Abstract
Background: Postoperative cerebrospinal fluid (CSF) leak is a life-threatening complication following endoscopic skull base surgery. This study describes a multilayered membrane reconstruction strategy for treating high-flow intraoperative CSF leaks during expanded endoscopic endonasal tumor resection (EEA) and presents the associated outcomes, supplemented by surgical video documentation. Methods: A retrospective review was performed on patients who underwent multilayered membrane reconstruction for high-flow CSF leaks during EEA. Results: From January 2019 to June 2023, 15 patients undergoing EEA experienced high-flow intraoperative CSF leaks and received multilayered membrane reconstruction. Tumor pathologies included pituitary adenoma, craniopharyngioma, and meningioma. After a median postoperative follow-up of 13 months, no postoperative CSF leakage, intracranial infection, meningitis, and pneumocephalus was detected. Conclusion: Our preliminary experience indicates that the multilayered membrane reconstruction technique may be a reliable method for achieving a watertight closure of high-flow intraoperative CSF leaks during EEA and warrants further study.
Keywords: Cerebrospinal fluid leak, endonasal transsphenoidal approach, endoscope, skull base reconstruction, repair technique
Introduction
Over the past decade, endoscopic endonasal approaches have made remarkable progress with the improvement of neuroendoscopic equipment and instruments and accumulation of surgical techniques and experience. Currently, expanded endoscopic endonasal approaches (EEA) are extensively utilized for resection of sellar, parasellar, and ventral skull base regions, including tuberculum sellae meningioma, craniopharyngioma, pituitary adenoma, Rathke’s cleft cyst, chordoma, chondrosarcoma, and others [1]. This approach enables accessing to lesions while avoiding brain retraction and minimizing neurovascular manipulation. It also provides a better illumination of the surgical field and the ability to a wider viewing angle that cannot be achieved with a microscope [1,2]. Thus, it is highly valued by neurosurgeons.
Cerebrospinal fluid (CSF) leak is a common and potential life-endangering complication due to inadequate closure of dural defects following EEA, particularly when intraoperative CSF leakage occurs [3]. Early case series reported leak rates often exceeded 15% [4-7]. Therefore, successful reconstructing skull base dural defects is a significant challenge. To achieve a watertight dural seal, multiple closure materials have been proposed for skull-base defect reconstruction, including the use of artificial dural substitutes, free autologous tissue grafts, various vascularized flaps, and other synthetic materials, alone or in combination [8,9]. The use of these materials alone or in combination, has dramatically decreased the rate of CSF leaks, although success rates vary greatly depending on the pathology, defect size, and personal surgical experience and technique [9]. A recent meta-analysis of 29 studies published between 2004 and April 2020 by Najafabadi et al. found a considerable decrease in the percentage of patients with CSF leaks over time, from 22% in early series published between 2004 and 2010 to 4% in recent studies published between 2016 and 2020 [10]. They also suggested that the decrease in CSF leaks could be attributed to not only a surgical learning curve but also to the use of multiple closure techniques [10]. Given that CSF leak carries potentially serious consequences of CSF leak, including pneumocephalus, meningitis, and prolonged hospitalization or readmission, a reliable method for achieving a durable watertight closure is still needed.
In this report, we present our preliminary experience with a small series of patients who underwent a novel, sample, multilayered membrane reconstruction technique for high-flow intraoperative leak during EEA.
Materials and methods
Patients. We retrospectively reviewed the demographic and outcome data of all adult patients who experienced a high-flow intraoperative CSF leakage and underwent EEA with multilayered membrane closure between 2019 and 2023. All procedures were performed by a senior surgeon. This project was approved by Chongqing General Hospital (KY S2023-097-01).
Key points of multilayered membrane reconstruction technique: Figure 1 shows a diagrammatic representation of skull base reconstruction and the order of the multilayered artificial dural substitutes placed in surgery. During the surgery, Gelfoam was used to eliminate the dead space, and then a piece of resorbable artificial dura mater was placed as the first layer, followed by a piece of un-resorbable artificial dura as the second layer. These two layers were placed in intradural space and covered the dural defect. Subsequently, another resorbable piece and a non-resorbable artificial dura were placed to overlay the defect of the skull base bone. In most cases, four-layered artificial dural substitutes were used. When there was a significant cerebrospinal fluid leaks obviously or more buttress was needed, additional artificial dural substitutes would be added. Notably, the artificial dura mater should be fashioned to be 0.5-1 cm larger than the dimensions of the dural defect and skull base bone. When there was no septal mucosal flap for recurrent tumors or reoperations, more SURGICEL FIBRILLAR and/or Gelfoam would be used to reinforce packing of the skull base bone. To provide a solid buttress, nasal packing was essential. Fibrin glue was not used in our surgery.
Figure 1.
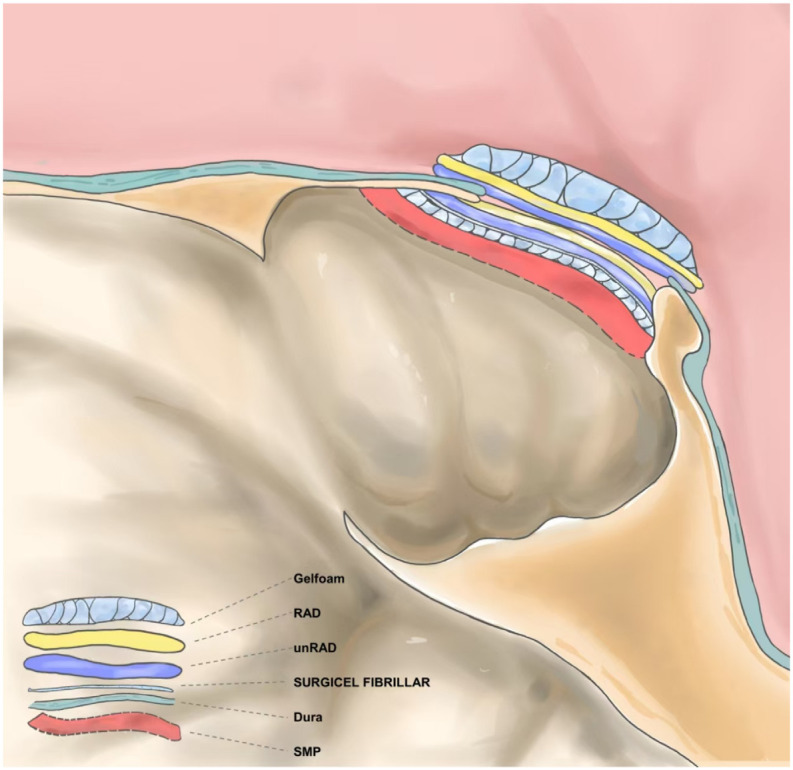
Diagram of skull base reconstruction and the order of the multilayered artificial dural substitutes placed in surgery. Abbreviations: RAD, resorbable artificial dura; unRAD, un-resorbable artificial dura; SMP, septal mucosal flap.
Postoperative management. General guidelines for managing CSF fistulas include routine CT or MRI reexamination within the first 24 hours after surgery, advising patients to avoid any activity that may increase intracranial pressure, and to use stool softeners. Lumbar drainage was not used. Prophylactic antibiotics were routinely administered for three days. In our practice, the iodoform gauze and French Foley were left in place for three weeks.
Results
Between January 2019 and June 2023, 15 patients with EEA experienced high-flow intraoperative CSF leaks. The current series included 7 males and 8 females, aged from 32 to 70 years. Tumor pathologies included pituitary adenomas, craniopharyngiomas, and meningiomas. In these patients, a septal mucosal flap was not used in 4 cases. No patients received postoperative lumbar subarachnoid drain treatment after surgery. After a median postoperative follow-up of 13 months, no postoperative CSF leakage, intracranial infection or/and meningitis, or pneumocephalus was observed.
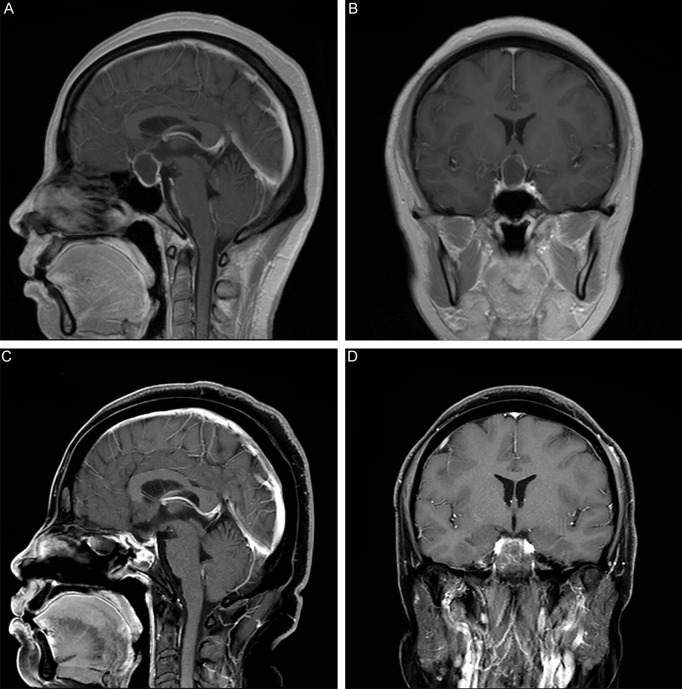
This report includes perioperative images and a surgical video to exemplify a representative case. A 41-year-old woman with complaints of headache, menstrual irregularities, and blurred vision was admitted to Chongqing General Hospital. Subsequent brain MRI scans revealed a cystic lesion in the suprasellar area, suggesting a craniopharyngioma (Figure 2A, 2B). After an in-depth discussion with the patient about potential surgical approaches, an EEA was decided upon. During the lesion removal, a high-flow intraoperative CSF leakage occurred, necessitating a multilayered membrane reconstruction technique (Supplementary Video 1). Because of the opening of the third ventricle, our initial step was to place the resorbable dura mater for protection, followed by Gelfoam to eliminate dead space. To mitigate CSF settling, a second layer of resorbable synthetic dura mater was added, succeeded by an outer layer of non-resorbable artificial dura within the intradural space. These three layers were strategically placed to cover the dural defect and ensure watertight closure. Subsequently, a resorbable piece and a non-resorbable artificial dura were placed to overlay the skull base bone defect and securely affixed with SURGICEL FIBRILLAR. Finally, the procedure also involved the fixation of a septal mucosal flap was fixed and nasal tampons were inserted to support the flap. A Foley catheter balloon was also placed to prevent the nasal tampons from migrating into the posterior nasal meatus. Pathological examinations confirmed the craniopharyngioma diagnosis. The 6-month postoperative MRI scans verified the complete removal of the lesion (Figure 2C, 2D).
Figure 2.
Preoperative and postoperative MRI. A, B. Coronal and Sagittal MRI scans show a cystic lesion located in suprasellar area. C, D. Coronal and Sagittal MRI examination at 6 months after operation suggested the complete removal of the lesion.
Discussion
Postoperative CSF fistulas remain a major complication of endonasal approaches, particularly the EEA for skull base tumors. Preventing CSF leakage is the primary goal of skull base reconstruction surgery. Currently, various multilayered repair technologies, such as pedicled nasoseptal flap [11], gasket seal technique [9], bilayer button technique [12], situ bone flap [13], and the 3F technique [14], have proposed and have greatly reduced the incidence of postoperative CSF leakage.
Our skull base repair techniques have undergone several modifications over the past 20 years. Similar to others, we have used artificial dural substitutes, free autologous tissue grafts, various vascularized flaps, and other synthetic materials, either alone or in combination during our previous operations. However, these technologies have some drawbacks. First, additional wounds and various donor site complications can occur after harvesting the autologous fascia lata and/or fat graft. Second, we cannot harvest pedicled nasoseptal flaps in patients with recurrent tumors, reoperation, or those who have undergone radiotherapy. Finally, hard repair materials, such as situ bone flaps, are difficult to apply to most patients, especially those with skull base bone infiltration. Moreover, hard repair materials are difficult to fashion to fit the defect of the skull base.
In this case report, we described a simple, multilayered membrane reconstruction technique for high-flow intraoperative CSF leaks during EEA in recent years. Using this multilayered membrane reconstruction technique, several precautions should be noted. First, absorbable and nonabsorbable artificial dura mater are alternately placed during surgery. Outside the skull base, a layered absorbable dural substitute is placed first, followed by nonabsorbable artificial dura mater. This placement sequence can prevent the migration of the artificial dura. Second, at least four layers of artificial dural substitutes should be were used to provide a solid buttress and should avoid wrinkles. Third, the first two layers of artificial dural substitutes placed in intradural space should be fashioned to be 0.5-1.0 cm larger than the dimensions of the dural defects. Fourth, from the outside to the inside, each layer of the dura mater is larger than the inner layer. Fifth, use of SURGICEL FIBRILLAR and Gelfoam fixes the artificial dural and promote adhesion to the surrounding nasal mucosa. Finally, a three-week period of nasal tamponades in practice is essential. Therefore, the multilayered membrane reconstruction technique provides a watertight dural seal and adequate biomechanical support. In our illustrate case with high-flow CSF leaking and an opened third ventricle, an additional artificial dural substitute was placed first before using Gelfoam to eliminate the dead space. The additional absorbable dural substitute not only prevents the Gelfoam from dislodging into the ventricle and blocking the Sylvius aqueduct but also decreases the CSF sedimentation.
Our technique has advantages, such as easy and simple operation, no dead space, and no extra damage. Two major concerns should be noted, however. One is that non-autologous grafts could be an infection source, increasing the risk of infection. Another is a three-week secured time with nasal tamponades, which may lead to unbearable nasal congestion and discomfort in some patients. Based on the author’s experience, the optimal time for removing the nasal packing is three weeks, especially in some patients without the septal mucosal flap. After three weeks of compression, the skull base is sufficiently sturdy.
Conclusions
In the current study with a limited number of cases, we presented our preliminary experience that a multilayered membrane reconstruction technique may be a reliable reconstructive technique for high-flow intraoperative CSF leak during EEA. This deserves further study.
Disclosure of conflict of interest
None.
Supplementary Video 1
References
- 1.de Divitiis E, Cavallo LM, Cappabianca P, Esposito F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: part 2. Neurosurgery. 2007;60:46–58. doi: 10.1227/01.NEU.0000249211.89096.25. discussion 58-59. [DOI] [PubMed] [Google Scholar]
- 2.Hannan CJ, Kelleher E, Javadpour M. Methods of skull base repair following endoscopic endonasal tumor resection: a review. Front Oncol. 2020;10:1614. doi: 10.3389/fonc.2020.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannan CJ, Kewlani B, Browne S, Javadpour M. Multi-layered repair of high-flow CSF fistulae following endoscopic skull base surgery without nasal packing or lumbar drains: technical refinements to optimise outcome. Acta Neurochir (Wien) 2023;165:2299–2307. doi: 10.1007/s00701-023-05581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitano M, Taneda M. Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery. 2004;54:653–660. doi: 10.1227/01.neu.0000108780.72365.dc. discussion 660-661. [DOI] [PubMed] [Google Scholar]
- 5.Dusick JR, Esposito F, Kelly DF, Cohan P, DeSalles A, Becker DP, Martin NA. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832–841. doi: 10.3171/jns.2005.102.5.0832. [DOI] [PubMed] [Google Scholar]
- 6.Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciarretta V, Farneti G, Calbucci F. The endoscopic extended transsphenoidal approach for craniopharyngiomas. Neurosurgery. 2006;59(Suppl 1):ONS75–83. doi: 10.1227/01.NEU.0000219897.98238.A3. discussion ONS75-83. [DOI] [PubMed] [Google Scholar]
- 7.Cavallo LM, Prevedello DM, Solari D, Gardner PA, Esposito F, Snyderman CH, Carrau RL, Kassam AB, Cappabianca P. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg. 2009;111:578–589. doi: 10.3171/2009.2.JNS081026. [DOI] [PubMed] [Google Scholar]
- 8.Hachem RA, Elkhatib A, Beer-Furlan A, Prevedello D, Carrau R. Reconstructive techniques in skull base surgery after resection of malignant lesions: a wide array of choices. Curr Opin Otolaryngol Head Neck Surg. 2016;24:91–97. doi: 10.1097/MOO.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg. 2013;80:563–568. doi: 10.1016/j.wneu.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 10.Zamanipoor Najafabadi AH, Khan DZ, Muskens IS, Broekman MLD, Dorward NL, van Furth WR, Marcus HJ. Trends in cerebrospinal fluid leak rates following the extended endoscopic endonasal approach for anterior skull base meningioma: a meta-analysis over the last 20 years. Acta Neurochir (Wien) 2021;163:711–719. doi: 10.1007/s00701-020-04641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- 12.Luginbuhl AJ, Campbell PG, Evans J, Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010;120:876–880. doi: 10.1002/lary.20861. [DOI] [PubMed] [Google Scholar]
- 13.Jin B, Wang XS, Huo G, Mou JM, Yang G. Reconstruction of skull base bone defects using an in situ bone flap after endoscopic endonasal transplanum-transtuberculum approaches. Eur Arch Otorhinolaryngol. 2020;277:2071–2080. doi: 10.1007/s00405-020-05911-1. [DOI] [PubMed] [Google Scholar]
- 14.Cavallo LM, Solari D, Somma T, Cappabianca P. The 3F (Fat, Flap, and Flash) technique for skull base reconstruction after endoscopic endonasal suprasellar approach. World Neurosurg. 2019;126:439–446. doi: 10.1016/j.wneu.2019.03.125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.