Abstract
O-Acetylserine(thiol) lyase (OASTL), a key enzyme of plant sulfur metabolism, catalyzes the formation of Cys from sulfide and O-acetylserine. The biosynthesis of Cys is regarded as the exclusive function of sulfur reduction in plants, and a key limiting step in the production of glutathione (GSH), a thiol implicated in various cellular functions, including sulfur transport, gene expression, scavenging of reactive oxygen species (ROS), and resistance to biotic and abiotic stresses. To examine whether an increased capacity for cysteine (Cys) biosynthesis alters cellular responses to such stresses, we studied the differential changes in thiol levels and ROS scavenging of transgenic tobacco (Nicotiana tabacum) plants expressing the wheat (Triticum aestivum) OASTL gene, cys1, to SO2 and to the ROS generator, methyl viologen. Intracellular Cys and GSH contents were generally higher in cys1 transgenics than in controls under normal growth conditions, but became especially elevated in transgenic plants after SO2 exposure. An examination of differences in the ROS scavenging system of the transgenic plants also demonstrated the specific accumulation of Cu/Zn superoxide dismutase transcripts, known to be induced by Cys or GSH, and elevated cellular superoxide dismutase activities. The transgenic plants accordingly showed dramatic reductions in the extent of both foliar and photooxidative damage in response to acute SO2, as well as reduced levels of chlorosis and membrane damage following methyl viologen treatment. Overall, our results imply that OASTL plays a pivotal role in the synthesis of Cys and GSH that are required for regulation of plant responses to oxidative stress.
The biosynthesis of Cys constitutes the final step of the sulfur reduction pathway in plants (for recent reviews, see Brunold and Rennenberg, 1997; Hell, 1997; Leustek and Saito, 1999). This pathway begins with the assimilation and reduction of sulfate first to sulfite and then to sulfide in chloroplasts, and ends with the coupling of the sulfide with O-acetyl-Ser (OAS) to form Cys in all three cellular compartments (cytosol, plastids, and mitochondria) by respective isoforms of O-acetyl-Ser(thiol) lyase (OASTL; EC 4.2.99.8). Thus, the Cys formed serves as a precursor for the synthesis of various sulfur-containing metabolites, of which glutathione (GSH) represents the major storage and transport form of reduced sulfur (Rennenberg, 1997; Noctor et al., 1998). In addition to this role, GSH has attracted considerable interest because of its proposed functions as a regulator of gene expression, in the control of cell proliferation, and most importantly in the context of oxidative stress resistance, as a donor of reducing equivalents for the scavenging of reactive oxygen species (ROS; May et al., 1998a).
All aerobic organisms produce ROS, such as superoxide, hydrogen peroxide, and hydroxyl radicals, during normal biological processes and they consequently have developed an elaborate system of antioxidants and enzymes to scavenge these ROS that would otherwise damage the cellular components (Alscher and Hess, 1993; Foyer et al., 1994). Nevertheless, under detrimental environmental conditions that lead to excessive ROS accumulation, the scavenging system is unable to cope and a state of oxidative stress damage arises. The importance of this scavenging system in plant stress tolerance has been amply demonstrated by ROS-mediated induction of genes encoding various scavenging enzymes (Willekens et al., 1994), and by the resistance of transgenic plants overexpressing such genes to ROS-induced stresses (Foyer et al., 1994; Aono et al., 1995; Pitcher and Zilinskas, 1996).
Accumulating evidence further suggests that these adaptive responses of plants to increased ROS levels are, at least in part, mediated by changes in cellular GSH concentrations, or in the redox state of the GSH pool (see May et al., 1998a; Noctor et al., 1998). The primary determinants of cellular GSH levels are thought to be the activity of the GSH biosynthetic enzymes and the availability of substrates. Of these, Cys availability has been shown to be the main factor limiting GSH production, both in normal plants and in those that overexpress genes for GSH biosynthesis (Strohm et al., 1995; Noctor et al., 1996).
As with GSH, the metabolic pathways involved in the biosynthesis of Cys are regulated with a high degree of complexity. For example, transcriptional and posttranscriptional control of several key enzymes are known be regulated by GSH and Cys, which appear to function as negative regulators of sulfur assimilation (Herschbach and Rennenberg, 1994; Bolchi et al., 1999), whereas the availability of OAS, synthesized by Ser acetyl transferase (SAT), is generally regarded as a limiting factor and a positive signal for sulfur assimilation and Cys biosynthesis (Rennenberg, 1984). A further level of control is also provided by the formation of a bi-enzyme complex between OASTL and SAT, in which the properties of the two enzymes are drastically modified, and the stability of which is dependent on the availability of the OASTL substrates, OAS and sulfide (Ruffet et al., 1994; Droux et al., 1998; Leustek and Saito, 1999).
Here, using a set of transgenic tobacco (Nicotiana tabacum) plants expressing the cys1 gene, which encodes a wheat (Triticum aestivum) OASTL, we set out to examine whether their high OASTL levels enhanced their capacity to synthesize Cys and GSH and, in so doing, their tolerance to specific oxidative stress conditions. Although these plants were previously found to possess enhanced resistance to sulfite (S. Youssefian, unpublished data) and hydrogen sulfide (Youssefian et al., 1993), consistent with proposals that OASTL is involved in sulfide fixation (De Kok et al., 1998), our current results clearly demonstrate that the plants possess additional traits that enhance their tolerance to oxidative stress.
RESULTS
Increased Thiol Accumulation in Transgenic Plants in Response to SO2
The T2 progeny of the C6 line of cys1 transgenic plants, with over 3-fold the OASTL activities of control plants (data not shown) as in the parental C6 lines (Youssefian et al., 1993), were used throughout these experiments. Although the cys1 gene was originally considered to encode a chloroplastic OASTL (Youssefian et al., 1993), sequence comparisons with other OASTL cDNA clones with demonstrated transit peptides (Saito et al., 1993) suggest that cys1 does not possess a transit peptide and so it most probably encodes a cytosolic OASTL isoform. Therefore, the elevated OASTL activities of these transgenic plants can be attributed to their increased cytosolic OASTL levels.
To examine whether these increased OASTL activities resulted in elevated foliar thiol contents, especially in response to an external sulfite supply in the form of SO2, the total foliar non-protein sulfhydryl (SH), Cys, and GSH contents were measured. Our use of gaseous SO2 was based on the premise that SO2 uptake would result in cellular accumulation of sulfite without the confounding effects of feedback mechanisms regulating the uptake of sulfur compounds by roots (Herschbach and Rennenberg, 1994), and partly because of the substantial amount of evidence linking SO2 exposure, thiol accumulation, and oxidative stress imposition.
In the first set of experiments, thiol contents in the fourth distal leaves of un-fumigated 7-week-old plants were determined. Despite some variation between independent replicate experiments, relative differences between control and transgenic plants were always consistent within replicates. Cellular thiol levels in control plants were within previously reported ranges with Cys levels (30 nmol g−1 fresh weight) comprising less than 10% of total SH contents (De Kok et al., 1988; Schütz et al., 1991). However, Cys levels in transgenic plants (71 nmol g−1 fresh weight) accounted for 17% of their total SH contents and were over 2-fold higher than control plant levels (Fig. 1A). These increases, together with consistently but nonsignificantly higher GSH levels, were reflected in higher overall SH contents of the transgenic plants.
Figure 1.
Total non-protein thiol contents in control and cys1 transgenic plants. A, The fourth distal leaf of unfumigated 7-week-old plants; B, the sixth distal leaf of 5-month-old tobacco plants either un-fumigated (U), or exposed to 1 μL L−1 SO2 (SO2) for 8 h in the light, after which no foliar damage to either control or transgenic plants was observed. Values are means (with se bars) of four independent plants with two duplicates each. SH, Total SH.
In the second set of experiments, the sixth distal leaves were sampled from 5-month-old tobacco plants either unfumigated or exposed to 1.0 μL L−1 SO2 for 8 h. Because of the age of plants, neither control nor transgenic plants showed any visible SO2 exposure-related damage. In the absence of fumigation, thiol levels were comparable to those in the first experiment although differences between these older control and transgenic plants were now not as distinct (Fig. 1B). Fumigation with SO2 resulted in increased thiol levels in control plants, but even more marked increases in the transgenic plants. Hence, in comparison with their unfumigated counterparts, fumigation of control and transgenic plants resulted in respective Cys increases of 10 and 53 nmol g−1 fresh weight, in GSH increases of 46 and 134 nmol g−1 fresh weight, and in SH increases of 56 and 187 nmol g−1 fresh weight (Fig. 1B). Overall, therefore, Cys levels were 2-fold higher and GSH contents 1.5-fold higher in transgenic than in control plants following SO2 fumigation.
Taken together, these results indicate that the cys1 transgenic plants possess higher levels of Cys and possibly GSH than control plants under normal growth conditions and, most importantly, that they have greater capacities than control plants to accumulate both Cys and GSH in response to SO2 exposure.
Reduced Foliar and Photooxidative Damage in Transgenic Plants in Response to SO2
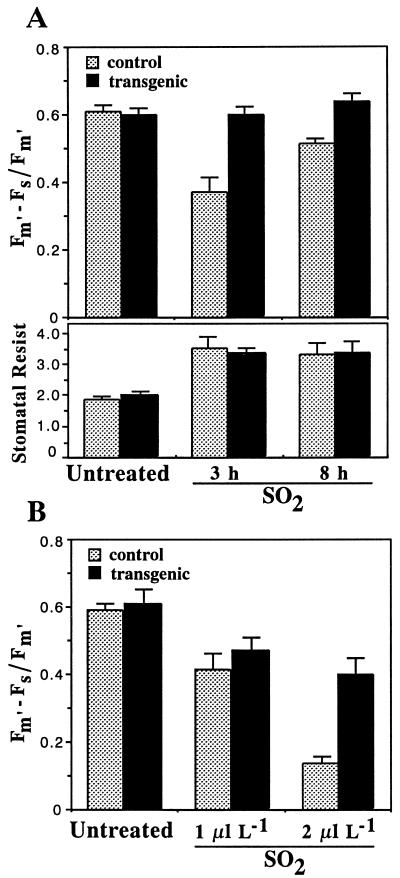
Control and cys1 transgenic plants, either 3 or 7 weeks of age, were maintained in the non-fumigated control cabinet, or were fumigated for 3, 6, or 8 h in the SO2 fumigation cabinet under light conditions before transfer back to the control cabinet for at least 18 h of recovery. Differential sensitivities of control and transgenic plants during SO2 exposure were quantified by measurements of pulse-modulated chlorophyll fluorescence in the light-adapted state; a measure of the effective quantum yield (Fm' − Fs/Fm') of photochemical energy conversion and, hence, of the overall photosynthetic performance of the plants. Under non-fumigated conditions, no differences in the effective quantum yields of control and transgenic plants were observed (Fig. 2, A and B). Exposure of 3-week-old plants to 1 μL L−1 SO2 for 3 h resulted in no visible foliar damage to either control or transgenic plants. However, in terms of photosynthetic performance, quantum yields were significantly reduced in controls but unaffected in transgenic plants (Fig. 2A, top). After exposure of these plants to 1 μL L−1 SO2 for 8 h, over 60% of control plants showed large areas of foliar necrosis, whereas less than 30% of transgenic plants showed only the most minimal signs of damage. In accordance, quantum yields in controls, despite having partially recovered, were still significantly reduced in comparison with those of transgenic plants (Fig. 2A, top). The differential SO2 sensitivities between control and transgenic plants were not due to differences in stomatal resistance either before or after fumigation (Fig. 2A, lower). In both sets of plants, stomatal resistance, a measure of stomatal closure, increased in response to SO2 and reached a maximum after 30 min from the start of fumigation (data not shown). The recovery of quantum yields of control plants after 8 h fumigation therefore may reflect the reduced levels of SO2 entering into the plant after stomatal closure, and may suggest that such SO2-induced effects on photosynthesis are reversible in the light (Veljovic-Jovanovic et al., 1993).
Figure 2.
Chlorophyll fluorescence and stomatal resistance in control and cys1 transgenic plants during SO2 fumigation. A, Upper, effective quantum yields (Fm' − Fs/Fm') in 3-week-old plants untreated or exposed to 1 μL L−1 SO2 (SO2) for 3 or 8 h in the light. A, Lower, stomatal resistance (Stomatal Resist; S cm−1), measured in the same plants after 3 or 8 h of 1 μL L−1 SO2. B, Quantum yields (Fm' − Fs/Fm') of 7-week-old plants untreated or exposed to 1 or 2 μL L−1 SO2 for 6 h in the light. Values for each experiment are means (with se bars) of five control and seven or eight transgenic plants, each with two independent measurements.
Fumigation of 7-week-old plants with 1 μL L−1 SO2 for either 6 or 8 h similarly resulted in no visible foliar damage to either control or transgenic plants. However, exposure to 2 μL L−1 SO2 for 8 h induced visible necrotic patches in all the control but not in any of the transgenic plants (Fig. 3). Again, in terms of photosynthetic performance, transgenic plants were significantly more resistant to both 1- and 2-μL L−1 SO2 exposure for 6 h than control plants, especially under conditions that induced the most extensive amount of foliar damage (Fig. 2B). The differential effects of SO2 on the photosynthetic activity of the control and transgenic plants were also examined by measurements of photosynthetic oxygen evolution using a Clark-type electrode in aqueous media as previously described (Shimazaki and Sugahara, 1979). Preliminary data indicate that although oxygen evolution (68 μmol O2 mg−1 chlorophyll [Chl] h−1) was equivalent in un-fumigated control and transgenic plants, oxygen evolution was reduced to 8.3 μmol O2 mg−1 Chl h−1 in controls (n = 2) and to 36.1 ± 9.2 μmol O2 mg−1 Chl h−1 in transgenic (n = 3) plants exposed to 2 μL L−1 SO2 for 3 h. Taken together, these results clearly demonstrate that SO2-induced damage to the foliar tissues and effects on photosynthetic performance were significantly alleviated in the cys1 transgenic plants.
Figure 3.
Extent of SO2-induced foliar damage in control and cys1 transgenic plants. The 7-week-old plants were exposed to 2 μL L−1 SO2 for 8 h in the light, and then allowed to recover for 24 h before being photographed. Punched leaf discs were used for oxygen evolution measurements (see text).
Enhanced Tolerance of Transgenic Plants to Methyl Viologen (MV)-Induced Oxidative Stress
Because SO2 exposure not only increases thiol contents but is also reported to result in the production of ROS, which are thought to be one of the main proponents of SO2-induced damage to the photosynthetic apparatus, we questioned whether the observed tolerance of the cys1 transgenic plants to SO2 was a result of their greater capacity to utilize the SO2 for Cys biosynthesis, and/or whether they possessed additional traits that enabled them to tolerate the SO2-generated ROS more effectively. To address this latter possibility, experiments utilizing MV, which generates ROS independently of the sulfur pathway, were performed. In repeated independent experiments, control plant leaf discs showed signs of chlorosis at 0.2 μm MV and almost complete chlorotic damage at concentrations above 0.5 μm. In contrast, leaf discs from independent cys1 plants showed almost no chlorosis at 0.2 μm MV, limited signs of chlorosis at 0.5 μm, and complete chlorotic damage at concentrations above 2.0 μm MV. A representative experiment with a few of the concentrations used is presented in Figure 4a.
Figure 4.
MV-induced chlorosis and electrolyte leakage in control and cys1 transgenic leaf discs. A, Leaf discs from three different 7-week-old control and transgenic plants were vacuum infiltrated with water (Water) or different MV concentrations (MV; 0.5 μm or 2.0 μm) and then illuminated for 18 h. B, Time course of electrolyte leakage from leaf discs of 7-week-old plants vacuum infiltrated with 20 μm MV or water and then transferred to water in the light for 18 h. Conductivity values (μS cm−1), a measure of cellular damage, were calculated as the difference in leakage between leaf discs exposed to MV and to water in the light. Maximum values for controls and transgenics exposed to water only in the light for 18 h were 26.2 ± 4.3 and 31.2 ± 1.0 μS cm−1, respectively. Values are means (with se bars) of 10 leaf discs from three independent control or transgenic plants.
To quantify these effects, the extent of light-dependent MV-induced membrane damage, used as an indicator of cellular injury, was determined by measuring the leakage of ionic solutes out of the cells. Untreated leaf discs showed only minimal damage in water in the light and even less damage in the dark, whereas MV treatment in the light resulted in photooxidative damage to leaf discs from both sets of plants. However, in repeated experiments, control plants consistently showed a greater extent of electrolyte leakage than transgenic plants, as shown by a representative time course (Fig. 4b). Such differences in electrolyte leakage are comparable to the data of Aono et al. (1995), who demonstrated similar increases in the MV resistance of superoxide dismutase (SOD) and GSH reductase overexpressing transgenic tobacco plants. Our results are clearly indicative, therefore, of the greater tolerance of the cys1 transgenic plants, not only to SO2, but also to MV-induced photooxidative damage through a mechanism that is independent of the primary effects on sulfur metabolism.
Elevated Cu/ZnSOD Transcript Levels and SOD Activities in Transgenic Plants
As changes in the cellular thiols, especially GSH content and redox state, are known to affect the ROS scavenging system, we questioned whether the increased capacity of transgenic plants to generate Cys and GSH in any way affected the expression of genes encoding enzymes of the antioxidant defense pathway. Thus, northern-blot analysis was performed using whole plants or leaf discs of 7-week-old control and transgenic plants exposed to various treatments, together with radiolabeled probes of cDNAs encoding four different tobacco SOD isoforms, two catalases, an ascorbate peroxidase, a GSH reductase, and a GSH-S-transferase.
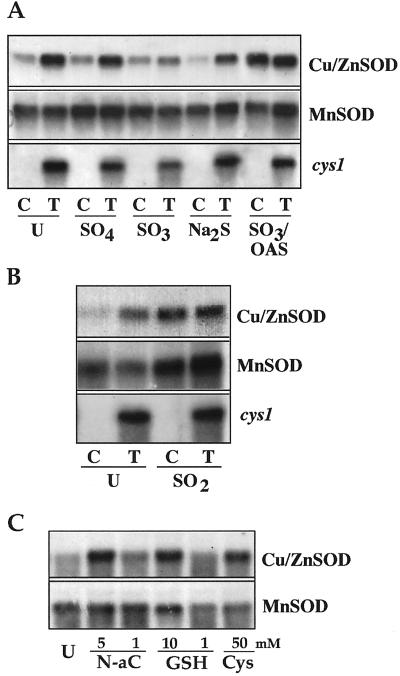
Of the nine probes used, only that encoding a cytosolic Cu/ZnSOD showed consistent differences in transcript levels between control and transgenic plants, whereas transcript levels of the other scavenging enzyme genes were comparable in the two sets of plants. Leaf discs of 7-week-old plants, exposed to either a control treatment lacking a sulfur source, or to that supplemented with sulfate or sulfide, which are known to increase internal SH but not necessarily Cys levels, showed 2- to 3-fold higher Cu/ZnSOD transcript levels in the transgenic plants than in control plants (Fig. 5A). Sulfite alone reduced Cu/ZnSOD transcript levels in transgenic samples to levels equivalent to control plants, whereas sulfite supplemented with OAS, which reportedly promotes efficient Cys biosynthesis in the light (Saito et al., 1994), increased Cu/ZnSOD transcripts in control plants to levels comparable to transgenic plants (Fig. 5A). Evidence that these differences in Cu/ZnSOD transcript levels were associated with cys1 expression, rather than with wound-induced responses, was obtained by an independent set of experiments using whole plants. Whereas Cu/ZnSOD transcripts were almost undetectable in untreated control plant leaves in comparison with the moderately expressed levels in transgenic plants, a 3-h exposure to 2 μL L−1 SO2 increased Cu/ZnSOD transcripts in both control and transgenic plants to comparable levels (Fig. 5B). To further confirm that the induction of Cu/ZnSOD transcripts in tobacco was directly associated with increased endogenous levels of reduced thiols, leaf discs of control plants were subjected to treatment with either 1, 5, or 50 mm Cys, N-aC, or GSH. Clear increases in Cu/ZnSOD transcript levels were observed after treatment of these control plant leaf discs with 5 mm N-aC, 10 mm GSH, and 50 mm Cys (Fig. 5C). The higher Cys levels required presumably reflect its instability and rapid oxidation.
Figure 5.
Northern-blot analysis of cytosolic SOD transcript accumulation in control and cys1 transgenic plants. A, Six leaf discs from the fourth distal leaf of 7-week-old plants exposed to one-fifth Murashige and Skoog media lacking a sulfur source (U), or that supplemented with 10 mm Na2SO4 (sulfate; SO4), 10 mm Na2SO3 (sulfite; SO3), 10 mm Na2S (sulfide; Na2S), or sulfite plus 1 mm O-acetyl-Ser (SO3/OAS) for 18 h in the light. B, Seven-week-old plants untreated (U) or fumigated for 3 h with 2 μL L−1 SO2 (SO2) in the light. C, Leaf discs of 7-week-old control plants exposed to one-fifth Murashige and Skoog (U), or that supplemented with the stated concentrations of Cys, GSH, or N-acetyl Cys (N-aC) for 18 h in the light. Leaf discs or leaf samples were immediately used for RNA extraction and northern hybridization using cDNA probes for tobacco cytosolic Cu/ZnSOD or MnSOD, or for wheat cys1. C, Control plants; T, cys1 transgenic plants.
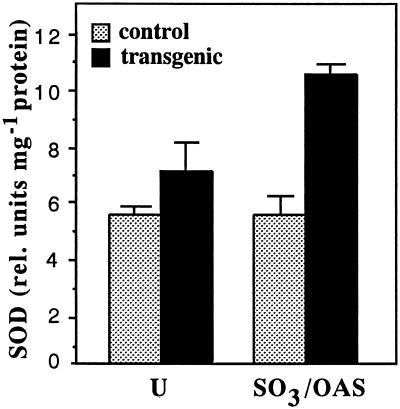
To examine whether these increased Cu/ZnSOD transcript levels were also reflected in higher SOD enzyme activities, leaf discs of control and transgenic plants subjected to either a control treatment or that supplemented with sulfite plus OAS were used for SOD activity analysis. Without treatment, SOD activities in transgenic plants were higher than control plant levels. However, following combined sulfite and OAS treatment, SOD activities in transgenic plants increased even further, whereas unlike their Cu/ZnSOD transcript levels, there was no further increase in control plant SOD activities. Overall, therefore, SOD activities following treatment were 2-fold higher in transgenic plants than in control plants (Fig. 6). These results not only clearly indicate that the transgenic plants have higher basal Cu/ZnSOD transcripts and slightly enhanced SOD activities than control plants, but that the overall SOD activities could be differentially activated in the transgenic plants by treatment that provides substrates for Cys biosynthesis.
Figure 6.
Comparison of relative total cellular SOD activities in control and cys1 transgenic plants. Leaf discs from fourth distal leaves of 7-week-old plants treated with one-fifth MS (U), or that supplemented with 10 mm Na2SO3 and 1 mm O-acetyl-Ser (SO3/OAS) for 18 h in the light. Leaf discs were used to prepare crude samples for SOD determination. Values are means (with se bars) of three plants with two duplicate measurements each.
DISCUSSION
In this study, we have shown that transgenic tobacco plants expressing the wheat cys1 gene have enhanced tolerance to both SO2 exposure and to MV-induced oxidative stress, and that they also possess higher basal levels of Cu/ZnSOD gene expression and SOD activity. The basis of these responses appears to be the increased capacity of the transgenic plants to generate Cys and GSH, especially after SO2 exposure, and below we describe the characteristics of these plants and the possible mechanisms that could be involved in these responses.
The primary effect of the enhanced cytosolic OASTL activities of the cys1 transgenic plants was to elevate Cys contents to over 2-fold those of control plants in the absence of any imposed stress. However, such Cys increases, together with the generally higher GSH levels that resulted in overall thiol increases in the transgenic plants, were more clearly discernible in younger transgenic plants even though older plant leaves showed the same general trends. This correlates with our observation that, despite equivalent cys1 transcript levels, OASTL activities were generally higher in younger transgenic plant leaves, suggesting that the synthesis and accumulation of these thiols were regulated by developmental events as also found in transgenic poplar plants overexpressing γ-glutamyl-Cys synthetase (Arisi et al., 1997).
In a different set of experiments using in vitro-grown transgenic tobacco plantlets expressing a spinach (Spinacia oleracea) OASTL, a similar 2-fold increase in Cys contents as in our results was also observed (Saito et al., 1994) although, possibly because of the artificial growth conditions used and the large errors involved, these effects were not significant. However, based on these findings and the observation that endogenous plant OASTL levels are far in excess of the levels of SAT (Ruffet et al., 1994), the enzyme that catalyzes the formation of OAS required for Cys biosynthesis, it was recently suggested that OASTL does not play an important role in regulating plant Cys biosynthesis (Harms et al., 2000). In contrast, analyses of the bi-enzyme complex that forms between OASTL and SAT in chloroplasts not only have clearly demonstrated that OASTL can act as a regulatory subunit of SAT activity, and hence Cys biosynthesis, but that because OASTL in this complex is almost inactive, a 400-fold excess of unbound auxiliary OASTL over SAT levels is required to convert the accumulating OAS and provide full Cys synthesis capacity (Ruffet et al., 1994, Droux et al., 1998). As cytosolic OASTL and SAT form similar complexes (Bogdanova and Hell, 1997), we consider it most likely that the wheat OASTL in the cys1 transgenic plants could augment the cytosolic tobacco OAST levels and thus provide an increased capacity for Cys biosynthesis.
Larger and more significant increases in the levels of Cys and GSH may also not have been observed in the cys1 transgenic plants due to the limited availability of the substrates of Cys biosynthesis, OAS, and sulfide. However, even though OAS availability is regarded as the main factor limiting Cys biosynthesis (Neuenschwander et al., 1991), bacterial SAT-overexpressing transgenic tobacco and potato (Solanum tuberosum) plants, with up to 20-fold higher SAT activities, also showed only up to 2- to 3-fold higher Cys and GSH levels than control plants (Blaszczyk et al., 1999, Harms et al., 2000). Similar levels of Cys increase were also observed in sulfite-exposed leaf discs of transgenic plants expressing a chloroplastic- but not a cytosolic-targeted spinach OASTL (Saito et al., 1994). Our findings that the most marked effects of OASTL overexpression, on both Cys and GSH accumulation, were obtained by exposure of the cys1 transgenic plants to non-damaging levels of SO2, are therefore partially consistent with these latter results. It is unclear why the cytosolic-targeted OASTLs should show such different responses in these two experiments, although it may be related to the SO2-fumigated whole plant system in our experiment and the sulfite-exposed leaf disc system of Saito et al. (1994).
Increased thiols in response to SO2 is a common phenomenon (see De Kok, 1990, and references therein), even though the mechanisms involved are not fully resolved. The weight of evidence suggests that most of the toxic sulfite that accumulates in the cytosol from hydration of SO2 enters the chloroplasts. There is only limited circumstantial evidence that the sulfite can be directly oxidized to sulfate or reduced to sulfide in the cytosol. In chloroplasts, the majority of the sulfite is oxidized by a light-induced process to sulfate and the remainder is reduced to sulfide and used for Cys biosynthesis in chloroplasts and presumably the cytosol. However, oxidation results in cellular acidification and ROS generation, both of which negatively affect cellular functions, whereas reduction to sulfide and subsequent Cys formation appear to constitute a detoxification mechanism that limits acidification and oxidative stress. Associated with this reduction pathway is the production, possibly from chloroplast-generated sulfide, and the emission of small quantities of H2S from SO2-exposed plants. However, such emissions are thought to have little physiological significance and to be rather indicative of the general reductive strategy by which plants remove excess sulfur compounds and prevent chloroplastic damage (Rennenberg and Herschbach, 1996).
Based on these observations, and the fact that there can be direct competition for sulfite between these oxidative and reduction pathways in chloroplasts (Ghisi et al., 1990), the simplest explanation of our results is that the high cytosolic OASTL activities in the transgenic plants, by providing an increased capacity for utilization of the chloroplast-derived sulfide, promoted reductive conversion of sulfite to sulfide and thus limited sulfite oxidation in chloroplasts. This would not only lead to the observed increases in cytosolic Cys, and hence GSH which is known to be primarily limited by Cys availability (Noctor et al., 1996), but would also limit acidification and ROS production in chloroplasts of the transgenic plants in response to SO2. Because SO2-induced acidification (Pfanz et al., 1987) may induce a low intrathylakoid pH, and a subsequently enhanced formation of zeaxanthin (Veljovic-Jovanovic et al., 1993) that would then increase dissipation of light energy as heat, the observed reductions in chlorophyll fluorescence in the control plants in response to SO2 could well be due to the induction of such protective photochemical quenching mechanisms resulting from cellular acidification. Furthermore, the observed recovery of the fluorescent yields in the control plants after several hours of SO2 exposure may also be suggestive of the induction of various pH-stabilizing mechanisms rather than simply resulting from SO2-induced stomatal closure. Finally, as inhibition of photosynthesis and damage to the cellular membranes appear to be primarily a consequence of the SO2-derived ROS than cellular acidification (Shimazaki et al., 1980; Veljovic-Jovanovic et al., 1993), the minimal extent of SO2-induced foliar damage in transgenic plants, compared with the extensive injury suffered by control plants, may be indicative of their limited ROS production as a direct consequence of their preferential reductive conversion of sulfite for Cys biosynthesis.
However, the increased tolerance of the cys1 transgenic plants to MV, in the absence of any SO2-imposed stress, precludes such a simple model. When the similarities between the modes of SO2 and MV phytotoxicity are considered, namely that their light-mediated superoxide generation in chloroplasts induces damage to the photosynthetic apparatus and cellular membranes, it appears more likely that the tolerance of the cys1 plants to SO2 and MV is associated more with their resistance to elevated ROS levels than with just their enhanced metabolism of sulfite.
Exposure to SO2, MV, and other oxidative stress conditions that result in ROS production, have been shown to result in changes in GSH contents and in the activation of several scavenging enzymes (Madamanchi and Alscher, 1991; Willekens et al., 1994; May et al., 1998b), presumably to protect cells from the harmful effects of ROS. The GSH functions as an important antioxidant in both enzymatic and nonenzymatic ROS scavenging reactions (Foyer and Halliwell, 1976; Noctor and Foyer, 1998), and both Cys and N-aC are commonly used as antioxidants to scavenge ROS and protect against oxidative damage (Bolwell et al., 1998). In support of the importance of these thiols in such protective mechanisms, several transgenic plants with elevated levels of GSH have been shown to be particularly resistant to oxidative stress (Foyer et al., 1995; Wellburn et al., 1998). Of specific interest is the observation that SAT-overexpressing transgenic plants, with 2- to 3-fold higher Cys and GSH levels than control plants, showed associated increases in hydrogen peroxide-imposed oxidative stress resistance (Blaszczyk et al., 1999). In accordance with these observations, our results therefore would imply that the increased capacity of the cys1 transgenic plants to generate Cys and GSH, especially under conditions of oxidative stress induced by SO2, and possibly even MV as shown by the rapid generation of GSH in response to superoxide-generating menadione (May et al., 1998b), directly provided antioxidant buffering against the generated ROS.
In addition to their antioxidant effects, however, thiols have been implicated in altered signaling events and in the expression of genes encoding particular enzymes of the scavenging system (Hérouart et al., 1993; Wingsle and Karpinski, 1996; May et al., 1998a). Of particular relevance is the study of Hérouart et al. (1993), which demonstrated that the promoter of the same cytosolic Cu/Zn SOD gene used in our present study, when coupled to a reporter gene and expressed in transgenic tobacco protoplasts could be induced only by reduced thiols, including Cys, N-aC, or GSH. Furthermore, using an inhibitor of GSH biosynthesis, Cys supplementation even without conversion to GSH could still induce the Cu/ZnSOD promoter, albeit not as effectively (Hérouart et al., 1993). These observations, which implicate Cys itself as an inducer of Cu/ZnSOD, concur with our findings that the 2- to 3-fold higher basal transcript levels of cytosolic Cu/ZnSOD were associated with the 2-fold higher Cys contents of the cys1 transgenic plants in the absence of imposed stress, and that similar Cu/ZnSOD transcript levels could be induced in control plants by conditions that increased internal Cys or GSH levels. Although the increased accumulation of Cu/ZnSOD transcripts in the cys1 transgenic plants was also reflected in higher basal total cellular SOD enzyme activities, we are uncertain why only SOD activities of the transgenic plants were further increased after feeding with sulfite and OAS.
From these results, and those of others showing that expression of Cu/ZnSOD only occurs after increases in GSH levels (Madamanchi and Alscher 1991) or the appearance of induced foliar injury (Willekens et al., 1994), we conclude that the increased capacity of the cys1 transgenic plants to generate Cys and GSH may not only have limited cellular acidification and ROS generation but, more importantly, by enhancing the levels of these antioxidants and by maintaining low basal SOD activities prior to the normal induction of GSH and Cu/ZnSOD by oxidative stress, afforded the transgenic plants a greater level of tolerance to such stress conditions.
MATERIALS AND METHODS
Transgenic Plants
Transgenic tobacco (Nicotiana tabacum cv Xanthi NC) plants, expressing the wheat (Triticum aestivum) cys1 gene under control of the cauliflower mosaic virus 35S promoter, have been described previously (Youssefian et al., 1993). Homozygous, kanamycin-resistant T2 lines of the C6 transformant, which showed the highest levels of cys1 transcripts, OASTL activity, and H2S resistance (Youssefian et al., 1993), were used throughout the experiments. Control plants were untransformed F2 Xanthi plants propagated from wild-type leaf discs.
Plant Growth and SO2 Fumigations
Transgenic and control plants were grown in a soil:vermiculite (1:1) mixture and watered on alternate days with tap water and twice a week with a 1,000-fold dilution of a commercial nutrient solution containing 0% sulfate (Hyponex 5-10-5, Hyponex Japan, Tokyo). The plants were maintained in controlled environment chambers at 25°C under a 14-h photoperiod with a photosynthetic photon flux density of 250 μmol m−2 s−1 and a constant 70% relative humidity. Next, plants were briefly maintained in greenhouse facilities of the National Institute for Environmental Studies (Tsukuba, Japan) before transfer to a walk-in control cabinet, identical to the cabinet to be used for fumigations, for 1 to 2 d of preconditioning. The fumigation experiments and thiol measurements were performed using either 3-week-old (control n = 19; transgenic n = 22) plantlets, 7-week-old (control n = 21; transgenic n = 25) plants, or 5-month-old (control n = 4; transgenic n = 4) plants. Fumigations were begun at least 4 h after start of the 14-h light cycle, and were conducted at 25°C, at a photosynthetic photon flux density of 400 μmol m−2 s−1, a 70% relative humidity, a wind velocity of 0.22 m s−1, and a constant flow of SO2 at 1 or 2 μL L−1. During or after set periods of fumigation, non-destructive measurements of stomatal resistance and chlorophyll fluorescence were performed, or leaves were immediately sampled and used for thiol measurements and RNA extraction. Plants were then returned to the un-fumigated control cabinet, and allowed to recover for over 18 h until the next day when injury to the leaves was visually assessed.
Determination of Thiol Contents
Whole leaves from control, and transgenic, either untreated or exposed to SO2 were sampled. Every effort was made to ensure sampled leaves from controls and transgenics were of similar developmental stage and position. The total water-soluble, non-protein SH content of tobacco leaf samples was determined colorimetrically with 5–5′dithiobis (2-nitrobenzoic acid) (DTNB), essentially as described by De Kok et al. (1988). In brief, 1-g tobacco leaf samples were homogenized in 10 mL of an ice-cold solution of 8 mm sodium ascorbate, 80 mm sulfosalicylic acid, and 1 mm EDTA (Maas et al., 1987), from which oxygen had been removed by saturation with N2. After filtering through one layer of Miracloth, samples were deproteinized by boiling for 4 min followed by centrifugation at 30,000g for 15 min at 0°C. For SH measurements, 1 mL of the deproteinized supernatant was added to 1 mL of 50 mm MES [2-(N-morpholino)ethanesulfonic acid], pH 5.8, and the mixture incubated for 10 min at 30°C. Next, 0.1 mL of 10 mm DTNB (in 80 mm potassium phosphate, pH 7.0) and 2 mL of 0.4 m Tris-HCl, pH 8.0, were added, and the yellow color that developed determined spectrophotometrically at 415 nm. Corrections for the absorbance of mixture components were made by replacement of DTNB and sample extracts with water, ascorbate solution, or both (Maas et al., 1987). The Cys content, determined on the basis of the reactivity of its SH group with methylglyoxal, was calculated as the difference between the total SH content and that of the sample incubated with 0.1 mL of 0.1 m methylglyoxal in addition to the 1 mL of 50 mm MES added above (De Kok et al., 1988). The GSH content of samples was calculated as the difference between their total SH and Cys contents (Schütz et al., 1991).
Stomatal Resistance and Chlorophyll Fluorescence
Measurements were made of plant leaves during SO2 fumigation and of plants in the unfumigated control cabinet. Stomatal resistance was determined using a steady-state porometer (model LI-1600; Li-COR A, Inc., Lincoln, NE) with a Parkinson clamp-on leaf chamber (PLC-B, ADC, GB-Hoddedon), as instructed by the manufacturers.
Modulated chlorophyll fluorescence was non-destructively determined on leaves with a portable chlorophyll fluorometer in conjunction with an attached leaf-clip holder as instructed by the manufacturer (PAM-2000 and 2030B, Walz, Effeltrich, Germany). The effective light intensity inside the leaf holder at the leaf surface was 215 μmol m−2 s−1, and the atmosphere inside the holder was equivalent to the ambient conditions of the growth chamber set at 25°C. Using the saturation-pulse method of the fluorometer (Schreiber et al., 1986), the overall photochemical quantum yield of PSII under steady-state conditions (Fm' − Fs/Fm'), where Fs denotes the steady-state yield of fluorescence and Fm' the yield of fluorescence induced by a saturating pulse in light-adapted leaves (Genty et al., 1989), was determined. This parameter of effective quantum yield was used here as a measure of the photosynthetic performance of the plants, and hence of their tolerance to SO2 exposure.
MV Treatments and Electrolyte Leakage
Measurements of the differential effects of MV (paraquat, Sigma Chemical Co., St. Louis) on control and transgenic plants essentially followed the methods of Aono et al. (1991; 1995). In brief, 7-mm circular leaf discs, from control and transgenic leaves of equivalent stage and position, were immersed with their abaxial sides up in 200 μL of a 0.1% Tween 20 solution containing MV at various (0.1–5.0 μm) concentrations. Leaf discs were then subjected to vacuum infiltration for 1 min, pre-incubated in the dark for 1 h, and then incubated at 25°C under either dark or light (300 μmol m−2 s−1) conditions for 18 to 24 h, after which the extent of damage was visually inspected.
For electrolyte leakage experiments, plant leaf discs were processed in a similar manner to that above except that the discs were vacuum infiltrated with water or a 20-μm MV solution, preincubated for 1 h in the dark, and then thoroughly washed with deionized water before being placed in 10 mL of water in a glass test tube in the dark or light. The conductivity of the solution was measured at 2-h intervals with a conductivity meter (model CD-35MII; M & S Instruments Inc., Tokyo).
RNA Isolation and Northern-Blot Analysis
Leaves of the same stage, size, and position from control and transgenic plants were used to prepare leaf discs that were subjected to various treatments. In an alternate manner, whole leaves from plants exposed to 2 μL L−1 SO2 for 3 h were sampled and immediately frozen in liquid nitrogen. Total RNA was extracted from the discs and whole leaves by a modified method of Nagy et al. (1988). In brief, 0.2 g of leaf was ground to a fine powder in an Eppendorf tube with carborundum and 900 μL extraction buffer (50 mm Tris-HCl, pH 8.0, 300 mm NaCl, 5 mm EDTA, 2 mm aurin tricarboxylic acid, 2% [w/v] SDS, and 15 mm 2-mercaptoethanol). The homogenate was brought to a final concentration of 0.4 m KCl, placed on ice for 15 min, and then subjected to centrifugation at 20,000g for 15 min at 4°C. The resulting supernatant was brought to a final concentration of 4 m LiCl, and kept on ice for 30 min before recentrifugation for 30 min at 4°C. The resulting pellet was resuspended in 400 μL water, subjected to phenol/chloroform extraction, and the RNA precipitated with 0.1 volume of 5 m NaCl and 2.5 volumes of ethanol. Aliquots of 25 μg of RNA were analyzed by northern-blot analysis as described previously (Youssefian et al., 1993) with a final high-stringency wash in 0.1 × 0.15 m sodium chloride and 0.015 m sodium citrate, 0.5% (w/v) SDS at 65°C for 30 min. After autoradiographic exposure, films were used for densitometric analysis (CS-9000, Shimadzu Co., Tokyo) and membranes were stripped for rehybridization with a new probe. The cys1 probe was the full-length cDNA fragment described previously (Youssefian et al., 1993). The SOD probes were made using Nicotiana plumbaginifolia cytosolic MnSOD and Cu/ZnSOD cDNAs (Willekens et al., 1994; kindly provided by Prof. M. Van Montagu), labeled by random primer extension.
SOD Assay
Leaf discs from control and transgenic plant leaves were subjected to a control treatment or that supplemented with 10 mm Na2SO3 and 1 mm OAS for 18 h in the light and used immediately for crude enzyme preparation and subsequent SOD assay. Here, 50 mg of leaf was homogenized with a glass rod at 4°C in 200 μL of protein homogenization buffer (50 mm potassium phosphate, pH 7.0, 1% [v/v] Triton X-100, and 10 mm 2-mercaptoethanol) and then subjected to two rounds of centrifugation at 20,000g for 20 min each at 4°C. The SOD activity of the resulting crude extract was measured by the nitro blue tetrazolium method of Beyer and Fridovich (1987), using an SOD test kit (Wako Chemical Co., Osaka), and data were linearized according to Giannopolitis and Ries (1977). Protein concentrations were determined according to Bradford (1976).
ACKNOWLEDGMENTS
We would like to thank Drs. Mitsuko Aono, Nobuyoshi Nakajima, and Hikaru Saji (National Institute for Environmental Studies, Tsukuba, Japan) for their valuable support in the fumigation studies and for discussion; Dr. Kenichiro Shimazaki (National Institute for Environmental Studies) for instruction in oxygen evolution measurements; Mariko Kudoh and Issei Sasaki (Akita Prefectural University, Akita, Japan) for plant maintenance; Prof. Marc Van Montagu (Universiteit Gent, Gent) for the tobacco SOD cDNA clones; and Drs. Hiroetsu Wabiko, Tomokazu Konishi, and Ivan Galis (Akita Prefectural University) for critical reading of the manuscript.
Footnotes
This work was supported in part by the Nissan Science Foundation (grant to S.Y.) and by the Sasagawa Science Foundation (grant to S.Y.).
LITERATURE CITED
- Alscher RG, Hess JL. Antioxidants in Higher Plants. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Aono M, Kubo A, Saji H, Natori T, Tanaka K, Kondo N. Resistance to active oxygen toxicity of transgenic Nicotiana tabacum that expresses the gene for glutathione reductase from Escherichia coli. Plant Cell Physiol. 1991;32:691–697. [Google Scholar]
- Aono M, Saji H, Sakamoto A, Tanaka K, Kondo N, Tanaka K. Paraquat tolerance of transgenic Nicotiana tabacum with enhanced activities of glutathione reductase and superoxide dismutase. Plant Cell Physiol. 1995;36:1687–1691. [PubMed] [Google Scholar]
- Arisi ACM, Noctor G, Foyer C, Jouanin L. Modifications of thiol contents in poplars (Poplar tremula × P. alba) overexpressing enzymes involved in glutathione synthesis. Planta. 1997;203:362–372. doi: 10.1007/s004250050202. [DOI] [PubMed] [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Blaszczyk A, Brodzik R, Sirko A. Increased resistance to oxidative stress in transgenic tobacco plants overexpressing bacterial serine acetyltransferase. Plant J. 1999;20:237–243. doi: 10.1046/j.1365-313x.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- Bogdanova N, Hell R. Cysteine synthesis in plants: protein-protein interactions of serine acetyltransferase from Arabidopsis thaliana. Plant J. 1997;11:251–262. doi: 10.1046/j.1365-313x.1997.11020251.x. [DOI] [PubMed] [Google Scholar]
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S. Coordinate modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down-regulation by L-cysteine. Plant Mol Biol. 1999;39:527–537. doi: 10.1023/a:1006148815106. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh C-K, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunold C, Rennenberg H. Regulation of sulphur metabolism in plants: first molecular approaches. Prog Bot. 1997;58:164–186. [Google Scholar]
- De Kok LJ. Sulfur metabolism in plants exposed to atmospheric sulfur. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 111–130. [Google Scholar]
- De Kok LJ, Buwulda F, Bosma W. Determination of cysteine and its accumulation in spinach leaf tissue upon exposure to excess sulfur. J Plant Physiol. 1988;133:502–505. [Google Scholar]
- De Kok LJ, Stuiver CEE, Stulen I. The impact of elevated levels of atmospheric H2S on plants. In: De Kok LJ, Stulen I, editors. Responses of Plant Metabolism to Air Pollution and Global Change. Leiden, The Netherlands: Backhuys Publishers; 1998. pp. 51–63. [Google Scholar]
- Droux M, Ruffet ML, Douce R, Job D. Interactions between serine acetyltransferase and O-acetylserine(thiol) lyase in higher plants: structural and kinetic properties of the free and bound enzymes. Eur J Biochem. 1998;255:235–245. doi: 10.1046/j.1432-1327.1998.2550235.x. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Descourvieres P, Kunert KJ. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert K-J, Pruvost C, Jouanin L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty BE, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Ghisi R, Dittrich APM, Heber U. Oxidation versus reductive detoxification of SO2 by chloroplasts. Plant Physiol. 1990;92:846–849. doi: 10.1104/pp.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms K, von Ballmoos P, Brunold C, Hofgen R, Hesse H. Expression of a bacterial serine acetyltransferase in transgenic potato plants leads to increased levels of cysteine and glutathione. Plant J. 2000;22:335–343. doi: 10.1046/j.1365-313x.2000.00743.x. [DOI] [PubMed] [Google Scholar]
- Hell R. Molecular physiology of plant sulphur metabolism. Planta. 1997;202:138–148. doi: 10.1007/s004250050112. [DOI] [PubMed] [Google Scholar]
- Hérouart D, Van Montagu M, Inze D. Redox-activated expression of the cytosolic copper/zinc superoxide dismutase gene in Nicotiana. Proc Natl Acad Sci USA. 1993;90:3108–3112. doi: 10.1073/pnas.90.7.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on net uptake of sulphate and sulphate transport in tobacco plants. J Exp Bot. 1994;45:1069–1076. [Google Scholar]
- Leustek T, Saito K. Sulphate transport and assimilation in plants. Plant Physiol. 1999;120:637–643. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas FM, De Kok LJ, Peters JL, Kuiper PJC. A comparative study of the effects of H2S and SO2 fumigation on the growth and accumulation of sulfate and sulfhydryl compounds in Trifolium pratense L., Glycine max MERR. and Phaseolus vulgaris L. J Exp Bot. 1987;38:1459–1469. [Google Scholar]
- Madamanchi NR, Alscher RG. Metabolic bases for differences in sensitivity of two pea cultivars to sulfur dioxide. Plant Physiol. 1991;97:88–93. doi: 10.1104/pp.97.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998a;49:649–667. [Google Scholar]
- May MJ, Vernoux T, Sanchez-Fernandez R, Van Montagu M, Inzé D. Evidence for posttranscriptional activation of γ-glutamylcysteine synthase during plant stress responses. Proc Nat Acad Sci USA. 1998b;95:12049–12054. doi: 10.1073/pnas.95.20.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F, Kay SA, Chua NH. Analysis of gene expression in transgenic plants. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual. Dordrect, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–29. [Google Scholar]
- Neuenschwander U, Suter M, Brunold C. Regulation of sulfate assimilation by light and O-acetyl-L-serine in Lemna minor L. Plant Physiol. 1991;106:1241–1255. doi: 10.1104/pp.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert KJ, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing γ-glutamylcysteine synthetase. Plant Physiol. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanz H, Martinoia E, Lange O-T, Heber U. Flux of SO2 into leaf cells and cellular acidification by SO2. Plant Physiol. 1987;85:928–933. doi: 10.1104/pp.85.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher LH, Zilinskas BA. Overexpression of copper/zinc superoxide dismutase in the cytosol of transgenic tobacco confers partial resistance to ozone-induced foliar necrosis. Plant Physiol. 1996;110:583–588. doi: 10.1104/pp.110.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg H. The fate of excess sulphur in higher plants. Annu Rev Plant Physiol. 1984;35:121–153. [Google Scholar]
- Rennenberg H. Molecular approaches to glutathione biosynthesis. In: Cram WJ, De Kok LJ, Stulen I, Brunold C, Rennenberg H, editors. Sulphur Metabolism in Higher Plants, Molecular, Ecophysiological and Nutritional Aspects. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 59–70. [Google Scholar]
- Rennenberg H, Herschbach C. Responses of plants to atmospheric sulphur. In: Yunus M, Iqbal M, editors. Plant Responses to Air Pollution. Chichester, UK: John Wiley & Sons; 1996. pp. 285–293. [Google Scholar]
- Ruffet M-L, Droux M, Douce R. Purification and kinetic properties of serine acetyltransferase free of O-acetylserine(thiol) lyase from spinach chloroplasts. Plant Physiol. 1994;104:597–604. doi: 10.1104/pp.104.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Kurosawa M, Tatsuguchi K, Takagi Y, Murakoshi I. Modulation of cysteine biosynthesis in chloroplasts of transgenic tobacco overexpressing cysteine synthase [O-acetylserine(thiol)-lyase] Plant Physiol. 1994;106:887–895. doi: 10.1104/pp.106.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Tatsuguchi K, Murakoshi I, Hirano H. cDNA cloning and expression of cysteine synthase B localized in chloroplasts of Spinacia oleracea. FEBS Lett. 1993;324:247–252. doi: 10.1016/0014-5793(93)80127-g. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Schütz B, De Kok LJ, Rennenberg H. Thiol accumulation and cysteine desulfhydrase activity in H2S-fumigated leaves and leaf homogenates of cucurbit plants. Plant Cell Physiol. 1991;32:733–736. [Google Scholar]
- Shimazaki K, Sakaki T, Kondo N, Sugahara K. Active oxygen participation in chlorophyll destruction and lipid peroxidation in SO2-fumigated leaves of spinach. Plant Cell Physiol. 1980;21:1193–1204. [Google Scholar]
- Shimazaki K, Sugahara K. Specific inhibition of photosystem II activity in chloroplasts by fumigation of spinach leaves with SO2. Plant Cell Physiol. 1979;20:947–955. [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing glutathione synthetase. Plant J. 1995;7:141–145. [Google Scholar]
- Veljovic-Jovanovic S, Bilger W, Heber U. Inhibition of photosynthesis, acidification and stimulation of zeaxanthin formation in leaves by sulfur dioxide and reversal of these effects. Planta. 1993;191:365–376. [Google Scholar]
- Wellburn FAM, Creissen GP, Lake JA, Mullineaux PM, Wellburn AR. Tolerance to atmospheric ozone in transgenic tobacco over-expressing glutathione synthetase in plastids. Physiol Plant. 1998;104:623–629. [Google Scholar]
- Willekens H, Van Camp W, Van Montagu M, Inzé D, Langerbartels C, Sandermann H. Ozone, sulfur dioxide, and ultraviolet B have similar effects on mRNA accumulation of antioxidant genes in Nicotiana plumbaginifolia L. Plant Physiol. 1994;106:1007–1014. doi: 10.1104/pp.106.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingsle G, Karpinski S. Differential redox regulation by glutathione of glutathione reductase and CuZn superoxide dismutase genes expression in Pinus sylvestris (L.) needles. Planta. 1996;198:151–157. doi: 10.1007/BF00197598. [DOI] [PubMed] [Google Scholar]
- Youssefian S, Nakamura M, Sano H. Tobacco plants transformed with the O-acetylserine (thiol) lyase gene of wheat are resistant to toxic levels of hydrogen sulphide gas. Plant J. 1993;4:759–769. doi: 10.1046/j.1365-313x.1993.04050759.x. [DOI] [PubMed] [Google Scholar]