Abstract
Objective: To investigate the effects of zoledronic acid (ZA) on periodontal indices, serum inflammatory markers, and bone metabolism in postmenopausal osteoporosis (PMO) patients with periodontitis (PD). Methods: A total of 113 PMO+PD cases were recruited between May 2021 and February 2024. Fifty-two cases in the control group received standard therapy, while 61 cases in the observation group were treated with ZA. Therapeutic efficacy, periodontal indices (attachment loss [AL], probing depth [PD], and gingival bleeding index [GBI]), serum inflammatory markers (interleukin-1β [IL-1β], tumor necrosis factor-α [TNF-α], and C-reactive protein [CRP]), bone metabolism markers (N-terminal midfragment of osteocalcin [N-MID], beta-CrossLaps [β-CTx], and human calcitonin [hCT]), safety (fever, constipation, muscle soreness), and bone mineral density (BMD) at the lumbar spine and proximal femur were analyzed. A multivariable binary logistic regression model was used to determine factors influencing therapeutic efficacy. Results: The observation group demonstrated significantly better therapeutic outcomes than the control group. Treatment type was identified as an independent factor influencing efficacy. In the observation group, AL, PD, GBI, IL-1β, TNF-α, CRP, N-MID, and β-CTX levels were significantly reduced post-intervention compared to pre-intervention levels and the control group (all P<0.05), with no significant inter-group differences in hCT levels or adverse event rates (both P>0.05). BMD in the lumbar spine and proximal femur improved significantly in the observation group compared to the control group (both P<0.05). Conclusions: ZA positively impacts periodontal health, reduces serum inflammation, and enhances bone metabolism in PMO patients with PD.
Keywords: Zoledronic acid, postmenopausal osteoporosis, periodontitis, periodontal indices
Introduction
Postmenopausal osteoporosis (PMO) is a metabolic disorder primarily linked to estrogen deficiency, a steroid hormone that declines with age as ovarian function ceases [1,2]. This deficiency increases fracture risk and is associated with higher mortality rates and economic burdens [3]. Statistics indicate that nearly one-third of women over 50 are affected by osteoporosis (OP), and women across all age groups exhibit significantly lower bone mass compared to men of the same age and race [4,5].
Periodontitis (PD) and OP are both skeletal diseases closely related to inflammation and aging. PD involves chronic inflammation of periodontal tissue and alveolar bone, leading to gingival sulcus deepening, as well as plaque and tartar accumulation [6]. Studies have shown a clinical link between PD and OP, suggesting that systemic and alveolar bone loss may contribute to this correlation [7]. However, research on treating PMO complicated by PD is limited, focusing mainly on the relationship between PMO and PD or the effects of ZA on either condition individually. This study aims to address this gap by providing relevant analyses and further clinical insights.
Zoledronic acid (ZA), typically administered intravenously, is widely used to treat OP, helping to prevent hip, vertebral, and non-vertebral fractures [8]. Its anti-resorptive action in OP likely involves inhibition of the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (AKT) pathway [9]. Additionally, ZA has been shown to induce osteoclast apoptosis in ovariectomized rats by activating the nuclear factor kappa-B (NF-κB) pathway, which suppresses osteoclast formation and promotes bone homeostasis [10]. ZA has also demonstrated benefits in increasing bone mineral density (BMD) across different age groups, partially preventing bone loss [11]. Its potential in treating PD has also been reported: Raj et al. [12] found that a 0.05% ZA gel, applied locally, significantly improved periodontal indices in patients with stage III, grade B PD. In another study, Leite de Marcelos et al. [13] observed that ZA intervention in experimental PD models reduced alveolar bone resorption and inhibited disease progression.
Current clinical research on using ZA for PMO+PD remains limited, and this study aims to contribute to this field by providing further analysis and clinical perspectives.
Materials and methods
Patient selection
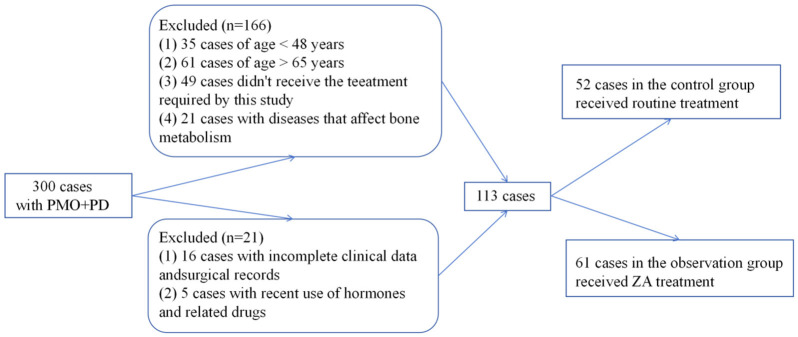
This study included 113 PMO+PD patients admitted from May 2021 to February 2024. Of these, 52 patients in the control group received routine treatment, while 61 patients in the observation group received ZA treatment. The study received approval from the Ethics Committee of the General Hospital of Shenzhen University, and relevant medical records were accessed via the hospital’s electronic medical record system. Inclusion and exclusion criteria are detailed below, and patient selection is illustrated in Figure 1.
Figure 1.
Flowchart of patient selection.
Inclusion Criteria: Diagnosis of PMO complicated by PD; no recent use of hormones or related medications; no history of limb immobilization or prolonged bed rest; no systemic abnormalities or diseases affecting bone metabolism; normal communication and cognitive abilities; and willingness to cooperate with the study.
Exclusion Criteria: Age <48 or >65; presence of uterine fibroids, endometriosis, endometrial cancer, or hematologic diseases; long-term use of steroids, anticonvulsants, or anticoagulants; use of bisphosphonates within the past year, calcium supplements within six months, or fluoride within one month; comorbidities including diabetes, cancer, enteritis, malabsorption, peptic ulcers, renal impairment, or liver injury; urinary calcium excretion >400 mg/day; pregnancy or lactation; and severe mental disorders.
Treatment protocol
All patients received conventional supragingival cleaning, with calculus removal via subgingival ultrasonic scaling. Periodontal pockets were irrigated with normal saline, and tinidazole muco-adhesive buccal tablets (0.5 mg each) were inserted into the pockets, with 2-3 tablets per tooth for a 7-day course. The control group received daily calcium carbonate (1500 mg, equivalent to 600 mg calcium) and 3200 IU of vitamin D. The observation group received a single 100 mL:5 mg intravenous infusion of ZA over at least 15 minutes, with 250 mL of physiological saline or 5% glucose administered for hydration before and after infusion. Treatment plans were individualized, allowing patients to choose their preferred method based on a full understanding of benefits and limitations, rather than by random selection.
Data collection
Patient data were extracted from the medical records, including therapeutic efficacy, periodontal indices, serum inflammatory markers, bone metabolism indicators, safety outcomes, and BMD measurements, to compare the clinical efficacy of the two treatment approaches.
Outcome measurement
Therapeutic Effectiveness: Patients were evaluated for treatment efficacy 3 months post-intervention. Marked effectiveness was defined as normalization of periodontal inflammatory markers and indices, with resolution of related symptoms. Effectiveness was defined as improvement in periodontal inflammatory markers and indices, with gradual symptom alleviation. Ineffectiveness referred to cases where periodontal inflammatory markers and indices failed to normalize, with no improvement or worsening of symptoms. The total effective rate was calculated as the percentage of cases showing either effectiveness or marked effectiveness.
Periodontal Indices: Periodontal indices, including attachment loss (AL), probing depth (PD), and gingival bleeding index (GBI), were assessed before and three months after initial periodontal treatment.
Serum Inflammation: Venous blood samples were collected before and three months post-treatment to measure levels of inflammatory markers, such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and C-reactive protein (CRP), using enzyme-linked immunosorbent assay.
Bone Metabolism: Bone metabolism markers - including N-terminal midfragment of osteocalcin (N-MID), beta-CrossLaps (β-CTx), and human calcitonin (hCT) - were analyzed pre-treatment and three months post-treatment. N-MID and β-CTx were measured using electrochemiluminescence immunoassay, while hCT was quantified by radioimmunoassay.
Safety: Adverse events, such as fever, constipation, and muscle soreness, were recorded throughout the treatment period, and incidence rates were calculated.
BMD: BMD of the lumbar spine (L1-L4) and proximal femur was measured using a bone densitometer before and three months postintervention.
The primary outcome measures included therapeutic effectiveness, periodontal indices, serum inflammation, bone metabolism, and safety, while the secondary measure was BMD. These metrics aimed to determine the clinical efficacy and advantages of ZA in treating PMO+PD.
Statistical analysis
Quantitative and categorical data were imported into SPSS 22.0 for analysis. Quantitative data were presented as mean ± SEM, with between-group comparisons conducted using t-tests and within-group comparisons using paired t-tests. Categorical data were expressed as ratios (percentages), and inter-group comparisons of categorical data were analyzed using χ2 tests. Factors influencing treatment efficacy were identified using a multivariable binary logistic regression model. Statistical significance was set at P<0.05. The sample size for this study was calculated to meet minimum requirements, with a threshold of 42 cases.
Results
Comparison of general information
The two groups showed no statistically significant differences in mean age, disease duration, body mass index (BMI), drinking/smoking history, or marital status (all P>0.05) (Table 1).
Table 1.
Comparison of general information [n (%), mean ± SD]
| Variables | n | Control group (n=52) | Observation group (n=61) | χ2/t | P |
|---|---|---|---|---|---|
| Mean age (years) | 113 | 55.42 ± 4.23 | 56.11 ± 4.29 | 0.858 | 0.393 |
| Disease course (months) | 113 | 15.62 ± 5.87 | 15.11 ± 7.32 | 0.404 | 0.687 |
| Body mass index (kg/m2) | 113 | 21.67 ± 2.37 | 22.30 ± 2.70 | 1.307 | 0.194 |
| History of drinking | 0.013 | 0.909 | |||
| No | 81 | 37 (71.15) | 44 (72.13) | ||
| Yes | 32 | 15 (28.85) | 17 (27.87) | ||
| History of smoking | 2.251 | 0.134 | |||
| No | 79 | 40 (76.92) | 39 (63.93) | ||
| Yes | 34 | 12 (23.08) | 22 (36.07) | ||
| Marital status | 0.776 | 0.378 | |||
| Single | 26 | 10 (19.23) | 16 (26.23) | ||
| Married | 87 | 42 (80.77) | 45 (73.77) |
Comparison of therapeutic effectiveness of ZA treatment
In the control group, there were 20 cases with marked effectiveness, 14 cases with effectiveness, and 18 cases deemed ineffective. In contrast, the observation group had 36 cases with marked effectiveness, 18 with effectiveness, and 7 cases of ineffectiveness. The observation group had a significantly higher overall treatment effectiveness rate compared to the control group (P<0.05, Table 2). Multivariate analysis further identified the treatment method as a significant factor affecting patient efficacy (P=0.003) (Table 3).
Table 2.
Comparison of effect of zoledronic acid on therapeutic effectiveness
| Variables | Control group (n=52) | Observation group (n=61) | χ2 | P |
|---|---|---|---|---|
| Marked effectiveness | 20 (38.46) | 36 (59.02) | ||
| Effectiveness | 14 (26.92) | 18 (29.51) | ||
| Ineffectiveness | 18 (34.62) | 7 (11.48) | ||
| Total effectiveness | 34 (65.38) | 54 (88.52) | 8.724 | 0.003 |
Table 3.
Multivariate analysis of factors influencing patient efficacy
| Factors | β | S.E. | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age (years) | 0.504 | 0.515 | 0.956 | 0.328 | 1.655 | 0.603-4.544 |
| Disease course (months) | 0.027 | 0.501 | 0.003 | 0.958 | 1.027 | 0.385-2.740 |
| Body mass index (kg/m2) | 0.422 | 0.517 | 0.666 | 0.414 | 1.525 | 0.554-4.198 |
| History of drinking | 0.396 | 0.525 | 0.569 | 0.450 | 1.486 | 0.531-4.162 |
| History of smoking | -0.090 | 0.562 | 0.025 | 0.873 | 0.914 | 0.304-2.753 |
| Marital status | 0.730 | 0.571 | 1.632 | 0.201 | 2.074 | 0.677-6.354 |
| Therapeutic method | 1.561 | 0.525 | 8.846 | 0.003 | 4.765 | 1.703-13.333 |
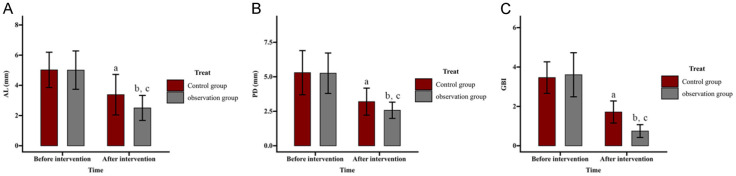
Comparison of effect of ZA on periodontal indices
Periodontal indices - AL, PD, and GBI, were evaluated (Figure 2). Before intervention, AL values were (5.02 ± 1.17) mm in the control group and (5.01 ± 1.27) mm in the observation group, which decreased post-intervention to (3.38 ± 1.34) mm and (2.50 ± 0.83) mm, respectively. The PD values in the control group were (5.30 ± 1.6) mm pre-intervention and (3.20 ± 0.98) mm post-intervention, while the observation showed a reduction from (5.26 ± 1.46) mm to (2.57 ± 0.59) mm. GBI values decreased from (3.46 ± 0.80) to (1.71 ± 0.56) in the control group and from (3.61 ± 1.12) to (0.75 ± 0.33) in the observation group. No significant differences were observed between the groups before intervention (all P>0.05). However, all indices improved significantly post-intervention in both groups, with the observation group showing even greater reductions (all P<0.05).
Figure 2.
Comparison of effect of zoledronic acid (ZA) on periodontal indices. A. Impact of ZA on AL in patients. B. Impact of ZA treatment on PD in patients. C. Impact of ZA treatment on GBI in patients. Note: aP<0.05 and bP<0.01 vs. before intervention; cP<0.05 vs. Control. AL, attachment loss; PD, probing depth; GBI, gingival bleeding index.
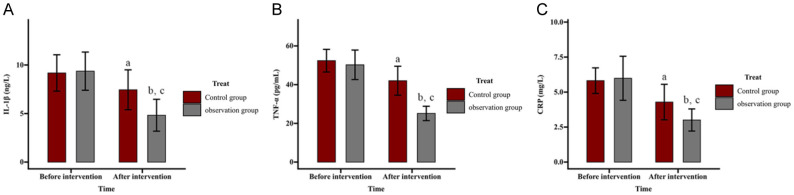
Comparison of effect of ZA on serum inflammatory markers
Serum levels of IL-1β, TNF-α, and CRP were measured to assess inflammation. Pre-intervention IL-1β levels were (9.18 ± 1.87) ng/L in the control group and (9.37 ± 1.97) ng/L in the observation group, reducing to (7.45 ± 2.06) ng/L and (4.83 ± 1.64) ng/L post-intervention, respectively. TNF-α levels in the control and observation groups were (52.38 ± 5.85) pg/mL and (50.25 ± 7.62) pg/mL pre-intervention, decreasing to (42.02 ± 7.48) pg/mL and (25.13 ± 3.71) pg/mL post-intervention, respectively. CRP levels also decreased from (5.82 ± 0.91) mg/L to (4.29 ± 1.27) mg/L in the control group and from (5.99 ± 1.57) mg/L to (3.01 ± 0.79) mg/L in the observation group. No significant inter-group differences were noted before intervention (all P>0.05). Post-intervention, all inflammatory markers showed significant reductions in both groups, with the observation group demonstrating lower levels than the control group (all P<0.05) (Figure 3).
Figure 3.
Comparison of effect of zoledronic acid (ZA) on serum inflammation. A. Influence of ZA on IL-1β in patients. B. Influence of ZA on TNF-α in patients. C. Influence of ZA on CRP. Note: aP<0.05 and bP<0.01 vs. before intervention; cP<0.05 vs. Control. IL, Interleukin; TNF, tumor necrosis factor; CRP, C-reactive protein.
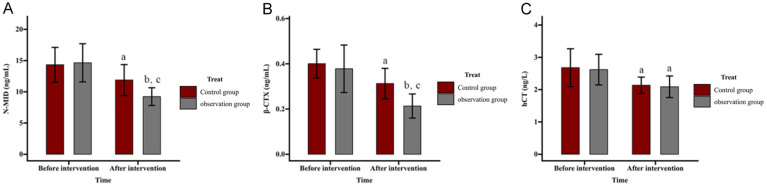
Comparison of effect of ZA on bone metabolism
Bone metabolism markers, including N-MID, β-CTX, and hCT, were measured, and the results are shown in Figure 4. Pre-intervention, N-MID levels were (14.33 ± 2.78) ng/mL in the control group and (14.64 ± 3.06) ng/mL in the observation group, decreasing post-intervention to (11.88 ± 2.48) ng/mL and (9.23 ± 1.42) ng/mL, respectively. β-CTX levels were (0.40 ± 0.06) ng/mL and (0.38 ± 0.10) ng/mL pre-intervention and dropped to (0.31 ± 0.07) ng/mL and (0.21 ± 0.05) ng/mL post-intervention in the control and observation groups, respectively. For hCT, levels decreased from (2.68 ± 0.59) ng/L to (2.14 ± 0.25) ng/L in the control group and from (2.62 ± 0.47) ng/L to (2.09 ± 0.33) ng/L in the observation group. No significant differences were observed between groups before intervention (all P>0.05). After intervention, N-MID, β-CTX, and hCT levels decreased significantly in both groups (all P<0.05), with greater reductions in N-MID and β-CTX in the observation group (both P<0.05), while hCT levels showed no significant inter-group difference (P>0.05).
Figure 4.
Comparison of effect of zoledronic acid (ZA) on bone metabolism. A. Influence of ZA on N-MID in patients. B. Influence of ZA on β-CTX in patients. C. Influence of ZA on hCT in patients. Note: aP<0.05 and bP<0.01 vs. before intervention; cP<0.05 vs. Control. N-MID, N-terminal midfragment of osteocalcin; β-CTX, beta-CrossLaps; hCT, human calcitonin.
Comparison of effect of ZA treatment on patient complications
The incidence of adverse events, including fever, constipation, and muscle soreness, was similar between the groups, with no significant difference in the overall rate of adverse reactions (7.69% vs. 13.11%, P>0.05) (Table 4).
Table 4.
Comparison of effect of zoledronic acid treatment on patient complications
| Safety | Control group (n=52) | Observation group (n=61) | χ2 | P |
|---|---|---|---|---|
| Fever | 0 (0.00) | 6 (9.84) | ||
| Constipation | 4 (7.69) | 0 (0.00) | ||
| Muscular soreness | 0 (0.00) | 2 (3.28) | ||
| Total | 4 (7.69) | 8 (13.11) | 0.870 | 0.351 |
Comparison of effect of ZA on BMD
BMD of the lumbar spine and proximal femur was assessed. Pre-intervention BMD of lumbar vertebrae L1-L4 was (0.74 ± 0.10) g/cm2 in the control group and (0.74 ± 0.08) g/cm2 in the observation group, which increased post-intervention to (0.83 ± 0.11) g/cm2 and (0.96 ± 0.14) g/cm2, respectively. The proximal femur BMD rose from (0.65 ± 0.06) g/cm2 to (0.70 ± 0.09) g/cm2 in the control group and from (0.67 ± 0.14) g/cm2 to (0.80 ± 0.12) g/cm2 in the observation group. No significant inter-group differences were found in lumbar and femur BMD pre-intervention (both P>0.05). Post-intervention, BMD increased significantly in both groups, with more pronounced improvements in the observation group (both P<0.05) (Figure 5).
Figure 5.
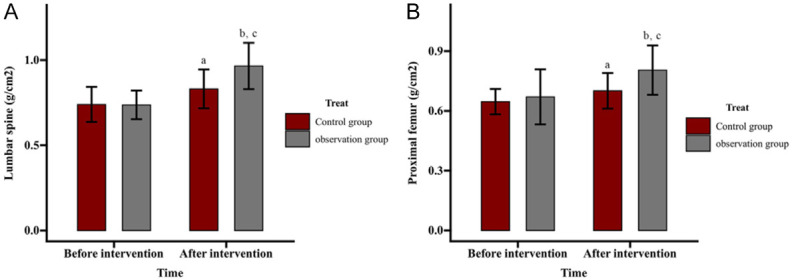
Comparison of effect of zoledronic acid (ZA) on bone mineral density. A. Effect of ZA on bone mineral density of the lumbar vertebrae in patients. B. Effect of ZA on bone mineral density of the proximal femur in patients. Note: aP<0.05 and bP<0.01 vs. before intervention; cP<0.05 vs. Control.
Discussion
PD is a chronic inflammatory condition affecting the supporting structures of the teeth, and its systemic effects may increase the risk of OP [14]. While the pathogenesis of PD involves dysbiotic biofilms, the progression of inflammation and disruption of bone homeostasis can predispose individuals to OP [15,16]. This study primarily evaluates the effects of ZA on periodontal indices, serum inflammation, and bone metabolism in patients with PMO complicated by PD, as detailed below.
In this study, the observation group showed a significantly higher total treatment effective rate than the control group (88.52% vs. 65.38%), indicating that ZA treatment in PMO patients with PD enhances therapeutic effectiveness. A meta-analysis of randomized controlled trials supports the efficacy of ZA in improving BMD in the lumbar spine, femoral neck, and trochanter in PMO patients, while reducing fracture risk with a favorable safety profile [17]. Additionally, a randomized clinical trial by Taguchi et al. [18] indicated that ZA use in PMO patients may help prevent symptomatic periodontal disease. The efficacy of ZA in treating PMO+PD appears partly due to its inhibition of osteoclast differentiation and induction of osteoclast apoptosis, which reduce bone resorption, increase BMD, and positively affect alveolar bone preservation [13,19].
This study further identified the treatment method as a significant independent factor influencing patient efficacy, underscoring the clinical effectiveness and reliability of ZA treatment. Additionally, improvements in periodontal indices - AL, PD, and GBI were observed in both the groups, with significantly lower AL, PD, and GBI levels in the observation group compared to the control group. This suggests that ZA treatment provides superior improvement in periodontal health for PMO+PD patients. Previous research indicates that ZA’s protective effects on periodontal tissues may be related to its inhibition of transforming growth factor β-induced fibrosis in human gingival fibroblasts [20]. Supporting this, Raj et al. [12] reported that ZA significantly reduced PD indices in patients with stage III, grade B periodontitis, consistent with our findings.
The observation group exhibited notably lower levels of inflammatory markers (IL-1β, TNF-α, and CRP) post-intervention compared to both pre-intervention levels and the control group, indicating that ZA treatment for PMO+PD patients effectively reduces serum hyperinflammation. These markers, including IL-1β, TNF-α, and CRP, are known to contribute to systemic bone loss and remodeling through inflammatory mediators [21,22]. Consistent with our findings, ZA significantly inhibited pro-inflammatory cytokines like IL-1β and TNF-α and reduced bone resorption in a mouse model of apical PD [23].
Post-intervention, the observation group also showed marked improvements in bone metabolism indices (N-MID, β-CTX, and hCT), with significantly greater improvements than the control group (except for hCT), indicating ZA’s positive effect on bone metabolism in PMO patients with PD. A meta-analysis by Zhuang et al. [24] supports our findings, demonstrating that ZA improved bone metabolism indices such as N-MID and β-CTX and increased BMD in patients with osteoporotic vertebral compression fractures. Huang et al. [25] also found that ZA, compared to pamidronic acid in treating bone metastases from non-small cell lung cancer, significantly reduced N-MID levels and lowered serum inflammatory markers like TNF-α and CRP, aligning with our observations.
In terms of safety, the overall incidence of adverse events, including fever, constipation, and muscle soreness, was similar between the two groups, suggesting that ZA is safe for treating PMO with PD. This aligns with findings from a meta-analysis by Wang et al. [26], which indicated that long-term ZA use in PMO patients has fewer gastrointestinal adverse events than alendronate, further supporting our results. Additionally, the observation group demonstrated a clear advantage in increasing BMD of the lumbar spine and proximal femur compared to the control group. Yoshizawa et al. [27] similarly reported that ZA improved BMD in OP patients undergoing distal radius fracture surgery. Furthermore, Huang et al. [28] highlighted that ZA improves appendicular skeletal muscle mass and index, benefiting muscle levels in OP patients. ZA has also been shown to be more cost-effective than alendronate for PMO treatment, regardless of age [29].
In summary, ZA treatment for PMO patients with PD enhances therapeutic effectiveness, improves periodontal indices, reduces serum inflammation, supports bone metabolism, and increases BMD, while maintaining a favorable safety profile, making it a valuable option for clinical application. Our findings offer an effective treatment choice for PMO patients with PD and provide new clinical insights for managing this patient population.
Acknowledgements
This study was supported by Guangdong scientific research foundation (No. A2022435); and Science, Technology and Innovation Commotion of Shenzhen Municipality, General project of Shenzhen University Stability Support Program (No. 20220810161720001).
Disclosure of conflict of interest
None.
References
- 1.Bhatnagar A, Kekatpure AL. Postmenopausal osteoporosis: a literature review. Cureus. 2022;14:e29367. doi: 10.7759/cureus.29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eghbali T, Abdi K, Nazari M, Mohammadnejad E, Gheshlagh RG. Prevalence of osteoporosis among Iranian postmenopausal women: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord. 2022;15:11795441211072471. doi: 10.1177/11795441211072471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migliorini F, Colarossi G, Baroncini A, Eschweiler J, Tingart M, Maffulli N. Pharmacological management of postmenopausal osteoporosis: a level I evidence based - expert opinion. Expert Rev Clin Pharmacol. 2021;14:105–119. doi: 10.1080/17512433.2021.1851192. [DOI] [PubMed] [Google Scholar]
- 4.Dionyssiotis Y, Paspati I, Trovas G, Galanos A, Lyritis GP. Association of physical exercise and calcium intake with bone mass measured by quantitative ultrasound. BMC Womens Health. 2010;10:12. doi: 10.1186/1472-6874-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemmati E, Mirghafourvand M, Mobasseri M, Shakouri SK, Mikaeli P, Farshbaf-Khalili A. Prevalence of primary osteoporosis and low bone mass in postmenopausal women and related risk factors. J Educ Health Promot. 2021;10:204. doi: 10.4103/jehp.jehp_945_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu B, Wang CY. Osteoporosis and periodontal diseases - an update on their association and mechanistic links. Periodontol 2000. 2022;89:99–113. doi: 10.1111/prd.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayusman PA, Nasruddin NS, Baharin B, Ibrahim N’, Ahmad Hairi H, Shuid AN. Overview on postmenopausal osteoporosis and periodontitis: the therapeutic potential of phytoestrogens against alveolar bone loss. Front Pharmacol. 2023;14:1120457. doi: 10.3389/fphar.2023.1120457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wang ZY, Ren N, Wei ZY, Hu WW, Gu JM, Zhang ZL, Yu XT, Wang C. Identifying therapeutic biomarkers of zoledronic acid by metabolomics. Front Pharmacol. 2023;14:1084453. doi: 10.3389/fphar.2023.1084453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng YT, Liao J, Zhou Q, Huo H, Zellmer L, Tang ZL, Ma H, Hong W, Liao DJ. Zoledronic acid modulates osteoclast apoptosis through activation of the NF-kappaB signaling pathway in ovariectomized rats. Exp Biol Med (Maywood) 2021;246:1727–1739. doi: 10.1177/15353702211011052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kostyshyn NM, Gzhegotskyi MR, Kostyshyn LP, Mudry SI. Effect of zoledronic acid on bone nanocomposites organization and prevention of bone mineral density loss in ovariectomized rats. Drug Metab Pers Ther. 2021;36:239–245. doi: 10.1515/dmpt-2020-0187. [DOI] [PubMed] [Google Scholar]
- 12.Raj SC, Mishra AK, Mohanty D, Katti N, Pattnaik S, Patra L, Pattanaik A. Comparative evaluation of the clinical and radiographic efficacy of 0.05% zoledronate gel as local drug delivery system in treating intrabony defects in stage III grade B periodontitis patients with and without type-2 diabetes mellitus-a randomized split-mouth clinical trial. Clin Adv Periodontics. 2024;14:211–222. doi: 10.1002/cap.10262. [DOI] [PubMed] [Google Scholar]
- 13.Leite de Marcelos PGC, Perez DEDCP, Soares DM, de Araujo SS, Evencio LB, Pontual MLDA, Ramos-Perez FMM. The effects of zoledronic acid on the progression of experimental periodontitis in rats: histological and microtomographic analyses. J Periodontal Implant Sci. 2021;51:264–275. doi: 10.5051/jpis.2001100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mau LP, Kuan YC, Tsai YC, Lin JJ, Huynh-Ba G, Weng PW, Shieh YS, Cheng WC, Huang RY. Patients with chronic periodontitis present increased risk for osteoporosis: a population-based cohort study in Taiwan. J Periodontal Res. 2017;52:922–929. doi: 10.1111/jre.12464. [DOI] [PubMed] [Google Scholar]
- 15.Samaranayake L, Matsubara VH. Normal oral flora and the oral ecosystem. Dent Clin North Am. 2017;61:199–215. doi: 10.1016/j.cden.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Wang CJ, McCauley LK. Osteoporosis and periodontitis. Curr Osteoporos Rep. 2016;14:284–291. doi: 10.1007/s11914-016-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Rahmati M, Xie W, Yang G, Ji B, Yon DK, Lee SW, Gyasi RM, Lopez Sanchez GF, Soysal P, Koyanagi A, Smith L, Shin JI, Li Y. Efficacy and safety of zoledronic acid in the treatment of osteoporosis: a meta-analysis of randomized controlled trials. Heliyon. 2024;10:e33871. doi: 10.1016/j.heliyon.2024.e33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taguchi A, Shiraki M, Tanaka S, Ohshige H, Nakamura T. Improved periodontal disease and prevention of tooth loss in osteoporosis patients receiving once-yearly zoledronic acid: a randomized clinical trial. Menopause. 2019;26:1277–1283. doi: 10.1097/GME.0000000000001393. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Zhan Y, Yan L, Hao D. How zoledronic acid improves osteoporosis by acting on osteoclasts. Front Pharmacol. 2022;13:961941. doi: 10.3389/fphar.2022.961941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsu Y, Ibi M, Chosa N, Kyakumoto S, Kamo M, Shibata T, Sugiyama Y, Ishisaki A. Zoledronic acid suppresses transforming growth factor-beta-induced fibrogenesis by human gingival fibroblasts. Int J Mol Med. 2016;38:139–147. doi: 10.3892/ijmm.2016.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S, Zhang G, Guo JF, Tan YH. Associations between osteoporosis and risk of periodontitis: a pooled analysis of observational studies. Oral Dis. 2021;27:357–369. doi: 10.1111/odi.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85:596–607. doi: 10.1177/154405910608500704. [DOI] [PubMed] [Google Scholar]
- 23.Maia CA, Chaves HGDS, Benetti F, de Menezes GB, Antunes MM, Pinto KP, Silva EJNL, Sobrinho APR, Tavares WLF. Zoledronic acid modulates cytokine expression and mitigates bone loss during the development of induced apical periodontitis in a mice model. J Endod. 2023;49:1522–1528. doi: 10.1016/j.joen.2023.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang M, Cai B, Wang F. Effectiveness and safety of percutaneous kyphoplasty combined with zoledronic acid in treatment of osteoporotic vertebral compression fractures: a meta-analysis. Arch Orthop Trauma Surg. 2022;142:2435–2443. doi: 10.1007/s00402-021-03858-4. [DOI] [PubMed] [Google Scholar]
- 25.Huang K, Tang X, Tang F. Combined chemotherapy of zoledronic acid and pamidronate in the treatment of bone metastases from nonsmall cell lung cancer and the effects on pain stress and bone metabolic indices. Drug Dev Res. 2024;85:e22147. doi: 10.1002/ddr.22147. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Yu Q, Zeng P, Ai W. Efficacy and safety of annual infusion of zoledronic acid and weekly oral alendronate in the treatment of primary osteoporosis: a meta-analysis. J Clin Pharmacol. 2023;63:455–465. doi: 10.1002/jcph.2181. [DOI] [PubMed] [Google Scholar]
- 27.Yoshizawa S, Shintaku T, Ishii H, Sakamoto M, Musha Y, Ikegami H. Zoledronic acid for osteoporosis after distal radius fracture surgery: prospective longitudinal study. J Orthop. 2023;43:109–114. doi: 10.1016/j.jor.2023.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CF, Shiao MS, Mao TY. Retrospective study of the effects of zoledronic acid on muscle mass in osteoporosis patients. Drug Des Devel Ther. 2021;15:3711–3715. doi: 10.2147/DDDT.S328858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You R, Zhang Y, Wu DB, Liu J, Qian X, Luo N, Mori T. Cost-effectiveness of zoledronic acid versus oral alendronate for postmenopausal osteoporotic women in China. Front Pharmacol. 2020;11:456. doi: 10.3389/fphar.2020.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]