Abstract
Polycystic ovary syndrome (PCOS) is a prevalent endocrine and metabolic disorder affecting women of reproductive age. We provide an overview of how mitochondrial DNA (mtDNA) copy number variation (CNVs) and gene mutations mediate PCOS, including current research findings and clinical trial data to underscore a theoretical basis for further exploring its pathogenesis and developing targeted therapy. Characterized by hyperandrogenism, oligo-ovulation or anovulation, and polycystic ovarian morphology, PCOS is often accompanied by insulin resistance, metabolic syndrome, and chronic inflammation, which reduce fertility throughout the reproductive lifespan. Despite its diverse phenotypes, the etiology and pathophysiologic mechanisms of PCOS remain unclear. As the center of energy metabolism, mitochondria have emerged as a key player. Evidence suggests their structural and functional abnormalities may underlie diverse manifestations of PCOS. Previous studies have highlighted the critical role of mitochondrial morphologic alterations, functional impairments, mtDNA mutations, and CNVs in the pathogenesis and progression of PCOS. This review systematically summarizes the latest research on mtDNA CNVs and gene mutations in PCOS, identifying them as promising targets for therapeutic intervention.
Keywords: Polycystic ovary syndrome, mitochondrial DNA, copy number variation, gene mutation, pathogenesis, therapy
Introduction
Polycystic ovary syndrome (PCOS), also known as Stein-Leventhal syndrome, is a complex endocrine and metabolic disorder prevalent among women of reproductive age, with a prevalence ranging from 5% to 15% based on population and diagnostic criteria [1,2]. The Rotterdam criteria, proposed by the European Society of Human Reproduction and Embryology and the American Society for Reproductive Medicine in 2003, remain the most widely recognized diagnostic standard. The criteria include three parts: (1) oligo-ovulation and/or anovulation; (2) clinical and/or laboratory evidence of hyperandrogenism; and (3) polycystic ovarian morphology on ultrasound. A PCOS diagnosis is confirmed when at least two of three criteria are met, excluding other causes of hyperandrogenism [3]. The clinical manifestations of PCOS are highly variable due to differences in geography, ethnicity, and lifestyle. In addition to menstrual disorders and infertility, PCOS can also predispose to hirsutism, acne, metabolic diseases (type 2 diabetes, non-alcoholic fatty liver disease, and metabolic syndrome), cardiovascular disease, cancer, various pregnancy complications (deep vein thrombosis, preeclampsia, gestational diabetes, macrosomia, fetal growth restriction, miscarriage, stillbirth, and preterm birth), and other psychological problems (anxiety and depression) [4]. Therefore, accurate diagnosis, effective treatment, and long-term management of PCOS are crucial for women’s health. Although the exact pathogenesis of PCOS remains unclear, evidence suggests that mitochondrial dysfunction may be a common mechanism underlying the heterogeneous features in PCOS women. Mitochondrial dysfunction is closely related to defects in oocyte developmental, hyperandrogenism, insulin resistance (IR), and chronic inflammation in PCOS women [5]. Given the critical role of mitochondrial DNA (mtDNA) in mitochondrial function, alterations in mtDNA--including copy number variations (CNVs) and mutations--could affect cellular energy metabolism and reactive oxygen species (ROS) levels, thereby influencing cellular functions. This review will focus on the mechanisms, early diagnosis, and therapeutic application of mtDNA, CNVs, and mutations in PCOS, providing a foundation for further exploration of the pathogenesis and targeted treatment of PCOS.
Mechanistic insight into PCOS
Emerging evidence suggests that PCOS is a complex endocrine disorder with a multifactorial etiology, involving a complex interplay of genetic, lifestyle, environment, and psychological factors [6]. Genetic predisposition plays a significant role in PCOS development, as evidenced by its familial clustering, with female relatives of PCOS patients at higher risk of developing the condition, highlighting the role of heritable factors [7,8]. The PCOSKB2 database has identified 241 genes and 114 single nucleotide polymorphisms (SNPs) implicated in PCOS pathogenesis, either directly or indirectly [9]. Genome-wide association studies (GWAS) have identified common genetic loci associated with PCOS phenotypes across various ethnic populations, potentially influencing processes such as hypothalamic-pituitary-ovarian axis function, insulin sensitivity, and androgen metabolism [10-12]. Additionally, PCOS is considered as a complex trait resulting from the interaction between inherited genetic variants and environmental factors. Lifestyle factors, including diet quality, physical activity, exposure to endocrine-disrupting chemicals (EDCs), light cycle alterations, sleep disturbances, and elevated stress levels, have been implicated in the development and progression of PCOS [13-17]. Furthermore, the higher concordance rate in monozygotic twins, familial clustering of PCOS cases, and the disease threshold phenomenon observed in long-term complications, along with the involvement of multiple organ systems and mitochondrial dysfunction, suggest a role of mtDNA in PCOS pathogenesis. These characteristics are often associated with mitochondrial disorders, indicating that mtDNA may be a significant factor in PCOS [18-21].
PCOS is characterized by chronic inflammation. Local ovarian inflammation disrupts ovulation and contributes to systemic inflammation, exacerbated by excess adipose tissue [22]. Adiponectin, an anti-inflammatory hormone, is reduced in PCOS patients [23-25]. Compared to healthy controls, women with PCOS exhibit elevated levels of inflammatory markers such as C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), interleukin (IL-18), interleukin (IL-6), Monocyte Chemotactic Protein (MCP-1), macrophage inflammatory protein-1α (MIP-1α), white blood cell (WBC), and oxidative stress markers [26-35]. Recent research highlights mitochondrial-derived reactive oxygen species (ROS) and chronic inflammation as core pathogenic factors in PCOS, associated with hyperglycemia, anovulation, hyperandrogenism, and IR [36,37]. Understanding mitochondrial function in the pathogenesis and etiology of PCOS will contribute to the development of better diagnostic tools and targeted therapy.
Biological properties of mitochondria
Mitochondria are crucial organelles in eukaryotic cells, comprising the outer membrane, inner membrane, and soluble matrix. The inner membrane folds inward to form cristae, which house the electron transport chain and ATP synthase, crucial for energy production through oxidative phosphorylation. Besides energy supply, mitochondria are involved in cellular apoptosis, ROS regulation, calcium signaling, and other crucial processes [38]. Moreover, mitochondria are controlled by both mtDNA and the nuclear genome, possessing semi-autonomous function and self-replication capacity. mtDNA is a double-stranded, closed-circular molecule of 16,569 base pairs, comprising coding and non-coding regions. The coding region of mtDNA encodes 13 essential polypeptides for ATP synthesis, 22 mitochondria transfer RNAs (mt-tRNAs), and 2 mitochondria ribosomal RNAs (mt-rRNAs). The non-coding region, known as D-loop, contains the origin of replication and transcription promoters for both the heavy and light strands of mtDNA, and regulates mtDNA replication and transcription. Unlike nuclear DNA, mtDNA lacks protective histones and is highly susceptible to mutations due to its proximity to ROS [39]. These mutations can impair mitochondrial function, leading to cellular energy deficits and oxidative stress [40]. Defects in mtDNA replication, including mutations, multiple deletions, and CNVs, are indicative of mitochondrial functional status. To date, over 200 pathogenic mutations, deletions, insertions, and CNVs have been identified in human mitochondria, contributing to various diseases, including those affecting oocyte maturation and PCOS. Studies have found that women with PCOS diabetes have abnormal mtDNA copy number and various types of mutations [41]. Aberrant mtDNA has become a central focus in PCOS research. Elucidating the patterns of mtDNA variation in PCOS is crucial for better prevention and treatment.
mtDNA abnormalities and PCOS
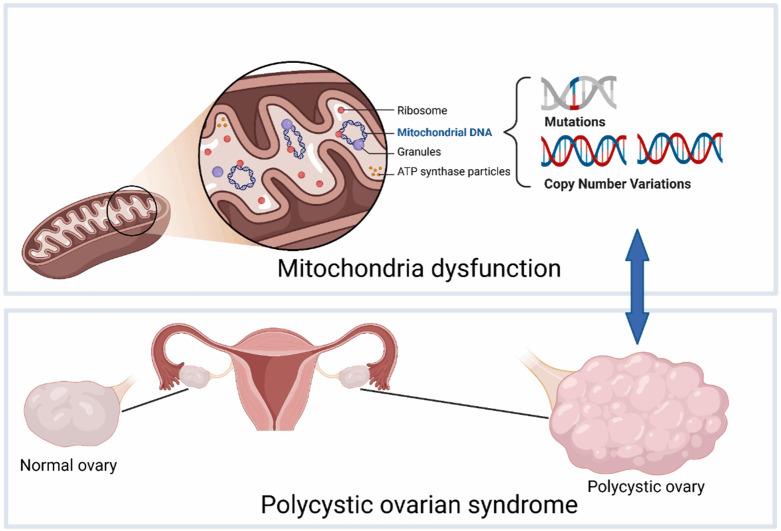
Mitochondrial defects are common in PCOS, characterized by morphologic alterations, accumulation of mtDNA mutation, and metabolic dysfunction. These defects contribute to the onset and development of PCOS through various mechanisms, including abnormal energy metabolism, impaired biosynthesis, and oxidative stress, which are reflected in PCOS clinical features. Ultrastructural studies have observed significant mitochondrial swelling and vacuolation in oocytes, myocytes, and granulosa cells of PCOS mice models [20,42-44]. Mitochondrial damage aggravates PCOS symptoms and can affect the offspring through meiotic inheritance, possibly contributing to the familial aggregation of PCOS and the formation of various mtDNA haplogroup clusters. These haplogroup clusters exhibit different biochemical cellular effects, which may further influence susceptibility to PCOS. We focus on the correlation between PCOS and functional disorders mediated by mtDNA gene mutations and copy number variations (Figure 1).
Figure 1.
Mitochondrial DNA copy number variation and gene mutations mediate polycystic ovary syndrome.
mtDNA mutations mediate PCOS
mtDNA mutations are implicated in the pathogenesis of PCOS by affecting protein synthesis, respiratory chain function, and IR. A study identified a significant association between a specific 9-bp deletion mutation in the mtDNA V region and PCOS, suggesting a link to the high heterogeneity of PCOS [45]. This finding was consistent with previous reports of mtDNA mutations in PCOS patients, encompassing the D-loop, 12S mt-rRNA, 16S mt-rRNA, and mt-tRNA genes, and genes involved in oxidative phosphorylation [46]. Pallavi Shukla’s use of next-generation sequencing (NGS) revealed numerous variants in PCOS patients, particularly within oxidative phosphorylation complexes, tRNAs, and rRNAs, supporting a role of mitochondrial dysfunction in PCOS [47]. Ding discovered that the relative mitochondrial count was significantly lower in the PCOS-IR group with mutant mt-tRNA compared to the control group. Specific mutations, such as mt-tRNALeu(UUR) C3275T, altered the secondary structure of mt-tRNA, resulting in decreased mtDNA copy number, reduced mitochondrial membrane potential, decreased ATP synthesis, and increased ROS production. Moreover, impaired mitochondrial membrane potential can further promote ROS production, forming a vicious cycle that leads to various clinical phenotypes of PCOS [48-50]. These mutations result in mitochondrial respiratory dysfunction and impaired mitochondrial protein synthesis in pancreatic β cells, possibly triggering or aggravating IR [41].
In addition to mt-tRNA gene alterations, mutations in the Nicotinamide adenine dinucleotide (NADH) dehydrogenase subunit 5 (ND5) gene are closely associated with IR in PCOS. Studies have identified mutations in ND5 T12338C and tRNASer(UCN) C7 492T in mtDNA of PCOS patients, which may reduce ND5 messenger RNA (mRNA) expression and alter the tertiary structure of tRNASer(UCN). These alterations could contribute to the development of IR [51]. Disruptions in mtDNA translation or transcription lead to oxidative stress and impaired adenosine triphosphate (ATP) production through oxidative phosphorylation. This mitochondrial dysfunction compromises pancreatic β-cell function, resulting in decreased insulin secretion. Consequently, insufficient insulin levels fail to suppress hepatic glucose production or stimulate peripheral glucose uptake, exacerbating IR in PCOS. This cascade of events represents a critical pathogenic mechanism underlying the disorder.
Mitochondrial dysfunction is a hallmark of PCOS, primarily attributed to defects in mitochondrial biogenesis, including mtDNA mutations and alterations in rRNA, tRNA, and mRNA. These aberrations collectively impair mitochondrial protein synthesis and function, leading to disruptions in the oxidative phosphorylation pathway, uncoupling of electron transport and ATP generation, and alterations in ATP and ROS levels. Consequently, cellular homeostasis is disrupted, culminating in IR and a myriad of clinical symptoms associated with PCOS.
CNVs of mtDNA mediate PCOS
Mitochondrial function is tightly associated with mtDNA copy number, making it a valuable indicator for assessing mitochondrial function. Each mammalian cell typically contains 1,000-10,000 copies of mtDNA, with transcription levels largely reflecting mtDNA copy number [52]. mtDNA copy number serves as an indicator of fertilization potential and oocyte maturation, with alterations linked to abnormal follicular development and metabolic disorders in PCOS patients. Primordial oocytes contain approximately 500 mtDNA copies, but this number increases dramatically to 150,000-700,000 in mature MII oocytes, highlighting substantial variability among individual oocytes. Optimal mtDNA copy number and ATP production are essential for successful oocyte maturation and embryo development [53-55]. Studies have revealed a strong association between decreased mtDNA copy number and PCOS, with analysis revealing mutations in mt-tRNAs and a significant reduction in mtDNA copy number in PCOS women [41]. Further research by Pallavi Shukla confirmed these findings, reporting a significantly lower mean mtDNA copy number in PCOS patients compared to controls (1.262 ± 0.33 vs. 1.662 ± 0.38, P < 0.0001) [47]. Under normal conditions, ROS produced during oxidative phosphorylation serve as signaling molecules. However, excessive ROS can damage mitochondria including mtDNA, leading to decreased energy production, impaired repair mechanisms, and ultimately, cell death [56]. Oxidative stress is widely recognized as a critical pathophysiologic mechanism underlying PCOS [37]. Decreased expression of NADH-ubiquinone oxidoreductase assembly factor 3 (NDUFA3), involved in oxidative phosphorylation, has been observed in PCOS patients, correlating with mtDNA copy number, follicular development, and IR [57]. Reduced mtDNA copy number leads to mitochondrial dysfunction due to insufficient mtDNA-encoded gene expression, characterized by loss of mitochondrial membrane potential (MMP), increased ROS production, and decreased ATP output. Moreover, the impaired MMP further promoted the generation of excessive cellular ROS, exacerbating the vicious cycle [5,58,59].
In conclusion, mtDNA alterations play a significant role in the pathogenesis of PCOS through perturbation of mitochondrial protein synthesis, induction of oxidative stress, and impairment of tissue and organ function. Further research is required to clarify the molecular mechanisms involved and to develop novel therapeutic strategies targeting mtDNA damage and mitochondrial dysfunction in PCOS.
mtRNA as a therapeutic target for PCOS
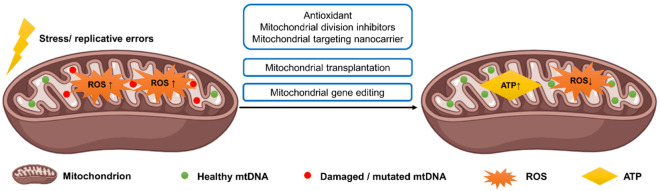
Mitochondrial dysfunction and its subsequent metabolic abnormalities in PCOS impose a double burden of physiologic and psychological distress on women. Due to the unclear pathogenesis of PCOS, individualized therapy is often based on patient symptoms and anticipated responses. Currently, primary treatment options for PCOS encompass lifestyle interventions, suppression of excessive androgen secretion, menstrual regulation, and amelioration of metabolic disorders [60]. As a novel therapeutic avenue, mitochondrial therapy holds promise through targeted approaches (Table 1), such as: (1) Repair of damaged mtDNA, attenuation of oxidative stress, inflammatory response, and mtDNA damage-induced cell apoptosis; (2) Augmentation of energy metabolism intermediates, ATP synthesis, respiratory chain function, and redox balance [61] (Figure 2).
Table 1.
mtRNA-targeted therapies for PCOS
| Treatment approach | Mechanism of Action | Description of Effects | |
|---|---|---|---|
| Antioxidants | Vitamin D [65-68] | Modulate hormone production, enhance antioxidant defenses, and improve morphology | Improve ovarian and uterine morphology in PCOS models, reduce inflammation and oxidative stress markers |
| Coenzyme Q10 [69] | Support mitochondrial function and fertility | Improve mitochondrial function and fertility in aged mice | |
| Melatonin [62,70,71] | Optimize mitochondrial distribution, increases mtDNA copy number, regulates transcription | Enhance mitochondrial function | |
| Inositol [72] | Improve metabolism and autophagy | Promote mitochondrial health | |
| Metformin [73] | Decrease mtDNA copy number, improve oxidative stress | Improve oxidative stress in PCOS patients | |
| Nanocarrier Systems [71,75-77] | Enhance delivery of mtDNA using targeting moieties | Enhance mtDNA delivery efficiency, improve mitochondrial function | |
| Mitochondrial Transplantation [72,78] | Replace defective mitochondria with healthy ones | Improve mitochondrial function and embryo development, increase fertilization rates and embryo quality | |
| Mitochondrial Gene Editing [79-84] | Deliver or repair mtDNA to correct genetic defects | Precisely correct mutations, enhance mitochondrial function, though challenges remain | |
Figure 2.
Mitochondrial function restoration as a therapeutic target for polycystic ovary syndrome.
Antioxidants, such as resveratrol, vitamin C and E, and glutathione, are effective in reducing oxidative damage to mtDNA by enhancing cellular antioxidant defenses and mitigating ROS-induced mitochondrial injury [62-64]. Vitamin D modulated hormone production by downregulating steroidogenesis, while simultaneously improving ovarian and uterine morphology in PCOS models [65-67]. Additionally, it enhances the defenses of antioxidants and hormone synthesis. Combined with MitoQ or probiotics, vitamin D further ameliorates PCOS symptoms by reducing inflammation and oxidative stress markers [68]. Coenzyme Q10, another antioxidant, supports mitochondrial function and fertility in aged mice [69]. Melatonin improved mitochondrial function by optimizing mitochondrial distribution, increasing mtDNA copy number, and regulating mtDNA transcription through the modulation of DNMT1 and mtDNA methylation [62,70,71]. Inositol also benefits mitochondrial health by improving metabolism and autophagy [72]. While metformin decreased mtDNA copy number in PCOS patients, it also improved oxidative stress [73]. Overall, these antioxidants play a crucial role in mitigating oxidative stress and supporting mitochondrial health, with varying mechanisms and benefits depending on the specific compound.
Conventional drugs for mitochondrial diseases often face issues with efficacy, solubility, and selectivity. Mitochondrial division inhibitors (MDIs) like Mdivi1, P110, and Dynasore safeguard mitochondrial structure and dynamics but struggle with mitochondrial membrane delivery [74]. Nanocarrier systems, enhanced with targeting moieties such as triphenylphosphine (TPP), rhodamine, mitochondrial penetrating peptides (MPPs), and specific sequences, can surmount these barriers. Notably, MPP-modified nanoparticles have shown potential in delivering mtDNA to the mitochondrial matrix of cancer cells, thereby reducing mutant mtDNA levels and restoring mitochondrial function [71,75-77].
Mitochondrial transplantation has promise as a therapeutic strategy for PCOS by directly replacing defective mitochondria with healthy ones, thereby correcting mtDNA mutations or quantity errors and restoring ovarian energy metabolism, ovulation, and hormone balance. Animal studies have demonstrated that transplanted mitochondria can effectively improve mitochondrial function and embryo development [72]. In a small study by Oktay, co-injecting sorted autologous mitochondria with sperm into oocytes by intracytoplasmic sperm injection (ICSI) improved fertilization rates (78.3% vs. 47.9%, P = 0.036) and embryo quality (3.1% vs. 2.3%, P = 0.082) compared to previous cycles [78]. Nonetheless, these findings were preliminary, and further investigation with larger sample sizes and standardized protocols is needed to fully determine the efficacy of mitochondrial transplantation for PCOS.
Mitochondrial gene editing offers a promising approach for treating mitochondrial heteroplasmy, having advantages over conventional therapies in precision and effectiveness. Key strategies include: (1) delivering wild-type mtDNA to correct or compensate for genetic defects by introducing functional mtDNA copies [79,80]; (2) eliminating heteroplasmic mutant mtDNA using gene editing tools to excise mutated mtDNA and facilitate the replication of wild-type mtDNA [81-83]; and (3) directly repairing mutated mtDNA with base editing technology precisely to correct pathogenic mutations without disrupting the mtDNA genome [84]. Mitochondria-targeted base editors represent powerful tools for research and therapeutic application. Although clinical mitochondrial gene therapy currently focuses on monogenic disorders with well-defined genetic causes [85,86], the complex and multifaceted nature of PCOS, with its various mtDNA mutations and unclear etiology, presents challenges for applying mitochondrial gene editing for its treatment.
Summary
Mitochondria are crucial organelles in human cells and their dysfunction results from morphologic alterations, genetic mutations, decreased biogenesis, or increased production of harmful byproducts. This contributes to oxidative stress and damage, thereby exacerbating PCOS. Conversely, PCOS can further aggravate mitochondrial dysfunction through various mechanisms, forming a vicious cycle. Preliminary research in animal models and human studies suggests that restoring mtDNA may offer a promising therapeutic approach for PCOS. However, further investigation is necessary to confirm its safety and efficacy. Screening and evaluating biomarkers to predict disease progression will provide essential evidence for developing targeted precision therapies aimed at mitochondria.
Disclosure of conflict of interest
None.
References
- 1.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–284. doi: 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 5.Cozzolino M, Seli E. Mitochondrial function in women with polycystic ovary syndrome. Curr Opin Obstet Gynecol. 2020;32:205–212. doi: 10.1097/GCO.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert EW, Tay CT, Hiam DS, Teede HJ, Moran LJ. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf) 2018;89:683–699. doi: 10.1111/cen.13828. [DOI] [PubMed] [Google Scholar]
- 7.Shabir I, Ganie MA, Zargar MA, Bhat D, Mir MM, Jan A, Shah ZA, Jan V, Rasool R, Naqati A. Prevalence of metabolic syndrome in the family members of women with polycystic ovary syndrome from North India. Indian J Endocrinol Metab. 2014;18:364–369. doi: 10.4103/2230-8210.131186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasidevi A, Vellanki P, Kunselman AR, Raja-Khan N, Dunaif A, Legro RS. Familial aggregation of circulating C-reactive protein in polycystic ovary syndrome. Hum Reprod. 2013;28:770–776. doi: 10.1093/humrep/des416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamanti-Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30:19–26. doi: 10.1385/ENDO:30:1:19. [DOI] [PubMed] [Google Scholar]
- 10.Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, Kraft P, Lin N, Huang H, Broer L, Magi R, Saxena R, Laisk T, Urbanek M, Hayes MG, Thorleifsson G, Fernandez-Tajes J, Mahajan A, Mullin BH, Stuckey BGA, Spector TD, Wilson SG, Goodarzi MO, Davis L, Obermayer-Pietsch B, Uitterlinden AG, Anttila V, Neale BM, Jarvelin MR, Fauser B, Kowalska I, Visser JA, Andersen M, Ong K, Stener-Victorin E, Ehrmann D, Legro RS, Salumets A, McCarthy MI, Morin-Papunen L, Thorsteinsdottir U, Stefansson K 23andMe Research Team. Styrkarsdottir U, Perry JRB, Dunaif A, Laven J, Franks S, Lindgren CM, Welt CK. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14:e1007813. doi: 10.1371/journal.pgen.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crespo RP, Bachega TASS, Mendonca BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018;62:352–361. doi: 10.20945/2359-3997000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones MR, Goodarzi MO. Genetic determinants of polycystic ovary syndrome: progress and future directions. Fertil Steril. 2016;106:25–32. doi: 10.1016/j.fertnstert.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Shao S, Zhao H, Lu Z, Lei X, Zhang Y. Circadian rhythms within the female HPG axis: from physiology to etiology. Endocrinology. 2021;162:bqab117. doi: 10.1210/endocr/bqab117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Xie N, Wu Y, Zhang Q, Zhu Y, Dai M, Zhou J, Pan J, Tang M, Cheng Q, Shi B, Guo Q, Li X, Xie L, Wang B, Yang D, Weng Q, Guo L, Ye J, Pan M, Zhang S, Zhou H, Zhen C, Liu P, Ning K, Brackenridge L, Hardiman PJ, Qu F. Association between circadian rhythm disruption and polycystic ovary syndrome. Fertil Steril. 2021;115:771–781. doi: 10.1016/j.fertnstert.2020.08.1425. [DOI] [PubMed] [Google Scholar]
- 15.Piazza MJ, Urbanetz AA. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assist Reprod. 2019;23:154–164. doi: 10.5935/1518-0557.20190016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu BR, Chowdhury O, Saha SK. Possible link between stress-related factors and altered body composition in women with polycystic ovarian syndrome. J Hum Reprod Sci. 2018;11:10–18. doi: 10.4103/jhrs.JHRS_78_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iovine JC, Claypool SM, Alder NN. Mitochondrial compartmentalization: emerging themes in structure and function. Trends Biochem Sci. 2021;46:902–917. doi: 10.1016/j.tibs.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinnery PF, Hudson G. Mitochondrial genetics. Br Med Bull. 2013;106:135–159. doi: 10.1093/bmb/ldt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chappell NR, Zhou B, Schutt AK, Gibbons WE, Blesson CS. Prenatal androgen induced lean PCOS impairs mitochondria and mRNA profiles in oocytes. Endocr Connect. 2020;9:261–270. doi: 10.1530/EC-19-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui HP, Zhao Z, Massart J, Ohlsson C, Lindgren E, Crisosto N, Maliqueo M, Echiburu B, Ladrón de Guevara A, Sir-Petermann T, Larsson H, Rosenqvist MA, Cesta CE, Benrick A, Deng Q, Stener-Victorin E. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25:1894–1904. doi: 10.1038/s41591-019-0666-1. [DOI] [PubMed] [Google Scholar]
- 22.Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. doi: 10.1016/j.jsbmb.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 24.Franik G, Sadlocha M, Madej P, Owczarek A, Skrzypulec-Plinta V, Plinta R, Chudek J, Olszanecka-Glinianowicz M. Circulating omentin-1 levels and inflammation in polycystic ovary syndrome. Ginekol Pol. 2020;91:308–312. doi: 10.5603/GP.2020.0057. [DOI] [PubMed] [Google Scholar]
- 25.Yang HY, Ma Y, Lu XH, Liang XH, Suo YJ, Huang ZX, Lu DC, Qin YF, Luo ZJ. The correlation of plasma omentin-1 with insulin resistance in non-obese polycystic ovary syndrome. Ann Endocrinol (Paris) 2015;76:620–627. doi: 10.1016/j.ando.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Abraham Gnanadass S, Divakar Prabhu Y, Valsala Gopalakrishnan A. Association of metabolic and inflammatory markers with polycystic ovarian syndrome (PCOS): an update. Arch Gynecol Obstet. 2021;303:631–643. doi: 10.1007/s00404-020-05951-2. [DOI] [PubMed] [Google Scholar]
- 27.Alissa EM, Algarni SA, Khaffji AJ, Al Mansouri NM. Role of inflammatory markers in polycystic ovaries syndrome: In relation to insulin resistance. J Obstet Gynaecol Res. 2021;47:1409–1415. doi: 10.1111/jog.14684. [DOI] [PubMed] [Google Scholar]
- 28.Artimani T, Karimi J, Mehdizadeh M, Yavangi M, Khanlarzadeh E, Ghorbani M, Asadi S, Kheiripour N. Evaluation of pro-oxidant-antioxidant balance (PAB) and its association with inflammatory cytokines in polycystic ovary syndrome (PCOS) Gynecol Endocrinol. 2018;34:148–152. doi: 10.1080/09513590.2017.1371691. [DOI] [PubMed] [Google Scholar]
- 29.Khashchenko E, Vysokikh M, Uvarova E, Krechetova L, Vtorushina V, Ivanets T, Volodina M, Tarasova N, Sukhanova I, Sukhikh G. Activation of systemic inflammation and oxidative stress in adolescent girls with polycystic ovary syndrome in combination with metabolic disorders and excessive body weight. J Clin Med. 2020;9:1399. doi: 10.3390/jcm9051399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millán JL. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. J Clin Endocrinol Metab. 2004;89:806–811. doi: 10.1210/jc.2003-031365. [DOI] [PubMed] [Google Scholar]
- 31.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95:1048–58. e1–2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism. 2009;58:954–962. doi: 10.1016/j.metabol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1α are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol (Oxf) 2009;71:652–658. doi: 10.1111/j.1365-2265.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- 34.Sabuncu T, Vural H, Harma M, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001;34:407–413. doi: 10.1016/s0009-9120(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 35.Ebejer K, Calleja-Agius J. The role of cytokines in polycystic ovarian syndrome. Gynecol Endocrinol. 2013;29:536–540. doi: 10.3109/09513590.2012.760195. [DOI] [PubMed] [Google Scholar]
- 36.González F, Considine RV, Abdelhadi OA, Acton AJ. Oxidative stress in response to saturated fat ingestion is linked to insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104:5360–5371. doi: 10.1210/jc.2019-00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammadi M. Oxidative stress and polycystic ovary syndrome: a brief review. Int J Prev Med. 2019;10:86. doi: 10.4103/ijpvm.IJPVM_576_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pernas L, Scorrano L. Mito-morphosis: mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol. 2016;78:505–31. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- 39.Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V. Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: mitochondria as a “chi”. Immun Ageing. 2013;10:15. doi: 10.1186/1742-4933-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babayev E, Seli E. Oocyte mitochondrial function and reproduction. Curr Opin Obstet Gynecol. 2015;27:175–181. doi: 10.1097/GCO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saeed NAAAH, Hamzah IH, Al-Gharrawi SAR. Polycystic ovary syndrome dependency on mtDNA mutation; copy Number and its association with insulin resistance. BMC Res Notes. 2019;12:455. doi: 10.1186/s13104-019-4453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leduc-Gaudet JP, Hussain SNA, Barreiro E, Gouspillou G. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int J Mol Sci. 2021;22:8179. doi: 10.3390/ijms22158179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H, Zhao Y, Li T, Li M, Li J, Li R, Liu P, Yu Y, Qiao J. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic Biol Med. 2015;86:295–307. doi: 10.1016/j.freeradbiomed.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Salehi R, Mazier HL, Nivet AL, Reunov AA, Lima P, Wang Q, Fiocco A, Isidoro C, Tsang BK. Ovarian mitochondrial dynamics and cell fate regulation in an androgen-induced rat model of polycystic ovarian syndrome. Sci Rep. 2020;10:1021. doi: 10.1038/s41598-020-57672-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhuo G, Feng G, Leng J, Yu L, Jiang Y. A 9-bp deletion homoplasmy in women with polycystic ovary syndrome revealed by mitochondrial genome-mutation screen. Biochem Genet. 2010;48:157–163. doi: 10.1007/s10528-009-9308-5. [DOI] [PubMed] [Google Scholar]
- 46.Zhuo G, Ding Y, Feng G, Yu L, Jiang Y. Analysis of mitochondrial DNA sequence variants in patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2012;286:653–659. doi: 10.1007/s00404-012-2358-7. [DOI] [PubMed] [Google Scholar]
- 47.Shukla P, Mukherjee S, Patil A, Joshi B. Molecular characterization of variants in mitochondrial DNA encoded genes using next generation sequencing analysis and mitochondrial dysfunction in women with PCOS. Gene. 2023;855:147126. doi: 10.1016/j.gene.2022.147126. [DOI] [PubMed] [Google Scholar]
- 48.Ding Y, Xia BH, Zhang CJ, Zhuo GC. Mutations in mitochondrial tRNA genes may be related to insulin resistance in women with polycystic ovary syndrome. Am J Transl Res. 2017;9:2984–2996. [PMC free article] [PubMed] [Google Scholar]
- 49.Ding Y, Xia BH, Zhang CJ, Zhuo GC. Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene. 2018;642:299–306. doi: 10.1016/j.gene.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 50.Ding Y, Zhuo G, Zhang C. The mitochondrial tRNALeu(UUR) A3302G mutation may be associated with insulin resistance in woman with polycystic ovary syndrome. Reprod Sci. 2016;23:228–233. doi: 10.1177/1933719115602777. [DOI] [PubMed] [Google Scholar]
- 51.Ding Y, Zhuo G, Zhang C, Leng J. Point mutation in mitochondrial tRNA gene is associated with polycystic ovary syndrome and insulin resistance. Mol Med Rep. 2016;13:3169–3172. doi: 10.3892/mmr.2016.4916. [DOI] [PubMed] [Google Scholar]
- 52.Fernández-Silva P, Enriquez JA, Montoya J. Replication and transcription of mammalian mitochondrial DNA. Exp Physiol. 2003;88:41–56. doi: 10.1113/eph8802514. [DOI] [PubMed] [Google Scholar]
- 53.Bentov Y, Esfandiari N, Burstein E, Casper RF. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril. 2010;93:272–275. doi: 10.1016/j.fertnstert.2009.07.988. [DOI] [PubMed] [Google Scholar]
- 54.Van Blerkom J, Davis PW, Lee J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:415–424. doi: 10.1093/oxfordjournals.humrep.a135954. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi T, Neri QV, Katagiri Y, Rosenwaks Z, Palermo GD. Effect of treating induced mitochondrial damage on embryonic development and epigenesis. Biol Reprod. 2005;72:584–592. doi: 10.1095/biolreprod.104.032391. [DOI] [PubMed] [Google Scholar]
- 56.Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malamouli M, Levinger I, McAinch AJ, Trewin AJ, Rodgers RJ, Moreno-Asso A. The mitochondrial profile in women with polycystic ovary syndrome: impact of exercise. J Mol Endocrinol. 2022;68:R11–R23. doi: 10.1530/JME-21-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy TV, Govatati S, Deenadayal M, Sisinthy S, Bhanoori M. Impact of mitochondrial DNA copy number and displacement loop alterations on polycystic ovary syndrome risk in south Indian women. Mitochondrion. 2019;44:35–40. doi: 10.1016/j.mito.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Lee SH, Chung DJ, Lee HS, Kim TJ, Kim MH, Jeong HJ, Im JA, Lee DC, Lee JW. Mitochondrial DNA copy number in peripheral blood in polycystic ovary syndrome. Metabolism. 2011;60:1677–1682. doi: 10.1016/j.metabol.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Alesi S, Ee C, Moran LJ, Rao V, Mousa A. Nutritional supplements and complementary therapies in polycystic ovary syndrome. Adv Nutr. 2022;13:1243–1266. doi: 10.1093/advances/nmab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dabravolski SA, Nikiforov NG, Eid AH, Nedosugova LV, Starodubova AV, Popkova TV, Bezsonov EE, Orekhov AN. Mitochondrial dysfunction and chronic inflammation in polycystic ovary syndrome. Int J Mol Sci. 2021;22:3923. doi: 10.3390/ijms22083923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yi S, Zheng B, Zhu Y, Cai Y, Sun H, Zhou J. Melatonin ameliorates excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS. Am J Physiol Endocrinol Metab. 2020;319:E91–E101. doi: 10.1152/ajpendo.00006.2020. [DOI] [PubMed] [Google Scholar]
- 63.Safaei Z, Bakhshalizadeh S, Nasr-Esfahani MH, Akbari Sene A, Najafzadeh V, Soleimani M, Shirazi R. Vitamin D3 affects mitochondrial biogenesis through mitogen-activated protein kinase in polycystic ovary syndrome mouse model. J Cell Physiol. 2020;235:6113–6126. doi: 10.1002/jcp.29540. [DOI] [PubMed] [Google Scholar]
- 64.Scheibye-Knudsen M, Fang EF, Croteau DL, Wilson DM 3rd, Bohr VA. Protecting the mitochondrial powerhouse. Trends Cell Biol. 2015;25:158–170. doi: 10.1016/j.tcb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masjedi F, Keshtgar S, Zal F, Talaei-Khozani T, Sameti S, Fallahi S, Kazeroni M. Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. J Steroid Biochem Mol Biol. 2020;197:105521. doi: 10.1016/j.jsbmb.2019.105521. [DOI] [PubMed] [Google Scholar]
- 66.Bakhshalizadeh S, Amidi F, Shirazi R, Shabani Nashtaei M. Vitamin D3 regulates steroidogenesis in granulosa cells through AMP-activated protein kinase (AMPK) activation in a mouse model of polycystic ovary syndrome. Cell Biochem Funct. 2018;36:183–193. doi: 10.1002/cbf.3330. [DOI] [PubMed] [Google Scholar]
- 67.Azhar A, Haider G, Naseem Z, Farooqui N, Farooqui MU, Rehman R. Morphological changes in the experimental model of polycystic ovary syndrome and effects of vitamin D treatment. J Obstet Gynaecol Res. 2021;47:1164–1171. doi: 10.1111/jog.14671. [DOI] [PubMed] [Google Scholar]
- 68.Kyei G, Sobhani A, Nekonam S, Shabani M, Ebrahimi F, Qasemi M, Salahi E, Fardin A. Assessing the effect of MitoQ10 and Vitamin D3 on ovarian oxidative stress, steroidogenesis and histomorphology in DHEA induced PCOS mouse model. Heliyon. 2020;6:e04279. doi: 10.1016/j.heliyon.2020.e04279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, Naranian T, Chi M, Wang Y, Bentov Y, Alexis J, Meriano J, Sung HK, Gasser DL, Moley KH, Hekimi S, Casper RF, Jurisicova A. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int J Mol Sci. 2016;17:939. doi: 10.3390/ijms17060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He B, Yin C, Gong Y, Liu J, Guo H, Zhao R. Melatonin-induced increase of lipid droplets accumulation and in vitro maturation in porcine oocytes is mediated by mitochondrial quiescence. J Cell Physiol. 2018;233:302–312. doi: 10.1002/jcp.25876. [DOI] [PubMed] [Google Scholar]
- 72.Visioli F, Ingram A, Beckman JS, Magnusson KR, Hagen TM. Strategies to protect against age-related mitochondrial decay: do natural products and their derivatives help? Free Radic Biol Med. 2022;178:330–346. doi: 10.1016/j.freeradbiomed.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 73.Yang PK, Chou CH, Chang CH, Chen SU, Ho HN, Chen MJ. Changes in peripheral mitochondrial DNA copy number in metformin-treated women with polycystic ovary syndrome: a longitudinal study. Reprod Biol Endocrinol. 2020;18:69. doi: 10.1186/s12958-020-00629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rodríguez-Varela C, Herraiz S, Labarta E. Mitochondrial enrichment in infertile patients: a review of different mitochondrial replacement therapies. Ther Adv Reprod Health. 2021;15:26334941211023544. doi: 10.1177/26334941211023544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gogada R, Amadori M, Zhang H, Jones A, Verone A, Pitarresi J, Jandhyam S, Prabhu V, Black JD, Chandra D. Curcumin induces Apaf-1-dependent, p21-mediated caspase activation and apoptosis. Cell Cycle. 2011;10:4128–4137. doi: 10.4161/cc.10.23.18292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gogada R, Prabhu V, Amadori M, Scott R, Hashmi S, Chandra D. Resveratrol induces p53-independent, X-linked inhibitor of apoptosis protein (XIAP)-mediated bax protein oligomerization on mitochondria to initiate cytochrome c release and caspase activation. J Biol Chem. 2011;286:28749–28760. doi: 10.1074/jbc.M110.202440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Q, Arnold RS, Sun CQ, Petros JA. A mitochondrial DNA mutation influences the apoptotic effect of statins on prostate cancer. Prostate. 2015;75:1916–1925. doi: 10.1002/pros.23089. [DOI] [PubMed] [Google Scholar]
- 78.Oktay K, Baltaci V, Sonmezer M, Turan V, Unsal E, Baltaci A, Aktuna S, Moy F. Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22:1612–1617. doi: 10.1177/1933719115612137. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Hu LF, Cui PF, Qi LY, Xing L, Jiang HL. Pathologically responsive mitochondrial gene therapy in an allotopic expression-independent manner cures Leber’s hereditary optic neuropathy. Adv Mater. 2021;33:e2103307. doi: 10.1002/adma.202103307. [DOI] [PubMed] [Google Scholar]
- 80.Yasuzaki Y, Yamada Y, Ishikawa T, Harashima H. Validation of mitochondrial gene delivery in liver and skeletal muscle via hydrodynamic injection using an artificial mitochondrial reporter DNA vector. Mol Pharm. 2015;12:4311–4320. doi: 10.1021/acs.molpharmaceut.5b00511. [DOI] [PubMed] [Google Scholar]
- 81.Minczuk M, Papworth MA, Kolasinska P, Murphy MP, Klug A. Sequence-specific modification of mitochondrial DNA using a chimeric zinc finger methylase. Proc Natl Acad Sci U S A. 2006;103:19689–19694. doi: 10.1073/pnas.0609502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reddy P, Ocampo A, Suzuki K, Luo J, Bacman SR, Williams SL, Sugawara A, Okamura D, Tsunekawa Y, Wu J, Lam D, Xiong X, Montserrat N, Esteban CR, Liu GH, Sancho-Martinez I, Manau D, Civico S, Cardellach F, Del Mar O’Callaghan M, Campistol J, Zhao H, Campistol JM, Moraes CT, Izpisua Belmonte JC. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell. 2015;161:459–469. doi: 10.1016/j.cell.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bian WP, Chen YL, Luo JJ, Wang C, Xie SL, Pei DS. Knock-in strategy for editing human and zebrafish mitochondrial dna using Mito-CRISPR/Cas9 system. ACS Synth Biol. 2019;8:621–632. doi: 10.1021/acssynbio.8b00411. [DOI] [PubMed] [Google Scholar]
- 84.Mok BY, de Moraes MH, Zeng J, Bosch DE, Kotrys AV, Raguram A, Hsu F, Radey MC, Peterson SB, Mootha VK, Mougous JD, Liu DR. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature. 2020;583:631–637. doi: 10.1038/s41586-020-2477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iyer S, Bergquist K, Young K, Gnaiger E, Rao RR, Bennett JP Jr. Mitochondrial gene therapy improves respiration, biogenesis, and transcription in G11778A Leber’s hereditary optic neuropathy and T8993G Leigh’s syndrome cells. Hum Gene Ther. 2012;23:647–657. doi: 10.1089/hum.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chakrabarty S, Govindaraj P, Sankaran BP, Nagappa M, Kabekkodu SP, Jayaram P, Mallya S, Deepha S, Ponmalar JNJ, Arivinda HR, Meena AK, Jha RK, Sinha S, Gayathri N, Taly AB, Thangaraj K, Satyamoorthy K. Contribution of nuclear and mitochondrial gene mutations in mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome. J Neurol. 2021;268:2192–2207. doi: 10.1007/s00415-020-10390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]