Abstract
The intestinal microbiota is vast in type and quantity and it plays a critical role in regulating various physiological functions in the host, including intestinal function, immune response and energy metabolism. Existing research shows that intestinal flora is associated with various hormones, cell cycles and ovarian function-related diseases in the female ovaries. Certain microorganisms within the intestinal flora can modulate the levels of hormones secreted by the ovary, such as estrogen and androgens. Furthermore, an imbalance in the gut microbiota can result in altered hormone levels in the host, potentially leading to related diseases. Studies have found that a variety of ovarian function-related diseases are closely related to intestinal flora, such as polycystic ovary syndrome (PCOS), ovarian insufficiency (POI), endometriosis (EMS) and ovarian cancer. Importantly, ovarian function-related diseases are notably difficult to diagnose early and often require prolonged treatment for effective management. The microbiota and its metabolites in patients with ovarian function-related diseases and cancers can serve as valuable biomarkers for early diagnosis, offering novel strategies for disease screening, treatment stratification, and prognosis.
Keywords: Intestinal flora, estrogen, polycystic ovary syndrome, premature ovarian insufficiency, endometriosis, ovarian tumors
Introduction
The ovary is one of the crucial organs within the female reproductive system and it has reproductive and endocrine functions [1]. Normal ovarian function has been proven to be closely related to women’s health, and ovarian dysfunction can cause a variety of diseases, such as PCOS (polycystic ovary syndrome), POI (premature ovarian insufficiency), infertility, osteoporosis and cardiovascular problems [2-4]. PCOS is the most common ovarian dysfunction which can affect 8-13% of women of childbearing age worldwide with 70% of cases remaining undiagnosed [5]. Diseases related to ovarian dysfunction are high-risk factors for humans that suffer from diabetes, endometrial cancer, cardiovascular and cerebrovascular diseases [6,7] and they seriously harm human health. The pathogenesis of diseases related to ovarian dysfunction is complex and it is affected by a variety of factors, such as genetics, inflammation, intestinal flora, endocrine hormones and environmental factors [8,9]. In recent years, with the study of intestinal flora attracting extensive attention, some researchers began to pay attention to the effect of intestinal flora on ovarian function [10], especially the role of intestinal flora in ovarian function-related diseases.
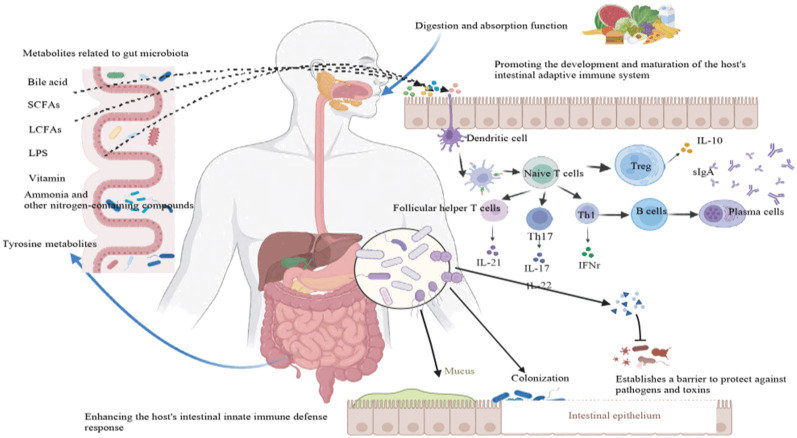
Intestinal flora is the community of microorganisms present in the human intestine, including bacteria, fungi, viruses and other microorganisms [11]. The intestinal flora coexists with the human body and forms a complex and huge ecosystem. Intestinal flora plays an extremely important role in the growth and development of the human body, participating in nutrient supply, metabolic regulation, immune regulation and other physiological processes [12-14] (Figure 1). Specific bacterial communities in the intestine can participate in the metabolism of estrogen through the microbial-intestinal-ovarian axis which can affect the hormone expression level and function of the ovaries [15]. Research has shown that women’s exposure to zearalenone significantly alters the intestinal flora of their offspring which can lead to changes in Bacteroidetes, Proteobacteria, and Firmicutes [16]. These changes cause adjustments in glutathione metabolism and antioxidant enzyme activity in the ovaries that in turn affects the development of oocytes in the offspring.
Figure 1.
Function of intestinal flora.
The intestinal flora is vital for the maturation and fertilization of female follicles and oocytes, as well as for embryo migration and implantation. At present, research on ovarian function mainly focuses on the biological functions of the ovary and various ovary-related diseases, some studies have shown that intestinal flora were closely related to patients’ metabolic indicators compared with healthy controls [17], such as insulin resistance and body weight. Intestinal flora plays a crucial role in the occurrence and regression of female reproductive endocrine diseases such as PCOS and premature ovarian insufficiency (POI) [2]. Intestinal flora can interact with a variety of hormones and plays an important role in the female reproductive endocrine system, such as estrogen, androgens, and insulin [18]. A study on intestinal flora found that Clostridium can transform glucocorticoids to androgens [19]. Therefore, it is imperative to conduct in-depth research on the relationship between ovarian function and intestinal flora.
The impact of intestinal flora on hormonal regulation and ovarian function
Intestinal flora and estrogen
Estrogen is a crucial hormone in the human body and is involved in various vital physiological functions, such as cell proliferation and death, lipid metabolism, energy balance, glucose metabolism, immune and cardiovascular regulation, gametogenesis, reproduction and bone growth [20-22]. Research indicates that intestinal flora is influenced by estrogen and can also regulate estrogen levels through enterohepatic circulation [19]. Some intestinal flora can secrete β-glucuronidase which is an enzyme that converts estrogen into its active form and allows it to bind to estrogen receptors. Imbalances in intestinal flora can alter β-glucuronidase levels that can lead to an excess or deficiency of free estrogen and potentially causing estrogen-related diseases [23]. A reduced diversity of gut flora can decrease β-glucuronidase activity which can lower estrogen levels and contributing to conditions like obesity, metabolic syndrome, cardiovascular disease, and cognitive decline [24]. Conversely, an increased presence of β-glucuronidase-secreting bacteria can raise estrogen levels that possibly trigger endometriosis (EMS) and cancer [25,26]. Studies have also found that specific gut bacteria, such as Clostridium, Ruminococcus, Bacteroides and Staphylococcus are correlated with varying levels of estrogen and its metabolites in the body [27]. Additionally, the liver produces conjugated estrogens that intestinal bacterial enzymes convert into their active forms which influence overall estrogen levels and their physiological effects.
A study on the E2 (estradiol) hormone in women found that those with elevated E2 levels had significantly increased diversity in their intestinal flora, specifically in species like Slackia and Butyricimonas [28]. In men and postmenopausal women, urinary estrogen levels strongly correlate with gut microbiota richness and α-diversity, with intestinal flora diversity being positively associated with the proportion of urinary estrogen metabolites [29]. Previous research has established a link between estrogen and the development of various cancers, including endometrial, ovarian, prostate, and breast cancer [30]. A recent investigation on breast cancer highlighted estrogen’s ability to induce DNA double-strand breaks in the estrogen receptor binding region, which can contribute to breast cancer development [30]. Furthermore, some intestinal flora plays a key role in the onset and progression of these cancers. A study examining the intestinal flora of adenocarcinoma patients found increased diversity and quantity of microorganisms such as Rikenellaceae, Alistipes, and Lachnospira compared to healthy individuals [31].
Intestinal flora and androgens
Androgen levels are one of the basic prerequisites for healthy women [32]. Androgen deficiency may cause individuals to experience symptoms of sexual dysfunction, such as decreased sexual desire, loss of sexual response, or weakened sexual arousal [33]. Women with POI not only lack estrogen but may also have reduced ovarian androgens due to ovarian cortical atrophy [9]. Studies have found that Clostridium can synthesize androgens using glucocorticoids as raw materials [34]. Some bacteria can also produce 5α reductase to convert testosterone into more active dihydrotestosterone [35]. Some intestinal flora regulates androgen levels through Deglucuronidation to release free dihydrotestosterone from the glucuronide conjugate [36]. Androgen levels can be affected by intestinal flora, and androgens in the host can also affect the composition of intestinal flora.
Hyperandrogenism (HA) is a key feature of PCOS that can lead to symptoms like acne, hirsutism and androgenic alopecia [37]. Various intestinal floras can produce enzymes involved in androgen metabolism, potentially affecting androgen levels in the body, while serum androgen levels can also impact gut flora composition. There is a strong link between gut microbiota and HA. Studies in patients with POI show that an increase in Campylobacter, Desulfobacteria, and Bacteroidetes can raise testosterone levels, while more Proteobacteria, Chloroflexi, and Actinobacteria can lower them [38]. Androgens, such as testosterone, support early follicular development and help improve ovarian reserves in women with reduced ovarian function [39]. Low testosterone levels have been linked to the development of EMS and POI [40]. Research also indicates that gut microbiota composition strongly correlates with circulating gonadal steroid levels, especially testosterone. For example, excessive prenatal testosterone injections in female rats led to reduced abundance of Akkermansia, Bacteroidetes, Lactobacilli, and Clostridium in their offspring, indicating an interaction between androgens and intestinal flora [41].
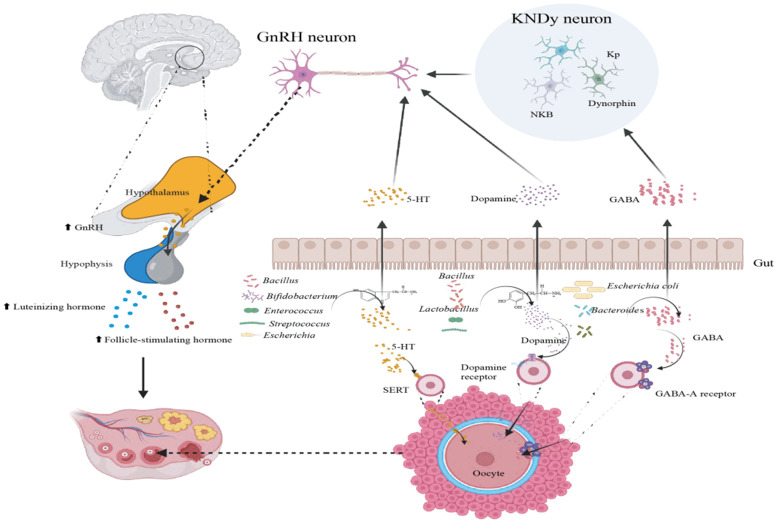
Intestinal flora and the hypothalamus-pituitary-ovarian (HPO) axis
Communication between intestinal flora and the brain forms a physiological network known as the “gut-brain” axis, involving the central nervous system (CNS), enteric nervous system (ENS), endocrine and immune systems [42] (Figure 2). This axis is closely linked to the neuroendocrine regulation of the hypothalamus-pituitary-ovarian (HPO) axis which is part of the hypothalamus-pituitary-gonadal (HPG) system [43]. The pituitary gland, located at the brain’s base, receives signals from the hypothalamus via gonadotropin-releasing hormone (GnRH) to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). FSH stimulates follicle maturation in the ovaries, leading to estrogen production, which then inhibits FSH secretion and triggers LH secretion. High LH levels ultimately lead to ovulation. Increasing evidence suggests that intestinal flora communicates with the CNS through the “gut-brain” axis, potentially influencing the HPO axis by modulating neurotransmitter synthesis and release [44].
Figure 2.
Gut-microbiota-brain axis and hypothalamus-pituitary-ovarian (HPO) axis.
Key neurotransmitters such as γ-aminobutyric acid (GABA), 5-hydroxytryptamine (5-HT) and dopamine (DA) can impact ovarian function through neural pathways [45]. Intestinal bacteria like Streptococcus, Enterococcus, Escherichia, Bifidobacterium, and Bacillus can synthesize 5-HT, which regulates gonadotropin secretion by affecting GnRH neuron activity [46]. 5-HT is involved in ovarian hormone production, follicle maturation, and ovulation [47]. Additionally, 5-HT can induce germ cell production and initiate egg maturation. Bacillus, Lactobacillus, and Streptococcus can synthesize DA in vitro, and Enterococcus faecalis expresses enzymes involved in DA biosynthesis [48,49]. Bacteroidetes and Escherichia produce GABA which can influences GnRH neurons and reproductive function [50]. Excessive GABA production by gut flora can interfere with KNDy (Kisspeptin Neurokinin B Dynorphin) neurons which can affect GnRH secretion, fertility, sex hormone levels and menstrual cycles [51].
Diseases related to intestinal flora and ovarian function
The human intestinal flora is primarily composed of three types: probiotics, neutral bacteria, and pathogenic bacteria [52]. Probiotics that mainly obligate anaerobic bacteria like Bacteroides, Eurobacterium, and Bifidobacterium play key roles in regulating intestinal flora, intestinal function and immune response, preventing intestinal infection, increasing mineral absorption and promoting bone health, regulating energy metabolism, maintaining weight and reducing obesity [52-54].
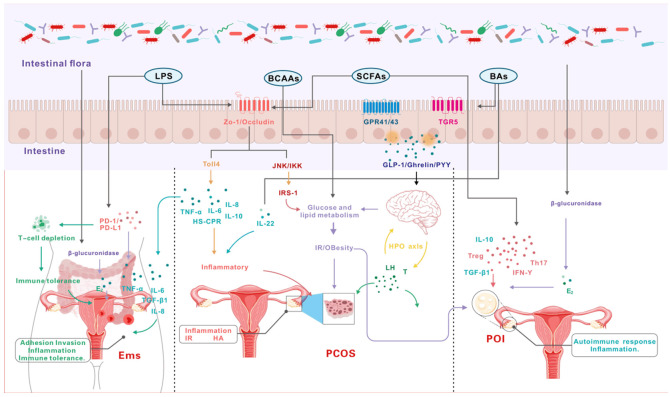
However, an imbalance in gut flora decreases the expression of ZO-1 (zonula occludens-1) and occludin in the intestinal mucosa, leading to increased intestinal permeability [55]. This increased permeability allows particles, bacteria and toxins to enter the bloodstream, potentially triggering chronic inflammation, insulin resistance (IR), and hyperandrogenism (HA) [56,57]. Short-chain fatty acids (SCFAs) that are key metabolites produced by intestinal flora are crucial for energy supply, immune regulation, intestinal mucosal protection and appetite control [58]. Research indicates that imbalances in intestinal flora contribute to various diseases, including autoimmune disorders, neurodegenerative conditions, cancer and ovarian function-related diseases [59,60]. Recent studies have focused on the connection between gut flora and ovarian function disorders like PCOS [61], POI [62] and EMS [63] (Figure 3; Table 1).
Figure 3.
Molecular mechanism of diseases related to intestinal flora and ovarian function.
Table 1.
Changes in intestinal flora content in the host and diseases related to ovarian function
| Disease | Species | Intestinal flora | |
|---|---|---|---|
|
| |||
| Increase | Descend | ||
| PCOS | Human | Bacteroides Escherichia coli | |
| Mouse | Firmicutes | Bacteroidetes | |
| POI | Human | Firmicutes, Brucella, Faecalis | Bacillus, Bacteroides |
| Mouse | Akkermansia, Bacteroidetes, Lactobacilli, Clostridium | ||
| EMS | Human | Leptothrix, Pasteurella, Gardnerella | |
| Mouse | Actinobacillus, Firmicutes, Bifidobacteria, Burkella | ||
PCOS and intestinal flora
PCOS is the most common endocrine disorder with diverse clinical manifestations [2]. Menstrual disorders, enlarged and polycystic ovaries, HA, IR, hirsutism, oligomenorrhea and no ovulation are the main clinical features of PCOS [64]. A low-grade chronic inflammatory response is a key factor in follicle development disorders, with inflammatory markers like tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) playing a role [65]. Patients with PCOS experience persistent low-grade inflammation which contributes to both its pathology and associated metabolic disorders. Diamine oxidase (DAO) is a highly active enzyme in the intestinal mucosa, and lipopolysaccharide (LPS) is a bacterial metabolite [66]. In healthy individuals, LPS levels remain relatively low, and both DAO and LPS are used clinically to assess intestinal barrier function [67]. In PCOS patients, serum DAO levels are significantly elevated, particularly in those with obesity-related intestinal flora imbalance. This imbalance can lead to the overexpression of zonulin, increasing intestinal permeability [68]. Higher permeability allows LPS to enter the bloodstream, binding to receptors and triggering a chronic inflammatory response that can exacerbate PCOS [69].
Studies have found a link between specific intestinal flora and PCOS. Beneficial bacteria like Bifidobacterium, Blautia, and Holdemania are linked to a reduced risk, while an increased abundance of Lachnospiraceae correlates with adverse PCOS outcomes [70]. PCOS patients show decreased alpha diversity in intestinal flora with notable shifts in microbial abundance. Alterations in beta diversity can also impact intestinal barrier function and inflammation, such as by increasing the relative abundance of Porphyromonas which can affect intestinal permeability [71]. In individuals with PCOS, especially those exhibiting insulin resistance, the diminished abundance of beneficial bacteria such as Prevotella correlates with disruptions in sex hormone balance and increased inflammatory responses [72]. This imbalance of intestinal flora impairs intestinal barrier function and exacerbates PCOS symptoms.
IR is a key clinical feature of PCOS, and abnormal glucose and lipid metabolism are common in these patients [73]. Studies suggest that intestinal flora imbalance may contribute to IR and compensatory hyperinsulinemia in PCOS, with Actinobacteria showing a strong correlation with the insulin resistance index (HOMA-IR) [74,75]. Patients with IR exhibit a more significant reduction in intestinal flora diversity, an increase in Bacteroidetes, and a notable decrease in Firmicutes [76]. Certain bacterial groups are closely related to blood lipids, glucose levels, endocrine and metabolic disorders, potentially playing a role in alleviating PCOS symptoms, such as Proteobacteria, Actinobacteria and Chloroflexi [77].
HA is a core pathological feature of PCOS, where excessive androgens disrupt follicle development that can lead to anovulation or infrequent ovulation [78]. Some studies have found that as the diversity of intestinal flora decreases, the balance of intestinal commensal bacteria is disrupted which may lead to corresponding changes in the abundance of some intestinal flora related to testosterone and metabolic disease markers [79]. In HA conditions, PCOS patients often exhibit gut flora imbalances. In a study using 5α-dihydrotestosterone (5α-DHT) to establish a PCOS rat model, both regular and high-fat diet groups demonstrated increased androgen levels and changes in ovarian morphology [80]. Compared to controls, these rats showed dysregulated gut flora, elevated inflammatory markers, and higher HOMA-IR than healthy rats. These findings suggest that gut flora imbalance and HA may interact in a vicious cycle, exacerbating the clinical symptoms of PCOS.
POI and intestinal flora
Primary ovarian insufficiency (POI) is characterized by the decline of ovarian function in women under the age of 40 and is often accompanied by hypergonadotropic amenorrhea resulting from estrogen deficiency [9]. Research on the gut microbiome in POI patients has revealed alterations in the microbial spectrum, including a decrease in Firmicutes, Brucella, and Fecalobacteria, alongside an increase in Bacteroidetes compared to healthy individuals [81]. Additionally, POI patients exhibited significantly reduced estradiol levels. Correlation analysis indicated associations between certain intestinal flora and serum levels of estradiol, FSH, luteinizing hormone, and anti-Müllerian hormone. POI patients not only lack estrogen but may also experience reduced ovarian androgens due to cortical atrophy. A meta-analysis further demonstrated that women with POI are at risk of lower concentrations of total testosterone, dehydroepiandrosterone sulfate and androstenedione [82].
The pathogenesis of POI is complex, but analyses have found that 10% to 55% of POI patients also suffer from autoimmune diseases [83]. Among POI cases, 4% to 30% may develop autoimmune conditions, such as autoimmune thyroiditis, type 1 diabetes, Addison’s disease, and systemic lupus erythematosus [83-85]. The intestinal flora plays a key role in autoimmune processes, including immune system regulation, immune cell development, anti-inflammatory effects, intestinal barrier function and immune tolerance modulation [86]. Autoimmune diseases in POI patients are related to the regulation of cytokines such as Treg, IFN-γ, and Th17 [87]. Short-chain fatty acids (SCFAs) produced by gut flora promote the expression and differentiation of Treg cells, facilitating anti-inflammatory responses that regulate immune function [88]. Notably, POI patients exhibit a significant increase in Treg cells after treatment, indicating changes in their immunomodulatory effects [89].
EMS and intestinal flora
EMS is a chronic estrogen-dependent disease caused by the retrograde entry of shed endometrial tissue into the lower abdominal cavity [90], and its incidence tends to be younger and rising [91]. The abnormal endocrine microenvironment of EMS lesions is considered to be its main feature [92]. Estrogen directly promotes anti-apoptotic and proliferative effects in EMS lesions, contributing to a pro-inflammatory environment [93]. Increased estrogen synthesis is linked to various enzymatic pathways, and intestinal flora can regulate estrogen levels through the secretion of β-glucuronidase [94]. Intestinal flora imbalance can lead to increased β-glucuronidase activity which can lead to increased estrogen levels. This rise in estrogen triggers the invasive growth of ectopic endometrial tissue, accelerating the proliferation of endometriotic lesions. Intestinal flora can also regulate estrogen levels by producing SCFA [95]. Butyrate is one of the more abundant SCFAs, and butyrate can regulate the synthesis of progesterone and E2 in primordial germ cells (PGCs) through the cAMP signaling pathway to promote the synthesis of estrogen. In vitro studies indicate that low concentrations of butyric acid stimulate progesterone secretion in PGCs, whereas higher concentrations significantly inhibit progesterone production [96].
The abnormal inflammatory microenvironment accelerates the colonization and invasion of ectopic endometrial tissue [92]. Normally, the intestinal flora maintains epithelial integrity, offering protection against bacterial invasion while exhibiting complex antibacterial and immunomodulatory functions. However, an imbalance in the intestinal flora can lead to the production of endotoxins like lipopolysaccharides (LPS) by Gram-negative bacteria [66]. LPS promotes the expression of adhesion molecules between the endometrium and pelvic peritoneal cells, facilitating ectopic endometrial adhesion and invasion. Dysbiosis in EMS patients can further trigger an inflammatory response, increasing the number of peritoneal macrophages [97]. These macrophages secrete large amounts of TGF-β that can promote the secretion of extracellular matrix proteins like fibronectin and collagen [98]. Additionally, an imbalance in the intestinal flora in EMS patients is associated with a significant increase in Streptococcus bovis, which releases toxic proteins with pro-inflammatory effects [99]. The persistent inflammation caused by the shedding of endometrial tissue disrupts the diversity of the gut microbiota and impairs intestinal barrier function. This disturbance creates a vicious cycle that contributes to disease progression and exacerbates gut dysbiosis.
The reflux of endometrial tissue into the abdominal cavity as foreign matter in healthy women triggers a response from immune cells in the peritoneal fluid [100]. This immune response clears up endometrial tissue or cells that cause reflux of menstrual blood. However, EMS patients have immune tolerance and refluxed EMS tissues or cells can escape immune clearance [101]. The escaped EMS tissue or cells can grow and develop into ectopic lesions in the pelvis or abdomen. Studies have found that a large number of bacterial endotoxins caused by intestinal flora disorders continue to stimulate and activate immune signaling-related pathways that leads to the overexpression of PD-1 and PD-L1 [102]. This induces immune T cell exhaustion and contributes to the occurrence and development of EMS.
Ovarian tumors and intestinal flora
Ovarian tumors are the most common gynecological tumors in women and the leading cause of gynecological cancer deaths worldwide [103]. The embryonic development and composition of ovarian tissue is complex that can result in many histological types, more than 30 [104]. Patients with ovarian tumors often exhibit heightened sensitivity to changes in the intestinal flora and may present with gastrointestinal symptoms in the early stages of the disease, such as abdominal pain, bloating, indigestion, constipation and early satiety [105,106].
LPS, lysophospholipids and tryptophan are all related products of intestinal flora metabolism, and these substances play a key role in the development of ovarian cancer [107,108]. LPS can stimulate Toll-like receptor 2 (TLR2), TLR4 and TLR5, activate phosphatidylinositol 3-kinase (PI3K) signaling, matrix metalloproteinases (MMP)-related family expression and tumor-associated macrophages and induce epithelial-mesenchymal transition (EMT) [109]. Lysophosphatidylserine and lysophosphatidylserine have been shown to induce protein kinase B (Akt), mitogen-activated protein kinase (MAPK) and Ca2+ signaling [110] which can upregulate the expression of angiogenesis and induce the proliferation, migration and invasion of ovarian cancer cells. Tryptophan as an energy source can support the growth of Lactobacillus, inhibit the expansion of pathogenic bacteria, regulate mucosal immunity by activating aryl hydrocarbon receptor (AHR) and pregnane X receptor (PXR) [111].
A study found that after using antibiotics to deplete the intestinal flora of human ovarian adenocarcinoma cell SKOV-3 cells in nude mice [112], the growth rate of ovarian cancer tumors was significantly accelerated which confirmed that dysbiosis of the intestinal microbiota promotes the progression of ovarian cancer. In addition, a study conducted high-throughput sequencing of 16S ribosomal ribonucleic acid (16SrRNA) on the peritoneal fluid of 10 patients with ovarian cancer and 20 patients with benign ovarian tumors and found that the peritoneal fluid of ovarian cancer patients was rich in gram-negative bacteria derived from the intestinal tract, and 18 microorganisms were identified as new markers for ovarian cancer [113].
Intestinal flora treats ovarian function-related diseases
Probiotics are active microorganisms that provide health benefits to the host, primarily including beneficial bacteria such as Lactobacilli and Bifidobacteria [114]. They are involved in various physiological processes in the human body, including maintaining intestinal flora balance, regulating the immune system, preventing and treating antibiotic-induced dysbiosis, exhibiting anti-inflammatory effects, and contributing to energy metabolism and weight management [115,116]. Probiotics have been extensively studied and are widely used to treat a range of health conditions, particularly those related to intestinal health, immune system regulation, diarrhea, digestive issues, allergic diseases, metabolic disorders, and psychiatric conditions [114,117]. The intestinal flora is known to be associated with several ovarian function-related diseases, such as polycystic ovary syndrome (PCOS), premature ovarian insufficiency (POI), and endometriosis (EMS), playing a significant role in the onset and progression of these conditions.
Ovarian function-related diseases are often difficult to diagnose in their early stages [118]. Most of these diseases are chronic diseases with long treatment and follow-up management cycles, making them difficult to treat with conventional treatments [119]. A study on the effects of probiotics in patients with PCOS found that after 12 weeks of supplementation, there was a significant decrease in both body weight and body mass index, along with notable reductions in blood sugar and lipid levels [120]. Probiotics can influence the host’s energy balance and metabolism by modulating the composition and quantity of the intestinal flora that can reduce body weight and BMI [121]. Given that PCOS patients are prone to insulin resistance and metabolic dysfunction, some research suggests that probiotic supplementation may also reduce insulin resistance in individuals with type II diabetes [122]. Studies using mouse models have found that probiotic supplementation can improve intestinal permeability, reduce plasma endotoxin levels, alleviate inflammation and decrease insulin resistance [122,123]. The imbalance of intestinal flora in POI patients is one of the main characteristics. In a study of guinea pigs, pretreating them with probiotics restored beneficial bacterial species, butyric acid production, and defecation [124]. The combination of gut microbiota and probiotics can influence glucose metabolism through immune system modulation and treat related diseases [125], through reduction of lipopolysaccharides and inflammation-causing bacterial endotoxins.
From 0% to 80% of patients with advanced ovarian cancer relapse within 2 years and develop chemotherapy resistance. Some studies have found that gut microbiota has a two-way effect in tumor chemotherapy and targeted therapy [126]. Microorganisms can mediate chemotherapy resistance and enhance anti-tumor activity. Studies have found that multidrug resistance proteins involved in paclitaxel resistance are downregulated upon TLR4 inactivation, further supporting the potential impact of the microbiota on chemotherapy resistance in ovarian cancer [127]. The microbiota affects the efficacy of commonly used drugs for ovarian cancer and has great potential to enhance immunotherapy responses.
Currently, fecal transplantation and probiotic supplementation are commonly used in the treatment of non-malignant diseases [128]. Research has found that changing the microbiota structure is expected to alleviate the adverse reactions of chemotherapy for ovarian cancer and provide new opportunities for its treatment [129]. In the treatment of platinum and anti-PD-1 monoclonal antibodies, combining probiotics can significantly improve the efficacy of ovarian cancer therapy [130]. Moreover, supplementation with Ackermannia or implantation of donor fecal bacteria with good drug response can reverse resistance to PD-1 therapy [131]. Antibiotics may have potential in treating ovarian cancer, but studies have been inconsistent in their conclusions. Chloramphenicol, salinomycin, and cisplatin were used in combination to inhibit tumor growth [132]. While combined treatment with ampicillin, vancomycin, neomycin and metronidazole promoted the growth and invasion of transplanted tumors in nude mice [133].
Conclusion
The ovaries are vital organs in the female reproductive system which can play a crucial role in maintaining overall health. This study focuses on the relationship between ovarian function-related diseases and intestinal flora in women with PCOS, POI and EMS. The findings suggest that an imbalance in intestinal flora can increase intestinal permeability, contributing to low-grade chronic inflammation, IR and HA. Imbalance of intestinal flora can affect the levels of estrogen and androgen that can promote the occurrence and development of PCOS, POI and EMS diseases. In ovarian cancer, the microbiota can also impact disease onset, progression and treatment response. While some studies have demonstrated that probiotics can be beneficial in treating ovarian function-related diseases, further research is needed to elucidate the specific mechanisms by which probiotics act in different conditions and to identify the most effective strains and treatment regimens. This study delves into the molecular mechanisms linking intestinal flora to ovarian function, highlighting that gut imbalance could be a contributing factor in these diseases. Additionally, the potential benefits of probiotics in treating ovarian function-related conditions and cancer offer a promising direction for future clinical applications.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81971354).
Disclosure of conflict of interest
None.
References
- 1.Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467–520. doi: 10.1210/er.2015-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szeliga A, Maciejewska-Jeske M, Męczekalski B. Bone health and evaluation of bone mineral density in patients with premature ovarian insufficiency. Prz Menopauzalny. 2018;17:112–116. doi: 10.5114/pm.2018.78552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24:302–312. doi: 10.1210/er.2003-0004. [DOI] [PubMed] [Google Scholar]
- 5.Safiri S, Noori M, Nejadghaderi SA, Karamzad N, Carson-Chahhoud K, Sullman MJM, Collins GS, Kolahi AA, Avery J. Prevalence, incidence and years lived with disability due to polycystic ovary syndrome in 204 countries and territories, 1990-2019. Hum Reprod. 2022;37:1919–1931. doi: 10.1093/humrep/deac091. [DOI] [PubMed] [Google Scholar]
- 6.Wellons MF, Matthews JJ, Kim C. Ovarian aging in women with diabetes: an overview. Maturitas. 2017;96:109–113. doi: 10.1016/j.maturitas.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Xu JH, Qu QQ, Zhong GQ. Risk of cardiovascular and cerebrovascular events in polycystic ovarian syndrome women: a meta-analysis of cohort studies. Front Cardiovasc Med. 2020;7:552421. doi: 10.3389/fcvm.2020.552421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huhtaniemi I, Hovatta O, La Marca A, Livera G, Monniaux D, Persani L, Heddar A, Jarzabek K, Laisk-Podar T, Salumets A, Tapanainen JS, Veitia RA, Visser JA, Wieacker P, Wolczynski S, Misrahi M. Advances in the molecular pathophysiology, genetics, and treatment of primary ovarian insufficiency. Trends Endocrinol Metab. 2018;29:400–419. doi: 10.1016/j.tem.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI) Front Endocrinol (Lausanne) 2021;12:626924. doi: 10.3389/fendo.2021.626924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Chen C, Yu W, Xu L, Jia H, Wang C, Pei N, Liu Z, Luo D, Wang J, Lv W, Yuan B, Zhang J, Jiang H. Colitis-mediated dysbiosis of the intestinal flora and impaired vitamin A absorption reduce ovarian function in mice. Nutrients. 2023;15:2425. doi: 10.3390/nu15112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunz C, Kuntz S, Rudloff S. Intestinal flora. Adv Exp Med Biol. 2009;639:67–79. doi: 10.1007/978-1-4020-8749-3_6. [DOI] [PubMed] [Google Scholar]
- 12.Yin L, Yang H, Li J, Li Y, Ding X, Wu G, Yin Y. Pig models on intestinal development and therapeutics. Amino Acids. 2017;49:2099–2106. doi: 10.1007/s00726-017-2497-z. [DOI] [PubMed] [Google Scholar]
- 13.Madan S, Mehra MR. Gut dysbiosis and heart failure: navigating the universe within. Eur J Heart Fail. 2020;22:629–637. doi: 10.1002/ejhf.1792. [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Cheng L, Zeng X, Zhang X, Wu Z, Weng P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int J Biol Macromol. 2020;164:1484–1492. doi: 10.1016/j.ijbiomac.2020.07.208. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Zhou J, Gober HJ, Leung WT, Huang Z, Pan X, Li C, Zhang N, Wang L. Alterations in the intestinal microbiome associated with PCOS affect the clinical phenotype. Biomed Pharmacother. 2021;133:110958. doi: 10.1016/j.biopha.2020.110958. [DOI] [PubMed] [Google Scholar]
- 16.Yan J, Kong L, Zhang X, Yu M, Zhu K, Zhao A, Shi D, Sun Y, Wang J, Shen W, Li L. Maternal zearalenone exposure affects gut microbiota and follicular development in suckled offspring. J Agric Food Chem. 2022;70:15570–15582. doi: 10.1021/acs.jafc.2c06457. [DOI] [PubMed] [Google Scholar]
- 17.Lüll K, Arffman RK, Sola-Leyva A, Molina NM, Aasmets O, Herzig KH, Plaza-Díaz J, Franks S, Morin-Papunen L, Tapanainen JS, Salumets A, Altmäe S, Piltonen TT, Org E. The gut microbiome in polycystic ovary syndrome and its association with metabolic traits. J Clin Endocrinol Metab. 2021;106:858–871. doi: 10.1210/clinem/dgaa848. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Liu Y, Li X. Effects of intestinal flora on polycystic ovary syndrome. Front Endocrinol (Lausanne) 2023;14:1151723. doi: 10.3389/fendo.2023.1151723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N. Sex difference of gut microbiota. Sex/Gender-Specific Medicine in the Gastrointestinal Diseases. 2022:363–377. [Google Scholar]
- 20.Hamilton KJ, Hewitt SC, Arao Y, Korach KS. Estrogen hormone biology. Curr Top Dev Biol. 2017;125:109–146. doi: 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JÅ. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 22.Leung KC, Johannsson G, Leong GM, Ho KK. Estrogen regulation of growth hormone action. Endocr Rev. 2004;25:693–721. doi: 10.1210/er.2003-0035. [DOI] [PubMed] [Google Scholar]
- 23.Sui Y, Wu J, Chen J. The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front Cell Dev Biol. 2021;9:631552. doi: 10.3389/fcell.2021.631552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66:487–495. doi: 10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 25.Guarner-Lans V, Rubio-Ruiz ME, Pérez-Torres I, Baños de MacCarthy G. Relation of aging and sex hormones to metabolic syndrome and cardiovascular disease. Exp Gerontol. 2011;46:517–523. doi: 10.1016/j.exger.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Lui LY, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 27.d’Afflitto M, Upadhyaya A, Green A, Peiris M. Association between sex hormone levels and gut microbiota composition and diversity-a systematic review. J Clin Gastroenterol. 2022;56:384–392. doi: 10.1097/MCG.0000000000001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 29.Flores R, Shi J, Fuhrman B, Xu X, Veenstra TD, Gail MH, Gajer P, Ravel J, Goedert JJ. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JJ, Jung YL, Cheong TC, Espejo Valle-Inclan J, Chu C, Gulhan DC, Ljungström V, Jin H, Viswanadham VV, Watson EV, Cortés-Ciriano I, Elledge SJ, Chiarle R, Pellman D, Park PJ. ERα-associated translocations underlie oncogene amplifications in breast cancer. Nature. 2023;618:1024–1032. doi: 10.1038/s41586-023-06057-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushita M, Fujita K, Motooka D, Hatano K, Fukae S, Kawamura N, Tomiyama E, Hayashi Y, Banno E, Takao T, Takada S, Yachida S, Uemura H, Nakamura S, Nonomura N. The gut microbiota associated with high-Gleason prostate cancer. Cancer Sci. 2021;112:3125–3135. doi: 10.1111/cas.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wylie K, Rees M, Hackett G, Anderson R, Bouloux PM, Cust M, Goldmeier D, Kell P, Terry T, Trinick T, Wu F. Androgens, health and sexuality in women and men. Maturitas. 2010;67:275–289. doi: 10.1016/j.maturitas.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Basson R, Brotto LA, Petkau AJ, Labrie F. Role of androgens in women’s sexual dysfunction. Menopause. 2010;17:962–971. doi: 10.1097/gme.0b013e3181d59765. [DOI] [PubMed] [Google Scholar]
- 34.Devendran S, Mythen SM, Ridlon JM. The desA and desB genes from Clostridium scindens ATCC 35704 encode steroid-17,20-desmolase. J Lipid Res. 2018;59:1005–1014. doi: 10.1194/jlr.M083949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambyal K, Singh RV. Production aspects of testosterone by microbial biotransformation and future prospects. Steroids. 2020;159:108651. doi: 10.1016/j.steroids.2020.108651. [DOI] [PubMed] [Google Scholar]
- 36.Colldén H, Landin A, Wallenius V, Elebring E, Fändriks L, Nilsson ME, Ryberg H, Poutanen M, Sjögren K, Vandenput L, Ohlsson C. The gut microbiota is a major regulator of androgen metabolism in intestinal contents. Am J Physiol Endocrinol Metab. 2019;317:E1182–E1192. doi: 10.1152/ajpendo.00338.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sardana K, Muddebihal A, Sehrawat M, Bansal P, Khurana A. An updated clinico-investigative approach to diagnosis of cutaneous hyperandrogenism in relation to adult female acne, female pattern alopecia & hirsutism a primer for dermatologists. Expert Rev Endocrinol Metab. 2024;19:111–128. doi: 10.1080/17446651.2023.2299400. [DOI] [PubMed] [Google Scholar]
- 38.Gulhan I, Bozkaya G, Uyar I, Oztekin D, Pamuk BO, Dogan E. Serum lipid levels in women with premature ovarian failure. Menopause. 2012;19:1231–1234. doi: 10.1097/gme.0b013e318254102b. [DOI] [PubMed] [Google Scholar]
- 39.Neves AR, Montoya-Botero P, Polyzos NP. The role of androgen supplementation in women with diminished ovarian reserve: time to randomize, not meta-analyze. Front Endocrinol (Lausanne) 2021;12:653857. doi: 10.3389/fendo.2021.653857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Wu K, Wu Y. The emerging role of ferroptosis in female reproductive disorders. Biomed Pharmacother. 2023;166:115415. doi: 10.1016/j.biopha.2023.115415. [DOI] [PubMed] [Google Scholar]
- 41.Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, Joe B, Hill JW. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes. 2018;9:400–421. doi: 10.1080/19490976.2018.1441664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang S, Wu X, Jin F. Gut-brain psychology: rethinking psychology from the microbiota-gut-brain axis. Front Integr Neurosci. 2018;12:33. doi: 10.3389/fnint.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson OE, Chukwuma EF. Association of body mass index with hypothalamus-pituitary-ovarian axis hormones in infertile women in the Niger Delta region, Nigeria. Open J Obstet Gynecol. 2022;12:671–685. [Google Scholar]
- 44.Acevedo-Rodriguez A, Kauffman AS, Cherrington BD, Borges CS, Roepke TA, Laconi M. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. J Neuroendocrinol. 2018;30:e12590. doi: 10.1111/jne.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilie IR. Neurotransmitter, neuropeptide and gut peptide profile in PCOS-pathways contributing to the pathophysiology, food intake and psychiatric manifestations of PCOS. Adv Clin Chem. 2020;96:85–135. doi: 10.1016/bs.acc.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Bacterial neuroactive compounds produced by psychobiotics. Adv Exp Med Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 47.Romero-Reyes J, Cárdenas M, Damián-Matsumura P, Domínguez R, Ayala ME. Inhibition of serotonin reuptake in the prepubertal rat ovary by fluoxetine and effects on ovarian functions. Reprod Toxicol. 2016;59:80–88. doi: 10.1016/j.reprotox.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Ruas-Madiedo P, Medrano M, Salazar N, De Los Reyes-Gavilán CG, Pérez PF, Abraham AG. Exopolysaccharides produced by Lactobacillus and Bifidobacterium strains abrogate in vitro the cytotoxic effect of bacterial toxins on eukaryotic cells. J Appl Microbiol. 2010;109:2079–2086. doi: 10.1111/j.1365-2672.2010.04839.x. [DOI] [PubMed] [Google Scholar]
- 49.Kuebutornye FK, Tang J, Cai J, Yu H, Wang Z, Abarike ED, Lu Y, Li Y, Afriyie G. In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture. 2020;527:735440. [Google Scholar]
- 50.Haque R, Das II, Sawant PB, Chadha NK, Sahoo L, Kumar R, Sundaray JK. Tenets in microbial endocrinology: a new vista in teleost reproduction. Front Physiol. 2022;13:871045. doi: 10.3389/fphys.2022.871045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai H, Cao X, Qin D, Liu Y, Liu Y, Hua J, Peng S. Gut microbiota supports male reproduction via nutrition, immunity, and signaling. Front Microbiol. 2022;13:977574. doi: 10.3389/fmicb.2022.977574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan Å, Nord CE. The place of probiotics in human intestinal infections. Int J Antimicrob Agents. 2002;20:313–319. doi: 10.1016/s0924-8579(02)00199-1. [DOI] [PubMed] [Google Scholar]
- 53.LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L, Fusek J, Rodrigo L, Kruzliak P. Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab (Lond) 2016;13:14. doi: 10.1186/s12986-016-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B, Guo Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br J Nutr. 2009;102:687–693. doi: 10.1017/S0007114509289033. [DOI] [PubMed] [Google Scholar]
- 56.Scheithauer TP, Dallinga-Thie GM, de Vos WM, Nieuwdorp M, van Raalte DH. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5:759–770. doi: 10.1016/j.molmet.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. 2020;13:73. doi: 10.1186/s13048-020-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Zhu Y, Li X, Sun B. Dynamic balancing of intestinal short-chain fatty acids: the crucial role of bacterial metabolism. Trends Food Sci Tech. 2020;100:118–130. [Google Scholar]
- 59.Drasar BS, Hill MJ. Intestinal bacteria and cancer. The Am J Clin Nutr. 1972;25:1399–1404. doi: 10.1093/ajcn/25.12.1399. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Jia R, Gong H, Celi P, Zhuo Y, Ding X, Bai S, Zeng Q, Yin H, Xu S, Liu J, Mao X, Zhang K. The effect of oxidative stress on the chicken ovary: involvement of microbiota and melatonin interventions. Antioxidants (Basel) 2021;10:1422. doi: 10.3390/antiox10091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang YL, Zhou WW, Wu S, Tang WL, Wang ZW, Zhou ZY, Li ZW, Huang QF, He Y, Zhou HW. Intestinal flora is a key factor in insulin resistance and contributes to the development of polycystic ovary syndrome. Endocrinology. 2021;162:bqab118. doi: 10.1210/endocr/bqab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu M, Yan J, Wu Y, Zhu H, Huang Y, Wu K. The impact of herbal medicine in regulating intestinal flora on female reproductive disorders. Front Pharmacol. 2022;13:1026141. doi: 10.3389/fphar.2022.1026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao QQ, Ni ZX, Bi YL, Sun S, Cheng W, Yu CQ. Huayu Jiedu prescription alleviates gut microbiota and fecal metabolites in mice with endometriosis. Chinese Journal of Experimental Traditional Medical Formulae. 2021:202–214. [Google Scholar]
- 64.Zehra B, Khursheed A. Polycystic ovarian syndrome: symptoms, treatment and diagnosis: a review. J Pharmacognosy Phytochem. 2018;7:875–880. [Google Scholar]
- 65.Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, Capeau J, Bastard JP. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women. Int J Obes Relat Metab Disord. 2004;28:993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 66.Sun X, Cui Y, Su Y, Gao Z, Diao X, Li J, Zhu X, Li D, Li Z, Wang C, Shi Y. Dietary fiber ameliorates lipopolysaccharide-induced intestinal barrier function damage in piglets by modulation of intestinal microbiome. mSystems. 2021;6:e01374–20. doi: 10.1128/mSystems.01374-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linsalata M, Riezzo G, D’Attoma B, Clemente C, Orlando A, Russo F. Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: a case-control study. BMC Gastroenterol. 2018;18:167. doi: 10.1186/s12876-018-0888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serek P, Oleksy-Wawrzyniak M. The effect of bacterial infections, probiotics and zonulin on intestinal barrier integrity. Int J Mol Sci. 2021;22:11359. doi: 10.3390/ijms222111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rahman MA, Pal RK, Islam N, Freeman R, Berthiaume F, Mazzeo A, Ashraf A. A facile graphene conductive polymer paper based biosensor for dopamine, TNF-α, and IL-6 detection. Sensors (Basel) 2023;23:8115. doi: 10.3390/s23198115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang T, Li G, Xu Y, He X, Song B, Cao Y. Characterization of the gut microbiota in polycystic ovary syndrome with dyslipidemia. BMC Microbiol. 2024;24:169. doi: 10.1186/s12866-024-03329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuzuno T, Takahashi N, Yamada-Hara M, Yokoji-Takeuchi M, Sulijaya B, Aoki-Nonaka Y, Matsugishi A, Katakura K, Tabeta K, Yamazaki K. Ingestion of Porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J Periodontal Res. 2021;56:275–288. doi: 10.1111/jre.12816. [DOI] [PubMed] [Google Scholar]
- 72.Guo Y, Qi Y, Yang X, Zhao L, Wen S, Liu Y, Tang L. Association between polycystic ovary syndrome and gut microbiota. PLoS One. 2016;11:e0153196. doi: 10.1371/journal.pone.0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: is there a difference? Clin Endocrinol (Oxf) 2002;57:343–350. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 74.Guney C, Bal NB, Akar F. The impact of dietary fructose on gut permeability, microbiota, abdominal adiposity, insulin signaling and reproductive function. Heliyon. 2023;9:e18896. doi: 10.1016/j.heliyon.2023.e18896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kosmalski M, Śliwińska A, Drzewoski J. Non-alcoholic fatty liver disease or type 2 diabetes mellitus-the chicken or the egg dilemma. Biomedicines. 2023;11:1097. doi: 10.3390/biomedicines11041097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Q, He R, Zhang F, Zhang J, Lian S, Liu H. Combination of oligofructose and metformin alters the gut microbiota and improves metabolic profiles, contributing to the potentiated therapeutic effects on diet-induced obese animals. Front Endocrinol (Lausanne) 2020;10:939. doi: 10.3389/fendo.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ni Z, Cheng W, Ding J, Yao R, Zhang D, Zhai D, Zhou L, Yu C. Impact of Buzhong Yiqi prescription on the gut microbiota of patients with obesity manifesting polycystic ovarian syndrome. Evid Based Complement Alternat Med. 2021;2021:6671367. doi: 10.1155/2021/6671367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Wu D, Guo H, Li M. Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 2019;236:116940. doi: 10.1016/j.lfs.2019.116940. [DOI] [PubMed] [Google Scholar]
- 79.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8:523–531. doi: 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- 80.Vojnović Milutinović D, Teofilović A, Veličković N, Brkljačić J, Jelača S, Djordjevic A, Macut D. Glucocorticoid signaling and lipid metabolism disturbances in the liver of rats treated with 5α-dihydrotestosterone in an animal model of polycystic ovary syndrome. Endocrine. 2021;72:562–572. doi: 10.1007/s12020-020-02600-1. [DOI] [PubMed] [Google Scholar]
- 81.Wu J, Zhuo Y, Liu Y, Chen Y, Ning Y, Yao J. Association between premature ovarian insufficiency and gut microbiota. BMC Pregnancy Childbirth. 2021;21:418. doi: 10.1186/s12884-021-03855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li M, Zhu Y, Wei J, Chen L, Chen S, Lai D. The global prevalence of premature ovarian insufficiency: a systematic review and meta-analysis. Climacteric. 2023;26:95–102. doi: 10.1080/13697137.2022.2153033. [DOI] [PubMed] [Google Scholar]
- 83.Szeliga A, Calik-Ksepka A, Maciejewska-Jeske M, Grymowicz M, Smolarczyk K, Kostrzak A, Smolarczyk R, Rudnicka E, Meczekalski B. Autoimmune diseases in patients with premature ovarian insufficiency-our current state of knowledge. Int J Mol Sci. 2021;22:2594. doi: 10.3390/ijms22052594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Domniz N, Meirow D. Premature ovarian insufficiency and autoimmune diseases. Best Pract Res Clin Obstet Gynaecol. 2019;60:42–55. doi: 10.1016/j.bpobgyn.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Kirshenbaum M, Orvieto R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J Assist Reprod Genet. 2019;36:2207–2215. doi: 10.1007/s10815-019-01572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E, Gasbarrini A. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 2013;17:323–33. [PubMed] [Google Scholar]
- 87.Jiao X, Zhang X, Li N, Zhang D, Zhao S, Dang Y, Zanvit P, Jin W, Chen ZJ, Chen W, Qin Y. Treg deficiency-mediated TH1 response causes human premature ovarian insufficiency through apoptosis and steroidogenesis dysfunction of granulosa cells. Clin Transl Med. 2021;11:e448. doi: 10.1002/ctm2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin SY, Hussain Z, Lee YJ, Park H. An altered composition of fecal microbiota, organic acids, and the effect of probiotics in the guinea pig model of postoperative ileus. Neurogastroenterol Motil. 2021;33:e13966. doi: 10.1111/nmo.13966. [DOI] [PubMed] [Google Scholar]
- 90.Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O’Malley BW. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163:960–974. doi: 10.1016/j.cell.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Medina-Perucha L, Pistillo A, Raventós B, Jacques-Aviñó C, Munrós-Feliu J, Martínez-Bueno C, Valls-Llobet C, Carmona F, López-Jiménez T, Pujolar-Díaz G, Flo Arcas E, Berenguera A, Duarte-Salles T. Endometriosis prevalence and incidence trends in a large population-based study in Catalonia (Spain) from 2009 to 2018. Womens Health (Lond) 2022;18:17455057221130566. doi: 10.1177/17455057221130566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Begum MIA, Chuan L, Hong ST, Chae HS. The pathological role of miRNAs in endometriosis. Biomedicines. 2023;11:3087. doi: 10.3390/biomedicines11113087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taghavipour M, Sadoughi F, Mirzaei H, Yousefi B, Moazzami B, Chaichian S, Mansournia MA, Asemi Z. Apoptotic functions of microRNAs in pathogenesis, diagnosis, and treatment of endometriosis. Cell Biosci. 2020;10:12. doi: 10.1186/s13578-020-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei Y, Tan H, Yang R, Yang F, Liu D, Huang B, OuYang L, Lei S, Wang Z, Jiang S, Cai H, Xie X, Yao S, Liang Y. Gut dysbiosis-derived β-glucuronidase promotes the development of endometriosis. Fertil Steril. 2023;120:682–694. doi: 10.1016/j.fertnstert.2023.03.032. [DOI] [PubMed] [Google Scholar]
- 95.Chadchan SB, Popli P, Ambati CR, Tycksen E, Han SJ, Bulun SE, Putluri N, Biest SW, Kommagani R. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci Alliance. 2021;4:e202101224. doi: 10.26508/lsa.202101224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ye Q, Zeng X, Wang S, Zeng X, Yang G, Ye C, Cai S, Chen M, Li S, Qiao S. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARγ and PGC1α pathways in ovarian granulosa cells. FASEB J. 2021;35:e21316. doi: 10.1096/fj.202000444R. [DOI] [PubMed] [Google Scholar]
- 97.Shan J, Ni Z, Cheng W, Zhou L, Zhai D, Sun S, Yu C. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch Gynecol Obstet. 2021;304:1363–1373. doi: 10.1007/s00404-021-06057-z. [DOI] [PubMed] [Google Scholar]
- 98.Kim JE, Kim SJ, Jeong HW, Lee BH, Choi JY, Park RW, Park JY, Kim IS. RGD peptides released from βig-h3, a TGF-β-induced cell-adhesive molecule, mediate apoptosis. Oncogene. 2003;22:2045–2053. doi: 10.1038/sj.onc.1206269. [DOI] [PubMed] [Google Scholar]
- 99.Tsai CE, Chiu CT, Rayner CK, Wu KL, Chiu YC, Hu ML, Chuah SK, Tai WC, Liang CM, Wang HM. Associated factors in Streptococcus bovis bacteremia and colorectal cancer. Kaohsiung J Med Sci. 2016;32:196–200. doi: 10.1016/j.kjms.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Christodoulakos G, Augoulea A, Lambrinoudaki I, Sioulas V, Creatsas G. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance’. Eur J Contracept Reprod Health Care. 2007;12:194–202. doi: 10.1080/13625180701387266. [DOI] [PubMed] [Google Scholar]
- 101.Vinatier D, Orazi G, Cosson M, Dufour P. Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2001;96:21–34. doi: 10.1016/s0301-2115(00)00405-x. [DOI] [PubMed] [Google Scholar]
- 102.Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl. 2001:29–40. doi: 10.1080/003655201753265082. [DOI] [PubMed] [Google Scholar]
- 103.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97(Suppl):2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 104.Yada-Hashimoto N, Yamamoto T, Kamiura S, Seino H, Ohira H, Sawai K, Kimura T, Saji F. Metastatic ovarian tumors: a review of 64 cases. Gynecol Oncol. 2003;89:314–317. doi: 10.1016/s0090-8258(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 105.Chase D, Goulder A, Zenhausern F, Monk B, Herbst-Kralovetz M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: a review of applications in etiology, symptoms and treatment. Gynecol Oncol. 2015;138:190–200. doi: 10.1016/j.ygyno.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 106.Houghton LA, Whorwell PJ. Towards a better understanding of abdominal bloating and distension in functional gastrointestinal disorders. Neurogastroenterol Motil. 2005;17:500–511. doi: 10.1111/j.1365-2982.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 107.Liu Q, Yang W, Luo N, Liu J, Wu Y, Ding J, Li C, Cheng Z. LPS and IL-8 activated umbilical cord blood-derived neutrophils inhibit the progression of ovarian cancer. J Cancer. 2020;11:4413–4420. doi: 10.7150/jca.41035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y, Tao F, Jiang J, Chen L, Du J, Cheng X, He Q, Zhong S, Chen W, Wu X, Ou R, Xu Y, Tang KF. Tryptophan 2, 3-dioxygenase promotes proliferation, migration and invasion of ovarian cancer cells. Mol Med Rep. 2021;23:445. doi: 10.3892/mmr.2021.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruze R, Song J, Yin X, Chen Y, Xu R, Wang C, Zhao Y. Mechanisms of obesity- and diabetes mellitus-related pancreatic carcinogenesis: a comprehensive and systematic review. Signal Transduct Target Ther. 2023;8:139. doi: 10.1038/s41392-023-01376-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yaginuma S, Omi J, Uwamizu A, Aoki J. Emerging roles of lysophosphatidylserine as an immune modulator. Immunol Rev. 2023;317:20–29. doi: 10.1111/imr.13204. [DOI] [PubMed] [Google Scholar]
- 111.Pan T, Pei Z, Fang Z, Wang H, Zhu J, Zhang H, Zhao J, Chen W, Lu W. Uncovering the specificity and predictability of tryptophan metabolism in lactic acid bacteria with genomics and metabolomics. Front Cell Infect Microbiol. 2023;13:1154346. doi: 10.3389/fcimb.2023.1154346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu S, Liu Z, Lv M, Chen Y, Liu Y. Intestinal dysbiosis promotes epithelial-mesenchymal transition by activating tumor-associated macrophages in ovarian cancer. Pathog Dis. 2019;77:ftz019. doi: 10.1093/femspd/ftz019. [DOI] [PubMed] [Google Scholar]
- 113.Miao R, Badger TC, Groesch K, Diaz-Sylvester PL, Wilson T, Ghareeb A, Martin JA, Cregger M, Welge M, Bushell C, Auvil L, Zhu R, Brard L, Braundmeier-Fleming A. Assessment of peritoneal microbial features and tumor marker levels as potential diagnostic tools for ovarian cancer. PLoS One. 2020;15:e0227707. doi: 10.1371/journal.pone.0227707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017:61. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 115.Tegegne BA, Kebede B. Probiotics, their prophylactic and therapeutic applications in human health development: a review of the literature. Heliyon. 2022;8:e09725. doi: 10.1016/j.heliyon.2022.e09725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duan H, Yu L, Tian F, Zhai Q, Fan L, Chen W. Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit Rev Food Sci Nutr. 2022;62:1427–1452. doi: 10.1080/10408398.2020.1843396. [DOI] [PubMed] [Google Scholar]
- 117.Alvarez-Olmos MI, Oberhelman RA. Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clin Infect Dis. 2001;32:1567–1576. doi: 10.1086/320518. [DOI] [PubMed] [Google Scholar]
- 118.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Jin P, Xie Y. Treatment strategies for women with polycystic ovary syndrome. Gynecol Endocrinol. 2018;34:272–277. doi: 10.1080/09513590.2017.1395841. [DOI] [PubMed] [Google Scholar]
- 120.Ahmadi S, Jamilian M, Karamali M, Tajabadi-Ebrahimi M, Jafari P, Taghizadeh M, Memarzadeh MR, Asemi Z. Probiotic supplementation and the effects on weight loss, glycaemia and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Hum Fertil (Camb) 2017;20:254–261. doi: 10.1080/14647273.2017.1283446. [DOI] [PubMed] [Google Scholar]
- 121.Delzenne NM, Knudsen C, Beaumont M, Rodriguez J, Neyrinck AM, Bindels LB. Contribution of the gut microbiota to the regulation of host metabolism and energy balance: a focus on the gut-liver axis. Proc Nutr Soc. 2019;78:319–328. doi: 10.1017/S0029665118002756. [DOI] [PubMed] [Google Scholar]
- 122.Salles BIM, Cioffi D, Ferreira SRG. Probiotics supplementation and insulin resistance: a systematic review. Diabetol Metab Syndr. 2020;12:98. doi: 10.1186/s13098-020-00603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang Y, Kang X, Yang H, Liu H, Yang X, Liu Q, Tian H, Xue Y, Ren P, Kuang X, Cai Y, Tong M, Li L, Fan W. Lactobacillus acidophilus ameliorates obesity in mice through modulation of gut microbiota dysbiosis and intestinal permeability. Pharmacol Res. 2022;175:106020. doi: 10.1016/j.phrs.2021.106020. [DOI] [PubMed] [Google Scholar]
- 124.López-Gómez L, Szymaszkiewicz A, Zielińska M, Abalo R. Nutraceuticals and enteric glial cells. Molecules. 2021;26:3762. doi: 10.3390/molecules26123762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bordalo Tonucci L, Dos Santos KM, De Luces Fortes Ferreira CL, Ribeiro SM, De Oliveira LL, Martino HS. Gut microbiota and probiotics: focus on diabetes mellitus. Crit Rev Food Sci Nutr. 2017;57:2296–2309. doi: 10.1080/10408398.2014.934438. [DOI] [PubMed] [Google Scholar]
- 126.Zhu R, Lang T, Yan W, Zhu X, Huang X, Yin Q, Li Y. Gut microbiota: influence on carcinogenesis and modulation strategies by drug delivery systems to improve cancer therapy. Adv Sci (Weinh) 2021;8:2003542. doi: 10.1002/advs.202003542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Borella F, Carosso AR, Cosma S, Preti M, Collemi G, Cassoni P, Bertero L, Benedetto C. Gut microbiota and gynecological cancers: a summary of pathogenetic mechanisms and future directions. ACS Infect Dis. 2021;7:987–1009. doi: 10.1021/acsinfecdis.0c00839. [DOI] [PubMed] [Google Scholar]
- 128.Zeng W, Shen J, Bo T, Peng L, Xu H, Nasser MI, Zhuang Q, Zhao M. Cutting edge: probiotics and fecal microbiota transplantation in immunomodulation. J Immunol Res. 2019;2019:1603758. doi: 10.1155/2019/1603758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng H, Wang Z, Cui L, Wen Y, Chen X, Gong F, Yi H. Opportunities and challenges of the human microbiome in ovarian cancer. Front Oncol. 2020;10:163. doi: 10.3389/fonc.2020.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Najafi S, Majidpoor J, Mortezaee K. The impact of microbiota on PD-1/PD-L1 inhibitor therapy outcomes: a focus on solid tumors. Life Sci. 2022;310:121138. doi: 10.1016/j.lfs.2022.121138. [DOI] [PubMed] [Google Scholar]
- 131.Tymoszuk P, Nairz M, Brigo N, Petzer V, Heeke S, Kircher B, Hermann-Kleiter N, Klepsch V, Theurl I, Weiss G, Pfeifhofer-Obermair C. Iron supplementation interferes with immune therapy of murine mammary carcinoma by inhibiting anti-tumor T cell function. Front Oncol. 2020;10:584477. doi: 10.3389/fonc.2020.584477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gao Y, Shang Q, Li W, Guo W, Stojadinovic A, Mannion C, Man YG, Chen T. Antibiotics for cancer treatment: a double-edged sword. J Cancer. 2020;11:5135–5149. doi: 10.7150/jca.47470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li Q, Ma L, Shen S, Guo Y, Cao Q, Cai X, Feng J, Yan Y, Hu T, Luo S, Zhou L, Peng B, Yang Z, Hua Y. Intestinal dysbacteriosis-induced IL-25 promotes development of HCC via alternative activation of macrophages in tumor microenvironment. J Exp Clin Cancer Res. 2019;38:303. doi: 10.1186/s13046-019-1271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]