This case-control study estimates vaccine effectiveness of RSVpreF for RSV-related lower respiratory tract disease hospitalizations and emergency department visits in older adults.
Key Points
Question
Does Respiratory Syncytial Virus Prefusion F (RSVpreF) protect older adults against respiratory syncytial virus (RSV)–related hospitalization and emergency department (ED) visits?
Findings
This test-negative case-control study in a large US health care network estimated 91% and 90% RSVpreF effectiveness using strict and broad control groups, respectively, against RSV-related hospitalization and ED events in adults aged 60 years and older, the majority of whom were older than 75 years and had a substantial burden of baseline comorbidities.
Meaning
These findings suggest that during the 2023 to 2024 RSV season, RSVpreF substantially reduced severe medically attended RSV disease in an older adult population with significant representation from the oldest age groups, those with comorbidities, and those who were immunocompromised.
Abstract
Importance
Clinical trials have demonstrated high vaccine efficacy (VE) against lower respiratory tract disease (LRTD) but enrolled a smaller proportion of persons aged 75 years or older and those with comorbidities than seen in highest-risk populations in clinical practice settings. Additionally, VE against respiratory syncytial virus (RSV)–related hospitalizations and emergency department (ED) visits is not yet fully described.
Objective
To estimate Respiratory Syncytial Virus Prefusion F (RSVpreF) effectiveness in older adults.
Design, Setting, and Participants
This was a retrospective case-control study with a test negative design. Cases were adults aged 60 years or older with hospitalizations or ED visits at Kaiser Permanente of Southern California for LRTD from November 24, 2023, to April 9, 2024, who had respiratory swabs collected and tested for RSV. Two control definitions were prespecified: (1) strict controls included RSV-negative LRTD events that were negative for human metapneumovirus, SARS-CoV-2, and influenza, and positive for a nonvaccine preventable cause (primary) and (2) broad controls included all RSV-negative LRTD events (sensitivity analysis). Enhanced specimen collection was conducted to salvage clinical respiratory swabs not tested for RSV during routine care. Data were analyzed from May to September 2024.
Exposure
RSVpreF vaccine receipt during the first RSV season after licensure and 21 or more days before LRTD event.
Main outcomes and measures
Estimated VE against first episode of RSV-related LRTD hospitalization or ED visit.
Results
A total of 7047 LRTD-related hospitalizations or ED encounters with RSV testing results were included. The mean (SD) age was 76.8 (9.6) years; 3819 (54.2%) were female; 839 (11.9%) were non-Hispanic Asian or Pacific Islander, 2323 (33.0%) were Hispanic, 1197 (17.0%) were non-Hispanic Black, and 2602 (36.9%) were non-Hispanic White; 998 (14.2%) were immunocompromised; and 6573 (93.3%) had 1 or more Charlson comorbidity. Using strict controls, estimated adjusted VE was 91% (95% CI, 59%-98%). Using broad controls, estimated adjusted VE was 90% (95% CI, 59%-97%).
Conclusions and Relevance
In a high-risk, general population, RSVpreF vaccination conferred protection against RSV-related LRTD in the hospital and ED settings among US adults aged 60 years or older, the majority of whom were aged 75 years or older and had comorbidities. These data support use of this vaccine in older adults.
Introduction
Respiratory syncytial virus (RSV) infection is a leading cause of severe acute respiratory disease in older adults and adults with underlying comorbidities1 and may also result in long-term sequelae and trigger cardiac events.2,3,4 Among US adults aged 65 years and older, approximately 160 000 RSV-related acute respiratory infection (ARI) hospitalizations and 120 000 emergency department (ED) visits are estimated to occur annually.1
On June 21, 2023, the US Advisory Committee on Immunization Practices recommended persons aged 60 years and older may receive a single dose of a vaccine licensed to prevent RSV lower respiratory tract disease (LRTD) using shared clinical decision-making,5 citing in part lack of vaccine effectiveness (VE) data from the phase 3 pivotal trials regarding RSV-related hospitalizations and among high risk groups.6 We evaluated, in a large US health care network, Respiratory Syncytial Virus Prefusion F (RSVpreF) effectiveness against first occurrence of RSV-related LRTD inpatient or ED visit.
Methods
Design, Setting, and Participants
We performed a test-negative case-control study comparing the odds of RSVpreF (Abrysvo) receipt between RSV cases and test-negative controls at Kaiser Permanente Southern California (KPSC) (providing care to approximately 4.8 million members, including 1.1 million aged ≥60 years). We included patients aged 60 years or older with an International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code consistent with LRTD in any position for a hospitalization or ED encounter who underwent molecular testing for RSV via respiratory swab from November 24, 2023, to April 9, 2024 (eTable 1 in Supplement 1). Participants were required to have 1 year or more of health plan membership to determine comorbidities. Non-RSVpreF vaccine recipients (eg, recipients of Arexvy) and those who received RSV vaccination less than 21 days before the LRTD encounter were excluded. KPSC’s institutional review board approved this study and waived the informed consent requirement because the research involved minimal risk to participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Enhanced Specimen Testing
To reduce bias associated with infrequent standard of care RSV testing, we enhanced specimen testing by salvaging respiratory swabs collected for standard-of-care molecular testing of SARS-CoV-2 and influenza. Swabs that were not SARS-CoV-2 or influenza positive and not already tested for RSV using the Roche Diagnostics Cobas eplex respiratory pathogen panel 2 (RP2) as part of routine care were defined as salvaged specimens and assayed using the same RP2 panel, which tests for 16 respiratory viral targets, and Chlamydia pneumoniae and Mycoplasma pneumoniae.
Outcomes
Outcomes were defined based on the results of Cobas RP2 testing 14 or fewer days before through 3 or fewer days after the LRTD encounter. Cases were RSV-positive LRTD events. Two sets of prespecified controls were used: (1) Strict (primary analysis): LRTD events that were negative for RSV, human metapneumovirus (HMPV), influenza, and SARS-CoV-2, and were positive for a nonvaccine preventable disease (VPD); adenovirus, coronavirus (229E, HKU1, NL63, or OC43), human rhinovirus/enterovirus, parainfluenza 1-4, Chlamydia pneumoniae, Mycoplasma pneumoniae; and (2) Broad (sensitivity analysis): RSV-negative LRTD events, regardless of other identified causes (and including those who tested negative for all pathogens). The strict approach accounted for potential bias associated with VPD controls7 and for potential RSVpreF VE against HMPV.8 Additional outcomes using only the strict control definition included RSV-related LRTD hospitalizations, RSV-related LRTD hospitalizations among patients with high-risk chronic medical conditions, RSV-related LRTD ED events, RSV-related severe (ie, supplemental use of oxygen) LTRD hospitalizations and ED events. Patients could contribute 1 or more LRTD event to the study if events were more than 30 days apart; deduplicated sensitivity analyses to estimate VE against LRTD hospitalization or ED events on only first event per patient were conducted.
Exposures
KPSC members aged 60 years or older were eligible for RSV vaccines at no cost based on FDA-authorized indications. KPSC electronic health records captured all vaccinations administered within the health system. Records were supplemented with vaccine administration data from California’s Immunization Registry, to which clinicians are required by law to report RSV vaccinations, rendering misclassification of vaccination status unlikely. RSVpreF was administered at 7 study hospitals in the KPSC network. Patients who received RSVpreF 21 or more days before the LRTD encounter were considered vaccinated.
Statistical Analysis
Odds ratios (ORs) and 95% CIs were calculated from multivariable logistic regression including prespecified and model-specified covariates: month of encounter, age, sex, self-reported race and ethnicity (Hispanic, non-Hispanic Asian or Pacific Islander, non-Hispanic Black, non-Hispanic White, or non-Hispanic multiple/other/unknown), modified Charlson score,9,10,11 and health care utilization in the year before encounter. The only variable with missing values was race and ethnicity, which was combined as a non-Hispanic multiple/other/unknown category. Race and ethnicity were assessed in this study due to association with vaccination and association with the outcomes of interest. A 2-sided α of .05 was used for logistic regression modeling. Corresponding 95% CIs were calculated using the Wald method. VE was calculated as 1 − OR × 100%. Analyses were performed using SAS version 9.4 (SAS Institute). Data were analyzed from May to September 2024.
Results
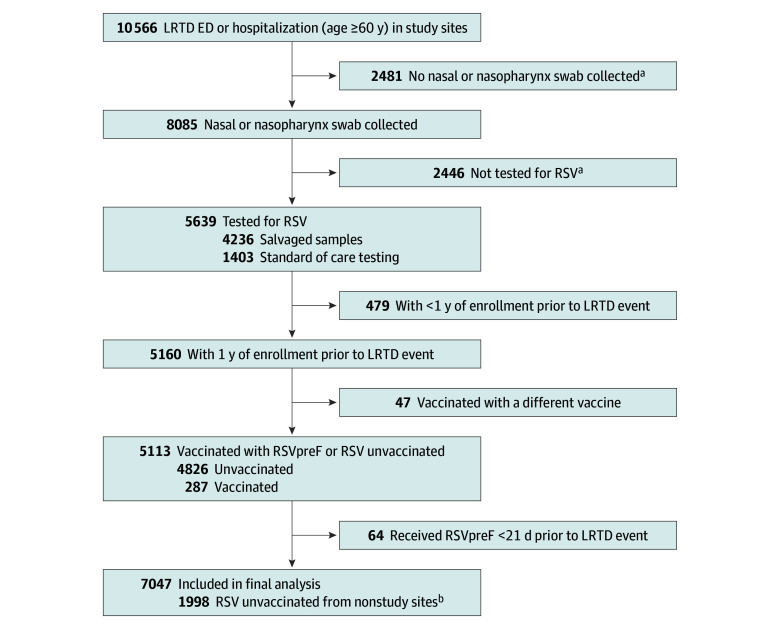
A total of 10 566 patients aged 60 years and older had LRTD hospitalizations or ED encounters at study sites. A total of 8085 (76.5%) had a nasal or nasopharyngeal swab, and of these, 5639 (69.7%) were tested for RSV (4236 salvage specimens and 1403 standard-of-care specimens). After applying eligibility criteria, 7047 LRTD events with tested specimens (64.2%) were included in the analysis (ie, met age, membership, and vaccination criteria) (Figure). The mean (SD) age was 76.8 (9.6) years, 3819 (54.2%) were female, 839 (11.9%) were non-Hispanic Asian or Pacific Islander, 2323 (33.0%) were Hispanic, 1197 (17.0%) were non-Hispanic Black, and 2602 (36.9%) were non-Hispanic White. A total of 998 (14.2%) were immunocompromised. The distribution of LRTD-defining ICD-10 codes was similar between RSV-positive and RSV-negative LRTD events, with pneumonia being the most common diagnosis in both groups (eTable 2 in Supplement 1). The study population included 725 specimens (10.3%) tested for RSV as part of standard of care, and 6322 salvaged specimens (89.7%). Overall, patient characteristics were similar between patients with salvaged vs standard of care specimens; however, salvaged specimens were more likely to be from encounters earlier in the season and among non-Hispanic White patients, those with 0 Charlson comorbidities, those with the least health care encounters in prior year, and those presenting in the ED; and less likely to be from those with select comorbidities (eTable 3 in Supplement 1). Compared with those in the study population and tested for RSV, LRTD inpatient or ED encounters with no swab collected or not tested for RSV were more likely to be ED visits, later in the RSV season, among younger patients, among patients with multiple/other/unknown or Hispanic race and ethnicity, among patients with lower health care utilization, among those with lower Charlson Index scores, and among those with fewer comorbidities (eTable 3 in Supplement 1). The majority of the study population had comorbidities (6573 [93.3%] had ≥1 Charlson comorbidity) and were aged 75 years or older (4046 [57.4%]) (Table 1). Overall, 8.8% (623 of 7047) tested positive for RSV and 3.2% (223 of 7047) received RSVpreF. Among all those who tested RSV-negative (6424 broad controls), 221 (3.4%) were vaccinated. A total of 27 RSV cases were coinfected with another VPD or non-VPD pathogen. Significant differences between vaccinated and unvaccinated persons in the broad analysis included a higher proportion vaccinated among older, non-Hispanic Asian or Pacific Islander and non-Hispanic White patients, those with 10 or more outpatient encounters in the year before LRTD encounter, and those with peripheral vascular disease, chronic obstructive pulmonary disease (COPD), or diabetes were less likely to receive the vaccine (eTable 4 in Supplement 1). To construct the strict control group (804 participants), VPD-positive specimens (185 HMPV, 186 SARS-CoV-2, and 164 influenza; nonmutually exclusive) and those that were not positive for a non-VPD pathogen (5266 participants) were removed from the broad control group (6424 participants).
Figure. Study Flowchart.
ED, emergency department; LRTD indicates lower respiratory tract disease; RSV, respiratory syncytial virus; RSVpreF, Respiratory Syncytial Virus Prefusion F.
aIncludes nonsalvageable point-of-care swabs and swabs positive for influenza and SARS-CoV-2 which were not salvaged for RSV testing.
bIncludes RSV unvaccinated ED/hospitalization events at Kaiser Permanente Southern California where RSVpreF was not available among a population meeting eligibility criteria.
Table 1. Characteristics of Patients Aged 60 Years or Older With Lower Respiratory Tract Disease Inpatient or Emergency Department (ED) Encounters.
| Characteristic | Patients, No. (%) | Strict analysis | Broad analysis | |||
|---|---|---|---|---|---|---|
| Total study population (N = 7047) | Cases (n = 623) | Control patients, No. (%) (n = 804) | P value | Control patients, No. (%) (n = 6424) | P value | |
| Age at index date | ||||||
| 60-74 | 3001 (42.6) | 250 (40.1) | 389 (48.4) | .002 | 2751 (42.8) | .19 |
| ≥75 | 4046 (57.4) | 373 (59.9) | 415 (51.6) | 3673 (57.2) | ||
| Sex | ||||||
| Female | 3819 (54.2) | 398 (63.9) | 418 (52.0) | <.001 | 3421 (53.3) | <.001 |
| Male | 3228 (45.8) | 225 (36.1) | 386 (48.0) | 3003 (46.7) | ||
| Months of encounter | ||||||
| November-December | 2275 (32.3) | 268 (43.0) | 237 (29.5) | <.001 | 2007 (31.2) | <.001 |
| January-February | 3733 (53.0) | 326 (52.3) | 396 (49.3) | 3407 (53) | ||
| March-April | 1039 (14.7) | 29 (4.7) | 171 (21.3) | 1010 (15.7) | ||
| Race and ethnicity | ||||||
| Hispanic | 2323 (33.0) | 225 (36.1) | 310 (38.6) | .14 | 2098 (32.7) | <.001 |
| Non-Hispanic Asian or Pacific Islander | 839 (11.9) | 93 (14.9) | 100 (12.4) | 746 (11.6) | ||
| Non-Hispanic Black | 1197 (17.0) | 70 (11.2) | 118 (14.7) | 1127 (17.5) | ||
| Non-Hispanic White | 2602 (36.9) | 226 (36.3) | 262 (32.6) | 2376 (37.0) | ||
| Multiple/other/unknowna | 86 (1.2) | 9 (1.4) | 14 (1.7) | 77 (1.2) | ||
| Modified Charlson Comorbidity Index Score | ||||||
| 0 | 474 (6.7) | 41 (6.6) | 70 (8.7) | .50 | 433 (6.7) | .48 |
| 1 | 660 (9.4) | 67 (10.8) | 89 (11.1) | 593 (9.2) | ||
| 2 | 856 (12.1) | 82 (13.2) | 101 (12.6) | 774 (12.0) | ||
| ≥3 | 5057 (71.8) | 433 (69.5) | 544 (67.7) | 4624 (72.0) | ||
| No. of inpatient encounters in the year before index date | ||||||
| 0 | 4331 (61.5) | 456 (73.2) | 542 (67.4) | .06 | 3875 (60.3) | <.001 |
| 1 | 1432 (20.3) | 91 (14.6) | 146 (18.2) | 1341 (20.9) | ||
| ≥2 | 1284 (18.2) | 76 (12.2) | 116 (14.4) | 1208 (18.8) | ||
| No. of ED encounters in the year before index date | ||||||
| 0 | 2698 (38.3) | 284 (45.6) | 317 (39.4) | .02 | 2414 (37.6) | <.001 |
| 1 | 1668 (23.7) | 151 (24.2) | 191 (23.8) | 1517 (23.6) | ||
| ≥2 | 2681 (38.0) | 188 (30.2) | 296 (36.8) | 2493 (38.8) | ||
| No. of outpatient encounters in the year before index date | ||||||
| <10 | 2033 (28.8) | 208 (33.4) | 245 (30.5) | .24 | 1825 (28.4) | .01 |
| ≥10 | 5014 (71.2) | 415 (66.6) | 559 (69.5) | 4599 (71.6) | ||
| Comorbidities | ||||||
| Myocardial infarction | 1126 (16.0) | 76 (12.2) | 115 (14.3) | .25 | 1050 (16.3) | .01 |
| Congestive heart failure | 2602 (36.9) | 215 (34.5) | 254 (31.6) | .24 | 2387 (37.2) | .19 |
| Cerebrovascular disease | 1016 (14.4) | 67 (10.8) | 105 (13.1) | .19 | 949 (14.8) | .01 |
| Peripheral vascular disease | 4823 (68.4) | 413 (66.3) | 540 (67.2) | .73 | 4410 (68.6) | .23 |
| Moderate or severe liver disease | 120 (1.7) | 9 (1.4) | 10 (1.2) | .74 | 111 (1.7) | .60 |
| Malignant neoplasm | 1219 (17.3) | 95 (15.2) | 133 (16.5) | .51 | 1124 (17.5) | .16 |
| Kidney disease | 2990 (42.4) | 274 (44.0) | 311 (38.7) | .04 | 2716 (42.3) | .41 |
| Chronic obstructive pulmonary disease | 3518 (49.9) | 292 (46.9) | 405 (50.4) | .19 | 3226 (50.2) | .11 |
| Dementia | 851 (12.1) | 86 (13.8) | 84 (10.4) | .05 | 765 (11.9) | .17 |
| AIDS/HIV | 23 (0.3) | 0 | 2 (0.2) | .21 | 23 (0.4) | .13 |
| Diabetes | ||||||
| Diabetes with HbA1c ≥7.5 | 1098 (15.6) | 90 (14.4) | 138 (17.2) | .45 | 1008 (15.7) | .52 |
| Diabetes with HbA1c <7.5 | 2398 (34.0) | 205 (32.9) | 256 (31.8) | 2193 (34.1) | ||
| Diabetes with unknown HbA1c | 152 (2.2) | 17 (2.7) | 16 (2.0) | 135 (2.1) | ||
| No diabetes diagnosis | 3399 (48.2) | 311 (49.9) | 394 (49.0) | 3088 (48.1) | ||
| RSVpreF vaccination status | ||||||
| Did not receive RSVpreF vaccine | 6824 (96.8) | 621 (99.7) | 775 (96.4) | <.001 | 6203 (96.6) | <.001 |
| Received RSVpreF vaccine | 223 (3.2) | 2 (0.3) | 29 (3.6) | 221 (3.4) | ||
| Encounter setting | ||||||
| Hospitalization | 4024 (57.1) | 300 (48.2) | 392 (48.8) | .82 | 3724 (58.0) | <.001 |
| ED | 3023 (42.9) | 323 (51.8) | 412 (51.2) | 2700 (42.0) | ||
| Immunocompromised | ||||||
| No | 6049 (85.8) | 544 (87.3) | 683 (85.0) | .20 | 5505 (85.7) | .27 |
| Yes | 998 (14.2) | 79 (12.7) | 121 (15.0) | 919 (14.3) | ||
Abbreviations: HbA1c, glycated hemoglobin; RSVpreF, Respiratory Syncytial Virus Prefusion F.
SI conversion: To convert HbA1c to proportion of total hemoglobin, multiply by 0.01.
Other includes individuals who self-identified as American Indian or multiple or other race and ethnicity categories.
In the analysis comparing cases with strictly defined controls (623 cases and 804 controls), adjusted VE was 91% (95% CI, 59%-98%) with vaccinees receiving RSVpreF a median (IQR) of 61 (36-83) days before LRTD encounter (Table 2). In the analysis comparing cases with any RSV-negative controls (623 cases and 6424 controls), adjusted VE was 90% (95% CI, 59%-97%) with vaccinees receiving RSVpreF a median (IQR) of 58 (39-78) days before LRTD encounter.
Table 2. RSVpreF Vaccine Effectiveness (VE) Against Respiratory Syncytial Virus (RSV)–Related Lower Respiratory Tract Disease Hospitalizations and Emergency Department Visits.
| Lower respiratory tract disease hospitalizations and emergency department visits | Patients, No. (%) | VE (95% CI) | ||||
|---|---|---|---|---|---|---|
| Test negative controls (strict n = 804; broad n = 6424) | Test positive cases (n = 623) | Crude | Adjusteda | |||
| Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | |||
| Strict definition/primary analysis (n = 1427)b | 775 (96.4) | 29 (3.6) | 621 (99.7) | 2 (0.3)c | 91 (64-98) | 91 (59-98) |
| Broad definition/sensitivity analysis (n = 7047)d | 6203 (96.6) | 221 (3.4) | 621 (99.7) | 2 (0.3)c | 91 (64-98) | 90 (59-97) |
Abbreviation: RSVpreF, Respiratory Syncytial Virus Prefusion F.
VE was adjusted for age, sex, encounter months, race andethnicity, Charlson index, previous outpatient encounters, previous inpatient encounters, and previous ED encounters.
A case is considered positive for RSV. A control is considered positive for a nonvaccine preventable disease and negative for RSV, human metapneumovirus, and vaccine preventable diseases. Identified pathogens included adenovirus, Chlamydia pneumoniae, coronavirus (229E, HKU1, NL63, and OC43), human rhinovirus/enterovirus, parainfluenza (serotypes 1-4), and Mycoplasma pneumoniae.
Patient 1: aged 93 years, Charlson index of 6; patient 2: aged 63 years, Charlson index of 4.
A case is considered positive for RSV. A control is considered negative for RSV.
Additional VE estimates against other LRTD end points are provided in Table 3. Notably, estimated VE against severe LRTD hospitalizations and ED events was 89% (95% CI, 13%-99%).
Table 3. Additional RSVpreF Vaccine Effectiveness (VE) Estimates Against Respiratory Syncytial Virus (RSV)–Related Lower Respiratory Tract Disease (LRTD) Hospitalizations or Emergency Department Visits by End Point Type, Kaiser Permanente Southern California Network, November 2023 to April 2024.
| Patients, No. (%) | VE (95% CI) | |||||
|---|---|---|---|---|---|---|
| Test negative (strict control definition)a | Test positive | Crude | Adjustedb | |||
| Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | |||
| VE against RSV-related LRTD hospitalizations (n = 692)c | 380 (96.9) | 12 (3.1) | 299 (99.7) | 1 (0.3) | 89 (18 to 99) | 87 (−8 to 98) |
| VE against RSV-related LRTD hospitalizations among patients with high-risk chronic medical conditions (n = 683)d | 375 (96.9) | 12 (3.1) | 295 (99.7) | 1 (0.3) | 89 (18 to 99) | 87 (−6 to 98) |
| VE against RSV-related LRTD ED visits (n = 735) | 395 (95.9) | 17 (4.1) | 322 (99.7) | 1 (0.3) | 93 (45 to 99) | 93 (45 to 99) |
| VE against severe RSV-related LRTD hospitalizations and ED events (n = 719) | 389 (96.8) | 13 (3.2) | 316 (99.7) | 1 (0.3) | 91 (27 to 99) | 89 (13 to 99) |
Abbreviation: RSVpreF, Respiratory Syncytial Virus Prefusion F.
A case is considered positive for RSV. A control is considered positive for a nonvaccine preventable disease and negative for RSV, human metapneumovirus, and vaccine preventable diseases. Identified pathogens included adenovirus, Chlamydia pneumoniae, coronavirus (229E, HKU1, NL63, and OC43), human rhinovirus/enterovirus, parainfluenza (serotypes 1-4), and Mycoplasma pneumoniae.
VE was adjusted for age, sex, encounter months, race and ethnicity, Charlson index, previous outpatient encounters, previous inpatient encounters, and previous ED encounters.
95% CI included for this end point provided for comparability with other end points; formal prespecified interim analysis for this end point used a 99.99% CI which was (−754 to 100).
This estimate is limited to those at high risk for severe disease based on underlying comorbidities, including asthma; chronic obstructive pulmonary disease; congestive heart failure; coronary artery disease; other chronic lung, cardiac, kidney, or liver diseases; diabetes; neurological conditions; stroke; autoimmune disorders; immunocompromising conditions and medications; HIV; AIDS; cancers; organ transplant; and blood disorders.
Overall, 232 patients contributed more than 1 LRTD event more than 30 days apart. Of those, 215 (92.7%) were RSV-negative, 17 (7.3%) were RSV-positive; 224 (96.6%) were unvaccinated, and 8 were vaccinated (3.5%). Sensitivity VE analyses including only the first LRTD event for each patient demonstrated similar results to the main analyses, (strict: adjusted VE [aVE], 91%; 95% CI, 59%-98%; broad: aVE, 90%; 95% CI, 59%-97%).
Discussion
Since licensure in 2023, there are few reports of RSVpreF effectiveness in adults in a clinical practice setting. In our primary analysis, RSVpreF effectiveness was 91% against RSV-related LRTD requiring hospitalization or ED visit among adults aged 60 years or older in the first 5 months of vaccine use.
RSVpreF’s pivotal clinical trial, RENOIR,12 reported similar VE of 88.9% (95% CI, 53.6%-98.7%) at the end of season 1 for RSV-related LRTD with 3 or more signs or symptoms overall, with a similar result of 84.6% (95% CI, 32.0%-98.3%) for medically attended events.5 Medically attended LRTD in RENOIR included a mix of health care encounters (ie, outpatient and inpatient), but consistent with other phase 3 trials, VE estimates for hospitalization could not be estimated due to few RSV-positive hospitalizations among enrolled participants. In RENOIR, among those with 1 or more high risk conditions, VE against RSV-LRTD with 3 or more symptoms was 81.8% (95% CI, 16.7%-98.0%) at the end of season 1.13 While this latter finding is promising, our study included a substantially larger proportion of persons at high risk for severe disease than RENOIR (eg, median age 77 vs 68 years, 50% vs 6% with COPD, and 14% vs 0% immunocompromised) and assesses VE against a severe clinical outcome of ED and hospitalization visits. Moreover, in our study, VE was maintained when evaluating severe LRTD, as defined by oxygen supplementation.
Results from this general population evaluation, therefore, expand on clinical trial data by documenting VE against more severe outcomes (hospitalization and ED encounters or need for oxygen supplementation) among an older patient population with a higher proportion of high risk conditions compared with those in the clinical trial.14 Indeed, in light of the 2024 Advisory Committee on Immunization Practices update to use of RSV vaccines,5 which now recommends their use for all adults aged 75 years and older, and for adults aged 60 to 74 years at increased risk of severe RSV, our results are particularly relevant as they address data gaps for groups called out in this guidance.
Our analytic approach allowed for minimization of bias from several sources. First, we salvaged remnant swabs collected for standard of care testing for influenza and SARS-CoV-2 to overcome low routine clinical RSV testing rates. Next, our strict control definition, which required a positive result for a non-VPD, was used to ensure effective specimen collection technique and mitigate underascertainment associated with low sensitivity of nasopharyngeal/nasal swab reverse transcription polymerase chain reaction testing in adults (ie, approximately 50% of RSV events not detected),5,15 leading to RSV cases being misclassified as controls and potentially biasing VE estimates toward a null value. Next, removal of VPD infections from the control group addressed the correlation of respiratory vaccines that can confound test-negative design VE estimates.7 Lastly, we designated HMPV as a possible VPD, based on literature describing the isolation of multiple cross-reactive neutralizing antibodies between RSV and HMPV from human donors8,16 While it is currently unknown if this may result in RSVpreF vaccination providing cross-protection against HMPV, removing this pathogen from the control group allowed us to minimize this potential bias in our VE estimates against RSV end points. Regardless of the control selection approach used, VE was similar (91% vs 90%).
Limitations and Strengths
A limitation of this initial analysis is that less than 5% of those with LRTD events were vaccinated in this first season. This may impact generalizability of estimates and prevent stable VE estimates for some subgroups. This vaccination rate is lower than what is reported in national estimates,17 which may be due to a later rollout of RSVpreF at KPSC, absence of an automated alert for clinicians to prompt RSV vaccination, or other issues related to implementation of shared clinical decision-making for RSV vaccination at KPSC in this first year of administration. These early first-season estimates miss the first part of RSV season, resulting in median follow-up times of 61 and 58 days for strict and broad control group analyses, respectively. If our vaccinated population is older and with more comorbidities than recipients nationwide, our VE estimates may be biased toward the null, or vice versa. Further case accrual and vaccine uptake after the 2024 to 2025 RSV season will continue and will power future stratified analyses. Multiseason future analyses will be better suited to capture potential waning of protection with time since vaccination.
Not all LRTD events in the ED or hospital setting were tested for RSV. In general, LRTD events without a test were in the ED and among a younger, healthier population with events later in the RSV season. While the inclusion of higher-risk patients in the study population may bias VE lower, the intended target population for RSV vaccines consists of those at highest risk for severe outcomes. Important strengths of our enhanced testing approach include substantial increase of our study size and more robust analyses in the ED setting.
Conclusions
This demonstration of the general population effectiveness of RSV vaccination among older adults provides insight into the potential public health impact of this strategy. Based on our study results and RSV incidence in older adults,1 for approximately every 250 persons vaccinated, 1 RSV-related ED or hospitalization encounter could be prevented in the first season after vaccination. These data suggest use of RSVpreF in older adults, providing an opportunity to reduce severe medically attended RSV disease burden.
eTable 1. Lower Respiratory Tract Disease Diagnosis Codes (International Classification of Diseases [ICD] codes)
eTable 2. LRTD ICD-10 Code Distribution Between RSV-Positive Cases and RSV-Negative Controls Lower Respiratory Tract Disease Diagnosis Codes
eTable 3. Characteristics of Patients Aged 60 Years or Older With LRTD Inpatient or ED Encounters by RSV Testing Approach, Kaiser Permanente Southern California, November 2023 to April 2024
eTable 4. Characteristics of Patients Aged 60 Years or Older With LRTD Inpatient or ED Encounters by Vaccination Status, Kaiser Permanente Southern California, November 2023 to April 2024
eReferences.
Data Sharing Statement
References
- 1.McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022;9(7):ofac300. doi: 10.1093/ofid/ofac300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodruff RC, Melgar M, Pham H, et al. ; Respiratory Syncytial Virus Hospitalization Surveillance Network (RSV-NET) . Acute cardiac events in hospitalized older adults with respiratory syncytial virus infection. JAMA Intern Med. 2024;184(6):602-611. doi: 10.1001/jamainternmed.2024.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivey KS, Edwards KM, Talbot HK. Respiratory syncytial virus and associations with cardiovascular disease in adults. J Am Coll Cardiol. 2018;71(14):1574-1583. doi: 10.1016/j.jacc.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Ubamadu E, Betancur E, Gessner BD, et al. Respiratory syncytial virus sequelae among adults in high-income countries. a systematic literature review and meta-analysis. Infect Dis Ther. 2024;13(7):1399-1417. doi: 10.1007/s40121-024-00974-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801. doi: 10.15585/mmwr.mm7229a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawood FS, Payne AB, McMorrow ML. Assessing the real-world effectiveness of immunizations for respiratory syncytial virus. JAMA. 2024;331(21):1799-1800. doi: 10.1001/jama.2024.5859 [DOI] [PubMed] [Google Scholar]
- 7.Doll MK, Pettigrew SM, Ma J, Verma A. Effects of confounding bias in Coronavirus Disease 2019 (COVID-19) and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis. 2022;75(1):e564-e571. doi: 10.1093/cid/ciac234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen X, Mousa JJ, Bates JT, Lamb RA, Crowe JE Jr, Jardetzky TS. Structural basis for antibody cross-neutralization of respiratory syncytial virus and human metapneumovirus. Nat Microbiol. 2017;2:16272. doi: 10.1038/nmicrobiol.2016.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 12.Walsh EE, Pérez Marc G, Zareba AM, et al. ; RENOIR Clinical Trial Group . Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med. 2023;388(16):1465-1477. doi: 10.1056/NEJMoa2213836 [DOI] [PubMed] [Google Scholar]
- 13.Walsh EE, Perez Marc G, Falsey AR, et al. RENOIR Trial—RSVpreF vaccine efficacy over two seasons. N Engl J Med. 2024;391(15):1459-1460. doi: 10.1056/NEJMc23115602024 [DOI] [PubMed] [Google Scholar]
- 14.Njue A, Nuabor W, Lyall M, et al. Systematic literature review of risk factors for poor outcomes among adults with respiratory syncytial virus infection in high-income countries. Open Forum Infect Dis. 2023;10(11):ofad513. doi: 10.1093/ofid/ofad513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez J, Carrico R, Wilde A, et al. Diagnosis of respiratory syncytial virus in adults substantially increases when adding sputum, saliva, and serology testing to nasopharyngeal swab RT-PCR. Infect Dis Ther. 2023;12(6):1593-1603. doi: 10.1007/s40121-023-00805-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster JE, Cox RG, Hastings AK, et al. A broadly neutralizing human monoclonal antibody exhibits in vivo efficacy against both human metapneumovirus and respiratory syncytial virus. J Infect Dis. 2015;211(2):216-225. doi: 10.1093/infdis/jiu307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu PJ, Srivastav A, Hung M, et al. National and state-specific estimates of settings where adults received influenza, updated COVID-19, and RSV vaccinations, 2023-2024 respiratory virus season, United States. Centers for Disease Control and Prevention . 2024. Accessed October 1, 2024. https://www.cdc.gov/vaccines/imz-managers/coverage/national-state-vaccination-estimates.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Lower Respiratory Tract Disease Diagnosis Codes (International Classification of Diseases [ICD] codes)
eTable 2. LRTD ICD-10 Code Distribution Between RSV-Positive Cases and RSV-Negative Controls Lower Respiratory Tract Disease Diagnosis Codes
eTable 3. Characteristics of Patients Aged 60 Years or Older With LRTD Inpatient or ED Encounters by RSV Testing Approach, Kaiser Permanente Southern California, November 2023 to April 2024
eTable 4. Characteristics of Patients Aged 60 Years or Older With LRTD Inpatient or ED Encounters by Vaccination Status, Kaiser Permanente Southern California, November 2023 to April 2024
eReferences.
Data Sharing Statement