Abstract
Introduction: ECMO is an advanced technology for extracorporeal respiratory and circulatory support. It involves the extraction of venous blood from the patient’s body, which is subsequently oxygenated within an oxygenator (or membrane lung). This oxygen-rich blood is reinfused either into veins or arteries, rapidly compensating for impaired lung and heart functionalities. ECMO mirrors the essential processes of cardiac output in facilitating tissue perfusion and gas exchange, thereby expanding the therapeutic window for critically ill patients with acute circulatory insufficiency, and enabling restoration of cardiopulmonary function. We report a 55-year-old woman with no prior significant health issues who suddenly experienced headache accompanied by nausea and vomiting while resting at home. A small amount of gastric content was vomited, and no specific treatment was administered. Two hours later, she was found unconscious on the bathroom floor, with her mouth and nose filled with vomit, loss of consciousness, dyspnea, and urinary incontinence. A cranial CT scan revealed “subarachnoid hemorrhage with left frontal lobe cerebral hemorrhage extending into the ventricular system”. Despite intervention, the peripheral blood oxygen saturation rapidly dropped to 70%. The electrocardiogram showed sinus rhythm with abnormal ST-T changes. Subsequently, the ECMO (Extracorporeal Membrane Oxygenation) specialized treatment team performed a bedside ultrasound-guided Veno-Arterial (V-A) ECMO implantation procedure for the patient. Given that cranial CTA imaging demonstrated a 5 mm × 5 mm × 7 mm wide-necked aneurysm with an irregular shape at the M1 bifurcation of the left middle cerebral artery, this aneurysm was deemed the culprit lesion responsible for the rupture and bleeding. Consequently, an ECMO-supported cerebral aneurysm stent-assisted embolization procedure was carried out. Following anticoagulation, anti-infection therapy, continuous cerebrospinal fluid drainage, nimodipine infusion to prevent cerebral vasospasm, and traditional Chinese medicine rehabilitation treatment, the patient regained spontaneous respiration on the 5th day after surgery. She was successfully discharged from the hospital on the 26th day post-surgery. Thus, ECMO-supported stent-assisted embolization treatment modality is feasible for patients with ruptured cerebral aneurysms and subarachnoid hemorrhage. However, the value of ECMO in recovering consciousness among patients with ruptured cerebral aneurysm and subarachnoid hemorrhage-induced coma requires further validation through a larger number of cases.
Keywords: Extracorporeal membrane oxygenation, blood circulation failure, nerve intervention, aneurysm embolization, case report
Introduction
Extracorporeal membrane oxygenation (ECMO) represents a novel technology for extracorporeal respiratory and circulatory support [1]. It involves draining venous blood from the body, oxygenating it through an oxygenator (membrane lung), and then reinfusing it into a vein or artery. This process temporarily replaces lung and heart function, providing vital support for critically ill patients. Since its introduction in 1972, peripheral VA-ECMO has increasingly been utilized for refractory cardiogenic shock. According to the Extracorporeal Life Support Registry, over 15,000 adult patients have received VA-ECMO support since 1990, with an approximate discharge survival rate of 40%. However, its application in China remains limited, though its use in clinical resuscitation and rescue of cardiac arrest patients appears promising. It is crucial to note that ECMO alone can only sustain vital signs; addressing the underlying cause of illness is equally important. In ECMO-supported patients, neurological complications are prevalent, and paralysis or hypoxic conditions may hinder neurological assessment. The combination of ECMO with interventional surgery, particularly nerve intervention therapy, is uncommon, with only a handful of cases involving thrombus removal under ECMO support reported.
We have previously documented a case involving basilar artery thrombectomy and left posterior artery stent thrombectomy under ECMO, though the patient ultimately succumbed to heart failure. To our knowledge, there have been no reported cases of aneurysm embolization supported by ECMO. In cases where patients experience acute circulatory failure, the heart may be unable to provide adequate perfusion pressure or blood supply to the brain, significantly reducing feasibility of nerve intervention. Early establishment of ECMO support can rapidly restore systemic blood circulation and oxygen supply, stabilizing hemodynamics and blood pressure during nerve intervention surgery, thereby facilitating successful nerve intervention procedures under ECMO support.
Case report
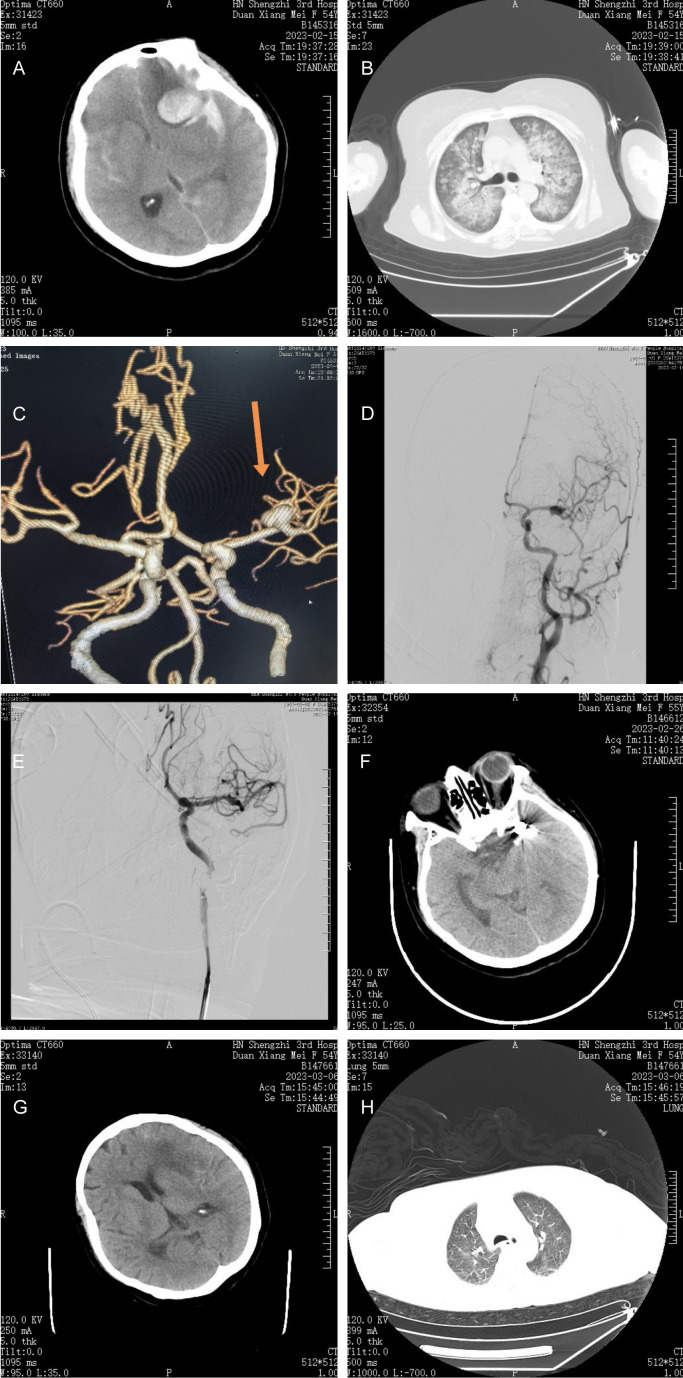
The patient was a 55-year-old female with no significant medical history. She suddenly developed headache accompanied by nausea and vomiting while resting at home. She vomited a small amount of gastric contents without receiving any specific treatment. Two hours later, she was found collapsed on the bathroom floor, with her mouth and nose filled with vomitus. She also experienced confusion, dyspnea, and urinary incontinence. Emergency medical services were called, and emergency physicians promptly arrived at the scene and initiated supportive symptomatic treatment immediately, including “cardiac monitoring, endotracheal intubation, and ventilator-assisted respiration”. Upon admission to the hospital, a head CT scan revealed “subarachnoid haemorrhage with left frontal lobe intracerebral haemorrhage extending into the ventricular system” (Figure 1A); a chest CT scan indicated “diffuse lesions in both lungs (white lungs), pulmonary edema, and aspiration pneumonia (Figure 1B)”. Despite ventilator-assisted respiration via tracheal intubation (ventilator parameters: inhaled oxygen concentration 100%, PEEP 8 cm H2O, Ps12 cm H2O, respiratory rate 18 breaths/min), peripheral oxygen saturation rapidly decreased to 70%. The electrocardiogram showed sinus rhythm with abnormal ST-T changes. Following expert consultation, severe hypoxemia due to the aspiration of gastric contents into the lungs was an indication for ECMO (Extracorporeal Membrane Oxygenation).
Figure 1.
A. Subarachnoid haemorrhage, left frontal lobe cerebral haemorrhage. B. Diffuse lesions in both lungs: white lungs. C. Wide carotid aneurysm at the M1 bifurcation of the left middle cerebral artery. D, E. Aneurysm stent-assisted dense spring coil tamponade and M1 upper trunk and lower trunk vessel patency. F, G. Subarachnoid hemorrhage and hematoma resorption with centred midline structures. H. Chest CT scan showing significant resorption of the original lung shadow.
Informed consent was obtained from the patient’s family members, and an ECMO specialist team initiated bedside ultrasound-guided V-A ECMO. The ECMO parameters were set as follows: flow rate, 2.39 L/min; rotation speed, 2186 rpm; no heparin anticoagulation; target APTT, 40-60 s; and ACT, 180-220 s (heparin dose adjusted based on the ACT and APTT values). The vital signs after ECMO initiation were as follows: T, 36.7°C; P, 102 beats/min; R, 18 beats/min (spontaneous + ventilator-controlled); BP, 95/54 mmHg; and SPO2, 99%. Despite the normalization of oxygen saturation, heart rate and blood pressure under ECMO support, the patient’s consciousness gradually deteriorated. Two hours after ECMO initiation, the Glasgow Coma Scale (GCS) score ranged from 13-14 points, indicating mildly impaired consciousness; scores ranging 9-12 points indicate moderately impaired consciousness, and scores ranging 3-8 points indicate severely impaired consciousness. A score less than 8 points indicates a comatose state, and a score less than 3 points indicates deep coma or brain death. The patient’s Hunt-Hess score was ≤ 4 points, indicating grade V haemorrhage, the bilateral pupils were dilated, and the patient was assessed to be in deep coma. After consultation with interventional radiologists and neurosurgeons, the patient was deemed unsuitable for active surgical craniotomy or intervention, and it was recommended that she continue her current medical treatment.
Fourteen hours after ECMO initiation, the patient could be aroused and was able to understand instructions, close eyes and turn her head when instructed. The Hunt-Hess grade was III. Repeat chest CT scan, head CT scan, and cerebral angiography (CTA) revealed no significant reduction in the extent of the lung lesion. The extents of subarachnoid haemorrhage and cerebral haemorrhage did not increase compared with those at admission. CTA revealed a wide 5 × 5 × 7 mm carotid aneurysm with an irregular morphology in the left middle cerebral artery at the bifurcation of M1 (Figure 1C). The aneurysm was judged to be the lesion responsible for the rupture and hemorrhage.
Decision-making process: After consulting with neurosurgeons, the interventional radiologist noted that the patient was previously in good health without other underlying diseases or obvious abnormalities in cardiac function, had improved consciousness and partially recovered spontaneous respiration, and had no increase in the extent of subarachnoid haemorrhage or cerebral haemorrhage compared to that at admission. Minimally invasive endovascular treatment was, therefore, more suitable for the patient who could undergo stent-assisted embolization of cerebral aneurysms under ECMO support.
Decision-making process: After obtaining the approval of the Hospital Medical Ethics Committee and communicating the patient’s condition with the family members, an informed consent form for cerebral angiography and aneurysm embolization surgery was signed. The ECMO operation team accompanied the patient to the catheterization room of the Department of Radiology and Interventional Medicine. The anesthesiologist administered intravenous general anaesthetics to the patient. Under monitoring by DSA (Siemens Artis Zeego), access was gained through the left femoral artery route (the right femoral artery had a VA-ECMO channel) for total cerebral angiography and 3D reconstruction of the left internal carotid artery. The images revealed a wide carotid aneurysm with an irregular morphology in the middle cerebral artery at the bifurcation of M1, with a close relationship between the neck of the aneurysm and the inferior trunk of M. Intraoperative discussion concluded that the aneurysm could be embolized with a spring coil assisted by a Neuroform Atlas stent. The 8F guiding catheter was exchanged into the left internal carotid artery with the 6F intermediate catheter, and the SL-10 microcatheter with a microguidewire was introduced into the distal part of the M1 lower trunk through the intermediate catheter. Then, the Echelon-10 microcatheter (EV3) was introduced into the aneurysm lumen under the assistance of the microguidewire, the AXIUM PRIME 6 mm × 15 cm 3D basket spring coil was released into the lumen of the aneurysm, and the Neuroform Atlas stent (3 mm × 24 mm from Stryker) was released through the SL-10. After the AXIUM PRIME 6 mm × 15 cm 3D basket spring coils were released into the aneurysm cavity, the Neuroform Atlas stent (Stryker 3 mm × 24 mm) was released via the SL-10 to cover the neck of the aneurysm, and 8 spring coils were subsequently released into the aneurysm cavity via the Echelon-10 microcatheter to densely pack the aneurysm. Imaging revealed that the aneurysm had disappeared, the adjacent M1 upper and lower trunk arterioles were smooth (Figure 1D, 1E), and the intervention procedure was completed. The intervention was completed. During the operation, the patient’s vital signs remained stable, all monitoring indices were normal, and the period of awakening from was uneventful. The left femoral artery catheter sheath was retained, and the patient was sent back to the intensive care center with ECMO support and a ventilator. Postoperative measures: 1. Control blood pressure at approximately 120/80 mmHg; 2. Provide “anticoagulation and anti-cerebral vasospasm” treatment; and 3. Implement blood cerebrospinal fluid replacement with a bedside lumbar cistern drainage tube. Postoperative assessments revealed a Glasgow Coma Scale (GCS) score of 12 and a Hunt-Hess grade of III.
Postoperative management: After anticoagulation, anti-infection treatment, continuous cerebrospinal fluid replacement, morphine administration to prevent cerebral vasospasm, and traditional Chinese medicine rehabilitation, the patient resumed voluntary respiratory treatment on the 5th day after stent-assisted embolization of the cerebral aneurysm. On the 6th day after the operation, the chest X-ray taken at the bedside showed a significant reduction in the extent of shadow in the lungs, and a repeat cranial CT scan demonstrated absorption of subarachnoid haemorrhage and haematomas in the brain parenchyma with the midline structure centred (Figure 1F, 1G). ECMO was successfully withdrawn on the 9th day, and the patient was discharged on the 26th day. Before discharge, a chest CT scan showed that the original lung shadow was almost completely absorbed (Figure 1H). Her Hess-Hunt grade was II, her mRS score was II, and her Glasgow Coma Scale (GCS) score was 15. Three months after discharge, during telephone follow-up, the patient’s general condition was good, and she was able to take care of herself.
Discussion
Extracorporeal membrane oxygenation (ECMO) provides powerful cardiopulmonary support and plays an important role in the treatment of patients with severe hypoxemia, respiratory failure, fulminant myocarditis, severe heart failure, and other acute and critical diseases [2]. However, ECMO, as a high-risk operation technique, is prone to various fatal complications during its implementation, among which acute brain injury is one of the common complications and the main cause of death in patients treated with VA-ECMO [3]. Recent literature shows that the incidence of intracranial complications is as high as 5%-21%. In ECMO patients, the extensive use of sedation and neuromuscular blockade to prevent ventilator dyssynchrony and cannula dislodgement can potentially delay the recognition of neurological deterioration and subsequent stroke assessment. Following the initiation of ECMO, there is a trend towards discontinuing neuromuscular blockade and reducing sedation levels. In previously reported cases of neurologic interventions performed with ECMO support, the majority were emergency procedures for ischemic stroke management (Table 1).
Table 1.
Literature review
| Case | Age/Gender | Indication for ECMO | ECMO | Treatment | Outcome |
|---|---|---|---|---|---|
| Gurkirat Kohli et al. 2023 [5] | 55/male | Cardiogenic shock | V-A ECMO | Internal carotid artery stent thrombectomy | Survival |
| Gurkirat Kohli et al. 2023 | 48/male | Cardiogenic shock | V-A ECMO | Internal carotid artery stent thrombectomy | Survival |
| Gurkirat Kohli et al. 2023 | 17/female | Cardiogenic shock | V-A ECMO | Basilar artery thrombus | Survival |
| Shan L et al. 2024 [6] | 81/female | Cardiogenic shock | V-A ECMO | Intracranial revascularization | Die |
| Loic Le Guennec et al. 2023 [7] | NA | Cardiogenic shock | V-A ECMO | Internal carotid artery stent thrombectomy | Survival |
| Loic Le Guennec et al. 2023 | NA | Cardiogenic shock | V-A ECMO | Internal carotid artery stent thrombectomy | Survival |
| Ali Sultan-Qurraie et al. 2023 [8] | 53/male | Cardiogenic shock | V-A ECMO | Basilar artery thrombus | Survival |
A ruptured intracranial aneurysm causing subarachnoid haemorrhage is also a common acute and critical clinical condition with a high mortality rate. Given the high complication rate of ECMO support in the treatment of patients with neurological disorders [4], ECMO is usually not recommended by clinicians for patients with ruptured aneurysms and subarachnoid haemorrhage (SAH).
In this case, after the patient developed sudden subarachnoid haemorrhage (SAH), the gastric contents were aspirated into the bilateral alveoli, resulting in the admission CT images showing “large white lungs” (Figure 1B). Despite the timely initiation of the ventilator-assisted breathing mode, the blood oxygen saturation level decreased rapidly. Without prompt correction of severe hypoxemia, the patient would have suffered irreversible severe damage to the heart, brain, and kidneys. The patient was 55 years old and previously healthy with normal cardiac function. Timely administration of VA-ECMO rapidly corrected hypoxemia, and the oxygen saturation increased from 70% before initiation to 99%, ensuring an adequate oxygen supply to the heart, kidneys, and brain tissues, thereby creating an opportunity for subsequent treatment of the ruptured aneurysm. After 14 hours of VA-ECMO support, consciousness improved, and spontaneous respiration was partially restored, meeting the criteria for active treatment of cerebral aneurysm. The minimally invasive endovascular treatment approach was more suitable for this patient. After densely packing the aneurysm for embolization with the assistance of a stent, lumbar cistern haemoperitoneum cerebrospinal fluid replacement was performed, which quickly diluted the blood within the cerebrospinal fluid and reduced the incidence of cerebral arterial spasm, thereby leading to relatively good clinical outcome. Although the current literature demonstrates that ECMO support has damaging neurological effects [9], severe hypoxemia resulting from aspiration of gastric contents poses a greater risk to the patient. Whether this patient’s rapid improvement in consciousness was a direct result of ECMO requires further confirmation from additional relevant cases, but at least part of the contribution of ECMO was the normalization of blood oxygen levels and the stabilization of the body’s internal environment.
Conclusion
Stent-assisted embolization with ECMO support is a feasible mode of treatment for patients with ruptured cerebral aneurysms and subarachnoid haemorrhage. The value of ECMO in promoting awakening in comatose patients with ruptured cerebral aneurysms and subarachnoid haemorrhage needs to be further confirmed in more cases.
Disclosure of conflict of interest
None.
References
- 1.Hadaya J, Benharash P. Extracorporeal membrane oxygenation. JAMA. 2020;323:2536. doi: 10.1001/jama.2020.9148. [DOI] [PubMed] [Google Scholar]
- 2.Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M, Volovar S, Seyfrydova M, Jarkovsky J, Svoboda M, Linhart A, Belohlavek J ECMO-CS Investigators. Extracorporeal membrane oxygenation oxygenation in the therapy of cardiogenic shock: results of the ECMO-CS randomized clinical trial. Circulation. 2023;147:454–464. doi: 10.1161/CIRCULATIONAHA.122.062949. [DOI] [PubMed] [Google Scholar]
- 3.Cho SM, Canner J, Chiarini G, Calligy K, Caturegli G, Rycus P, Barbaro RP, Tonna J, Lorusso R, Kilic A, Choi CW, Ziai W, Geocadin R, Whitman G. Modifiable risk factors and mortality from ischemic and hemorrhagic strokes in patients receiving venoarterial extracorporeal membrane oxygenation: results from the extracorporeal life support organization registry. Crit Care Med. 2020;48:e897–e905. doi: 10.1097/CCM.0000000000004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavayas YA, Del Sorbo L, Fan E. Intracranial hemorrhage in adults on ECMO. Perfusion. 2018;33:42–50. doi: 10.1177/0267659118766435. [DOI] [PubMed] [Google Scholar]
- 5.Kohli G, George DD, Grenga A, Santangelo G, Gosev I, Schartz D, Kessler A, Khan I, Barrus B, Gu Y, Bhalla T, Mattingly TK, Bender MT. Mechanical thrombectomy for ischemic stroke secondary to large vessel occlusions in patients on extracorporeal membrane oxygenation. Cerebrovasc Dis. 2023;52:532–538. doi: 10.1159/000528218. [DOI] [PubMed] [Google Scholar]
- 6.Ding LS, Liang H, Zheng M, Shen M, Li ZJ, Song RP, Chen QL. Extracorporeal membrane oxygenation states basilar artery thrombectomy and left posterior cerebral artery stent thrombectomy: a case report. World J Clin Cases. 2024;12:3589–3595. doi: 10.12998/wjcc.v12.i18.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Guennec L, Schmidt M, Clarençon F, Elhfnawy AM, Baronnet F, Kalamarides M, Lebreton G, Luyt CE. Mechanical thrombectomy in acute ischemic stroke patients under venoarterial extracorporeal membrane oxygenation. J Neurointerv Surg. 2020;12:486–488. doi: 10.1136/neurintsurg-2019-015407. [DOI] [PubMed] [Google Scholar]
- 8.Sultan-Qurraie A, Rozansky G, Cox JA, Lazzaro M. Cross-circulation thrombectomy with use of a stent retriever: a case report. Interv Neuroradiol. 2017;23:422–426. doi: 10.1177/1591019917706191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prinz V, Manekeller L, Menk M, Hecht N, Weber-Carstens S, Vajkoczy P, Finger T. Clinical management and outcome of adult patients with extracorporeal life support device-associated intracerebral hemorrhage-a neurocritical perspective and grading. Neurosurg Rev. 2021;44:2879–2888. doi: 10.1007/s10143-020-01471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]