Abstract
The ingestion of cotyledons or seeds of cocklebur (Xanthium strumarium) causes poisoning as a result of acute liver failure. Here we describe a spontaneous outbreak of X. strumarium toxicity in dairy cows in Uruguay. The outbreak occurred in the winter when the cows were fed sorghum silage contaminated with X. strumarium seeds. Clinical signs of depression, anorexia, paddling, opisthotonos, muscle tremors, sternal recumbency, and death were observed 2–12 h following ingestion. Of 160 Holstein cows, 30 (19%) animals were ill, and 6 (4%) died. At autopsy, the liver had a diffuse mottled appearance with intercalated red and yellow areas. Histologically, centrilobular hemorrhagic coagulative necrosis was found. The diagnosis of this natural outbreak of intoxication was based on the clinical signs observed, finding the fruits of X. strumarium in the silage, and the characteristic macroscopic and histologic lesions.
Keywords: cocklebur, dairy cattle, poisoning, sorghum silage, toxic seeds, Uruguay, Xanthium strumarium
Stored fodder, such as hay and silage, is commonly used in intensive production systems to complement times of forage scarcity. Contamination with weeds, either in hay or silage, can significantly diminish the nutritional value of the stored fodder.3,20 In addition, if the incorporated weeds are toxic, they can cause major problems. Toxic plants are responsible for many of the submissions to diagnostic laboratories in Uruguay 13 and in our region.1,7,10 In turn, hepatotoxic clinical signs due to plants and mycotoxins are the most numerous among the diagnosed intoxications. 8
Here we describe a spontaneous outbreak of poisoning of dairy cattle by cocklebur (syns. South American burr, “abrojo,” “carrapicho”; Asteraceae, Xanthium strumarium) fruits that contaminated whole-plant sorghum silage. The spontaneous poisoning outbreak took place during July and August 2015, on a 185-hectare dairy farm located in San José County, Uruguay. We had a telephone consultation with the farm veterinarian following the death of 3 cows. Two of 6 affected cows were examined clinically on the farm by 3 of the authors (S. Sosa, A. Capelli, C. García y Santos). Clinical signs included anorexia, ruminal atony, rectal tenesmus, muscle tremors, opisthotonos, sternal recumbency, and death. A postmortem examination was performed on the farm.
We collected epidemiologic data on the crop, sorghum harvest and silage, feed consumption, cattle category, and incident morbidity and mortality. Additionally, samples of feed and feces were taken for analysis of possible weed contamination; we obtained three 6-kg silage bag samples to search and separate weeds, seeds, or other contaminants. Other than X. strumarium, we found no other hepatotoxic plants, such as Cestrum parqui, Wedelia glauca (Pascalia glauca), Cycas revoluta, Sessea vestioides, or Vernonia plantaginoides, 8 in the fields where the cows were grazing at the time at which deaths began or during the clinical course of the outbreak. Among the disease-management measures, diets were supplemented with prairie hay. Contrary to the advice suggested to the dairy farm managers after the death of the first 3 animals, more silage was fed per animal; the managers erroneously believed that animals would select the feed and avoid consuming fruits of the poisonous Xanthium plants.
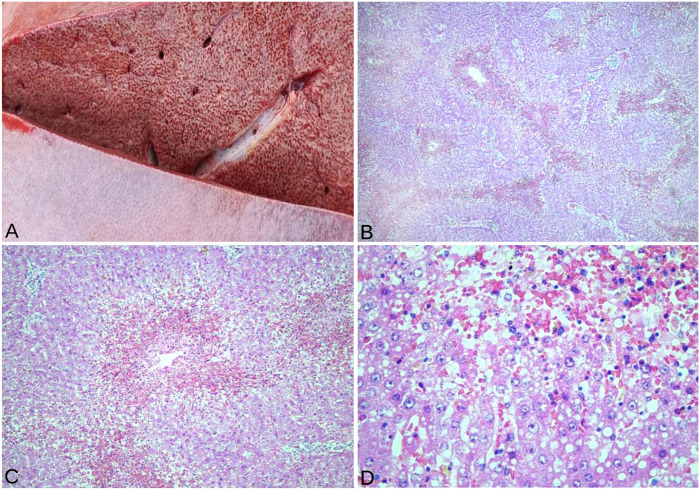
At autopsy, we noted ascites; slight jaundice; subcutaneous edema; congestion, edema, and hemorrhage in the rumen and abomasum; and lack of intestinal content. The most prominent lesions were in the liver, which had an enhanced lobular pattern, and rounded edges, with alternating red and yellow areas (Fig. 1A). The gall bladder was dilated, with an edematous wall. Fruits of X. strumarium were found in ruminal content. Samples of abomasum, rumen, small intestine, cecum, colon, rectum, liver, lymph nodes, heart, lung, kidney, urinary bladder, and brain were collected and fixed in 10% neutral-buffered formalin.
Figure 1.
Gross findings and microscopic lesions in a milking cow naturally intoxicated with Xanthium strumarium. A. The cut surface of the liver has an enhanced lobular pattern. B. Centrilobular-to-massive coagulative necrosis in the liver. H&E. C. Centrilobular hepatic necrosis mainly affecting zones 2 and 3. H&E. D. Necrotic hepatocytes are swollen and of different sizes, with cytoplasmic vacuolation and pyknosis, karyolysis, and karyorrhexis. H&E.
Histologically, the liver had foci of centrilobular-to-massive coagulative necrosis (Fig. 1B) that converged at some points, affecting zones 2 and 3 of the hepatic lobule (Fig. 1C). The necrotic hepatocytes were of different sizes, with cytoplasmic vacuolation and pyknosis, karyolysis, and karyorrhexis (Fig. 1D). In addition, dilated sinusoids, hemorrhages, and congestion were observed with a few diffusely distributed mononuclear inflammatory cells. No significant lesions were observed in other organs.
A 14.5-ha paddock had been sown with sorghum variety ACA 730 for whole-plant silage. X. strumarium plants were observed in high densities in some areas at harvest time. The plants were 60–80 cm tall, and fruits were at maximum maturity at the time of harvest (Fig. 2A). Plants were identified as X. strumarium Schouw, which matched voucher specimens kept in the herbarium of Cátedra de Botánica, Facultad de Química, Universidad de la República (MVFQ 1208).
Figure 2.
Xanthium strumarium and sorghum silage with fruits. A. A specimen of X. strumarium. B. Opened silage bag. C. Fruits of X. strumarium (arrows) in sorghum silage.
Silage bags were 60 m long, 1.7 m high, and 2.5 m wide, and each of 5 bags was filled with ~180 tonnes of plant material (Fig. 2B). The irregular presence of X. strumarium within the paddock at the time of silage made its distribution in the silage bags non-uniform. Some bags were more contaminated with fruits than others, leading to discontinuous consumption. Silage administration had begun in the first half of May. At that time, each of the 160 Holstein milk cows consumed 25 kg of contaminated sorghum silage, 6 kg of dry ground corn, 2 kg of rice husk, and 6 kg of grazing of grassland or ryegrass dry matter per day. During July and August, when the animals were consuming feed from the third silage bag, 30 of 160 (19%) animals were ill; 6 of 160 (4%) died (3 pregnant heifers, 3 milking cows).
From 6 kg of silage material, 94 g of whole and split X. strumarium fruits were separated and weighed, accounting for 1.6% of the total sample. No other weeds were found in the silage bag. Each X. strumarium plant can produce >500 fruits at temperatures of 20–33°C. 19 The temperature before silage harvest was higher than 28°C. This could explain the high concentration of fruit in the silage bag evaluated. Silage was made with the whole plant; hence, X. strumarium seeds and sorghum plants were collected simultaneously during harvest. The fact that animals became sick or died demonstrates that the silage process did not decrease the toxicity of the fruits in our case. Natural poisonings have been associated with consuming hay contaminated with seeds and plants of Xanthium5,20 and soybean crop residues contaminated with fruits of this weed. 6 The careful management of crops before ensilage is essential to obtain good nutritional quality, free of weeds that may be toxic.3,11,18
Silage samples were ground to a size of 1 mm and analyzed according to the Association of Official Analytical Chemists for dry matter (DM; methods 934.01), 2 after drying at 100°C to constant weight and pH (EW-05991-36 digital pH meter; Cole Parmer). The silage samples had 31% DM and a pH of 4.5, and they contained X. strumarium fruits (Fig. 2C). Toxin concentration in fruits can vary greatly (0.1–4.6 mg/kg).10,20 In our case, silage administration was carried out in bunk feeders, which made the consumption of fruits by each animal difficult to estimate. In addition, the distribution of fruits was uneven within the silage bags. In the analyzed silage sample, we estimated a concentration of 392 g of fruits per cow (94 g in 6,000 g = 1.6%). Assuming an average body weight of the dairy cow of 600 kg, the calculation of the consumed dose was 0.65 g of fruit, which is within the range of toxicity noted in the literature.10,20
X. strumarium Schouw is among the plants that cause hepatotoxicity in South America. The Xanthium species was initially called Xanthium cavanillesii Schouw. In a 2021 publication about cattle intoxication by this species, the species was named X. strumarium L. 10 Cocklebur is not native but naturalized in South America and is an invasive weed in all types of crops, paddocks, floodplains, and lake and river shores. Xanthium can reach 1–2 m in height, has an annual cycle, and its fruits (cocklefruits) maintain their germination capacity for many years. 17 Spontaneous intoxications by Xanthium have been reported in swine, 16 cattle,11,12,20 and sheep. 12
The active principles of Xanthium responsible for intoxication are sulfated glycoside carboxyatractylosides (CATs), which inhibit mitochondrial respiration and the synthesis of ATP by disturbing the oxidative phosphorylation process.9,12,15 CATs are found in greater concentrations in the cotyledons, which are palatable; as plants grow, toxicity decreases. 12 Fruits are another source of poisoning when they are accidentally mixed in the feed.5,6,20 The experimental lethal dose (LD50) of fruits in cattle is 5 g/kg; at doses of 3 g/kg, animals have clinical signs, with subsequent recovery. Animals administered doses of 1.5 g/kg did not have clinical manifestations; clinical signs appeared 6–12 h after the ingestion of fruits and had a 5–8 h clinical course. 4
Clinically, as in our case, ruminant poisoning causes depression, anorexia, drooling, ruminal atony, rectal tenesmus, abdominal pain, and nervous signs of generalized muscle tremors, hindlimb instability, aggressiveness, opisthotonos, seizures, sternal recumbency, and death. The main postmortem findings are hepatomegaly with an enhanced lobular pattern, gallbladder wall edema, ascites, dry stools in the rectum, petechiae, and subcutaneous ecchymoses.4,6,12,20 Xanthium fruits can be found in the rumen or reticulum.5,6,20 Histologic lesions are severe hepatic centrilobular necrosis, accompanied by congestion, hemorrhage, and hepatocellular vacuolation.6,12,20
The diagnosis of Xanthium intoxication is based on the presence of cotyledons or fruits in the feed, the observed clinical signs, and characteristic macroscopic and histologic lesions. 17 In a Korean native cow that died the day after consuming new silage containing X. strumarium, CATs were detected in gastric contents using high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. 14 In the differential diagnosis of such cases, poisoning caused by C. parqui, W. glauca, C. revoluta, S. vestioides, and V. plantaginoides 8 should be considered given that they produce similar acute liver disorders.4,6
Acknowledgments
We thank Drs. Rosmari Domínguez, Jorge Martínez, Carlos Morón, and Alejandro Britos for collaborating in the study of this outbreak, Ana Ingold for assistance with English correction, and Drs. Jose Manuel Verdes and Carlos Schild for their critical reading of the histologic studies.
Footnotes
The authors declared no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Carmen García y Santos  https://orcid.org/0000-0002-2485-0099
https://orcid.org/0000-0002-2485-0099
Contributor Information
Santiago Sosa, Departamento Patobiología, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay.
Alejandra Capelli, Departamento Patobiología, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay.
Ana C. Corro, Departamento Patobiología, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay
Fernando Dutra, Laboratorio Regional Este, Ministerio de Ganadería, Agricultura y Pesca (MGAP) DILAVE “Miguel C. Rubino”, Treinta y Tres, Uruguay.
Carmen García y Santos, Departamento Patobiología, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay.
References
- 1. Alberti T, dos S, et al. Intoxicação espontânea por brotos de Xanthium spp. em bovinos no sul do Rio Grande do Sul [Spontaneous poisoning by sprouts of Xanthium spp. in cattle in southern Rio Grande do Sul]. Acta Sci Vet 2020;48(Suppl 1):507. Portuguese. [Google Scholar]
- 2. AOAC International. Loss on drying (Moisture) at 95-100°C for feeds, Dry matter on oven drying at 95-100°C for feeds. Method 934.01. In: Latimer GW Jr, ed. Official Methods of Analysis of AOAC International. 22nd ed. AOAC Publications, Jan 2023. [cited 2024 Feb 1]. 10.1093/9780197610145.003.1381 [DOI] [Google Scholar]
- 3. Bagley CV. Toxic contaminants in harvested forages. Utah State University Extension, July 1997:AH/Beef/16. [Google Scholar]
- 4. Colodel EM, et al. Intoxicação experimental pelos fruto de Xanthium cavanillesii (Asteraceae) em bovinas [Experimental poisoning by the burs of Xanthium cavanillesii (Asteraceae) in cattle]. Pesq Vet Bras 2000;20:31–38. Portuguese. [Google Scholar]
- 5. Di Paolo LA, et al. Intoxicación natural en terneros por consumo de frutos de Xanthium cavanilliesii (abrojo grande) en un establecimiento de Pipinas, Buenos Aires, Argentina [Natural toxicosis in calves by consumption of Xanthium cavanilliesii burs (cocklebur) in a Pipinas’ farm, Buenos Aires, Argentine]. Rev Med Vet (B. Aires) 2011;92:33–38. Spanish. [Google Scholar]
- 6. Driemeier D, et al. Intoxicação espontânea pelos frutos de Xanthium cavanillesii (Asteraceae) em bovinos no Rio Grande do Sul [Spontaneous poisoning by the burs of Xanthium cavanillesii (Asteraceae) in cattle in Rio Grande do Sul, southern Brazil]. Pesq Vet Bras 1999;19:12–18. Portuguese. [Google Scholar]
- 7. García JA, et al. Retrospective analysis of cattle poisoning in Argentina (2000–2013). Pesq Vet Bras 2017;37:210–214. [Google Scholar]
- 8. García y, Santos C, Capelli A. Intoxicaciones por plantas y micotoxinas en rumiantes diagnosticadas en Uruguay [Plant and mycotoxin poisonings in ruminants diagnosed in Uruguay]. Veterinaria (Montevideo) 2016;52:28–42. Spanish. [Google Scholar]
- 9. Luciani S, et al. Effects of carboxyatractyloside, a structural analogue of atractyloside, on mitochondrial oxidative phosphorylation. Life Sci 1971;10:961–968. [DOI] [PubMed] [Google Scholar]
- 10. Machado M, et al. Endemic Xanthium strumarium poisoning in cattle in flooded areas of the Araguari River, Minas Gerais, Brazil. Toxicon 2021;200:23–29. [DOI] [PubMed] [Google Scholar]
- 11. Martin T, et al. Cocklebur poisoning in cattle. J Am Vet Med Assoc 1986;189:562–563. [PubMed] [Google Scholar]
- 12. Mendez MC, et al. Intoxication by Xanthium cavanillesii in cattle and sheep in southern Brazil. Vet Hum Toxicol 1998;40:144–147. [PubMed] [Google Scholar]
- 13. Rivero R, et al. Toxic plants and mycotoxins affecting cattle and sheep in Uruguay. In: Riet-Correa F, et al., eds. Poisoning by Plants, Mycotoxins and Related Toxins. CABI, 2011:25–34. [Google Scholar]
- 14. Roh SG, et al. A case of carboxyatractyloside intoxication by ingestion of the cocklebur (Xanthium strumarium) in a Korean native cow. Vet Med (Praha) 2022;67:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santos JCA, et al. Patogênese, sinais clínicos e patologia das doenças causadas por plantas hepatotóxicas em ruminantes e eqüinos no Brasil [Pathogenesis, clinical signs and pathology of diseases caused by hepatotoxic plants in ruminants and horses in Brazil]. Pesq Vet Bras 2008;28:1–14. Portuguese. [Google Scholar]
- 16. Stuart BP, et al. Cocklebur (Xanthium strumarium, L. var. strumarium) intoxication in swine: review and redefinition of the toxic principle. Vet Pathol 1981;18:368–383. [DOI] [PubMed] [Google Scholar]
- 17. Tokarnia CH, et al. Plantas/micotoxinas que afetan o figado [Plants/mycotoxins that affect the liver]. In: Tokarnia CH, et al. eds. Plantas Tóxicas do Brasil para animais de Prodção. 2nd ed. Ed Helianthus, 2012:148–204. Portuguese. [Google Scholar]
- 18. Van Barneveld RJ. Physical and chemical contaminants in grains used in livestock feeds. Aust J Agric Res 1990;50:807–824. [Google Scholar]
- 19. Weaver SE, Lechowicz MJ. The biology of Canadian weeds. 56. Xanthium strumarium L. Can J Plant Sci 1983;63:211–225. [Google Scholar]
- 20. Witte ST, et al. Cocklebur toxicosis in cattle associated with the consumption of mature Xanthium strumarium. J Vet Diagn Invest 1990;2:263–267. [DOI] [PubMed] [Google Scholar]