Abstract
Objective: To evaluate the efficacy of transcatheter arterial chemoembolization (TACE) alone or in combination with radiotherapy or anlotinib for treating locally advanced hepatocellular carcinoma. Methods: A retrospective analysis was conducted on 72 patients with locally advanced hepatocellular carcinoma, divided into three groups: TACE alone (n = 20), TACE + anlotinib (TACE+AH, n = 34), and TACE + intensity-modulated radiotherapy (TACE+IMRT, n = 18). TACE was administered every 30 days. For TACE+AH, patients received 12 mg of anlotinib daily for 14 days per cycle. TACE+IMRT involved 400-500 cGy radiotherapy sessions three times weekly, with a total dose of 5000-6000 cGy. Results: No significant differences in Eastern Cooperative Oncology Group (ECOG) performance scores were observed among the groupspost-treatment. The TACE+IMRT group exhibited the highest objective response rate (ORR) (83.33%) and disease control rate (DCR) (88.89%). Progression-free survival (PFS) at 3, 6, and 12 months was also highest in the TACE+IMRT group, indicating superior outcome compared to the TACE+AH and TACE-alone groups. Independent predictors of PFS included the TACE+IMRT combination and Child-Pugh B grade. Conclusion: TACE combined with radiotherapy is a safe and effective treatment for locally advanced hepatocellular carcinoma, significantly improving PFS and serving as a protective factor. While TACE combined with anlotinib showed moderate efficacy and manageable adverse events, its therapeutic effect was less pronounced than that of TACE+IMRT.
Keywords: Liver cancer, transcatheter arterial chemoembolization, anlotinib hydrochloride, radiotherapy, clinical efficacy
Introduction
According to recent data, approximately 870,000 individuals worldwide were diagnosed with liver cancer in 2022, making it the sixth most common cancer [1]. During the same period, nearly 760,000 deaths were attributed to liver cancer, ranking it third in cancer-related mortality [2]. In China, the primary cause of liver cancer is well-established, with the hepatitis B virus (HBV) being the most prevalent etiological factor; approximately 85% of patients show signs of HBV infection [3,4]. Due to the lack of obvious symptoms during the early stages, many patients miss the optimal window for surgical intervention. Consequently, typical lesions in the middle and late stages, such as portal vein tumor thrombus and extensive intrahepatic metastasis, are commonly observed in hepatocellular carcinoma (HCC) cases [5].
Transcatheter arterial chemoembolization (TACE) is a widely used treatment for advanced liver cancer [6,7]. This interventional therapy delivers localized medication directly to the tumor, inhibiting disease progression, reducing tumor volume, and extending patient survival. However, complete tumor necrosis is rarely achieved with TACE alone, leading to unsatisfactory median survival times and a high recurrence rate [8,9].
Radiotherapy, a fundamental treatment modality for malignant tumors, complements TACE by targeting residual cancer cells. The liver’s dual blood supply can render TACE insufficient for completely blocking tumor blood flow, leaving residual tumors. Radiotherapy can address this by eradicating residual cancer cells through radiation. Studies indicate that combining TACE with radiotherapy significantly improves survival rates compared to TACE alone [10,11].
Additionally, molecular targeted therapies are increasingly recognized for treating advanced, unresectable liver cancer [12]. Anlotinib, a small-molecule, multi-target tyrosine kinase inhibitor, blocks vascular endothelial growth factor receptors and fibroblast growth factor receptors, inhibiting tumor angiogenesis and exerting anti-tumor effects. Anlotinib has demonstrated efficacy and safety in advanced liver cancer patients [13].
Patients with locally advanced liver cancer often have poor tolerance of conventional chemotherapy due to compromised immunity and malnutrition, resulting in low one-year survival rates [14]. Current treatment approaches for locally advanced liver cancer include TACE, molecular targeted therapy, and radiotherapy. Studies suggest that combination therapies can significantly alleviate symptoms and improve outcome [15-17]. However, anlotinib is not yet recommended in treatment guidelines due to insufficient clinical data [18].
This study aims to evaluate the efficacy of TACE alone, TACE combined with radiotherapy, and TACE combined with anlotinib for locally advanced liver cancer. Our objective is to optimize treatment protocols, improve therapeutic outcome and patient prognosis, and provide evidence to inform future clinical practice, advancing the field of liver cancer management.
Materials and methods
Case selection
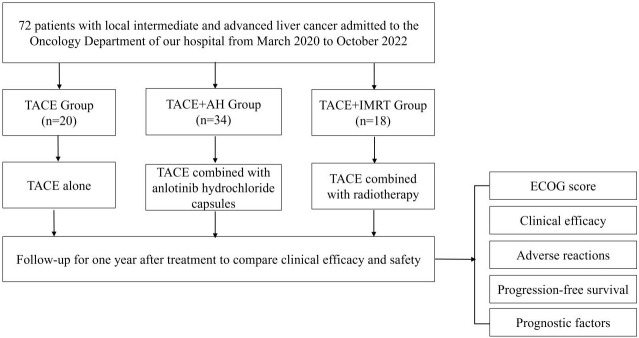
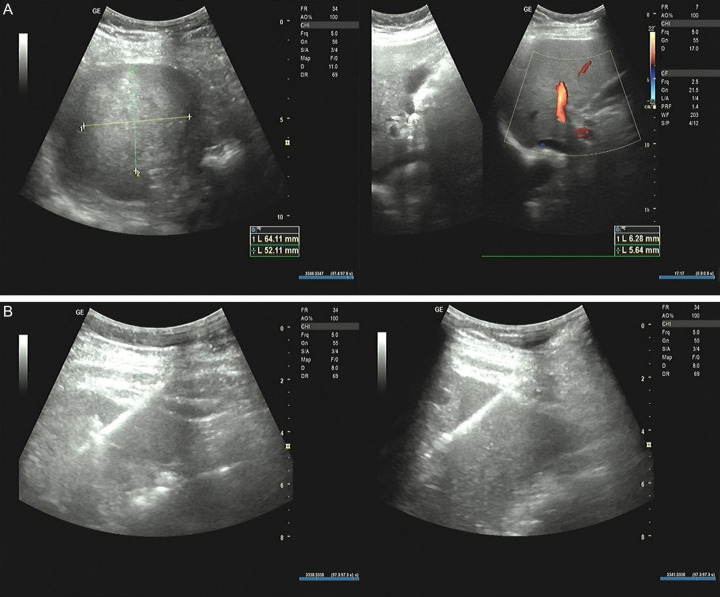
This study employed a case-control design. The research flow chart is presented in Figure 1. Patients diagnosed with locally advanced liver cancer and admitted to the Oncology Department of People’s Hospital of Hechi between March 2020 and October 2022 were included. All participants met the diagnostic criteria for liver cancer [19]. The diagnosis was confirmed through a comprehensive assessment of clinical symptoms, imaging findings, laboratory tests, and, when necessary, histologic examination. The diagnostic puncture procedure is illustrated in Figure 2. After applying the inclusion and exclusion criteria, a total of 72 patients were enrolled. This study was approved by the Ethics Committee of People’s Hospital of Hechi.
Figure 1.
The flow chart of this study. Abbreviations: TACE, transcatheter arterial chemoembolization; AH, anlotinib hydrochloride; IMRT, intensity-modulated radiotherapy; ECOG, Eastern Cooperative Oncology Group.
Figure 2.
Liver biopsy case diagram. A. To measure the size of the tumor: a mixed mass was detected in the parenchyma of the right lobe of the liver, the size was about 64×52 mm, the boundary was not clear, and the color blood flow signal could be seen around it. B. Puncture process: under the guidance of real-time B ultrasound, the automatic biopsy gun was used to eject the inner groove cutting needle, and the solid echo mass of the right lobe of the liver was punctured and biopsied four times.
Inclusion criteria
(1) Patients with unresectable stage IIb or locally advanced stage IIIa/IIIb liver cancer, as per the Chinese Liver Cancer Staging Program (CNLC). (2) Age range: 18-75 years. (3) Karnofsky Performance Status (KPS) score > 70, Child-Pugh grade A/B, and an expected survival time of more than three months. (4) Presence of at least one detectable lesion. (5) No prior exposure to chemotherapy, anti-vascular molecular targeted therapy, or small-molecule tyrosine kinase inhibitor (TKI) therapy.
Exclusion criteria
(1) The complete embolization of the main portal vein with poorly developed collateral circulation. (2) Extrahepatic metastases, including inferior vena cava invasion. (3) Patients planning to undergo liver transplantation. (4) Severe dysfunction of the heart, lungs, kidneys, brain, or other organs. (5) Leukopenia or thrombocytopenia induced by chemotherapy or other drugs that could not be corrected. (6) Tumor volume exceeding 70% of the total liver volume. (7) Allergy to any component of anlotinib. (8) Active bleeding, peptic ulcer, intestinal perforation, or intestinal obstruction. (9) Major surgery within the past 30 days. (10) Uncontrolled hypertension despite treatment with antihypertensive drugs. (11) Pregnant or lactating women. (12) Patients have a history of mental illness or psychotropic drug usage. (13) Incomplete clinical or laboratory data.
Intervention methods
In the TACE group, 20 patients received only TACE treatment. Seldinger’s technique was used to perform selective hepatic arteriography following femoral artery puncture and catheterization to assess the tumor’s location, size, number, and feeding arteries. A chemotherapeutic solution consisting of 5-fluorouracil (Shanghai Xudong Haipu Pharmaceutical Co., Ltd., H31020593), oxaliplatin (Sichuan Huiyu Pharmaceutical Co., Ltd., H20213060), and pirarubicin (Shenzhen Wanle Pharmaceutical Co., Ltd., H10930105) was diluted in 150-200 mL of normal saline and injected into the tumor through the tumor-feeding artery using a microcatheter. After drug perfusion, embolization was performed using a lipiodol emulsion and gelatin sponge. After that, angiography confirmed successful vascular embolization. Once target embolization was achieved, the catheter was removed, and local compression bandaging at the puncture site was applied for 15 minutes to control bleeding. Treatment was administered every 30 days, with the interval adjusted based on patient condition. Each patient underwent 1-3 treatment sessions.
In the TACE combined with intensity-modulated radiotherapy group (TACE+IMRT), 18 patients received TACE followed by IMRT after a 2-4 week interval, depending on liver function recovery. Using the Elekta Synergy accelerator with 6MV-X and the Pinical three-dimensional treatment planning system, three-dimensional conformal IMRT was performed. Patients were positioned supine on a phantom, with arms crossed and raised. A scanning positioning frame was used for enhanced CT imaging, covering the region from the diaphragm’s upper edge to the lower part of both kidneys. The tumor site was scanned with a slice thickness of 0.5 cm, and the images were transmitted to the planning system for gross tumor volume (GTV) and critical organ delineation.
The planning target volume (PTV) was extended by 10-15 mm above and below the GTV and 5 mm in all other directions. An isocentric plan with 5-7 irradiation fields was designed, ensuring that a 90-95% isodose curve covered the lesion’s edge. A dose-volume histogram (DVH) evaluation was performed, and treatment plans were optimized to minimize exposure to surrounding critical organs while maintaining target coverage. Large fractionated radiotherapy was administered three times weekly at 400-500 cGy per session, with a total dose of 5000-6000 cGy. Liver protection and supportive care were provided throughout the treatment course.
In the TACE Combined with Anlotinib Group (TACE+AH), 34 patients received TACE followed by oral anlotinib (Zhengda Tianqing Pharmaceutical Group Co., Ltd., H20180004) 1-2 weeks post-TACE, depending on liver function recovery. Anlotinib was administered at 12 mg once daily for 14 consecutive days, followed by a 7-day rest period, comprising a 21-day treatment cycle. Treatment was discontinued upon disease progression or occurrence of severe adverse reactions. TACE was performed 1-3 times during the treatment period.
Data collection
Following treatment, patients were monitored for one year, with monthly evaluations to assess quality of life, clinical efficacy, and other relevant data based on their initial assessment.
Primary indicator: The clinical efficacy of the three groups was evaluated according to RECIST 1.1 criteria: Complete remission (CR): Disappearance of the target lesion. Partial Remission (PR): Reduction of the target lesion’s maximum diameter by more than 30%. Progressive disease (PD): Increase of more than 20% in the maximum diameter of the target lesion or the appearance of new lesions. Stable disease (SD): Changes in the target lesion’s diameter not meeting PR or PD criteria.
The objective response rate (ORR) was calculated as the proportion of patients achieving CR and PR, while the Disease Control Rate (DCR) included patients with CR, PR, and SD.
Secondary indicators: (1) Quality of life: Assessed using the ECOG performance status score post-treatment. (2) Long-term outcomes: Progression-free survival (PFS) was tracked for one year, and the PFS rates at 3, 6, and 12 months were compared across the three groups. Prognostic factors influencing 12-month PFS were analyzed. (3) Adverse reactions: The incidence of adverse reactions, including gastrointestinal symptoms, liver dysfunction, hand-foot syndrome, and myelosuppression, was recorded for each group.
Statistical methods
Data analysis was performed using SPSS version 26.0.
Continuous variables: For normally distributed data, results were expressed as mean ± standard deviation (SD). Group comparisons were conducted using one-way ANOVA, with post-hoc pairwise comparisons performed using the Bonferroni correction. Non-normally distributed data are presented as median (interquartile range) and analyzed using non-parametric tests.
Categorical variables: Presented as frequencies (percentages) and analyzed using chi-square tests.
Survival analysis: Kaplan-Meier curves and Log-rank tests were used to compare PFS between groups. Cox regression analysis was performed to identify factors influencing PFS in patients with liver cancer.
A significance level of α = 0.05 was applied throughout the analyses.
Results
Comparison of patients’ baseline data
Table 1 presents the baseline characteristics of all patients. No significant differences were observed among the three groups in terms of age, sex, pathological diagnosis, history of alcohol consumption, family history, history of hepatitis, antiviral therapy, Child-Pugh score, tumor diameter, AFP, CA125, or CA19-9 levels (all P > 0.05).
Table 1.
Comparison of baseline data
| Baseline data | TACE (n = 20) | TACE+AH (n = 34) | TACE+IMRT (n = 18) | χ2 | P |
|---|---|---|---|---|---|
| Age | 0.201 | 0.905 | |||
| < 55 years old | 11 (55.00) | 17 (50.00) | 10 (55.56) | ||
| ≥ 55 years old | 9 (45.00) | 17 (50.00) | 8 (44.44) | ||
| Sex | 4.578 | 0.101 | |||
| Female | 1 (5.00) | 10 (29.41) | 4 (22.22) | ||
| Male | 19 (95.00) | 24 (70.59) | 14 (77.78) | ||
| Pathologic diagnosis | 0.195 | 0.907 | |||
| HCC | 12 (60.00) | 21 (61.76) | 12 (66.67) | ||
| ICC | 8 (40.00) | 13 (38.24) | 6 (33.33) | ||
| Drinking history | 2.848 | 0.241 | |||
| No | 16 (80.00) | 21 (61.76) | 10 (55.56) | ||
| Yes | 4 (20.00) | 13 (38.24) | 8 (44.44) | ||
| Family history | 0.899 | 0.638 | |||
| No | 7 (35.00) | 15 (44.12) | 9 (50.00) | ||
| Yes | 13 (65.00) | 19 (55.88) | 9 (50.00) | ||
| History of hepatitis | 4.665 | 0.097 | |||
| No | 6 (30.00) | 9 (26.47) | 10 (55.56) | ||
| Yes | 14 (70.00) | 25 (73.53) | 8 (44.44) | ||
| Antiviral therapy | 2.430 | 0.297 | |||
| No | 6 (30.00) | 8 (23.53) | 8 (44.44) | ||
| Yes | 14 (70.00) | 26 (76.47) | 10 (55.56) | ||
| Child-Pugh | 3.275 | 0.194 | |||
| A | 11 (55.00) | 18 (52.94) | 14 (77.78) | ||
| B | 9 (45.00) | 16 (47.06) | 4 (22.22) | ||
| Tumor diameter | 6.068 | 0.194 | |||
| ≥ 5 cm | 18 (90.00) | 24 (70.59) | 10 (55.56) | ||
| 3-5 cm | 2 (10.00) | 8 (23.53) | 6 (33.33) | ||
| ≤ 3 cm | 0 (0.00) | 2 (5.88) | 2 (11.11) | ||
| AFP | 2.552 | 0.279 | |||
| Negative | 6 (30.00) | 14 (41.18) | 10 (55.56) | ||
| Positive | 14 (70.00) | 20 (58.82) | 8 (44.44) | ||
| CA125 | 4.314 | 0.116 | |||
| Negative | 14 (70.00) | 16 (47.06) | 13 (72.22) | ||
| Positive | 6 (30.00) | 18 (52.94) | 5 (27.78) | ||
| CA19-9 | 5.278 | 0.071 | |||
| Negative | 12 (60.00) | 10 (29.41) | 9 (50.00) | ||
| Positive | 8 (40.00) | 24 (70.59) | 9 (50.00) |
Note: Abbreviations: TACE, transcatheter arterial chemoembolization; AH, anlotinib hydrochloride; IMRT, intensity-modulated radiotherapy; HCC, hepatocellular-cancer; ICC, intrahepatic cholangiocarcinoma; AFP, alpha-fetoprotein; CA125, carbohydrate antigen 125; CA19-9, carbohydrate antigen 19-9.
Comparison of ECOG scores
Post-treatment evaluations of ECOG scores revealed no significant differences among the three groups (P > 0.05). However, all groups demonstrated noticeable improvements in quality of life (Table 2).
Table 2.
Comparison of recovery status
| Treatment | TACE (n = 20) | TACE+AH (n = 34) | TACE+IMRT (n = 18) |
|---|---|---|---|
| ECOG score | 1 (1, 2) | 1 (1, 1) | 1 (1, 1) |
| H value | 1.515 | ||
| P value | 0.469 | ||
Note: Abbreviations: TACE, transcatheter arterial chemoembolization; AH, anlotinib hydrochloride; IMRT, intensity-modulated radiotherapy; ECOG, Eastern Cooperative Oncology Group.
Comparison of clinical efficacy
The ORR in the TACE+IMRT group (83.33%) was significantly higher compared to the TACE group (25.00%) and the TACE+AH group (44.12%). Similarly, the DCR in the TACE+IMRT group (88.89%) was also significantly greater than in the TACE group (50.00%), although no significant difference was found between the TACE+IMRT and TACE+AH groups (P > 0.05) (Table 3).
Table 3.
Comparison of clinical efficacy
| Group | CR | PR | SD | PD | ORR | DCR |
|---|---|---|---|---|---|---|
| TACE (n = 20) | 0 (0.00) | 5 (25.00) | 5 (25.00) | 10 (50.00) | 5 (25.00)* | 10 (50.00)* |
| TACE+AH (n = 34) | 0 (0.00) | 15 (44.12) | 7 (20.59) | 12 (35.29) | 15 (44.12)* | 22 (64.71) |
| TACE+IMRT (n = 18) | 2 (11.11) | 13 (72.22) | 1 (5.56) | 2 (11.11) | 15 (83.33) | 16 (88.89) |
| χ2 value | 6.068 | 6.559 | ||||
| P value | 0.194 | 0.038 |
Note: Compared with the TACE+IMRT group;
P < 0.05.
Abbreviations: TACE, transcatheter arterial chemoembolization; AH, anlotinib hydrochloride; IMRT, intensity-modulated radiotherapy. The disappearance of the target lesion was assessed as complete remission (CR). PR, partial remission; PD, progressive disease; SD, stable disease; ORR, objective response rate; DCR, disease control rate.
Comparison of adverse reactions
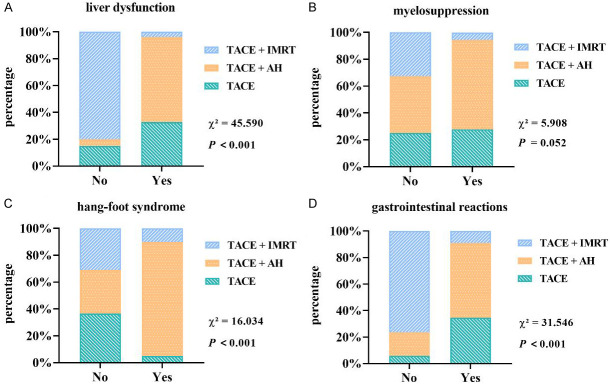
All patients experienced liver dysfunction, myelosuppression, hand-foot syndrome, and gastrointestinal reactions to varying degrees. The incidence of liver dysfunction (11.11%) and gastrointestinal reactions (27.78%) was significantly lower in the TACE+IMRT group compared to the TACE group (85%, 95%) and the TACE+AH group (97.06%, 91.18%). However, the TACE+AH group exhibited a markedly higher incidence of hand-foot syndrome (50%) than the other two groups. No significant differences were observed in the incidence of myelosuppression across the three groups (P > 0.05) (Figure 3).
Figure 3.
Comparison of adverse reactions. A. Liver dysfunction; B. Myelosuppression; C. Hand-foot syndrome; D. Gastrointestinal reactions. Abbreviations: TACE, transcatheter arterial chemoembolization; AH, anlotinib hydrochloride; IMRT, intensity-modulated radiotherapy.
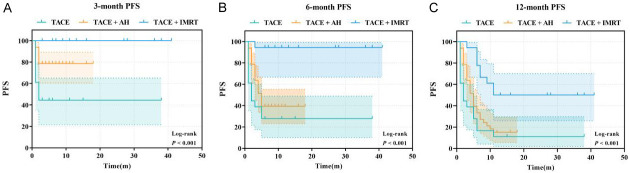
Comparison of PFS
Three months post-treatment, the TACE+IMRT group exhibited the highest PFS, followed by the TACE+AH and TACE groups. At 6 and 12 months post-treatment, PFS in the TACE+IMRT group remained significantly greater compared to the TACE+AH and TACE groups (P < 0.05) (Figure 4).
Figure 4.
Progression-free survival curve. A. 3-month progression-free survival (PFS); B. 6-month PFS; C. 12-month PFS. Abbreviations: TACE, transcatheter arterial chemoembolization; AH, anlotinib hydrochloride; IMRT, intensity-modulated radiotherapy.
Comparison of analysis of PFS influencing factors
Univariate Cox analysis showed that 12-month PFS was significantly associated with the treatment regimen, liver function grade, and tumor size (P < 0.05). These factors were further analyzed using a multivariate Cox regression model. The results showed that the TACE+IMRT regimen was significantly associated with prolonged PFS (HR = 0.226, 95% CI: 0.095-0.537, P = 0.001), while liver function grade B was associated with shorter PFS (HR = 2.916, 95% CI: 1.650-5.154, P < 0.001) (Table 4).
Table 4.
Univariate and multivariate analysis of progression-free survival (12-month PFS)
| Item | Univariate Cox | Multivariate Cox | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Treatment | < 0.001 | 0.003 | ||
| TACE | Reference | Reference | ||
| TACE+AH | 0.645 (0.357-1.165) | 0.146 | 0.628 (0.338-1.167) | 0.141 |
| TACE+IMRT | 0.218 (0.097-0.490) | < 0.001 | 0.226 (0.095-0.537) | 0.001 |
| Age | ||||
| < 55 years old | Reference | |||
| ≥ 55 years old | 0.667 (0.392-1.136) | 0.136 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.209 (0.625-2.340) | 0.573 | ||
| Pathologic diagnosis | ||||
| HCC | Reference | |||
| ICC | 1.076 (0.631-1.837) | 0.787 | ||
| Drinking history | ||||
| No | Reference | |||
| Yes | 0.850 (0.488-1.478) | 0.564 | ||
| Family history | ||||
| No | Reference | |||
| Yes | 1.287 (0.756-2.190) | 0.352 | ||
| History of hepatitis | ||||
| No | Reference | |||
| Yes | 1.107 (0.636-1.927) | 0.719 | ||
| Antiviral therapy | ||||
| No | Reference | |||
| Yes | 1.352 (0.753-2.427) | 0.313 | ||
| Child-Pugh | ||||
| A | Reference | Reference | ||
| B | 2.664 (1.550-4.576) | < 0.001 | 2.916 (1.650-5.154) | < 0.001 |
| Tumor diameter | 0.047 | 0.430 | ||
| ≥ 5 cm | Reference | Reference | ||
| 3-5 cm | 0.449 (0.211-0.955) | 0.038 | 0.624 (0.284-1.368) | 0.239 |
| ≤ 3 cm | 0.370 (0.090-1.528) | 0.170 | 0.599 (0.138-2.596) | 0.493 |
| AFP | ||||
| Negative | Reference | |||
| Positive | 1.641 (0.946-2.845) | 0.078 | ||
| CA125 | ||||
| Negative | Reference | |||
| Positive | 1.231 (0.725-2.090) | 0.442 | ||
| CA19-9 | ||||
| Negative | Reference | |||
| Positive | 1.206 (0.701-2.074) | 0.498 | ||
Note: Abbreviations: TACE, transcatheter arterial chemoembolization; AH, anlotinib hydrochloride; IMRT, intensity-modulated radiotherapy; HCC, hepatocellular-cancer; ICC, intrahepatic cholangiocarcinoma; AFP, alpha-fetoprotein; CA125, carbohydrate antigen 125; CA19-9, carbohydrate antigen 19-9.
Discussion
TACE is a primary treatment for patients with unresectable primary liver cancer in intermediate to advanced stages. Since the hepatic artery serves as the primary blood supply for liver tumors, TACE disrupts the tumor’s blood flow by embolizing the feeding arteries, leading to ischemia, necrosis, and subsequent tumor shrinkage. This approach effectively targets cancer cells [20-22].
However, TACE has notable limitations. Incomplete embolization and the formation of tumor collateral vessels often prevent complete pathologic necrosis. Additionally, ischemia and hypoxia within the tumor tissue stimulate the upregulation of hypoxia-inducible factor (HIF), which promotes the overexpression of vascular endothelial growth factor (VEGF). This mechanism can lead to intrahepatic tumor recurrence and distant metastasis [23-25]. Thus, supplementary therapies are necessary to address residual tumor lesions and maximize patient survival.
Anlotinib, a novel oral multi-target tyrosine kinase inhibitor developed in China, exerts antitumor effects by targeting pathways associated with angiogenesis and cell proliferation. It selectively inhibits VEGF receptor, stem cell factor receptor, and platelet-derived growth factor receptor, effectively reducing tumor vascular density and suppressing angiogenesis [26,27].
Liu et al. demonstrated that combining anlotinib with TACE effectively inhibits tumor angiogenesis, significantly improving treatment efficacy while maintaining a favorable safety profile [28]. This combination therapy has shown promise in prolonging survival [28]. In our study, TACE combined with anlotinib achieved a DCR of 64.71%, with 3-month PFS notably higher than TACE alone.
Although patients in the TACE+AH group experienced adverse reactions, including liver dysfunction, gastrointestinal symptoms, and hand-foot syndrome, these were generally mild. This highlights the importance of dietary guidance, drug intervention, and comprehensive evaluation of a patient’s physical status before initiating treatment.
Radiotherapy for liver cancer has been explored extensively over time. Traditionally, it was considered of limited value due to the inability to deliver radical doses to liver cancer lesions without causing significant adverse reactions, including severe liver injury, which discouraged clinical use [29,30]. However, advancements in radiotherapy techniques, such as three-dimensional conformal radiotherapy, IMRT, and stereotactic radiotherapy, have significantly improved targeting accuracy while minimizing radiation exposure to surrounding healthy tissues. As a result, radiotherapy has become an integral part of liver cancer treatment [31-33].
Studies demonstrate that combining IMRT with TACE markedly improves the prognosis of HCC patients. For example, in a cohort of 26 liver cancer patients (87% at stage III-IV), the ORR for IMRT combined with TACE was 64.8%, with a mean survival duration of 20.2 months and a PFS of 10.5 months [34].
Multivariate Cox regression analysis in this study confirmed that the treatment plan significantly affects 12-month PFS, with TACE+IMRT showing the most substantial improvement in PFS. Retrospective studies support these findings, indicating that TACE combined with IMRT is a strong prognostic factor for PFS [35,36]. While TACE combined with anlotinib exhibited a non-significant trend toward extending PFS, further research is needed to validate this effect. Additionally, patients with Child-Pugh grade B liver function showed significantly shorter PFS compared to grade A, underscoring liver function as a critical prognostic factor.
In this study, TACE combined with radiotherapy demonstrated the best clinical outcomes for treating locally advanced liver cancer. The ORR was 83.33%, and the DCR was 88.89%. The PFS at 3, 6, and 12 months was significantly higher in the TACE+IMRT group compared to the TACE and TACE+AH groups, reflecting improved serum tumor marker levels and enhanced quality of life.
Adverse reactions were also reduced in the TACE+IMRT group, with fewer instances of liver dysfunction (2/18) and gastrointestinal reactions (5/18). Radiotherapy-associated liver injury remains a primary concern. However, IMRT employs advanced optimization techniques to deliver a uniform radiation dose to the target area while sparing adjacent healthy tissues. This technique minimizes damage to sensitive organs and improves safety [37,38]. Liver protection and symptomatic treatments implemented before and after radiotherapy further reduced the incidence of adverse reactions. Consistent with these findings, Jang et al. reported that IMRT reduces gastrointestinal toxicity and liver function loss in advanced liver cancer patients [39].
However, this research has limitations. The small sample size and short follow-up period prevented evaluation of overall survival rates. Future studies should involve larger sample sizes and longer follow-up periods to further investigate treatment efficacy and long-term outcome.
In conclusion, this study confirms that the combination of TACE and radiotherapy is both safe and effective for managing locally advanced liver cancer, significantly prolonging PFS and identifying TACE+IMRT as a protective factor. In contrast, the therapeutic efficacy of TACE+AH was moderate, with manageable adverse reactions.
Acknowledgements
This work has been supported by grants from Hechi City Science and Technology Plan Project (2020AB3266).
Disclosure of conflict of interest
None.
References
- 1.Ye J, Cheng XD, Cheng B, Cheng YF, Chen XJ, Lu WG. MiRNA detection in cervical exfoliated cells for missed high-grade lesions in women with LSIL/CIN1 diagnosis after colposcopy-guided biopsy. BMC Cancer. 2019;19:112. doi: 10.1186/s12885-019-5311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 3.Toh MR, Wong EYT, Wong SH, Ng AWT, Loo LH, Chow PK, Ngeow J. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. 2023;164:766–782. doi: 10.1053/j.gastro.2023.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Alenezi AO, Krishna S, Mendiratta-Lala M, Kielar AZ. Imaging and management of liver cancer. Semin Ultrasound CT MR. 2020;41:122–138. doi: 10.1053/j.sult.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, Wang S, Cai J, Ke A, Fan J. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188638. doi: 10.1016/j.bbcan.2021.188638. [DOI] [PubMed] [Google Scholar]
- 6.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganesan P, Kulik LM. Hepatocellular carcinoma: new developments. Clin Liver Dis. 2023;27:85–102. doi: 10.1016/j.cld.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Su TH, Wu CH, Liu TH, Ho CM, Liu CJ. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Taiwan perspective. Clin Mol Hepatol. 2023;29:230–241. doi: 10.3350/cmh.2022.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Y, Huang X, Zhao C, Wang X, Mi G, Liu J. Summary of the evidence of best practices for the prevention and treatment of embolism syndrome after TACE in primary liver cancer. Front Oncol. 2024;13:1274235. doi: 10.3389/fonc.2023.1274235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreher C, Linde P, Boda-Heggemann J, Baessler B. Radiomics for liver tumours. Strahlenther Onkol. 2020;196:888–899. doi: 10.1007/s00066-020-01615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Zhang Z, Liao W, Hu K, Wang Z. Combination of sorafenib, camrelizumab, transcatheter arterial chemoembolization, and stereotactic body radiation therapy as a novel downstaging strategy in advanced hepatocellular carcinoma with portal vein tumor thrombus: a case series study. Front Oncol. 2021;11:650394. doi: 10.3389/fonc.2021.650394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao L, Li S, Ye F, Wang H, Zhong Y, Zhang X, Hu X, Huang X. The current status and future of targeted-immune combination for hepatocellular carcinoma. Front Immunol. 2024;15:1418965. doi: 10.3389/fimmu.2024.1418965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W, Chen S, Wu Z, Zhuang W, Yang J. Efficacy and safety of transarterial chemoembolization combined with anlotinib for unresectable hepatocellular carcinoma: a retrospective study. Technol Cancer Res Treat. 2020;19:1533033820965587. doi: 10.1177/1533033820965587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karachaliou GS, Dimitrokallis N, Moris DP. Downstaging strategies for unresectable hepatocellular carcinoma. World J Gastroenterol. 2024;30:2731–2733. doi: 10.3748/wjg.v30.i20.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown ZJ, Hewitt DB, Pawlik TM. Combination therapies plus transarterial chemoembolization in hepatocellular carcinoma: a snapshot of clinical trial progress. Expert Opin Investig Drugs. 2022;31:379–391. doi: 10.1080/13543784.2022.2008355. [DOI] [PubMed] [Google Scholar]
- 16.Lu H, Liang B, Xia X, Zheng C. Efficacy and safety analysis of TACE + donafenib + toripalimab versus TACE + sorafenib in the treatment of unresectable hepatocellular carcinoma: a retrospective study. BMC Cancer. 2023;23:1033. doi: 10.1186/s12885-023-11535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Song T. Conversion therapy and maintenance therapy for primary hepatocellular carcinoma. Biosci Trends. 2021;15:155–160. doi: 10.5582/bst.2021.01091. [DOI] [PubMed] [Google Scholar]
- 18.Wen N, Cai Y, Li F, Ye H, Tang W, Song P, Cheng N. The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines: 2022 update. Biosci Trends. 2022;16:20–30. doi: 10.5582/bst.2022.01061. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Wu QW, Li XK, Ye YA. Interpretation of Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition) Journal of Clinical Hepatology. 2020;36:996–999. [Google Scholar]
- 20.Dadrass F, Sher A, Kim E. Update on locoregional therapies for liver cancer: radiation segmentectomy. Curr Oncol. 2023;30:10075–10084. doi: 10.3390/curroncol30120732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson C, Chin HM, Hoverson J, Court C, Wagner T, Newman NB. Treatment of localized hepatocellular carcinoma: resection vs. ablation vs. radiation. Ann Palliat Med. 2024;13:344–354. doi: 10.21037/apm-23-486. [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Zhong BY, Zhu HD, Guo JH, Teng GJ. Embolotherapy of unresectable hepatocellular carcinoma: eastern perspective. Chin Clin Oncol. 2019;8:60. doi: 10.21037/cco.2019.11.01. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding ZN, Meng GX, Xue JS, Liu H, Yang LS, Li RZ, Mao XC, Yan YC, Wang DX, Dong ZR, Li T. Systemic therapy with or without locoregional therapy for advanced hepatocellular carcinoma: a systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2023;184:103940. doi: 10.1016/j.critrevonc.2023.103940. [DOI] [PubMed] [Google Scholar]
- 25.Zong Z, Tang R, Li M, Xiong X, Li D, Fan J, Ye W, Xue C. Efficiency and stability of transarterial chemoembolization combined with or without lenvatinib for unresectable hepatocellular carcinoma. Turk J Gastroenterol. 2024;35:212–222. doi: 10.5152/tjg.2024.23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu SH, Chang PY, Shih YL, Huang WY, Ko KH, Chang WC, Huang GS. Efficacy and safety of supplemental transarterial chemoembolization through extrahepatic collateral arteries with drug-eluting beads: treatment for unresectable hepatocellular carcinoma. Drug Des Devel Ther. 2020;14:5029–5041. doi: 10.2147/DDDT.S266470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song F, Hu B, Cheng JW, Sun YF, Zhou KQ, Wang PX, Guo W, Zhou J, Fan J, Chen Z, Yang XR. Anlotinib suppresses tumor progression via blocking the VEGFR2/PI3K/AKT cascade in intrahepatic cholangiocarcinoma. Cell Death Dis. 2020;11:573. doi: 10.1038/s41419-020-02749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Zhang K, Zhou LJ. Clinical efficacy of anlotinib in combination with TACE in the treatment of advanced primary hepatic carcinoma. China Licensed Pharmacist. 2023;20:59–65. [Google Scholar]
- 29.Miften M, Vinogradskiy Y, Moiseenko V, Grimm J, Yorke E, Jackson A, Tome WA, Ten Haken RK, Ohri N, Mendez Romero A, Goodman KA, Marks LB, Kavanagh B, Dawson LA. Radiation dose-volume effects for liver SBRT. Int J Radiat Oncol Biol Phys. 2021;110:196–205. doi: 10.1016/j.ijrobp.2017.12.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, Truty MJ, Kurup AN, Go RS. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J. Clin. Oncol. 2018;36:600–608. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Chiang CL, Dawson LA. Efficacy and safety of radiotherapy for primary liver cancer. Chin Clin Oncol. 2021;10:9. doi: 10.21037/cco-20-89. [DOI] [PubMed] [Google Scholar]
- 32.Shanker MD, Moodaley P, Soon W, Liu HY, Lee YY, Pryor DI. Stereotactic ablative radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis of local control, survival and toxicity outcomes. J Med Imaging Radiat Oncol. 2021;65:956–968. doi: 10.1111/1754-9485.13309. [DOI] [PubMed] [Google Scholar]
- 33.Lewis S, Barry A, Hawkins MA. Hypofractionation in hepatocellular carcinoma - the effect of fractionation size. Clin Oncol (R Coll Radiol) 2022;34:e195–e209. doi: 10.1016/j.clon.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 34.Zhang T, Zhao YT, Wang Z, Li CR, Jin J, Jia AY, Wang SL, Song YW, Liu YP, Ren H, Fang H, Bao H, Liu XF, Yu ZH, Li YX, Wang WH. Efficacy and safety of intensity-modulated radiotherapy following transarterial chemoembolization in patients with unresectable hepatocellular carcinoma. Medicine (Baltimore) 2016;95:e3789. doi: 10.1097/MD.0000000000003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, Huang X, Chen J, Zhang S. Evaluation of the clinical efficacy of intensity-modulated radiotherapy combined with transcatheter arterial chemoembolization for hepatocellular carcinoma with extrahepatic oligometastasis and prognostic factors for patient survival. Int J Gen Med. 2023;16:1271–1278. doi: 10.2147/IJGM.S403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang D, Du J, Nie W, Wang C, Ma Z. Combination treatment of transcatheter arterial chemoembolization, intensity-modulated radiotherapy, and sorafenib for hepatocellular carcinoma with macrovascular invasion. Medicine (Baltimore) 2023;102:e35713. doi: 10.1097/MD.0000000000035713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong VJW, Shelat VG, Chao YK. Clinical application of advances and innovation in radiation treatment of hepatocellular carcinoma. J Clin Transl Res. 2021;7:811–833. [PMC free article] [PubMed] [Google Scholar]
- 38.Wu F, Chen B, Dong D, Rong W, Wang H, Wang L, Wang S, Jin J, Song Y, Liu Y, Fang H, Tang Y, Li N, Zhu X, Li Y, Wang W, Wu J. Phase 2 evaluation of neoadjuvant intensity-modulated radiotherapy in centrally located hepatocellular carcinoma: a nonrandomized controlled trial. JAMA Surg. 2022;157:1089–1096. doi: 10.1001/jamasurg.2022.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang WI, Jo S, Moon JE, Bae SH, Park HC. The current evidence of intensity-modulated radiotherapy for hepatocellular carcinoma: a systematic review and meta-analysis. Cancers (Basel) 2023;15:4914. doi: 10.3390/cancers15204914. [DOI] [PMC free article] [PubMed] [Google Scholar]