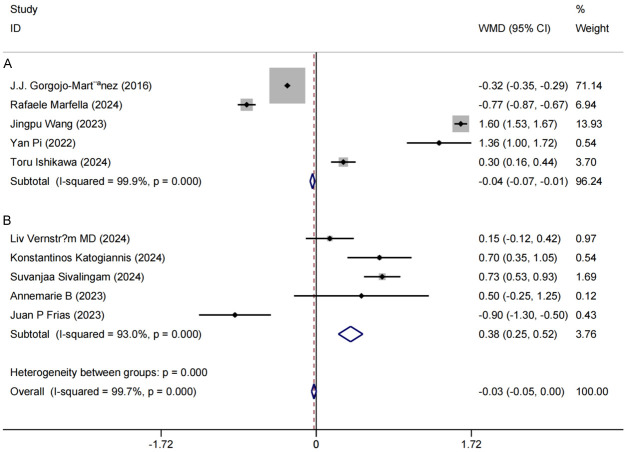
Among the 10 included studies [11,13-15,17-19,21,22,25], HbA1c was used as the primary endpoint to assess changes in HbA1c levels between the control and experimental groups before and after intervention. Post-intervention, substantial reductions in HbA1c were observed in both groups compared to baseline (P < 0.05). High heterogeneity was detected among studies (P < 0.0001, I2 = 99.7%), likely due to variables such as intervention duration. To explore the source of heterogeneity, a subgroup analysis was conducted based on intervention duration. Patients undergoing interventions shorter than one year were grouped separately. The results indicated that the combined use of GLP-1RA and SGLT-2i significantly lowered HbA1c levels in elderly T2DM patients, regardless of intervention length. Specifically, for interventions under one year, HbA1c showed a mean reduction of 0.04 [MD = -0.04, 95% CI (-0.07, -0.01), P < 0.0001]; for interventions lasting one year or more, the mean reduction was 0.38 [MD = 0.38, 95% CI (0.25, 0.52), P < 0.0001]. These findings suggest that combined GLP-1RA and SGLT-2i therapy produces a robust hypoglycemic effect in elderly T2DM patients, with consistent efficacy across varied intervention durations (Figure 3). This analysis provides evidence-based support for the clinical utility of this concurrent therapy in lowering HbA1c levels in elderly T2DM patients.
Figure 3.
Forest plot of combined hemoglobin A1c levels and meta-analysis of study participants. A: Intervention duration ≥ 1 year group; B: Intervention duration < 1 year group.
Body mass index (BMI) analysis
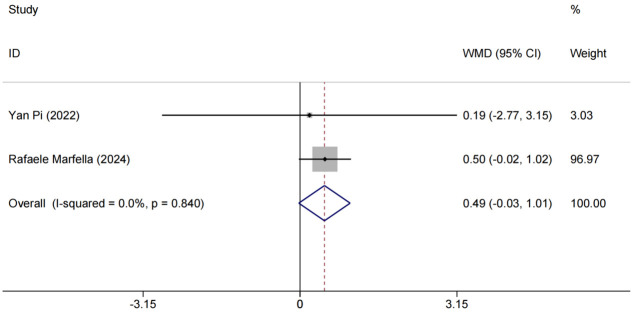
Data on changes in body mass index (BMI) were synthesized and analyzed for two studies [13,15]. The results demonstrated homogeneity (P = 0.840, I2 = 0.0%), supporting the use of a fixed-effects model. No statistically significant change in BMI was observed between the experimental and control groups [RR = 0.49, 95% CI (-0.03, 1.01), P > 0.05]; although differences were observed (Figure 4). Given that BMI alone may not fully capture the therapy’s impact, further research with larger sample sizes and longer follow-up is needed. Additionally, incorporating other metrics, such as body fat distribution and waist-to-hip ratio, would provide a more comprehensive view of the effects of combined SGLT-2i and GLP-1RA therapy on body composition in elderly T2DM patients.
Figure 4.
Forest plot of combined BMI and meta-analysis. BMI: Body Mass Index.
Estimated Glomerular Filtration Rate (eGFR) analysis
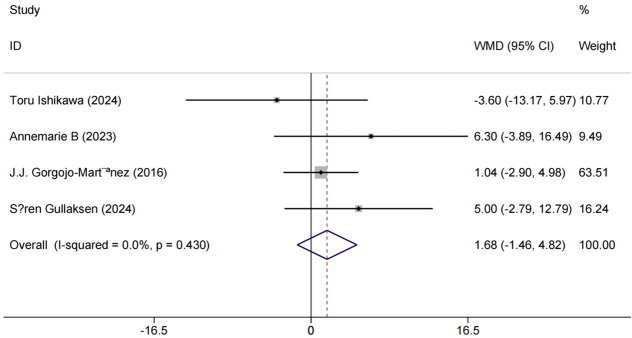
Four studies [11,17,22,24] used eGFR as an outcome measure. No meaningful variation was found among studies (P = 0.430, I2 = 0.0%), indicating collinearity. An RR of 1.68 indicated that patients treated with GLP-1RA and SGLT-2i were more likely to show improved renal function compared to the control group. Although the [95% CI (-1.46, 4.82), P < 0.001] was wide, with a lower bound close to 0 and an upper bound significantly above 1, this interval suggests a positive treatment effect. These results are shown in Figure 5.
Figure 5.
Forest plot of combined eGFR and meta-analysis. eGFR: estimated Glomerular Filtration Rate.
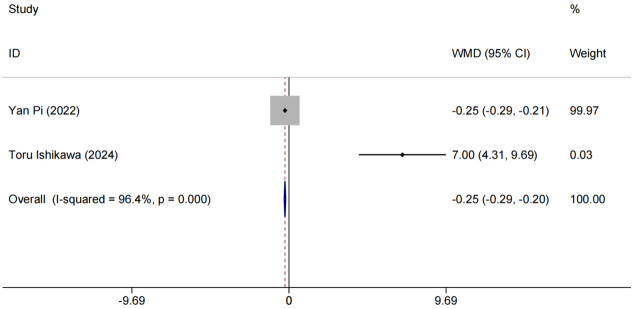
High-Density Lipoprotein Cholesterol (HDL-C) analysis
Two studies [15,17] evaluated HDL-C as an outcome measure. After treatment, HDL-C levels were highly heterogeneous among studies (P < 0.0001, I2 = 96.4%), possibly due to differences in study methods, sample characteristics, or other unknown factors, leading to large differences in study results. A random-effects model was used to account for this variability, providing more accurate and reliable results. Findings indicated that HDL-C levels in the experimental group improved significantly more than in the control group, with a clinically meaningful difference [MD = -0.25, 95% CI (-0.29, -0.20), P < 0.001], as shown in Figure 6.
Figure 6.
Forest plot of combined HDL-C levels and meta-analysis. HDL-C: High-Density Lipoprotein Cholesterol.
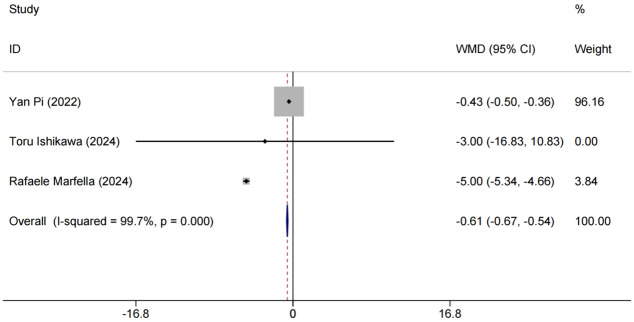
Low-Density Lipoprotein Cholesterol (LDL-C) analysis
Three [13,15,17] papers reported LDL-C as an outcome indicator. A high level of heterogeneity existed among these studies (P < 0.0001, I2 = 99.7%). Random effects model analysis showed that the experimental group experienced better LDL-C reduction [MD = -0.61, 95% CI (-0.64,-0.57), P < 0.001]. Since LDL-C is an important cardiovascular risk factor, this combination regimen may help to decrease cardiovascular complications in elderly T2DM patients, as shown in Figure 7.
Figure 7.
Forest plot of combined LDL-C levels and meta-analysis. LDL-C: Low-Density Lipoprotein Cholesterol.
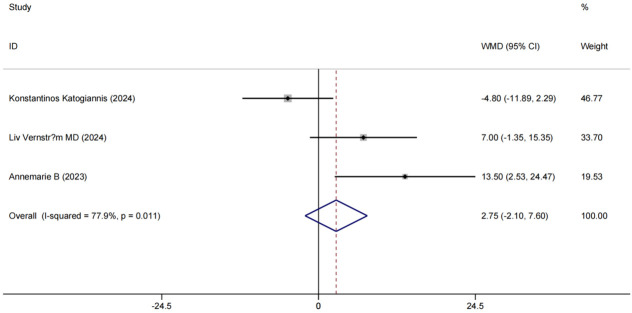
Systolic Blood Pressure (SBP) analysis
Three studies [18,19,22] examined SBP as an outcome measure, showing significant SBP improvements post-treatment. There was substantial heterogeneity among these studies (P < 0.1, I2 = 77.9%). Random-effects modeling showed that patients in the experimental group had more substantial SBP improvement [MD = 2.75, 95% CI (-2.10 7.60), P < 0.001], as shown in Figure 8.
Figure 8.
Forest plot of combined SBP levels and meta-analysis. SBP: Systolic Blood Pressure.
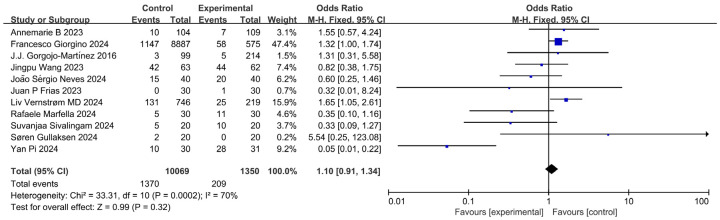
Adverse reaction analysis
Of the 15 included studies, 11 [11-15,18,20-22,24,25] reported on adverse reactions, primarily genital fungal infections, renal function deterioration, hypoglycemia, gastrointestinal effects, nausea, vomiting, and infections. Adverse reactions occurred in 1,370 out of 10,069 cases in the control group and 209 out of 1,350 cases in the experimental group. High heterogeneity was noted between study cohorts (P < 0.1, I2 = 70.0%), and the modeling analysis showed no statistically significant difference in adverse reaction occurrence between the two groups [MD = 1.10, 95% CI (0.91, 1.34), P = 0.32 > 0.05], as shown in Figure 9.
Figure 9.
Forest plot of combined adverse reactions and meta-analysis.
Sensitivity analysis
Sensitivity analysis did not identify any studies with a significant impact on the overall effect size or findings outside the confidence interval, indicating that the results of this study are stable and reliable. See Figures 10 and 11 for detailed sensitivity analysis results.
Figure 10.
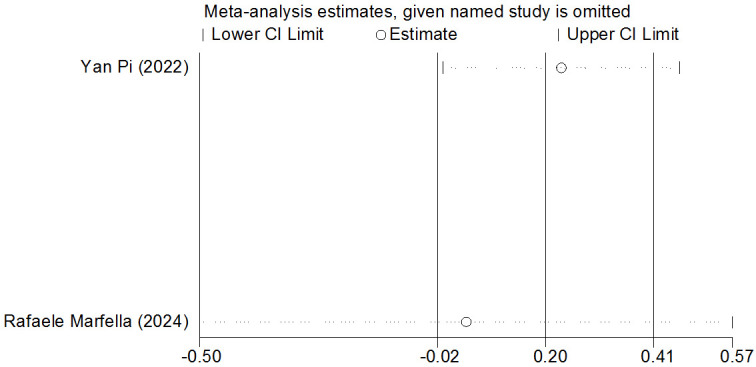
Sensitivity analysis of BMI. BMI: Body Mass Index.
Figure 11.
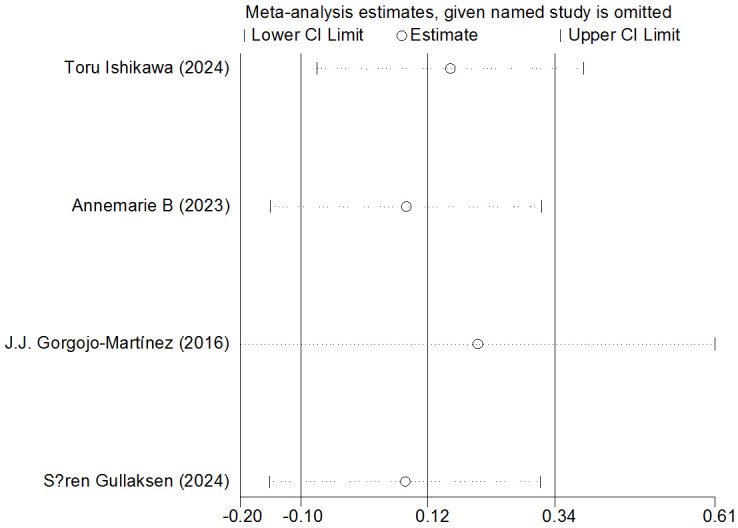
Sensitivity analysis of eGFR. eGFR: estimated Glomerular Filtration Rate.
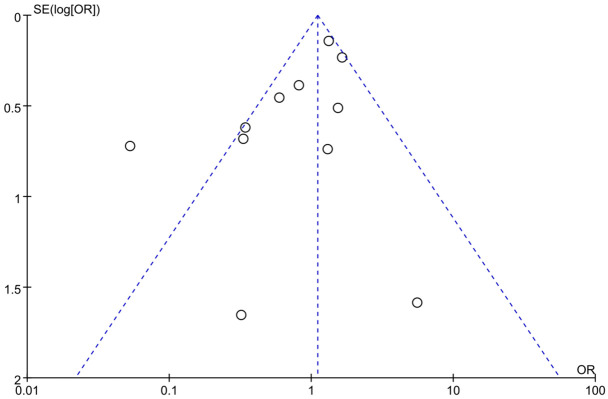
Evaluation of publication bias
A funnel plot and Egger’s test were used to assess publication bias in adverse reaction incidence. All outcome indices exceeded 0.05, indicating a risk of publication bias. However, as shown in Figure 12, the bias risk in the included literature was relatively low, supporting its inclusion in this study.
Figure 12.
Funnel plot of adverse reactions in control and experimental groups.
Discussion
With rapid economic development, increased life expectancy, and an aging population, diabetes prevalence is rising worldwide, posing a significant public health challenge [1]. T2DM is especially prevalent, accounting for approximately 90% to 95% of all diabetes cases [27]. Characterized by insulin resistance and relative insulin deficiency, T2DM significantly affects patient quality of life and increases disability and mortality rates. Thus, finding safe and effective treatments to enhance quality of life and reduce complications is of substantial clinical importance.
SGLT-2i, a newer class of glucose-lowering agents, act independently of insulin by inhibiting glucose reabsorption in renal tubules and promoting urinary glucose excretion. These drugs have garnered attention not only for their unique glucose-lowering mechanisms but also for their ability to reduce blood pressure, support weight loss, and lower uric acid levels. Numerous clinical studies have demonstrated significant benefits of SGLT-2i for heart and kidney health in T2DM patients, particularly those with cardiovascular disease or high cardiovascular risk [28].
GLP-1RA, secreted by intestinal L-cells, act on receptors distributed in the liver, gastrointestinal tract, skeletal muscle, heart, and other tissues. They stimulate insulin secretion by pancreatic beta cells, support beta-cell regeneration, inhibit beta-cell apoptosis, and reduce glucagon secretion, effectively aiding in blood sugar control. GLP-1RA also promotes weight loss by delaying gastric emptying and acting on the hypothalamus to increase satiety [29]. These agents have shown strong hypoglycemic and weight loss effects [13-15].
In a large multinational study involving 1.5 million T2DM patients starting second-line hypoglycemic therapy after metformin monotherapy, the risk of cardiovascular events was found to be 11% and 17% lower with SGLT-2i and GLP-1RA, respectively, compared to DPP-4 inhibitors [30]. However, this study did not evaluate the potential benefits of combining the two therapies. Sims-Williams et al. [31] reported in a meta-analysis that combining SGLT-2i with GLP-1RA reduced the risk of major adverse cardiovascular events by 30% and the risk of serious renal events by 57% compared to GLP-1RA alone, and reduced cardiovascular risk by 29% compared to SGLT-2i alone. Additionally, Guo M et al. [32] confirmed in a meta-analysis that combination therapy was more effective at lowering blood glucose levels than monotherapy, without increasing hypoglycemia risk, though its effectiveness in elderly patients and its protective cardiovascular and renal effects were not fully addressed.
Of note, gastrointestinal discomfort is the most common side effect associated with these drugs, and it remains unclear whether combination therapy raises the risk of adverse reactions in older patients. This study aims to comprehensively evaluate the efficacy of combined SGLT-2i and GLP-1RA therapy in elderly T2DM patients through a meta-analysis of clinical outcomes, including HbA1c levels, renal function, lipid metabolism, and cardiovascular parameters. We also examined potential synergistic effects arising from the distinct mechanisms of action of these agents to inform clinical practice and support the development of more effective and rational treatment strategies for elderly patients.
Patoulias et al. [33] conducted a meta-analysis showing that liraglutide, a GLP-1RA, significantly reduces waist circumference, with a more pronounced effect in patients with larger baseline waist measurements. Additionally, the SGLT2i dapagliflozin has been shown to reduce body adiposity and visceral fat in T2DM patients without affecting muscle mass. Combining GLP-1RA and SGLT2i resulted in weight loss ranging from 2.69 to 4.60 kg [34,35]. In contrast, a meta-analysis by Tuersun et al. [36] found that combination therapy did not significantly reduce fasting plasma glucose (P = 0.35), body weight (P = 1.00), HbA1c (P = 0.22), or systolic blood pressure (P = 0.21), which differs from the findings of the present study. Dai et al. [37] demonstrated that GLP-1RA was more effective than SGLT2i in reducing weight among adolescents with T2DM under 18 years but did not assess effects in older populations.
This study’s meta-analysis confirmed that post-treatment BMI improvements in elderly patients were significantly higher than pre-treatment values. However, the degree of weight loss with combination therapy was less than the sum of the individual effects of the monotherapies, likely due to the differing mechanisms: SGLT2 inhibitors promote weight loss through increased urinary glucose excretion, while GLP-1RA suppress appetite and reduces energy intake. This indicates that combining the two therapies does not produce an additive effect on body mass or lead to rapid weight reduction.
Moreover, the meta-analysis revealed a significant improvement in eGFR with SGLT-2i and GLP-1RA combination therapy, suggesting renal protective benefits. SGLT2, responsible for glucose reabsorption in the kidney, is inhibited by SGLT2i at the proximal renal tubules, leading to increased urinary glucose excretion of approximately 80 g/d [38]. In contrast, GLP-1RA improves urinary protein levels and enhances glomerular filtration rate. It also aids in blood glucose control by stimulating insulin secretion, inhibiting glucagon release, promoting satiety, and delaying gastric emptying. Research indicates [39] that short-acting GLP-1RA intermittently activates GLP-1 receptors, exerting an inhibitory effect on gastric emptying, which primarily affects postprandial blood glucose levels. Long-acting GLP-1RA, however, has a quicker gastric emptying response, showing a lesser effect on postprandial glucose but a more substantial impact on fasting glucose levels and HbA1c reduction.
Neves et al. [12] found that SGLT2i combined with GLP-1RA yielded greater reductions in blood glucose levels, body weight, and cardiovascular risk factors compared to monotherapy, consistent with the findings of this study. The present meta-analysis showed considerable heterogeneity in HbA1c data across studies, reflecting inconsistencies in existing research. Subgroup analyses based on intervention duration also revealed high heterogeneity, suggesting that differences in study design, patient characteristics, and intervention protocols may be influential factors.
Therefore, further high-quality studies are needed to precisely determine the effects of combining GLP-1RA agents with SGLT-2i treatment, to clarify their application scope, reduce heterogeneity sources, and provide more reliable evidence. SGLT2i contributes to lowering blood pressure and improving arterial stiffness, leading to enhanced subendocardial blood flow, particularly beneficial for individuals with heart failure. Key mechanisms involved include natriuresis and osmotic diuresis: natriuresis eliminates excess sodium, while osmotic diuresis increases urine production due to osmotically active substances, collectively reducing plasma volume and cardiac workload across heart regions.
Beyond cardiovascular risk factor reduction, SGLT2i induces mild but sustained hyperketonemia, marked by elevated ketone bodies like β-hydroxybutyrate in the blood. This mild hyperketonemic state may offer cardiovascular benefits, including improved insulin sensitivity and reduced inflammation. Ketones are preferentially absorbed by the heart and oxidized over fatty acids [40], enhancing mitochondrial efficiency in converting oxygen into work. Although subtle, this shift can yield substantial functional improvements, sustaining better cardiac function for months or even years with SGLT2i therapy.
GLP-1RA directly impacts atrial cardiomyocytes, stimulating the release of atrial natriuretic peptide, which counteracts angiotensin II effects. In this study, combination therapy significantly improved blood pressure control in T2DM patients, reducing cardiovascular event risk and enhancing metabolic profiles overall. The meta-analysis results also indicated that combined therapy did not increase the risk of adverse effects, such as gastrointestinal discomfort or hypoglycemia, in elderly T2DM patients. Prior clinical investigations [41-43] have highlighted the increased hypoglycemia risk in diabetic patients under intensive glycemic control, posing a major barrier to effective glucose regulation, with severe episodes linked to elevated mortality.
This analysis suggests that GLP-1RA and SGLT-2i therapies facilitate insulin secretion via a glucose-dependent mechanism, effectively reducing blood glucose and HbA1c levels with minimal hypoglycemia risk. These findings highlight a safe and effective therapeutic strategy for elderly T2DM patients, enabling tight glycemic control without hypoglycemic events.
This study provides robust, evidence-based support for optimizing treatment strategies for elderly patients with T2DM. Focusing on the unique physiological characteristics and common comorbidities of elderly T2DM patients, we examined treatment options tailored to this group. Our innovative evaluation of GLP-1RA combined with SGLT-2i opens new possibilities and provides a theoretical foundation for treatment strategies in elderly patients. Key therapeutic effects of the combination therapy were systematically assessed across multiple indicators, including HbA1c, blood pressure, blood lipids, glomerular filtration rate, and BMI. The focus on adverse effects further strengthens the evidence supporting the safety of this therapy in elderly patients.
Despite the value of this study, several limitations should be noted. Firstly, the inclusion of studies with smaller sample sizes may introduce heterogeneity, potentially affecting the consistency and robustness of findings. Secondly, methodological differences across studies-such as variations in dosages, administration methods, treatment durations, and outcome measures-may introduce bias. Additionally, unaddressed factors like disease duration, severity, and comorbidities could confound data interpretation. Lastly, the reviewed literature may lack comprehensive evaluations of treatment effects, specific adverse events, and safety concerns, which could limit the overall assessment of the safety and efficacy of combining SGLT-2i with GLP-1RA in elderly T2DM patients. However, sensitivity analysis indicates a stable overall effect and a low risk of publication bias. Despite limitations related to literature quality, consistency of outcome metrics, and adverse effect reporting, this study provides strong evidence supporting the safety and efficacy of combining SGLT-2i with GLP-1RA for T2DM treatment in older adults.
In conclusion, this comprehensive meta-analysis assessed the safety and efficacy of combining SGLT-2i with GLP-11RA in elderly T2DM patients. The findings indicate significant improvements in HbA1c levels, renal function, and lipid metabolism, as well as a reduced risk of cardiovascular events, without increasing the risk of hypoglycemia. These results provide critical evidence-based insights for managing T2DM in elderly patients. Further research is needed to explore the broader implications and safety profile of this combined therapy to validate its clinical applicability across diverse patient populations.
Disclosure of conflict of interest
None.
References
- 1.Sanz-Cánovas J, López-Sampalo A, Cobos-Palacios L, Ricci M, Hernández-Negrín H, Mancebo-Sevilla JJ, Álvarez-Recio E, López-Carmona MD, Pérez-Belmonte LM, Gómez-Huelgas R, Bernal-López MR. Management of type 2 diabetes mellitus in elderly patients with frailty and/or sarcopenia. Int J Environ Res Public Health. 2022;19:8677. doi: 10.3390/ijerph19148677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izzo A, Massimino E, Riccardi G, Della Pepa G. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients. 2021;13:183. doi: 10.3390/nu13010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan Y, Wu T, Zhang M, Li C, Liu Q, Li F. Prevalence, awareness and control of type 2 diabetes mellitus and risk factors in Chinese elderly population. BMC Public Health. 2022;22:1382. doi: 10.1186/s12889-022-13759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin H, Schneeweiss S, Glynn RJ, Patorno E. Cardiovascular outcomes in patients initiating first-line treatment of type 2 diabetes with sodium-glucose cotransporter-2 inhibitors versus metformin: a cohort study. Ann Intern Med. 2022;175:927–937. doi: 10.7326/M21-4012. [DOI] [PubMed] [Google Scholar]
- 5.Kutz A, Kim DH, Wexler DJ, Liu J, Schneeweiss S, Glynn RJ, Patorno E. Comparative cardiovascular effectiveness and safety of sglt-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors according to frailty in type 2 diabetes. Diabetes Care. 2023;46:2004–2014. doi: 10.2337/dc23-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuo M, D’Andrea E, Paik JM, Wexler DJ, Everett BM, Glynn RJ, Kim SC, Patorno E. Association of sodium-glucose cotransporter-2 inhibitors with incident atrial fibrillation in older adults with type 2 diabetes. JAMA Netw Open. 2022;5:e2235995. doi: 10.1001/jamanetworkopen.2022.35995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin H, Schneeweiss S, Glynn RJ, Patorno E. Trends in first-line glucose-lowering drug use in adults with type 2 diabetes in light of emerging evidence for SGLT-2i and GLP-1RA. Diabetes Care. 2021;44:1774–1782. doi: 10.2337/dc20-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2021;106:388–396. doi: 10.1210/clinem/dgaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuo M, Paik JM, Wexler DJ, Bonventre JV, Kim SC, Patorno E. SGLT2 inhibitors and the risk of acute kidney injury in older adults with type 2 diabetes. Am J Kidney Dis. 2022;79:858–867. e1. doi: 10.1053/j.ajkd.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin H, Schneeweiss S, Glynn RJ, Patorno E. Evolving channeling in prescribing SGLT-2 inhibitors as first-line treatment for type 2 diabetes. Pharmacoepidemiol Drug Saf. 2022;31:566–576. doi: 10.1002/pds.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorgojo-Martínez JJ, Serrano-Moreno C, Sanz-Velasco A, Feo-Ortega G, Almodóvar-Ruiz F. Real-world effectiveness and safety of dapagliflozin therapy added to a GLP1 receptor agonist in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:129–137. doi: 10.1016/j.numecd.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Neves JS, Borges-Canha M, Vasques-Nóvoa F, Green JB, Leiter LA, Granger CB, Carvalho D, Leite-Moreira A, Hernandez AF, Del Prato S, McMurray JJV, Ferreira JP. GLP-1 receptor agonist therapy with and without SGLT2 inhibitors in patients with type 2 diabetes. J Am Coll Cardiol. 2023;82:517–525. doi: 10.1016/j.jacc.2023.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Marfella R, Prattichizzo F, Sardu C, Rambaldi PF, Fumagalli C, Marfella LV, La Grotta R, Frigé C, Pellegrini V, D’Andrea D, Cesaro A, Calabrò P, Pizzi C, Antonicelli R, Ceriello A, Mauro C, Paolisso G. GLP-1 receptor agonists-SGLT-2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: an observational study in patients with type 2 diabetes. Cardiovasc Diabetol. 2024;23:10. doi: 10.1186/s12933-023-02118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Chen Y, Lu Y, Wang H, Zhao Y. Efficacy and safety of glucagon-like peptide-1 receptor agonist combined with sodium-glucose co-transporter-2 inhibitor in the treatment of type 2 diabetes mellitus patients with obesity: a retrospective analysis study. Am J Transl Res. 2023;15:2949–2956. [PMC free article] [PubMed] [Google Scholar]
- 15.Pi Y, Du TT, Zhao P. Clinical efficacy evaluation and long-term prognosis of glucagon-like peptide-1 combined with sodium glucose cotransporter-2 inhibitor in diabetes. Indian J Pharm Sci. 2022 n. pag. [Google Scholar]
- 16.Lin YH, Zhang ZJ, Zhong JQ, Wang ZY, Peng YT, Lin YM, Zhang HP, Tian JQ. Semaglutide combined with empagliflozin vs. monotherapy for non-alcoholic fatty liver disease in type 2 diabetes: study protocol for a randomized clinical trial. PLoS One. 2024;19:e0302155. doi: 10.1371/journal.pone.0302155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa T, Terai N, Sato R, Jimbo R, Kobayashi Y, Sato T, Iwanaga A, Sano T, Yokoyama J, Honma T. Clinical efficacy and body composition changes with sodium glucose cotransporter 2 inhibitor/glucagon-like peptide-1 antagonist combination therapy in patients with type 2 diabetes mellitus-associated nonalcoholic fatty liver disease. Intern Med. 2024;63:2491–2497. doi: 10.2169/internalmedicine.3259-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vernstrøm L, Gullaksen S, Sørensen SS, Funck KL, Laugesen E, Poulsen PL. Separate and combined effects of empagliflozin and semaglutide on vascular function: a 32-week randomized trial. Diabetes Obes Metab. 2024;26:1624–1635. doi: 10.1111/dom.15464. [DOI] [PubMed] [Google Scholar]
- 19.Katogiannis K, Thymis J, Kousathana F, Pavlidis G, Korakas E, Kountouri A, Balampanis K, Prentza V, Kostelli G, Michalopoulou H, Tsilivarakis D, Lambadiari V, Ikonomidis I. Effects of liraglutide, empagliflozin and their combination on left atrial strain and arterial function. Medicina (Kaunas) 2024;60:395. doi: 10.3390/medicina60030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgino F, Guja C, Aydın H, Lauand F, Melas-Melt L, Rosenstock J. Consistent glycaemic efficacy and safety of concomitant use of iGlarLixi and sodium-glucose co-transporter-2 inhibitor therapy for type 2 diabetes: a patient-level pooled analysis of three randomised clinical trials. Diabetes Res Clin Pract. 2024;209:111604. doi: 10.1016/j.diabres.2024.111604. [DOI] [PubMed] [Google Scholar]
- 21.Sivalingam S, Wasehuus VS, Rotbain Curovic V, Blond MB, Hansen TW, Persson F, Rossing P. Albuminuria-lowering effect of adding semaglutide on top of empagliflozin in individuals with type 2 diabetes: a randomized and placebo-controlled study. Diabetes Obes Metab. 2024;26:54–64. doi: 10.1111/dom.15287. [DOI] [PubMed] [Google Scholar]
- 22.van der Aart-van der Beek AB, Apperloo E, Jongs N, Rouw DB, Sjöström CD, Friedli I, Johansson L, van Raalte DH, Hoogenberg K, Heerspink HJL. Albuminuria-lowering effect of dapagliflozin, exenatide, and their combination in patients with type 2 diabetes: a randomized cross-over clinical study. Diabetes Obes Metab. 2023;25:1758–1768. doi: 10.1111/dom.15033. [DOI] [PubMed] [Google Scholar]
- 23.Gullaksen S, Vernstrøm L, Sørensen SS, Funck KL, Petersen L, Bek T, Poulsen PL, Laugesen E. Effects of semaglutide and empagliflozin on oxygenation, vascular autoregulation, and central thickness of the retina in people with type 2 diabetes: a prespecified secondary analysis of a randomised clinical trial. J Diabetes Complications. 2023;37:108472. doi: 10.1016/j.jdiacomp.2023.108472. [DOI] [PubMed] [Google Scholar]
- 24.Gullaksen S, Vernstrøm L, Sørensen SS, Ringgaard S, Laustsen C, Funck KL, Poulsen PL, Laugesen E. Correction: separate and combined effects of semaglutide and empagliflozin on kidney oxygenation and perfusion in people with type 2 diabetes: a randomised trial. Diabetologia. 2024;67:1451. doi: 10.1007/s00125-024-06167-8. [DOI] [PubMed] [Google Scholar]
- 25.Frias JP, Deenadayalan S, Erichsen L, Knop FK, Lingvay I, Macura S, Mathieu C, Pedersen SD, Davies M. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402:720–730. doi: 10.1016/S0140-6736(23)01163-7. [DOI] [PubMed] [Google Scholar]
- 26.Chinese Elderly Type 2 Diabetes Prevention and Treatment of Clinical Guidelines Writing Group; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Society; Geriatric Endocrinology and Metabolism Branch of Chinese Geriatric Health Care Society; Geriatric Professional Committee of Beijing Medical Award Foundation; National Clinical Medical Research Center for Geriatric Diseases (PLA General Hospital) Clinical guidelines for prevention and treatment of type 2 diabetes mellitus in the elderly in China (2022 edition) Zhonghua Nei Ke Za Zhi. 2022;61:12–50. doi: 10.3760/cma.j.cn112138-20211027-00751. [DOI] [PubMed] [Google Scholar]
- 27.Daly A, Hovorka R. Technology in the management of type 2 diabetes: present status and future prospects. Diabetes Obes Metab. 2021;23:1722–1732. doi: 10.1111/dom.14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Androutsakos T, Nasiri-Ansari N, Bakasis AD, Kyrou I, Efstathopoulos E, Randeva HS, Kassi E. SGLT-2 inhibitors in NAFLD: expanding their role beyond diabetes and cardioprotection. Int J Mol Sci. 2022;23:3107. doi: 10.3390/ijms23063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Marco L, Morillo V, Gorriz JL, Suarez MK, Nava M, Ortega Á, Parra H, Villasmil N, Rojas-Quintero J, Bermúdez V. SGLT2i and GLP-1RA in cardiometabolic and renal diseases: from glycemic control to adipose tissue inflammation and senescence. J Diabetes Res. 2021;2021:9032378. doi: 10.1155/2021/9032378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khera R, Aminorroaya A, Dhingra LS, Thangaraj PM, Camargos AP, Bu F, Ding X, Nishimura A, Anand TV, Arshad F, Blacketer C, Chai Y, Chattopadhyay S, Cook M, Dorr DA, Duarte-Salles T, DuVall SL, Falconer T, French TE, Hanchrow EE, Kaur G, Lau WC, Li J, Li K, Liu Y, Lu Y, Man KK, Matheny ME, Mathioudakis N, McLeggon JA, McLemore MF, Minty E, Morales DR, Nagy P, Ostropolets A, Pistillo A, Phan TP, Pratt N, Reyes C, Richter L, Ross J, Ruan E, Seager SL, Simon KR, Viernes B, Yang J, Yin C, You SC, Zhou JJ, Ryan PB, Schuemie MJ, Krumholz HM, Hripcsak G, Suchard MA. Comparative effectiveness of second-line antihyperglycemic agents for cardiovascular outcomes: a large-scale, multinational, federated analysis of the LEGEND-T2DM study. medRxiv. 2024 doi: 10.1016/j.jacc.2024.05.069. [DOI] [PubMed] [Google Scholar]
- 31.Simms-Williams N, Treves N, Yin H, Lu S, Yu O, Pradhan R, Renoux C, Suissa S, Azoulay L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: population based cohort study. BMJ. 2024;385:e078242. doi: 10.1136/bmj-2023-078242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo M, Gu J, Teng F, Chen J, Ma X, Chen Q, Pu Y, Jiang Z, Long Y, Xu Y. The efficacy and safety of combinations of SGLT2 inhibitors and GLP-1 receptor agonists in the treatment of type 2 diabetes or obese adults: a systematic review and meta-analysis. Endocrine. 2020;67:294–304. doi: 10.1007/s12020-019-02175-6. [DOI] [PubMed] [Google Scholar]
- 33.Patoulias D, Michailidis T, Dimosiari A, Fragakis N, Tse G, Rizzo M. Effect of glucagon-like peptide-1 receptor agonists on cardio-metabolic risk factors among obese/overweight individuals treated with antipsychotic drug classes: an updated systematic review and meta-analysis of randomized controlled trials. Biomedicines. 2023;11:669. doi: 10.3390/biomedicines11030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubino DM, Greenway FL, Khalid U, O’Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT STEP 8 Investigators. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327:138–150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y, Si Y, Fu R, Zhang M, Jiang K, Dai W, Shen J, Li X, Yuan Y. Efficacy and safety of SGLT-2i in overweight/obese, non-diabetic individuals: a meta-analysis of randomized controlled trials. Endokrynol Pol. 2022;73:71–80. doi: 10.5603/EP.a2021.0102. [DOI] [PubMed] [Google Scholar]
- 36.Tuersun A, Hou G, Cheng G. Efficacy and safety of the combination or monotherapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: an update systematic review and meta-analysis. Am J Med Sci. 2024;368:579–588. doi: 10.1016/j.amjms.2024.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Dai M, Dai S, Gu L, Xiang Z, Xu A, Lu S, Yang Y, Zhou C. Efficacy of glucagon-like peptide-1 receptor agonists in overweight/obese and/or T2DM adolescents: a meta-analysis based on randomized controlled trials. J Clin Res Pediatr Endocrinol. 2024;16:323–333. doi: 10.4274/jcrpe.galenos.2024.2024-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong M, Chen H, Wen S, Yuan Y, Yang L, Xu D, Zhou L. The mechanism of sodium-glucose cotransporter-2 inhibitors in reducing uric acid in type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2023;16:437–445. doi: 10.2147/DMSO.S399343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caruso I, Di Gioia L, Di Molfetta S, Cignarelli A, Palmer SC, Natale P, Strippoli GFM, Perrini S, Natalicchio A, Laviola L, Giorgino F. Glucometabolic outcomes of GLP-1 receptor agonist-based therapies in patients with type 2 diabetes: a systematic review and network meta-analysis. EClinicalMedicine. 2023;64:102181. doi: 10.1016/j.eclinm.2023.102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forst T, Mathieu C, Giorgino F, Wheeler DC, Papanas N, Schmieder RE, Halabi A, Schnell O, Streckbein M, Tuttle KR. New strategies to improve clinical outcomes for diabetic kidney disease. BMC Med. 2022;20:337. doi: 10.1186/s12916-022-02539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz P. Inpatient hypoglycemia: the challenge remains. J Diabetes Sci Technol. 2020;14:560–566. doi: 10.1177/1932296820918540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019;7:385–396. doi: 10.1016/S2213-8587(18)30315-2. [DOI] [PubMed] [Google Scholar]
- 43.Przezak A, Bielka W, Molęda P. Fear of hypoglycemia-an underestimated problem. Brain Behav. 2022;12:e2633. doi: 10.1002/brb3.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]