Abstract
Purpose: To investigate the use of short-term postoperative endotamponade with perfluorocarbon liquids (PFCLs) for the treatment of giant retinal tear–associated rhegmatogenous retinal detachment (RRD). Methods: This retrospective study evaluated patients who had 2-stage surgery, which entailed pars plana vitrectomy (PPV) completed in 2 consecutive surgeries spaced 5 days apart, during which a short-term tamponade with PFCL was used (Group 1), and patients who had conventional single-stage PPV with long-term silicone oil (SO) tamponade (Group 2). Results: The study comprised 74 eyes of 68 patients, 52 in Group 1 and 22 in Group 2. The mean (±SD) patient age at presentation was 48.19 ± 15.73 years. Of the cases, 18.9% had high myopia and 13.5% had previous trauma. The improvement in best-corrected visual acuity (BCVA) was significantly better in Group 1 than in Group 2 at all postoperative visits (P = .004, postoperative day [POD] 15; P = .002, POD 90; P = .00006, final follow-up). Anatomic success (an attached retina) was achieved in 82.7% of patients in Group 1 and in 72.7% of patients in Group 2 (P = .33). At the 6-month postoperative follow-up, 54.5% of patients and 50% of patients, respectively, had a logMAR BCVA of 1.00 or better (P = .721). The mean change in intraocular pressure from baseline was statistically significant in both groups (Group 1, P = .012; Group 2, P = .018). Conclusions: Anatomic and functional outcomes in giant retinal tear–associated RRD can be improved with short-term postoperative endotamponade with PFCLs.
Keywords: rhegmatogenous retinal detachment, giant retinal tear, perfluorocarbon liquid, short-term perfluoro-N-octane tamponade, silicone oil tamponade
Introduction
A giant retinal tear is a circumferential full-thickness retinal break of at least 3 clock hours (90 degrees) associated with posterior vitreous detachment.1,2 Giant retinal tears are considered rare, although the exact incidence is not well established. The literature reports a yearly incidence of giant retinal tears of 0.05 cases per 100 000 in the general population and 0.5% to 8.3% of all cases of rhegmatogenous retinal detachment (RRD).3 –6 In 2010, the British Giant Retinal Tear Epidemiology Eye Study Group published an incidence of 0.094 per 100 000 in the general population. 7 Most cases of giant retinal tears are idiopathic in nature, with Stickler syndrome considered one of the most significant risk factors. 8 However, retinal tears resulting from trauma, high myopia, laser photocoagulation, cryotherapy, and refractive surgery have been documented.9 –11
The main challenge in the surgical management of RRD with giant retinal tears is the high risk for proliferative vitreoretinopathy (PVR). 12 A variety of methods for improving surgical outcomes have been presented. Over the years, pars plana vitrectomy (PPV) has evolved as the mainstay surgical treatment for the condition. Despite the advent of newer techniques and better tamponade agents, the failure rate remains high. Limited case series in the existing literature with relatively small samples have reported variable success rates of 48% to 100%.3 –6,13 Recently, a large retrospective study of 396 eyes had a success rate of 64% after the initial surgery, a rate that increased to 78% after multiple surgeries for recurrent RD. 14 PVR is the principal factor responsible for surgical failure in these cases, resulting in the formation of new retinal tears or the reopening or extension of existing tears. 15
Here, we describe a relatively new surgical technique for giant retinal tear–associated RRD that encompasses 2 closely spaced surgeries (2-stage surgical approach). Our retrospective study evaluated the demographics, morphologic presentation, and other associations of giant retinal tear–associated RRD (eg, clinical findings, patient-related history, etc) to compare the efficacy of 2-stage surgery and short-term tamponade with conventional single-stage PPV and long-term tamponade. Although the 2-stage procedure has been described in the literature with a smaller sample, to our knowledge no statistical comparisons with the conventional surgical method have published. 16
Methods
This retrospective study comprised patients who had surgery for giant retinal tear–associated RRD at our tertiary eye care institute between January 1, 2018, and December 31, 2022. Informed consent was obtained for the surgical procedure, and the potential for a guarded visual prognosis was explained. The study conformed to the tenets of the Declaration of Helsinki, and permission from the institutional research board (RET202400455) was obtained.
A thorough search of the medical records was performed. Patients were included in the analysis if they were 18 years or older, presented with giant retinal tear–associated RRD in the specified time period, were managed with PPV and long-term silicone oil (SO) tamponade or 2-stage surgery by performed by experienced surgeons (ie, no less than 10 years of experience in vitreoretinal surgery), and had at least 6 months of postoperative follow-up. The surgical technique was chosen at the surgeons’ discretion based on their cumulative years of experience and the patients’ intraoperative evaluation. Patients with associated significant ocular comorbidities (eg, diabetic retinopathy, glaucoma, macular degeneration) were excluded.
Baseline parameters, including age, sex, lens status (phakic, pseudophakic, or aphakic), laterality (right or left eye), axial length, interval between the onset of symptoms and surgery, best-corrected visual acuity (BCVA), intraocular pressure (IOP), and factors related to the morphologic appearance of the giant retinal tear–associated RRD (clock hours, quadrant localization, total or subtotal RRD, macular status, degree of PVR at presentation, choroidal detachment) were recorded. The patients were divided into 2 groups. Group 1 included patients who had 2-stage surgery and short-term tamponade with perfluorocarbon liquids (PFCLs). Group 2 included patients who had conventional single-stage PPV and SO tamponade.
The BCVA and IOP were recorded on postoperative day (POD) 15, at 3 months (POD 90), and at 6 months. The total duration of follow-up and final status of the retina (attached or detached) were noted. The Snellen preoperative VA and postoperative VA were determined; for statistical analysis, the VA was converted to logMAR notation.17,18
Two-Stage Surgical Technique
For the 2-stage surgical technique, PPV was performed in 2 consecutive surgeries and a short-term (5-day) tamponade (preferably using a high-gravity liquid such as PFCLs) was placed. In the first stage of the surgery, a 23-gauge PPV was performed after sub-Tenon anesthesia was administered. Core vitrectomy was followed by injection of triamcinolone acetonide (0.4 mg/0.1 mL). Because complete PVD can be present with giant retinal tears, triamcinolone helped detect anomalous vitreous adhesion at the posterior hyaloid face. Perfluoro-N-octane was injected up to the posterior margin of the retinal tear, and vitreous base dissection was completed with scleral indentation. Adequate flattening of the peripheral retina and removal of full-thickness retinal folds were performed with gentle sweeping using a Tanos diamond-dusted membrane scraper. Heavy Brilliant Blue G dye (with 10% dextrose) was injected, staining the internal limiting membrane (ILM), and ILM peeling was performed with an end-grasping forceps. Sixty percent of the vitreous cavity was filled with PFCL, and the remaining 40% was filled with SO (1000 cs). The port sites were then sutured with 7-0 polyglactin (Vicryl).
The second stage of surgery was planned for 5 days later; during this period, patients were advised to adopt strict supine positioning. After 5 days, the second surgery began in the same way as the first. After insertion of the ports, active extrusion of SO was performed and an extensive barrage of laser to the retinal tear was followed by the completion of a 360-degree barrage with retained PFCL. Caution was exercised while barraging the horns of the retinal tear to prevent encroachment of subretinal fluid (SRF) or PFCL toward the posterior pole. The residual PFCL was then aspirated using a Charles flute needle after fluid–air exchange. The SRF was completely removed, and the laser was applied as required. The surgeon made sure the SO fill was complete to provide effective tamponade, after which the port sites were sutured with 7-0 polyglactin. The patient was told that it was necessary to maintain strict prone positioning for 15 days and to attend regular follow-up visits. The SO was removed 3 months after the initial surgery.
Safety Analysis
The safety of the surgical procedure was assessed using reports of ocular and nonocular adverse events (AEs), systemic AEs, and ocular assessments detailed in the patient’s medical record.
Statistical Analysis
Statistical analyses were performed using SPSS software (version 20, IBM Corp). The results of descriptive analyses were expressed as the mean ± SD for quantitative variables and as the number and percentage for categorical variables. A χ2 test was used to determine the association between categorical variables. Intergroup variability of outcomes was compared using the Student t test of unequal variances depending on the sample size. Between-group comparisons of preoperative and postoperative outcomes were performed using Wilcoxon rank test. Statistical significance was set at P < .05.
Results
The study comprised 74 eyes of 68 patients, 52 in Group 1 and 22 in Group 2. The mean patient age was 48.2 ± 15.7 years. Most patients were men (n = 67 [90.5%]). Table 1 shows the patients’ demographics and baseline characteristics.
Table 1.
Patient Demographic and Baseline Data. a
| Characteristic | Group 1 | Group 2 | P Value |
|---|---|---|---|
| (n = 52) | (n = 22) | ||
| Mean age (y) ± SD | 46.4 ± 16.3 | 52.5 ± 13.4 | .062 |
| Sex, n (%) | |||
| Male | 46 (88.5) | 21 (95.5) | .232 |
| Female | 6 (11.5) | 1 (4.6) | .117 |
| Eye, n (%) | .347 | ||
| Right | 28 (53.9) | 12 (54.5) | |
| Left | 24 (46.2) | 10 (45.5) | |
| Lens status, n (%) | .384 | ||
| Phakic | 22 (42.3) | 09 (40.9) | |
| Pseudophakic | 28 (53.9) | 13 (59.1) | |
| Aphakic | 2 (3.8) | 0 | |
| Axial length (mm), n (%) b | .544 | ||
| Emmetropic | 35 (67.3) | 13 (59.1) | |
| Moderately myopic | 8 (15.4) | 4 (18.2) | |
| Highly myopic | 9 (17.3) | 5 (22.7) | |
| Mean time from presentation to surgery (d) ± SD | 23.8 ± 12.5 | 19.0 ± 10.8 | .175 |
| Mean baseline logMAR BCVA (SD) | 1.6 ± 0.6 | 1.1 ± 0.8 | .0009 |
| Mean baseline IOP (mm Hg) | 13.8 ± 4.4 | 15.6 ± 4.0 | .077 |
| Mean postoperative follow-up (mo) | 11.7 ± 2.4 | 11.0 ± 3.4 | .783 |
| History of trauma, n (%) | 9 (17.3) | 1 (4.5) | .142 |
Abbreviations: BCVA, best-corrected visual acuity; IOP, intraocular pressure.
Group 1 patients received short-term tamponade with perfluoro-N-octane. Group 2 patients received tamponade with silicone oil.
Emmetropic, ≤24.5 mm; moderately myopic, >24.5-26.25 mm; highly myopic, ≥26.5 mm.
Of the cases, 18.9% had high myopia and 13.5% had previous trauma. The time between the onset of symptoms and surgery was comparable between Group 1 and Group 2 (P = .175). There was no history of hereditary vitreoretinopathy in the study eyes as per the medical records. Most patients presented with a temporal great retinal tear (54.1%) followed by a superotemporal tear (23.0%). Great retinal tears involving 180 degrees or more of the retinal circumference were observed in 24.3% patients, and 17.6% of patients presented with an associated choroidal detachment. Table 2 shows the features of the giant retinal tear–associated RRD at presentation by group.
Table 2.
Features of the GRT-Associated RRD at Presentation. a
| Characteristic | Group 1 | Group 2 | P Value |
|---|---|---|---|
| (n = 52) | (n = 22) | ||
| Mean number of clock hours (extent of GRT) ± SD | 4.9 ± 2.0 | 4.5 ± 1.3 | .193 |
| Quadrant, n (%) | .47 | ||
| Temporal | 27 (51.9) | 13 (59.1) | |
| Superotemporal | 13 (25.0) | 4 (18.2) | |
| Superior | 7 (13.5) | 1 (4.5) | |
| Other | 5 (9.6) | 4 (18.2) | |
| Macula status, n (%) | .0002 | ||
| On | 3 (5.8) | 9 (41.0) | |
| Off | 49 (94.2) | 13 (59.1) | |
| PVR changes at presentation, n (%) | .142 | ||
| Grade C | 9 (17.3) | 1 (4.5) | |
| Less than grade C | 43 (82.7) | 21 (95.5) | |
| RRD type, n (%) | .005 | ||
| Total | 35 (67.3) | 7 (31.8) | |
| Partial | 17 (32.7) | 15 (68.2) | |
| Choroidal detachment, n (%) | 11 (21.2) | 2 (9.1) | .212 |
Abbreviations: GRT, giant retinal tear; PVR, proliferative vitreoretinopathy; RRD, rhegmatogenous retinal detachment.
Group 1 patients received short-term tamponade with perfluoro-N-octane. Group 2 patients received tamponade with silicone oil.
At clinical presentation, 22 patients in Group 1 and 9 patients in Group 2 were phakic. Most patients had no significant cataracts on presentation. Six patients in Group 1 and 2 patients in Group 2 required phacoemulsification with intraocular lens implantation during the initial surgery. Over the follow-up period, 10 additional patients in Group 1 and 6 additional patients in Group 2 developed cataracts and subsequently had phacoemulsification.
Table 3 shows a comparison of outcomes between the 2 groups. A steady improvement in logMAR BCVA from baseline occurred in both groups. In Group 1, the improvement in BCVA was statistically significant at all follow-ups (P < .00001 at POD 15 and at final follow-up) while the improvement in BCVA in Group 2 was significant at the final follow-up (P = .015) but not on POD 15 (P = .131). The logMAR BCVA was 1.00 (Snellen equivalent, 6/60) or better at the final follow-up in 54.5% of patients in Group 2 and 50% of patients in Group 1 (Figure 1). The improvement in BCVA was significantly greater in Group 1 than in Group 2 at all postoperative follow-ups (P = .004, POD 15; P = .002, POD 90; P = .00006, last follow-up).
Table 3.
Comparison of Outcomes Between the 2 Groups. a
| Characteristic | Group 1 | Group 2 | P Value |
|---|---|---|---|
| (n = 52) | (n = 22) | ||
| Mean logMAR BCVA ± SD | |||
| POD 15 | 1.1 ± 0.5 | 0.8 ± 0.5 | .004 |
| POD 90 | 0.9 ± 0.5 | 0.7 ± 0.5 | .002 |
| Final follow-up | 0.8 ± 0.5 | 0.7 ± 0.5 | .0006 |
| Mean BCVA improvement vs baseline ± SD | |||
| POD 15 | 0.6 ± 0.6 | 0.2 ± 0.2 | .004 |
| POD 90 | 0.8 ± 0.7 | 0.3 ± 0.3 | .002 |
| Final follow-up | 0.8 ± 0.6 | 0.3 ± 0.2 | .0005 |
| Mean IOP (mm Hg) ± SD | |||
| POD 15 | 21.0 ± 8.9 | 18.4 ± 5.3 | .064 |
| Final follow-up | 20.2 ± 7.7 | 16.5 ± 4.3 | .006 |
| IOP ≥21 mm Hg b at final follow-up, n (%) | 5 (9.6) | 4 (18.2) | .303 |
| Snellen BCVA better than 6/60, n (%) | 26 (50.0) | 12 (54.5) | .721 |
| Silicone oil removal, n (%) | .546 | ||
| Yes | 39 (75.0) | 15 (68.2) | |
| Not until last follow-up/recurrent RD | 13 (25.0) | 7 (31.8) | |
| Final retina status, n (%) | .33 | ||
| Macula-on | 43 (82.69) | 16 (72.72) | |
| Recurrent RD, macula-on | 2 (3.84) | 2 (9.10) | |
| Recurrent RD, macula-off | 7 (13.46) | 4 (18.18) | |
Abbreviations: BCVA, best-corrected visual acuity; IOP, intraocular pressure; POD, postoperative day; RD, retinal detachment.
Group 1 patients received short-term tamponade with perfluoro-N-octane. Group 2 patients received tamponade with silicone oil.
With or without antiglaucoma medication.
Figure 1.
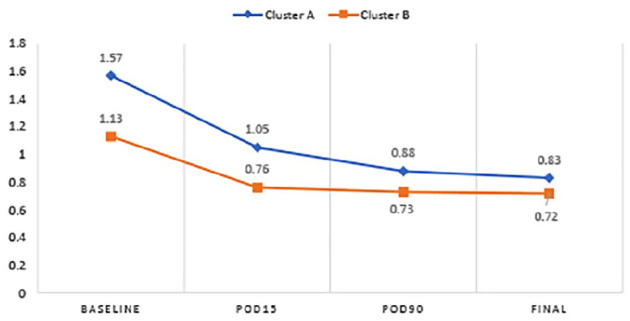
Between-group comparison of BCVA over time.
Abbreviations: BCVA, best-corrected visual acuity; Cluster A, Group 1 patients; Cluster B, Group 2 patients; POD, postoperative day.
Compared with the baseline, the variation in IOP 6 months postoperatively in both groups was significant (Group 1, P = .012; Group 2, P = .018), although the mean IOP remained within normal limits. Eighteen patients (34.6%) in Group 1 and 8 patients (36.4%) in Group 2 had interim spikes in IOP and required antiglaucoma therapy. Three patients in Group 2 had a severe increase in IOP (≥40 mm Hg), probably as a result of a steroid response or secondary to the SO, resulting in a skewed mean IOP value (Table 3).
At the 6-month follow-up, 82.7% of patients in Group 1 and 72.7% of patients in Group 2 achieved anatomic success (an attached retina). The difference between groups was not statistically significant (P = .33).
In Group 1, 7 (92.3%) of 9 patients with recurrent RDs had repeat surgeries, with 5 of those cases maintaining anatomic success at the final follow-up; the other 2 patients developed recurrent RDs despite multiple surgical attempts. In Group 2, 6 patients (86.4%) with recurrent RDs had repeat surgeries; 50% of them achieved anatomic reattachment that was maintained at the final follow-up (P = .424). No patient required concurrent scleral buckling during their surgical procedures. No serious AEs occurred as a result of the surgical technique.
Further multivariate regression analyses found that the interval between clinical presentation and the surgical procedure was significantly correlated with the postoperative BCVA at 6 months (considered the final outcome) in Group 2. For every 1-day increase in the interval between presentation and surgery, there was a 0.30-unit decrease in the postoperative BCVA at 6 months. Age was significantly correlated with the postoperative BCVA at 6 months in Group 1. For every 1-year decrease in age, there was a 0.01-unit improvement in the postoperative BCVA at 6 months (Table 4).
Table 4.
Multivariate Regression Analysis of Demographic and Preoperative Parameters With 6-Month Postoperative BCVA.
| Parameter | Group 2 (n = 22) a | Group 1 (n = 52) a | ||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | SE | P Value | β | (95% CI) | SE | P Value | |
| Age | −0.008 | −0.03 to 0.01 | 0.09 | .42 | −0.01 | −0.02 to −0.001 | 0.003 | .02 |
| Female sex | 0.74 | −0.20 to 1.69 | 0.43 | .11 | 0.18 | −0.22 to 0.58 | 0.19 | .36 |
| Time from presentation to surgery | −0.30 | −0.06 to −0.002 | 0.01 | .03 | 0.01 | −0.01 to 0.02 | 0.01 | .36 |
| Duration of presentation | 0.02 | −0.01 to 0.04 | 0.01 | .15 | 0.0001 | −0.01 to 0.01 | 0.02 | .96 |
| IOP | −0.01 | −0.06 to 0.05 | 0.02 | .77 | −0.02 | −0.04 to 0.01 | 0.01 | .26 |
| Preoperative BCVA | 0.31 | −0.03 to 0.65 | 0.16 | .07 | 0.09 | −0.13 to 0.33 | 0.111 | .38 |
| Macula-on status | −0.15 | −0.83 to 0.53 | 0.31 | .63 | −0.19 | −0.77 to 0.39 | 0.29 | .52 |
| Total RD on presentation | 0.09 | −0.49 to 0.67 | 0.27 | .73 | 0.22 | −0.08 to 0.52 | 0.15 | .14 |
| Myopic refractive status | 0.02 | −0.41 to 0.45 | 0.19 | .93 | 0.05 | −2.15 to 0.31 | 0.13 | .70 |
Abbreviations: BCVA, best-corrected visual acuity; IOP, intraocular pressure; RD, retinal detachment.
Group 1 patients received short-term tamponade with perfluoro-N-octane. Group 2 patients received tamponade with silicone oil.
Conclusions
This study compared the outcomes of conventional PPV and those of PPV with short-term PFCL and explored the ability of PFCL to enhance the anatomic and functional outcomes in eyes with a giant retinal tear–associated RRD. Giant retinal tears are most commonly categorized as idiopathic or spontaneous (28%-78%).1,7 High myopia (12%-47%)19 –22 and trauma (10%-40%)8,9 account for a considerable number of cases, occurring in 18.9% and 13.5% of cases, respectively, in our study. Severe PVR coexisting with giant retinal tears, ranging from 9% to 62%, has been documented in the literature.13,20 Ghasemi Falavarjani et al 23 described baseline PVR changes in 22.6% of patients with giant retinal tear–associated RRD, whereas in our study 13.5% presented with PVR grade C changes. In a study by Mehdizadeh et al 11 of giant retinal tear–associated RRD, younger age was the most significant risk factor. This is in contrast with our study, in which a minority (18.9%) of the sample was 30 years or younger and the median age at presentation was 48 years.
Recent advances in wide-angle viewing systems with innovations in microincision vitrectomy instrumentation have paved the way for a safer surgical procedure for giant retinal tear–associated RRD. 24 In their randomized controlled trial, Ang et al 7 found no difference in the long-term outcomes between perfluoropropane and SO as tamponading agents in eyes with giant retinal tears and severe PVR. In all our cases, SO was the preferred long-term tamponading agent irrespective of the degree of PVR.
With the advent of modern vitreoretinal surgery, the use of PFCLs has increased significantly, resulting in more predictable outcomes. Considered a third hand as an intraoperative tool, PFCL is indispensable for reattaching the retina in giant retinal tear–associated RRD. The fluttering retina is stabilized by the PFCL during vitrectomy, displacing the SRF more peripherally during intraoperative maneuvering. 10 We believe that short-term endotamponade with PFCL provides a steamroller effect over the retina that prevents slippage by enabling the edges and the horns of the retinal tear to reattach better. Furthermore, compared with conventional PPV, the adherence of giant retinal tears to the underlying RPE enables better uptake of barrage laser treatment, thereby improving the final surgical outcome without a significant toxic effect on the retina.
In a study including 17 eyes with a giant retinal tear–associated RRD associated with severe PVR, Zhioua et al 25 reported that the anatomic and functional outcomes were enhanced by the intraoperative injection of PFCL. In 2002, Scott et al 9 used intraoperative PFCL as a tamponading agent in 212 eyes with giant retinal tears and reported a retinal reattachment rate of 76% at the 6-month follow-up.
Although PFCL is considered an excellent material for intraoperative tamponade, its role as a short-term and intermediate-term postoperative tamponade is still emerging. The downside is related to its mechanical toxicity that is directed at the inner retinal layers and to pressure necrosis caused by PFCL’s high specific gravity. The progressive narrowing of the outer plexiform layer and degenerative thinning of the outer nuclear layer have been observed with prolonged intraocular retention of PFCL. 26 There is limited evidence in the literature that PFCL is well tolerated by the retinal tissue for the first 1 to 2 weeks, during which time it assists in flattening the retina.27,28
PFCL was first described by Bottoni et al 29 as a short-term or intermediate-term tamponade for surgical management of giant retinal tear–associated RRD. Sirimaharaj et al 30 found that PFCL as a short-term tamponading agent (median, 7.5 days) improved the outcomes in 62 eyes of 61 patients over a mean of 24.5 months, with 93.5% of patients attaining successful reattachment of the retina and 45.2% of patients having an improvement in Snellen VA of at least 2 lines. Another study by Mikhail et al 16 evaluated the use of PFCL as a short-term tamponading agent in 30 eyes of 29 patients. The PFCL was retained in the eye for a mean of 6.7 days and the success rate was 90.9% at the final follow-up.
Sheridan et al 31 investigated the safety of PFCL as a postoperative tamponading agent in macula-on RRD cases associated with giant retinal tears. The outcome was acceptable in most patients, with only 2 of 25 patients developing recurrent detachment. When PFCL was used for a mean duration of 14.6 days, there were no AEs, such as intraocular inflammation or inner retinal necrosis. Sheridan et al concluded that the use of PFCL likely mitigated the high rates of unexplained visual loss after SO removal in these macula-on RRD cases.
Another study by Randolph et al 32 described the outcomes of intermediate-term tamponade with PFCL in 23 patients with giant retinal tear–associated RRD. Of the patients, an initial 91.3% had successful reattachment, although 5 patients developed recurrent detachment secondary to PVR changes over a long-term follow-up. Associated complications included a transient elevation in IOP, a foreign-body response with increased intraocular inflammation, and cataractogenesis.
To our knowledge, our study is the first to directly compare the anatomic and functional outcomes of 2-stage surgery with single-stage conventional PPV in patients with giant retinal tear–associated RRD. In the 2-stage surgery group, PFCL was used as a short-term postoperative tamponading agent for a median of 5 days. The rate of successful reattachment was higher in the 2-stage surgery group than in the conventional-surgery group (82.69% vs 72.73%) after a 6-month follow. The BCVA had improved significantly in both groups by 6 months postoperatively, although the improvement was significantly better in the 2-stage surgery group than in the conventional-surgery group.
A major limitation of this study is its retrospective nature. A randomized controlled trial would have yielded a more specific and unbiased comparison between the 2 surgical techniques. In addition, a larger sample would have elucidated a more holistic outcome measure when comparing the 2 surgical techniques. Although treating giant retinal tear–associated RRDs may create an added economic burden to the patient or the institution, it is an extremely challenging surgical scenario that necessitates meticulous interventions to prevent recurrent RDs; thus, the marginal loss in terms of expenditure is justified. This unique technique of short-term tamponade offers excellent outcomes compared with conventional surgical modalities.
In conclusion, the outcomes of 2-stage surgery with short-term postoperative PFCL endotamponade for giant retinal tear–associated RRD have thus far been encouraging. This technique improves the anatomic and functional outcomes and would enable vitreoretinal surgeons to manage these challenging scenarios more efficiently.
Footnotes
Ethical Approval: The study conformed to the tenets of the Declaration of Helsinki, and permission from the institutional research board (RET202400455) was obtained.
Statement of Informed Consent: Informed consent was obtained for the surgical procedure, and the potential for a guarded visual prognosis was explained.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Naresh Babu Kannan  https://orcid.org/0000-0002-3993-9790
https://orcid.org/0000-0002-3993-9790
Avik Dey Sarkar  https://orcid.org/0000-0002-7404-0959
https://orcid.org/0000-0002-7404-0959
Supplemental Material: Supplemental material is available online with this article.
References
- 1. Schepens CL, Dobble JG, McMeel JW. Retinal detachments with giant breaks: preliminary report. Trans Am Acad Ophthalmol Otolaryngol. 1962;66:471-479. [PubMed] [Google Scholar]
- 2. Taleb EA, Nagpal MP, Mehrotra NS, Bhatt K, Goswami S, Noman A. Giant retinal tear retinal detachment etiologies, surgical outcome, and incidence of recurrent retinal detachment after silicone oil removal. Oman J Ophthalmol. 2020;13(3):117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freeman HM. Fellow eyes of giant retinal breaks. Trans Am Ophthalmol Soc. 1978;76:343-382. [PMC free article] [PubMed] [Google Scholar]
- 4. Yorston DB, Wood ML, Gilbert C. Retinal detachment in East Africa. Ophthalmology. 2002;109:2279-2283. [DOI] [PubMed] [Google Scholar]
- 5. Chou SC, Yang CH, Lee CH, et al. Characteristics of primary rhegmatogenous retinal detachment in Taiwan. Eye (Lond). 2007;21:1056-1061. [DOI] [PubMed] [Google Scholar]
- 6. Malbran E, Dodds RA, Hulsbus R, Charles DE, Buonsanti JL, Adrogue E. Retinal break type and proliferative vitreoretinopathy in nontraumatic retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1990;228:423-425. [DOI] [PubMed] [Google Scholar]
- 7. Ang GS, Townend J, Lois N. Epidemiology of giant retinal tears in the United Kingdom: the British Giant Retinal Tear Epidemiology Eye Study (BGEES). Invest Ophthalmol Vis Sci. 2010;51(9):4781-4787. [DOI] [PubMed] [Google Scholar]
- 8. Ang A, Poulson AV, Goodburn SF, Richards AJ, Scott JD, Snead MP. Retinal detachment and prophylaxis in type 1 Stickler syndrome. Ophthalmology. 2008;115(1):164-168. [DOI] [PubMed] [Google Scholar]
- 9. Scott IU, Murray TG, Flynn HW, Feuer WJ, Schiffman JC; Perfluoron Study Group. Outcomes and complications associated with giant retinal tear management using perfluoro-n-octane. Ophthalmology. 2002;109(10):1828-1833. [DOI] [PubMed] [Google Scholar]
- 10. Ambresin A, Wolfensberger TJ, Bovey EH. Management of giant retinal tears with vitrectomy, internal tamponade, and peripheral 360 degrees retinal photocoagulation. Retina. 2003;23:622-628. [DOI] [PubMed] [Google Scholar]
- 11. Mehdizadeh M, Afarid M, Haqiqi MS. Risk factors for giant retinal tears. J Ophthalmic Vis Res. 2010;5:246-249. [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh YK, Banerjee S, Savant V, et al. Surgical treatment and outcome of patients with giant retinal tears. Eye (Lond). 2004;18:996-1000. [DOI] [PubMed] [Google Scholar]
- 13. Kertes PJ, Wafapoor H, Peyman GA, Calixto N, Jr, Thompson H. The management of giant retinal tears using perfluoroperhydrophenanthrene: a multicentre case series. Vitreon Collaborative Study Group. Ophthalmology. 1997;104:1159-1165. [DOI] [PubMed] [Google Scholar]
- 14. Ramamurthy S, Raval V, Ali H, et al. Giant retinal tear detachment: clinical presentation and treatment outcomes in 396 patients. Retina. 2023;43(5):784-792. [DOI] [PubMed] [Google Scholar]
- 15. Chang S, Lincoff H, Zimmerman NJ, Fuchs W. Giant retinal tears. Surgical techniques and results using perfluorocarbon liquids. Arch Ophthalmol. 1989;107:761-766. [DOI] [PubMed] [Google Scholar]
- 16. Mikhail MA, Mangioris G, Best RM, McGimpsey S, Chan WC. Management of giant retinal tears with vitrectomy and perfluorocarbon liquid postoperatively as a short-term tamponade. Eye (Lond). 2017;31(9):1290-1295. doi: 10.1038/eye.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91-96. [PubMed] [Google Scholar]
- 18. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236-1240. [DOI] [PubMed] [Google Scholar]
- 19. Al-Khairi AM, Al-Kahtani E, Kangave D, Abu El-Asrar AM. Prognostic factors associated with outcomes after giant retinal tear management using perfluorocarbon liquids. Eur J Ophthalmol. 2008;18:270-277. [DOI] [PubMed] [Google Scholar]
- 20. Lee SY, Ong SG, Wong DWK, Ang CL. Giant retinal tear management: an Asian experience. Eye (Lond). 2009;23(3):601-605. [DOI] [PubMed] [Google Scholar]
- 21. Dabour SA. The outcome of surgical management for giant retinal tear more than 180°. BMC Ophthalmol. 2014;27(14):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goezinne F, LA Heij EC, Berendschot TT, et al. Low redetachment rate due to encircling scleral buckle in giant retinal tears treated with vitrectomy and silicone oil. Retina. 2008;28(3):485-492. [DOI] [PubMed] [Google Scholar]
- 23. Ghasemi Falavarjani K, Alemzadeh SA, Modarres M, Alemzadeh SA, Parvarash MM, Naseripour M, Hashemi M, Robatmeili M. Outcome of surgery in patients with giant retinal tear: 10 years experience. Eye (Lond). 2017;31(9):1284-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gonzalez MA, Flynn HW, Smiddy WE, Albini TA, Tenzel P. Surgery for retinal detachment in patients with giant retinal tear: etiologies, management strategies, and outcomes. Ophthalmic Surg Lasers Imaging Retina. 2013;44(3):232-237. [DOI] [PubMed] [Google Scholar]
- 25. Zhioua R, Malek I, Kria L, Ouertani A. Retinal detachment associated with giant retinal tear: surgical procedures and results of the perfluorocarbon liquid-silicone oil exchange with scleral buckling. J Fr Ophtalmol. 2005;28(4):366-370. [DOI] [PubMed] [Google Scholar]
- 26. Figueroa MS, Casas DR. Inflammation induced by perfluorocarbon liquid: intra- and postoperative use. Biomed Res Int. 2014;2014:907816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mackiewicz J, Maaijwee K, Lüke C, et al. Effect of gravity in long-term vitreous tamponade: in vivo investigation using perfluorocarbon liquids and semi-fluorinated alkanes. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):665-675. [DOI] [PubMed] [Google Scholar]
- 28. Chang S, Sparrow JR, Iwamoto T, Gershbein A, Ross R, Ortiz R. Experimental studies of tolerance to intravitreal perfluoro-n-octane liquid. Retina. 1991;11(4):367-374. [PubMed] [Google Scholar]
- 29. Bottoni F, Bailo G, Arpa P, Prussiani A, Monticelli M, de Molfetta V. Management of giant retinal tears using perfluorodecalin as a postoperative short-term vitreoretinal tamponade: a long-term follow-up study. Ophthalmic Surg. 1994;25(6):365-373. [PubMed] [Google Scholar]
- 30. Sirimaharaj M, Balachandran C, Chan WC, et al. Vitrectomy with short term postoperative tamponade using perfluorocarbon liquid for giant retinal tears. Br J Ophthalmol. 2005;89(9):1176-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheridan AM, Essex RW, Yeoh J, Allen P, Campbell WG, Edwards TL. Is post-operative perfluorocarbon liquid tamponade for macula-on giant retinal tear safer than silicone oil? Eye (Lond). 2019;33(4):689-691. doi: 10.1038/s41433-018-0287-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Randolph JC, Diaz RI, Sigler EJ, Calzada JI, Charles S. 25-gauge pars plana vitrectomy with medium-term postoperative perfluoro-n-octane for the repair of giant retinal tears. Graefes Arch Clin Exp Ophthalmol. 2016;254(2):253-257. [DOI] [PubMed] [Google Scholar]


