Abstract
Objective: To investigate the predictive value of placental growth factor (PlGF) for adverse pregnancy outcome in twin pregnancies at advanced maternal age. Methods: A retrospective analysis was conducted on 387 women with twin pregnancies who delivered at Northwest Women’s and Children’s Hospital between March 2020 and March 2024. The women were divided into a favorable outcome group (n = 249) and an adverse outcome group (n = 138) based on their pregnancy outcome. Clinical data and laboratory indicators were compared between the two groups. Logistic regression analysis was used to identify independent risk factors for adverse pregnancy outcome. Receiver Operating Characteristic (ROC) curve analysis was performed to evaluate the predictive value of these independent risk factors. Additionally, the interaction between PlGF and other independent risk factors was analyzed. Results: Significant differences were observed between the favorable and adverse outcome groups in terms of age, pre-pregnancy Body Mass Index (BMI), mode of conception, gestational hypertension, and gestational diabetes (all P < 0.05). Laboratory indicators revealed that the levels of White Blood Cells (WBC), Neutrophils (Neut), Alpha-Fetoprotein (AFP), Beta Human Chorionic Gonadotropin (β-HCG), and 24-hour urine protein quantification were lower in the favorable outcome group, while the levels of Lymphocytes (Lym) and PlGF were higher (all P < 0.05). Multivariate logistic regression analysis identified mode of conception, Neut, Lym, AFP, β-HCG, 24-hour urine protein quantification, and PlGF as independent risk factors for adverse pregnancy outcome (all P < 0.05). The Area Under the Curve (AUC) for PlGF in predicting adverse pregnancy outcomes was 0.874. Furthermore, an interaction was found between PlGF and adverse pregnancy outcome (P < 0.001) as well as between PlGF and 24-hour urine protein level (P = 0.035). Conclusion: PlGF has significant clinical value for predicting adverse pregnancy outcome in twin pregnancies among women of advanced maternal age. Its levels are strongly correlated with pregnancy outcome and may serve as an effective tool for early screening and intervention in high-risk pregnancies.
Keywords: Advanced maternal age, twin pregnancies, placental growth factor, pregnancy outcomes, predictive value
Introduction
Twin pregnancy is a complex condition characterized by simultaneous gestation of two fetuses, which poses heightened risks and challenges for both the mother and the fetuses throughout the pregnancy [1]. Complications associated with twin pregnancies, such as gestational hypertension, gestational diabetes, preterm birth, and placental complications, can significantly affect maternal and neonatal health [2]. The placenta plays a particularly crucial role in twin pregnancies, as its morphologic and functional abnormalities can directly influence fetal growth and pregnancy outcomes [3]. Advanced maternal age further elevates the risk of adverse outcome in twin pregnancies due to the decline in physiological functions [4]. Studies have shown that advanced maternal age is strongly associated with the development of gestational hypertension and pre-eclampsia, both of which can severely affect maternal and neonatal prognosis [4]. Additionally, older mothers are more likely to develop gestational diabetes, complicating the pregnancy and increasing the risk of adverse outcomes, such as fetal growth restriction and preterm labor. Placental abnormalities, including insufficiency and dysfunction, are more prevalent in twin pregnancies among older women, leading to an increased risk of intrauterine distress, preterm labor, and low birth weight infants. These factors collectively contribute to the higher incidence of adverse pregnancy outcome in twin pregnancies among women of advanced maternal age [5]. Therefore, effective management and monitoring of twin pregnancies are particularly important in older women.
Placental Growth Factor (PlGF) is a protein secreted by the placenta that belongs to the vascular endothelial growth factor (VEGF) family [6]. Its primary role during pregnancy is to promote placental angiogenesis and maintain normal placental function, thereby ensuring proper fetal development [7]. PlGF regulates placental blood supply, ensuring that the fetus receives adequate oxygen and nutrients to support healthy growth and development [8]. Research has shown that abnormal PlGF levels are often associated with various pregnancy complications, such as preeclampsia [9] and fetal growth restriction [10]. Low levels of PlGF are closely linked to the development of preeclampsia, a phenomenon extensively studied and validated in singleton pregnancies [11]. Moreover, PlGF levels can serve as an important biomarker for predicting pregnancy outcome, particularly in the context of gestational hypertensive diseases and fetal growth restriction [12]. However, research on PlGF in twin pregnancies is relatively limited, with most studies focusing on PlGF levels in singleton pregnancies and their predictive value for adverse outcomes [13]. The levels of PlGF in twin pregnancies and their predictive significance, particularly in women of advanced maternal age, warrant further investigation.
Therefore, this study aims to explore the predictive value of maternal plasma PlGF levels for adverse pregnancy outcomes in twin pregnancies at advanced maternal age and to analyze their correlation. By monitoring and analyzing PlGF levels, we seek to evaluate its effectiveness in predicting adverse pregnancy outcome, thereby providing a basis for early intervention and management.
Materials and methods
Ethical statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Northwest Women’s and Children’s Hospital.
Case source and grouping
A retrospective cohort study was performed on women with twin pregnancies who delivered at Northwest Women’s and Children’s Hospital between March 2020 and March 2024. A total of 387 eligible cases were identified based on predefined inclusion and exclusion criteria. The participants were categorized into two groups based on their pregnancy outcomes: a favorable outcome group (n = 249) and an adverse outcome group (n = 138). Grouping was determined by reviewing medical records for specific maternal and neonatal outcomes, ensuring consistent and accurate classification.
Inclusion and exclusion criteria
Inclusion criteria: (1) Confirmed twin pregnancy [14]; (2) Age ≥ 35 years; (3) Gestational age of 14-28 weeks; (4) All prenatal examinations conducted at Northwest Women’s and Children’s Hospital; (5) Complete clinical data.
Exclusion criteria: (1) Presence of congenital diseases; (2) Concurrent malignant tumors; (3) Congenital cardiovascular diseases; (4) Presence of underlying diseases or reproductive system diseases; (5) Use of corticosteroids or other medications during pregnancy that may affect serum marker levels.
Definition of adverse pregnancy outcome
Adverse pregnancy outcomes were meticulously defined to include both maternal and neonatal complications. For maternal outcomes, adverse events included preterm birth (delivery before 37 weeks of gestation), premature rupture of membranes (PROM), and postpartum hemorrhage (blood loss exceeding 500 mL within 24 hours after delivery). For neonatal outcomes, adverse events included intrauterine distress (non-reassuring fetal status), neonatal asphyxia (Apgar score of 3 or less at 1 minute or 5 or less at 5 minutes), and neonatal death (death within the first 28 days of life) [15].
Collection of clinical data
Clinical data and laboratory indicators were collected from the hospital’s electronic medical record system. The collected clinical data encompassed a wide range of variables, including maternal age, parity (number of deliveries), gravidity (number of pregnancies), history of miscarriage, pre-pregnancy Body Mass Index (BMI), gestational weight gain, education level, mode of conception (assisted reproduction/natural conception), family history of diabetes, gestational hypertension, gestational diabetes, and chorionicity (monochorionic or dichorionic twins). Laboratory indicators included complete blood counts (White Blood Cells [WBC], Platelets [PLT], Neutrophils [Neut], Lymphocytes [Lym]), serum levels of Alpha-Fetoprotein (AFP), Beta Human Chorionic Gonadotropin (β-HCG), Hemoglobin (Hb), and Placental Growth Factor (PlGF). Blood routine indicators, AFP, and PlGF were measured between 16-20 weeks of gestation, while 24-hour urine protein quantification was performed between 24-28 weeks, and β-HCG was measured between 20-28 weeks if an abnormal pregnancy was suspected.
Detection methods
Venous blood samples (5 mL) were collected from each participant before delivery and processed immediately to ensure the accuracy of laboratory results. Blood routine indicators were analyzed using a Sysmex XN-3000 automatic hematology analyzer, following standard operating procedures. Serum AFP levels were determined through chemiluminescence immunoassay using a Roche Cobas80 analyzer, which is known for its high sensitivity and specificity. β-HCG levels were measured using a double antibody sandwich method, with reagents provided by Shanghai Initial Biotechnology Co., Ltd. Serum PlGF levels were quantified using an enzyme-linked immunosorbent assay (ELISA), with kits supplied by Shanghai Enzyme-linked Biotechnology Co., Ltd. All measurements were carried out in strict accordance with the manufacturers’ protocols, and quality control was consistently maintained to ensure reliable data.
Outcome measures
Primary outcome
The independent risk factors for adverse pregnancy outcome were identified through logistic regression analysis. Receiver Operating Characteristic (ROC) curve analysis was utilized to evaluate the predictive value of these risk factors, with a particular focus on PlGF. Additionally, potential interactions between PlGF and other independent risk factors were assessed to explore their combined effect on pregnancy outcome.
Secondary outcomes
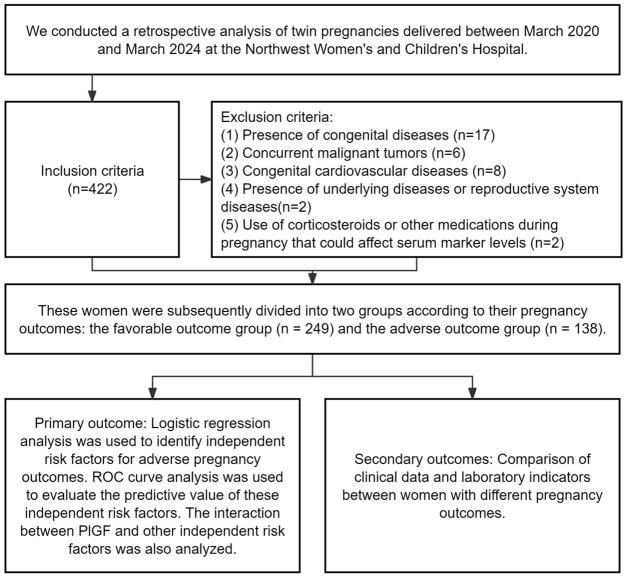
The clinical data and laboratory indicators were compared between women with favorable and adverse pregnancy outcome groups. The study flow chart is presented in Figure 1.
Figure 1.
Study flow chart.
Statistical analysis
Statistical analyses were performed using SPSS version 26.0 (IBM Corp, Armonk, NY). Categorical data were compared using the chi-square test. The distribution of continuous data was evaluated using the Kolmogorov-Smirnov test. For normally distributed data, independent sample t-tests were employed, and results were reported as mean ± standard deviation (SD). Non-normally distributed data were analyzed using the rank-sum test, with results expressed as median [interquartile range]. Multivariate logistic regression analysis was conducted using the stats package in R (version 4.3.2) to identify independent risk factors for adverse pregnancy outcomes. The interaction between PlGF and other independent risk factors was analyzed using the rms package in R software. ROC curve analysis was performed with the pROC package to determine the cut-off values for predicting adverse outcome. Data visualization was carried out using the ggplot2 package. Statistical significance was set at P < 0.05 for all analyses.
Results
Comparison of clinical data between different outcome groups
Patients were grouped into a favorable outcome group and an adverse outcome group based on their pregnancy outcome. The comparison of clinical data between these two groups revealed no significant differences in parity (P = 0.206), gravidity (P = 0.883), history of miscarriage (P = 0.485), gestational weight gain (P = 0.181), education level (P = 0.201), family history of diabetes (P = 0.482), or chorionicity (P = 0.295) (Table 1). However, significant differences were observed in age (P = 0.003), pre-pregnancy BMI (P = 0.002), mode of conception (P = 0.011), gestational hypertension (P = 0.025), and gestational diabetes (P = 0.018) between the two groups, suggesting that these factors may influence pregnancy outcome (Table 1).
Table 1.
Comparison of clinical data between the favorable and adverse outcome groups
| Factor | Favorable outcome group (n = 249) | Adverse outcome group (n = 138) | χ2 Value | P Value |
|---|---|---|---|---|
| Age | ||||
| ≥ 40 years | 105 (42.17%) | 80 (57.97%) | 8.886 | 0.003 |
| < 40 years | 144 (57.83%) | 58 (42.03%) | ||
| Parity | ||||
| Primiparous | 115 (46.18%) | 73 (52.9%) | 1.602 | 0.206 |
| Multiparous | 134 (53.82%) | 65 (47.1%) | ||
| Gravidity | ||||
| ≥ 2 times | 137 (55.02%) | 77 (55.8%) | 0.022 | 0.883 |
| < 2 times | 112 (44.98%) | 61 (44.2%) | ||
| History of miscarriage | ||||
| Yes | 85 (34.14%) | 52 (37.68%) | 0.488 | 0.485 |
| No | 164 (65.86%) | 86 (62.32%) | ||
| Pre-pregnancy BMI | ||||
| < 18 kg/m2 | 47 (18.88%) | 19 (13.77%) | 12.232 | 0.002 |
| 18-24.9 kg/m2 | 162 (65.06%) | 76 (55.07%) | ||
| ≥ 25 kg/m2 | 40 (16.06%) | 43 (31.16%) | ||
| Gestational weight gain | ||||
| < 15 kg | 70 (28.11%) | 50 (36.23%) | 3.42 | 0.181 |
| 15-20 kg | 119 (47.79%) | 63 (45.65%) | ||
| > 20 kg | 60 (24.1%) | 25 (18.12%) | ||
| Education level | ||||
| High school or below | 63 (25.3%) | 39 (28.26%) | 3.212 | 0.201 |
| College/Undergraduate | 164 (65.86%) | 80 (57.97%) | ||
| Graduate or above | 22 (8.84%) | 19 (13.77%) | ||
| Mode of conception | ||||
| Assisted reproduction | 129 (51.81%) | 90 (65.22%) | 6.5 | 0.011 |
| Natural conception | 120 (48.19%) | 48 (34.78%) | ||
| Family history of diabetes | ||||
| Yes | 20 (8.03%) | 14 (10.14%) | 0.495 | 0.482 |
| No | 229 (91.97%) | 124 (89.86%) | ||
| Gestational hypertension | ||||
| Yes | 12 (4.82%) | 15 (10.87%) | 5.008 | 0.025 |
| No | 237 (95.18%) | 123 (89.13%) | ||
| Gestational diabetes | ||||
| Yes | 15 (6.02%) | 18 (13.04%) | 5.609 | 0.018 |
| No | 234 (93.98%) | 120 (86.96%) | ||
| Chorionicity | ||||
| Dichorionic twins | 209 (83.94%) | 110 (79.71%) | 1.095 | 0.295 |
| Monochorionic twins | 40 (16.06%) | 28 (20.29%) |
Note: BMI, Body Mass Index.
Comparison of laboratory indicators between different outcome groups
Laboratory indicators were also compared between the favorable and adverse outcome groups. The analysis showed that levels of WBC (P = 0.047), Neut (P < 0.001), AFP (P < 0.001), β-HCG (P < 0.001), and 24-hour urine protein quantification (P < 0.001) were significantly lower in the favorable outcome group. In contrast, higher levels of Lym (P < 0.001) and PlGF (P < 0.001) were observed in the favorable outcome group compared to the adverse outcome group (Table 2). These findings suggest that these laboratory indicators may be associated with pregnancy outcome.
Table 2.
Comparison of laboratory indicators between the favorable and adverse outcome groups
| Indicator | Favorable outcome group (n = 249) | Adverse outcome group (n = 138) | Statistical Value | P Value |
|---|---|---|---|---|
| WBC (×10^9/L) | 9.80 ± 1.96 | 10.20 ± 1.89 | 1.993 | 0.047 |
| PLT (×10^9/L) | 224.13 ± 55.15 | 231.85 ± 43.01 | 1.525 | 0.128 |
| Neut (×10^9/L) | 7.07 ± 0.64 | 7.56 ± 0.96 | 5.416 | < 0.001 |
| Lym (×10^9/L) | 1.90 ± 0.54 | 1.62 ± 0.51 | -5.068 | < 0.001 |
| AFP (ng/mL) | 93.82 ± 7.92 | 98.87 ± 6.62 | 6.687 | < 0.001 |
| β-HCG (mU/L) | 3.34 ± 0.51 | 3.70 ± 0.50 | 6.671 | < 0.001 |
| 24 h urine protein quantification (g/L) | 2.05 [1.56, 2.66] | 2.62 [2.13, 3.10] | 5.242 | < 0.001 |
| Hb (g/L) | 115.13 ± 12.04 | 115.73 ± 15.59 | 0.393 | 0.695 |
| PlGF (pg/ml) | 225.49 ± 19.65 | 195.74 ± 17.72 | -15.211 | < 0.001 |
Note: WBC, White Blood Cells; PLT, Platelets; Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; Hb, Hemoglobin; PlGF, Placental Growth Factor.
Independent risk factors for adverse pregnancy outcome
Multivariate logistic regression analysis was conducted on the indicators that showed significant differences in the univariate analysis, including mode of conception, Neut, Lym, AFP, β-HCG, 24-hour urine protein quantification, and PlGF. Continuous variables were dichotomized using the cut-off values obtained from the ROC curve (Table 3). The results of the multivariate analysis identified mode of conception (P = 0.007, OR = 2.606), Neut (P < 0.001, OR = 5.595), Lym (P = 0.002, OR = 0.261), AFP (P < 0.001, OR = 4.261), β-HCG (P = 0.001, OR = 3.268), 24-hour urine protein quantification (P < 0.001, OR = 8.859), and PlGF (P < 0.001, OR = 0.044) as independent risk factors for adverse pregnancy outcome (Table 4).
Table 3.
Value assignment table
| Factor | Assignment Contents |
|---|---|
| Age | ≥ 40 years = 1, < 40 years = 0 |
| Pre-pregnancy BMI | < 18 kg/m2 = 0, 18-24.9 kg/m2 = 1, ≥ 25 kg/m2 = 2 |
| Mode of conception | Assisted Reproduction = 1, Natural Conception = 0 |
| Gestational hypertension | Yes = 1, No = 0 |
| Gestational diabetes | Yes = 1, No = 0 |
| WBC (×10^9/L) | ≤ 9.145 = 0, > 9.145 = 1 |
| Neut (×10^9/L) | ≤ 7.725 = 0, > 7.725 = 1 |
| Lym (×10^9/L) | ≤ 2.055 = 0, > 2.055 = 1 |
| AFP (ng/mL) | ≤ 96.04 = 0, > 96.04 = 1 |
| β-HCG (mU/L) | ≤ 3.405 = 0, > 3.405 = 1 |
| 24 h urine protein quantification (g/L) | ≤ 2.095 = 0, > 2.095 = 1 |
| PlGF (pg/ml) | ≤ 208.595 = 0, > 208.595 = 1 |
| Pregnancy Outcome | Favorable Outcome = 0, Adverse Outcome = 1 |
Note: BMI, Body Mass Index; WBC, White Blood Cells; Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; PlGF, Placental Growth Factor.
Table 4.
Multivariate logistics regression analysis
| Factor | Estimate | Std. Error | P Value | OR | Lower 95 CI | Upper 95 CI |
|---|---|---|---|---|---|---|
| Age | 0.428 | 0.345 | 0.214 | 1.535 | 0.781 | 3.036 |
| Pre pregnancy BMI | 0.499 | 0.273 | 0.068 | 1.647 | 0.970 | 2.844 |
| Mode of conception | 0.958 | 0.353 | 0.007 | 2.606 | 1.321 | 5.307 |
| Gestational hypertension | 0.421 | 0.663 | 0.526 | 1.523 | 0.424 | 5.711 |
| WBC | 0.254 | 0.373 | 0.495 | 1.290 | 0.622 | 2.700 |
| Neut | 1.722 | 0.386 | < 0.001 | 5.595 | 2.677 | 12.224 |
| Lym | -1.342 | 0.423 | 0.002 | 0.261 | 0.110 | 0.585 |
| AFP | 1.449 | 0.350 | < 0.001 | 4.261 | 2.180 | 8.662 |
| β-HCG | 1.184 | 0.359 | 0.001 | 3.268 | 1.639 | 6.731 |
| 24 h urine protein quantification | 2.181 | 0.394 | < 0.001 | 8.859 | 4.229 | 19.946 |
| PlGF | -3.120 | 0.372 | < 0.001 | 0.044 | 0.020 | 0.089 |
Note: BMI, Body Mass Index; WBC, White Blood Cells; Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; PlGF, Placental Growth Factor.
Predictive value of independent risk factors for adverse pregnancy outcome
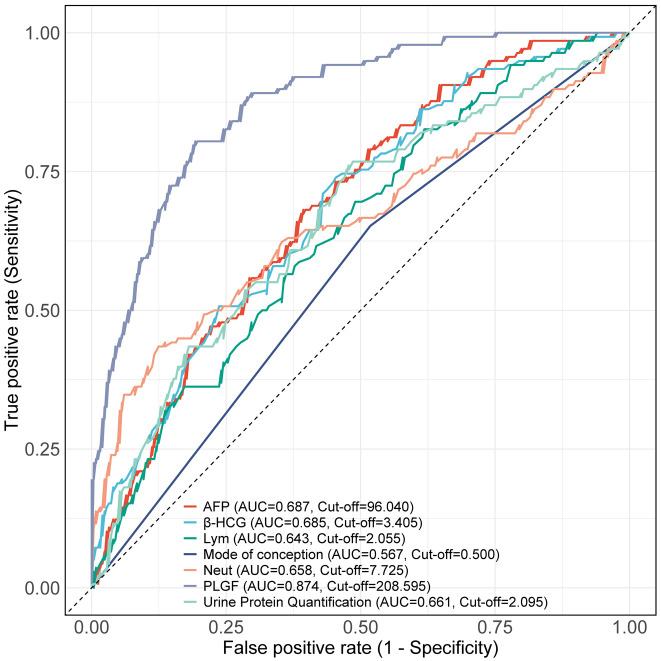
ROC curve analysis was performed to assess the predictive value of independent risk factors for adverse pregnancy outcome. The results revealed that the Area Under the Curve (AUC) for PlGF in predicting adverse pregnancy outcome was 0.874, which was significantly higher than that of other indicators (all P < 0.001, Figure 2; Tables 5 and 6). The optimal cut-off value for PlGF at 208.595 pg/ml provided a sensitivity of 80.43% and a specificity of 80.72%, indicating that PlGF has a strong predictive ability for adverse outcome in this patient population.
Figure 2.
ROC curves of independent risk factors for predicting adverse pregnancy outcome Note: Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; PlGF, Placental Growth Factor.
Table 5.
ROC curve parameters of independent risk factors in predicting adverse maternal and infant outcomes
| Marker | AUC | 95% CI | Specificity | Sensitivity | Youden index | Cut off | Accuracy | Precision | F1 Score |
|---|---|---|---|---|---|---|---|---|---|
| Mode of conception | 0.567 | 0.516-0.618 | 0.4819 | 0.6522 | 0.1341 | 0.5 | 0.5426 | 0.6522 | 0.5042 |
| Neut (×109/L) | 0.658 | 0.597-0.720 | 0.8755 | 0.4348 | 0.3103 | 7.725 | 0.7183 | 0.4348 | 0.524 |
| Lym (×109/L) | 0.643 | 0.587-0.699 | 0.3815 | 0.8261 | 0.2076 | 2.055 | 0.5401 | 0.8261 | 0.5616 |
| AFP (ng/mL) | 0.687 | 0.634-0.741 | 0.6064 | 0.6812 | 0.2876 | 96.04 | 0.6331 | 0.6812 | 0.5697 |
| β-HCG (mU/L) | 0.685 | 0.631-0.739 | 0.5462 | 0.7391 | 0.2853 | 3.405 | 0.615 | 0.7391 | 0.5779 |
| 24 h urine protein quantification (g/L) | 0.661 | 0.603-0.718 | 0.5221 | 0.7609 | 0.283 | 2.095 | 0.6072 | 0.7609 | 0.5801 |
| PlGF (pg/ml) | 0.874 | 0.840-0.909 | 0.8072 | 0.8043 | 0.6116 | 208.595 | 0.8062 | 0.8043 | 0.7475 |
Note: WBC, White Blood Cells; Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; PlGF, Placental Growth Factor.
Table 6.
Comparison of the AUCs between PlGF and other independent risk factors
| Marker1 | Marker2 | Z value | P value | AUC difference | 95% CI |
|---|---|---|---|---|---|
| Mode of conception | PlGF | -9.423 | < 0.001 | -0.307 | -0.371 - -0.243 |
| Neut | PlGF | -5.8 | < 0.001 | -0.216 | -0.289 - -0.143 |
| Lym | PlGF | -7.077 | < 0.001 | -0.231 | -0.295 - -0.167 |
| AFP | PlGF | -5.685 | < 0.001 | -0.187 | -0.251 - -0.123 |
| B HCG | PlGF | -5.645 | < 0.001 | -0.189 | -0.255 - -0.124 |
| Urine Protein Quantification | PlGF | -6.167 | < 0.001 | -0.214 | -0.281 - -0.146 |
Note: WBC, White Blood Cells; Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; PlGF, Placental Growth Factor; AUC, Area under the curve.
Interaction analysis between PlGF and other independent risk factors
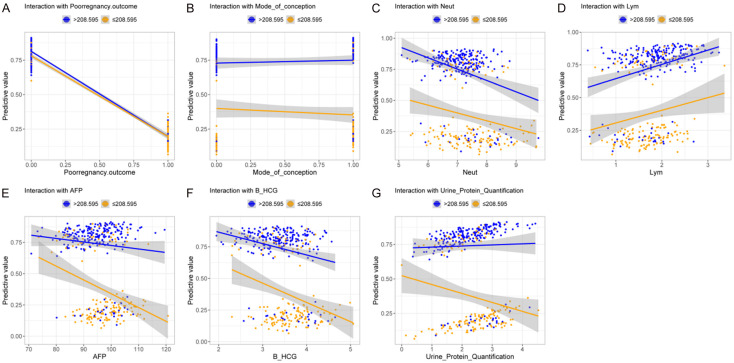
Finally, an interaction analysis was performed to explore the relationship between PlGF and other independent risk factors. The results showed significant interactions between PlGF and adverse pregnancy outcome (P < 0.001, Figure 3A, 3G), as well as between PlGF and 24-hour urine protein level (P = 0.035) as well. However, no significant interactions were observed between PlGF and other factors such as mode of conception, Neut, Lym, AFP, or β-HCG (all P > 0.05, Figure 3B-F; Table 7). These findings suggest that PlGF, in conjunction with certain other factors, may play a critical role in predicting adverse pregnancy outcome.
Figure 3.
Correlation between PlGF and other independent risk factors. A. Correlation between PlGF and adverse pregnancy outcome. B. Correlation between PlGF and mode of conception. C. Correlation between PlGF and Neut. D. Correlation between PlGF and Lym. E. Correlation between PlGF and AFP. F. Correlation between PlGF and β-HCG. G. Correlation between PlGF and 24 h Urine Protein Quantification. Note: The blue line represents the predictive value when PlGF level is less than or equal to 208.595, while the yellow line represents the predictive value when PlGF level is greater than 208.595. Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; PlGF, Placental Growth Factor.
Table 7.
Correlation between PlGF and other independent risk factors
| Factor | Estimate | Std. Error | Z value | P value |
|---|---|---|---|---|
| Pregnancy outcome | -3.186 | 0.356 | -8.962 | < 0.001 |
| Mode of conception | 0.403 | 0.270 | 1.489 | 0.136 |
| Neut | 0.012 | 0.171 | 0.069 | 0.945 |
| Lym | 0.168 | 0.248 | 0.679 | 0.497 |
| AFP | 0.016 | 0.018 | 0.916 | 0.360 |
| β-HCG | 0.016 | 0.257 | 0.063 | 0.949 |
| 24 h Urine Protein Quantification | 0.369 | 0.174 | 2.112 | 0.035 |
Note: Neut, Neutrophils; Lym, Lymphocytes; AFP, Alpha-Fetoprotein; β-HCG, Beta Human Chorionic Gonadotropin; PlGF, Placental Growth Factor.
Discussion
The combination of advanced maternal age and twin pregnancy significantly increases the risks for both the mother and the fetuses [16]. The decline in physiological function of the uterus and placenta in older pregnant women further elevate the risk of adverse outcomes in twin pregnancies [17]. Therefore, predicting adverse pregnancy outcome in twin pregnancies at an advanced maternal age is crucial for improving maternal and neonatal health, formulating personalized interventions, and reducing the incidence of pregnancy complications. Effective predictive tools can help clinicians identify high-risk pregnancies early and implement timely interventions.
The results of this study indicate that PlGF levels play a significant role in predicting adverse outcome in twin pregnancies at advanced maternal age. Specifically, there were significant differences in PlGF levels between the favorable outcome group and the adverse outcome group. The study also found that the AUC for PlGF in predicting adverse pregnancy outcome was 0.874, significantly higher than other indicators such as Neut, Lym, AFP, β-HCG, and 24-hour urine protein quantification. This suggests that PlGF has higher sensitivity and specificity in predicting adverse outcome. PlGF is an important angiogenic factor that plays a critical role in placental angiogenesis and maintaining placental function [18]. Elevated levels of PlGF can promote placental vascular formation, ensuring that the fetus receives adequate oxygen and nutrients, thereby supporting healthy fetal development [19]. In twin pregnancies, maintaining effective placental function is particularly important. Low levels of PlGF may lead to placental insufficiency, increasing the risk of adverse outcomes such as gestational hypertension, fetal growth restriction, and preterm birth. Previous research by Ekelund et al., involving 11,758 pregnant women, found that low levels of PlGF were associated with adverse outcome and were an independent risk factor [13]. Additionally, Bligh et al. discovered that lower PlGF levels were related to adverse neonatal outcomes [20]. Binder et al. proposed that the sFlt-1/PlGF ratio is significant in predicting composite adverse perinatal outcome and is superior to some conventional laboratory data [21]. These findings align with our results, further supporting the importance of PlGF in predicting pregnancy outcome. In this study, we demonstrated that PlGF has significant predictive value for pregnancy outcome in twin pregnancies at advanced maternal age. By measuring PlGF levels in these pregnancies, clinicians can identify high-risk pregnancies early and implement timely interventions to improve outcomes, and reduce complications.
This study further identified PlGF, Neut, Lym, AFP, β-HCG, and 24-hour urine protein quantification as important independent risk factors for adverse pregnancy outcome in this population. These risk factors were categorized into routine blood indicators, biomarkers, and urine indicators for discussion. Neut and Lym are important indicators in routine blood tests, reflecting maternal inflammation and immune status [22]. In advanced maternal age twin pregnancies, an increase in neutrophils may indicate chronic inflammation or acute infection in the mother, leading to adverse outcomes such as preterm birth and premature rupture of membranes [23]. Lymphocytes play a vital role immune response, and changes in the immune system during pregnancy significantly affect maternal and fetal health [24]. Low lymphocytes count may indicate impaired maternal immune function, increasing the risk of infection and pregnancy complications, which can result in adverse pregnancy outcomes. Previous studies by Christoforaki et al. found that the ratio of Neut to Lym in early pregnancy can predict adverse pregnancy outcomes [25]. Additionally, Ata et al. found that the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios can predict threatened miscarriage and early pregnancy loss [26].
PlGF, AFP, and β-HCG are key biomarkers identified in this study. PlGF is an important angiogenic factor that promotes placental vascular formation and maintains normal placental function [27]. Low levels of PlGF may lead to placental insufficiency, increasing the risk of gestational hypertension, fetal growth restriction, and preterm birth. Previous study demonstrated that PlGF levels were significantly associated with late preterm birth and adverse perinatal outcome in twin pregnancies, with the PlGF/sFlt-1 ratio showing strong predictive ability at 32 weeks [28]. Another study reported that low levels of PlGF were closely related to composite adverse pregnancy outcome in twin pregnancies and were independent risk factors [29]. AFP is mainly produced by the fetal liver and yolk sac, and its abnormal elevation is often associated with fetal structural abnormalities, such as neural tube defects and abdominal wall defects, thereby increasing the risk of adverse pregnancy outcome [30]. Research indicates that elevated AFP levels may suggest placental dysfunction, increasing the risk of gestational hypertension and fetal development issues [31]. β-HCG is a hormone secreted by the placenta, and fluctuations in its levels can reflect placental function and pregnancy status [32]. Abnormal β-HCG levels may be associated with placental dysfunction or fetal development issues, with both elevated and diminished β-HCG levels indicating adverse pregnancy outcomes, such as placental abruption and preeclampsia [33]. A systematic review and meta-analysis found that low β-HCG levels were significantly associated with adverse pregnancy outcomes, such as preterm birth, small-for-gestational-age infants, and placental abruption [34]. Another study showed that high levels of β-HCG in the mid-trimester were associated with increased risks of preeclampsia and fetal developmental abnormalities, while changes in β-HCG levels in early pregnancy could help predict early miscarriage and fetal death [35].
The 24-hour urine protein quantification is primarily used to detect kidney function and assess the risk of preeclampsia [36]. Older pregnant women are particularly prone to gestational hypertension and preeclampsia, both of which can lead to elevated urine protein levels [37]. High levels of urine protein indicate kidney damage, which may lead to placental dysfunction, increasing the risk of adverse outcome, such as preterm birth and fetal growth restriction. For example, a study by Lei et al. suggested that in patients with gestational hypertension, a higher 24-hour urine protein level correlated with an increased incidence of adverse outcomes for both the mother and the fetus. This study also pointed out that increased 24-hour urine protein was associated with gestational kidney dysfunction, indicating that kidney damage may compromise placental function and contribute to the risk of adverse pregnancy outcome [38]. Overall, monitoring these indicators can facilitate the early identification of high-risk pregnancies and enable prompt intervention.
Finally, we analyzed the interactions between PlGF and other risk factors. Significant interactions were observed between PlGF and adverse pregnancy outcomes, as well as 24-hour urine protein level. We speculate that decreased PlGF levels and increased urine protein reflect placental and kidney dysfunction, where a low level of PlGF may lead to endothelial dysfunction, increasing the filtration burden on the kidneys and resulting in elevated urine protein [39]. This interaction is significant in the diagnosis, treatment monitoring, and prognosis of of high-risk pregnancies and for early intervention to improve outcome.
Although this study provides valuable insights, it has several limitations. First, the sample size is relatively small, which may affect the representativeness and generalizability of the results. Future studies should include larger sample sizes to enhance the reliability and applicability of the findings. Second, as a retrospective study, there is a possibility of selection bias and information bias, and confounding factors cannot be fully controlled. Future prospective studies can help reduce bias and better control confounding factors. Additionally, this is a single-center study, and the data only come from one medical center, which may limit the external validity of the results and may not fully represent other regions or hospitals. Future multi-center studies are needed to broaden the applicability of the findings. Finally, the study lacks long-term follow-up data, so the impact of PlGF levels on long-term maternal and neonatal health outcomes cannot be assessed. Future studies should conduct longer follow-up to show the impact of PlGF levels on long-term maternal and neonatal health.
Conclusion
PlGF has significant clinical value for predicting adverse pregnancy outcome in twin pregnancies at advanced maternal age. PlGF level is significantly correlated with pregnancy outcome, making it an effective marker for early screening and intervention in high-risk pregnancies.
Acknowledgements
This study was supported by the Northwest Women and Children’s Hospital Project (No. 2022YN06).
Disclosure of conflict of interest
None.
References
- 1.D’Antonio F, Prasad S, Masciullo L, Eltaweel N, Khalil A. Selective fetal growth restriction in dichorionic diamniotic twin pregnancy: systematic review and meta-analysis of pregnancy and perinatal outcomes. Ultrasound Obstet Gynecol. 2024;63:164–172. doi: 10.1002/uog.26302. [DOI] [PubMed] [Google Scholar]
- 2.Yang M, Xiao LL, Wang JM. Association between maternal age and adverse pregnancy outcome in twin pregnancy. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:238–244. doi: 10.7499/j.issn.1008-8830.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greatholder I, Tomlinson E, Wilkinson J, Higgins LE, Kilby MD, Heazell AEP. Evaluating antenatal risk in twin pregnancies-a feasibility study to identify modifiable factors associated with adverse pregnancy outcomes. Acta Obstet Gynecol Scand. 2023;102:585–596. doi: 10.1111/aogs.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Yan J, Ma L, Huang Y, Zhu M, Jiang W. Effect of gestational diabetes mellitus on pregnancy outcomes among younger and older women and its additive interaction with advanced maternal age. Front Endocrinol (Lausanne) 2023;14:1158969. doi: 10.3389/fendo.2023.1158969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao J, Xu W, Liu Y, Zhang B, Zhang Y, Yu T, Huang T, Zou Y, Zhang B. Trends in maternal age and the relationship between advanced age and adverse pregnancy outcomes: a population-based register study in Wuhan, China, 2010-2017. Public Health. 2022;206:8–14. doi: 10.1016/j.puhe.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Cerdeira AS, Karumanchi SA. Serial placental growth factor-based testing in pre-eclampsia. Lancet. 2024;403:588–589. doi: 10.1016/S0140-6736(23)02578-3. [DOI] [PubMed] [Google Scholar]
- 7.Hurrell A, Webster L, Sparkes J, Battersby C, Brockbank A, Clark K, Duhig KE, Gill C, Green M, Hunter RM, Seed PT, Vowles Z, Myers J, Shennan AH, Chappell LC PARROT-2 trial group. Repeat placental growth factor-based testing in women with suspected preterm pre-eclampsia (PARROT-2): a multicentre, parallel-group, superiority, randomised controlled trial. Lancet. 2024;403:619–631. doi: 10.1016/S0140-6736(23)02357-7. [DOI] [PubMed] [Google Scholar]
- 8.Slomski A. Placental growth factor testing for faster preeclampsia diagnosis. JAMA. 2019;321:2396. doi: 10.1001/jama.2019.8397. [DOI] [PubMed] [Google Scholar]
- 9.Giardini V, Rovelli R, Algeri P, Giunti L, Lazzarin S, Callegari C, Roncaglia N, Vergani P. Placental growth factor as a predictive marker of preeclampsia - PREBIO study - PREeclampsia BIOchemical study. J Matern Fetal Neonatal Med. 2022;35:3029–3035. doi: 10.1080/14767058.2020.1792878. [DOI] [PubMed] [Google Scholar]
- 10.Rodríguez-Calvo J, Villalaín C, Gómez-Arriaga PI, Quezada MS, Herraiz I, Galindo A. Prediction of perinatal survival in early-onset fetal growth restriction: role of placental growth factor. Ultrasound Obstet Gynecol. 2023;61:181–190. doi: 10.1002/uog.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciciu E, Paṣatu-Cornea AM, Dumitru S, Petcu LC, Salim C, Tuta LA. Utility of sFtl-1 and placental growth factor ratio for adequate preeclampsia management. Healthcare (Basel) 2023;11:381. doi: 10.3390/healthcare11030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graupner O, Enzensberger C. Prediction of adverse pregnancy outcome related to placental dysfunction using the sFlt-1/PlGF ratio: a narrative review. Geburtshilfe Frauenheilkd. 2021;81:948–954. doi: 10.1055/a-1403-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekelund CK, Rode L, Tabor A, Hyett J, McLennan A. Placental growth factor and adverse obstetric outcomes in a mixed-risk cohort of women screened for preeclampsia in the first trimester of pregnancy. Fetal Diagn Ther. 2021;48:304–312. doi: 10.1159/000514201. [DOI] [PubMed] [Google Scholar]
- 14.Gardosi J. Toward safe standards for assessment of fetal growth in twin pregnancy. Am J Obstet Gynecol. 2017;216:431–433. doi: 10.1016/j.ajog.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Lei L, Feng F, Shao Y. Distribution of endotoxin in maternal and fetal body with intrahepatic cholestasis of pregnancy and its association with adverse fetal outcome. BMC Pregnancy Childbirth. 2022;22:920. doi: 10.1186/s12884-022-05235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gluck O, Mizrachi Y, Bar J, Barda G. The impact of advanced maternal age on the outcome of twin pregnancies. Arch Gynecol Obstet. 2018;297:891–895. doi: 10.1007/s00404-018-4656-1. [DOI] [PubMed] [Google Scholar]
- 17.Toussia-Cohen S, Mohr-Sasson A, Tsur A, Levin G, Orvieto R, Machtinger R, Meyer R. Pregnancy and neonatal outcomes of twin pregnancies - the role of maternal age. J Perinat Med. 2021;49:559–565. doi: 10.1515/jpm-2020-0386. [DOI] [PubMed] [Google Scholar]
- 18.Stepan H, Galindo A, Hund M, Schlembach D, Sillman J, Surbek D, Vatish M. Clinical utility of sFlt-1 and PlGF in screening, prediction, diagnosis and monitoring of pre-eclampsia and fetal growth restriction. Ultrasound Obstet Gynecol. 2023;61:168–180. doi: 10.1002/uog.26032. [DOI] [PubMed] [Google Scholar]
- 19.Satorres E, Martínez-Varea A, Diago-Almela V. sFlt-1/PlGF ratio as a predictor of pregnancy outcomes in twin pregnancies: a systematic review. J Matern Fetal Neonatal Med. 2023;36:2230514. doi: 10.1080/14767058.2023.2230514. [DOI] [PubMed] [Google Scholar]
- 20.Bligh LN, Greer RM, Kumar S. Screening performance of placental growth factor for the prediction of low birth weight and adverse intrapartum and neonatal outcomes in a term low-risk population. Fetal Diagn Ther. 2018;44:194–201. doi: 10.1159/000480381. [DOI] [PubMed] [Google Scholar]
- 21.Binder J, Palmrich P, Kalafat E, Haberl C, Schirwani N, Pateisky P, Khalil A. Longitudinal assessment of angiogenic markers in prediction of adverse outcome in women with confirmed pre-eclampsia. Ultrasound Obstet Gynecol. 2023;62:843–851. doi: 10.1002/uog.26276. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Sun H, Li P. Association of circulating inflammatory cells and platelets with gestational diabetes and pregnancy outcomes. Clin Chim Acta. 2021;523:87–96. doi: 10.1016/j.cca.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Y, Li L, Mao M, Liang X, Chen M, Xia Y, He W. Establishment of reference intervals of complete blood count for twin pregnancy. BMC Pregnancy Childbirth. 2021;21:714. doi: 10.1186/s12884-021-04192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amjad M. Placental biomarkers in the first trimester and adverse pregnancy outcome. BJOG. 2024 doi: 10.1111/1471-0528.17868. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Christoforaki V, Zafeiriou Z, Daskalakis G, Katasos T, Siristatidis C. First trimester neutrophil to lymphocyte ratio (NLR) and pregnancy outcome. J Obstet Gynaecol. 2020;40:59–64. doi: 10.1080/01443615.2019.1606171. [DOI] [PubMed] [Google Scholar]
- 26.Ata N, Kulhan M, Kulhan NG, Turkler C. Can neutrophil-lymphocyte and platelet-lymphocyte ratios predict threatened abortion and early pregnancy loss? Ginekol Pol. 2020;91:210–215. doi: 10.5603/GP.2020.0042. [DOI] [PubMed] [Google Scholar]
- 27.Sovio U, Gaccioli F, Cook E, Charnock-Jones DS, Smith GCS. Association between adverse pregnancy outcome and placental biomarkers in the first trimester: a prospective cohort study. BJOG. 2024;131:823–831. doi: 10.1111/1471-0528.17691. [DOI] [PubMed] [Google Scholar]
- 28.Satorres-Pérez E, Martínez-Varea A, Novillo-Del Álamo B, Morales-Roselló J, Diago-Almela V. The sFlt-1/PlGF ratio at 12, 24, and 32 weeks gestation in twin pregnancies as a predictor of late preterm birth and perinatal event secondary to prematurity. J Clin Med. 2024;13:2699. doi: 10.3390/jcm13092699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Wu K, Zhou S, Yin B, Bai X, Zhu B. Predictive value of maternal serum placental growth factor levels for discordant fetal growth in twins: a retrospective cohort study. BMC Pregnancy Childbirth. 2024;24:10. doi: 10.1186/s12884-023-06212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes AE, Sovio U, Gaccioli F, Cook E, Charnock-Jones DS, Smith GCS. The association between first trimester AFP to PAPP-A ratio and placentally-related adverse pregnancy outcome. Placenta. 2019;81:25–31. doi: 10.1016/j.placenta.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Erol SA, Altinboga O, Yakistiran B, Halici Ozturk F, Baser E, Caglar AT. Evaluation of actual maternal serum alpha-fetoprotein (MSAFP) levels in placental abruption and associations with adverse outcomes. SN Compr Clin Med. 2021;3:611–617. [Google Scholar]
- 32.Skogler J, Moberg T, Tancredi L, Styrmisdóttir L, Hedayati E, Alarcon-Ruiz CA, Khamis A, Persad E, Iskandarani G, Hansson SR, Bruschettini M. Association between human chorionic gonadotropin (hCG) levels and adverse pregnancy outcomes: a systematic review and meta-analysis. Pregnancy Hypertens. 2023;34:124–137. doi: 10.1016/j.preghy.2023.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Shen X, Shi Q, Shan H, Yan Y, Liu J, Kong N. Adverse impact of elevated serum progesterone and luteinizing hormone levels on the hCG trigger day on clinical pregnancy outcomes of modified natural frozen-thawed embryo transfer cycles. Front Endocrinol (Lausanne) 2022;13:1000047. doi: 10.3389/fendo.2022.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Queirós A, Gomes L, Pereira I, Charepe N, Plancha M, Rodrigues S, Cohen Á, Alves M, Papoila AL, Simões T. First-trimester serum biomarkers in twin pregnancies and adverse obstetric outcomes-a single center cohort study. Arch Gynecol Obstet. 2024;310:315–325. doi: 10.1007/s00404-024-07547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Li Z, Ren B, Wu W, Liu Y, Wang X, Guan Y, Jia L. Diagnostic value of a single β-hCG test in predicting reproductive outcomes in women undergoing cleavage embryo transfer: a retrospective analysis from a single center. Reprod Health. 2022;19:145. doi: 10.1186/s12978-022-01455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouzari Z, Javadiankutenai M, Darzi A, Barat S. Does proteinura in preeclampsia have enough value to predict pregnancy outcome? Clin Exp Obstet Gynecol. 2014;41:163–168. [PubMed] [Google Scholar]
- 37.Cheung HC, Leung KY, Choi CH. Diagnostic accuracy of spot urine protein-to-creatinine ratio for proteinuria and its association with adverse pregnancy outcomes in Chinese pregnant patients with pre-eclampsia. Hong Kong Med J. 2016;22:249–255. doi: 10.12809/hkmj154659. [DOI] [PubMed] [Google Scholar]
- 38.Lei T, Qiu T, Liao W, Li K, Lai X, Huang H, Yuan R, Chen L. Proteinuria may be an indicator of adverse pregnancy outcomes in patients with preeclampsia: a retrospective study. Reprod Biol Endocrinol. 2021;19:71. doi: 10.1186/s12958-021-00751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye S, Jiang Y, Lu Z. Correlation of 24-h urinary protein excretion, serum indicators, and placental growth factor in patients with preeclampsia and their adverse outcome. Altern Ther Health Med. 2024:AT8329. [PubMed] [Google Scholar]