Abstract
Purpose
To investigate the toxicity of terpinen-4-ol (T4O) on primary cultured human corneal epithelial cells (HCECs).
Methods
HCECs were exposed to various concentrations (0%–0.1%) of T4O for 15 minutes to 72 hours. Cell viability was assessed using the CCK8 kit and cell counting. Intracellular reactive oxygen species (ROS) were measured after 15 to 60 minutes of T4O exposure. Changes in cell morphology and cytoplasmic actin filaments were observed using phase contrast microscopy and immunocytochemistry. The expression levels of proteins involved in cell survival pathways (mTOR, Akt, Bcl-xL, and BAX) were evaluated by Western blot analysis.
Results
T4O induced dose-dependent toxicity in HCECs. Exposure to 0.05% T4O for 15 minutes significantly decreased cell viability. Lower concentrations (0.025% and 0.0125%) also caused significant toxicity with longer exposure times. Prolonged exposure enhanced cytotoxicity, with 0.05% T4O reducing viability by half after 24 hours and 0.1% T4O causing complete cell death. Increased intracellular ROS and decreased levels of phosphorylated mTOR, phosphorylated Akt, and Bcl-xL, along with increased BAX expression, accompanied this toxicity. F-actin staining revealed significant changes in cell adhesion.
Conclusions
Our study demonstrates that T4O exposure causes significant toxicity in HCECs, depending on concentration and incubation time. This toxic response is associated with increased ROS and decreased cell survival pathway activity.
Translational Relevance
The corneal epithelial toxicity data of T4O revealed in this study may be useful in the future use of tea tree oil or the development of tee tree oil–containing eyelid scrub products for treating eyelid demodex infestation.
Keywords: cornea, demodex, terpinen, tee tree oil, corneal epithelium
Introduction
Demodex are small skin mites (0.15–0.4 mm in length) that live in the hair follicle, including eyelashes, and are considered part of skin normal flora. Demodex folliculorum and Demodex brevis are the two main species that live in human skin.1 However, in certain circumstances, demodex can contribute to pathologic condition, and demodex infestation is one of the major contributors of chronic eyelid inflammation.2–4 Blepharitis combined with demodex infestation is characterized by burning sense, itchiness, redness, and scales, and it usually shows poor response to the conventional treatment, including warm compression and lid hygiene with commercial eyelid cleansing lotion.5
Tee tree oil is derived from the leaves and terminal branches of an Australian tee tree, Melaeuca alternifolia.5 Tee tree oil contains over 100 different components, and terpinen-4-ol (T4O) is the most abundant and active molecular component that kills demodex.5,6 T4O accounts for 30% to 40% of pure tee tree oil.6 Tee tree oil containing T4O is widely used as the effective treatment for eyelid demodex infestation.7,8 The mechanism about how T4O kill demodex has not been fully elucidated. However, some studies suggested that anticholinesterase activity of tee tree oil components including T4O can cause lethal muscular contraction and spastic paralysis of the parasite.7,9 Based on the antidemodex effect, eyelid scrub products containing various concentrations of tee tree oil were commercialized. It was reported that weekly eyelid scrub with 50% tee tree oil commercial shampoo combined with daily lid hygiene was effective in the treatment of eyelid demodex infestation.10 However, there was also a concern that using 50% concentration tee tree oil for eyelid demodex eradication resulted in corneal epithelial defect.11 In addition, the experiment using immortalized human meibomian gland epithelial cells (IHMGECs) demonstrated that a 15-minute exposure of 0.1% or 1.0% of T4O induced significant changes of the morphology and viability of cells.12 Of note, 5 days of culture of immortalized human meibomian gland epithelial cells with 1.0% T4O resulted in total cell death in the same report. In the clinical settings, there always exists a risk that scrub containing T4O may flow into the ocular surface and contact with corneal epithelial cells. Given this toxicity report, it is necessary to evaluate the potential toxicity of T4O on corneal epithelial cells. Corneal epithelial cells are important to maintain the homeostasis of the ocular surface and visual acuity.
In this study, we used primary cultured human corneal epithelial cells (HCECs) and evaluated the dose-dependent effect of T4O, the main component of tee tree oil, on cell viability. In addition, the change of cellular reactive oxygen species (ROS) and cell survival pathways were investigated.
Materials and Methods
Human Primary Corneal Epithelial Cell Culture
The primary culture of HCECs (cat. PCS-700-010) was purchased from American type culture collection (ATCC), Manassas, VA, USA. The cells were resuspended in corneal epithelial cell basal medium (serum and calcium free) and supplemented with a growth kit supplied by ATCC. The cells were plated in 75-cm2 tissue flasks with FNC coating mix (cat. 0407; Athena Enzyme Systems, Baltimore, MD, USA) and then were maintained at 37°C in a 5% CO2 and 95% air-humidified atmosphere. The culture medium was changed every 3 days, and the cells were passed using 0.05% Trypsin-EDTA (GibcoBRL, Grandisland, NY, USA). Cells with a passage number of ≤5 were used in this study.
Preparation of T4O
T4O (Merck KGaA, Darmstadt, Germany) was purchased and dissolved in ethanol (Merck KGaA) for 1/2 serial dilution; thus, the vehicle for T4O was 1.0 % (v/v) of ethanol. All stock and working solution of T4O were made freshly on the date of the experiment and treated.
Cell Viability Assay
The viability of HCECs was carried out using a commercial cell viability reagent, Cell Counting Kit-8 (CCK8) (Dojindo Laboratories, Kumamoto, Japan). HCECs were cultured and plated in the 96-well plate at a density of 1 × 104 cells/well. Following the adherence of the cells, T4O was treated at concentrations of 0, 0.00078125, 0.0015625, 0.003125, 0.00625, 0.0125, 0.025, 0.05, and 0.1 % (v/v) for 15 to 60 minutes or 24 to 72 hours. After appropriate incubation, 10 µL CCK8 reagent was added to each culture well. After a 4-hour incubation with the reagent at 37°C, absorbance at 450 nm was measured using a microplate reader. Triton X-100 (0.1%) was used as a positive control for cytotoxicity in a viability test.
ROS Assay
Intracellular ROS were measured using a 2′,7′-dichlorofluorescin diacetate (DCFDA)/H2DCFDA Cellular ROS Assay Kit (cat. ab113851: Abcam, Cambridge, UK). According to the manufacturer's protocol, briefly, T4O was treated for 15, 30, and 60 minutes in HCECs at 1.0% started 1/2 serial dilution: 0%, 0.00078125%, 0.0015625%, 0.003125%, 0.00625%, 0.0125%, 0.025%, 0.05%, and 0.1 % (v/v). After treatment, the media were removed and cells were washed using 100 µL/well of 1× ROS buffer. Cells were stained at 37°C in the dark for 45 minutes by adding 100 µL/well of a diluted 20-µM solution of DCFDA. They were then removed from the solution, and 100 µL/well of 1 × ROS buffer was added. Finally, the fluorescence was measured immediately at 485 nm excitation/535 nm emission. Tert-butyl hydrogen peroxide solution (55 mM) dissolved in complete media was used for positive control of the ROS assay.
Immunocytochemistry
HCECs were cultured at a density of 4 × 104 cells/mL, grown on 4-well Nunc Lab-Tek II chamber slides (Thermo Fisher Scientific, Rochester, NY, USA), and treated with T4O for 1 hour. HCECs were fixed with 10% formaldehyde at room temperature (RT) for 10 minutes. Permeabilization with 0.1% Triton X-100 was performed for 5 minutes at RT. After washing with dulbecco's phosphate buffered saline (DPBS), nonspecific antigen sites were blocked with 1% bovine serum albumin (BSA) in DPBS at RT for 30 minutes. Chamber slides were incubated with Phalloidin-Atto 488 (0.1 µM; cat. 49409; Sigma-Aldrich, St. Louis, MO, USA), which was used to stain F-actin. Cell nuclei were counterstained using 4′,6-diamidino-2′ phenylindole (cat. 10236276001; Roche, Mannheim, Germany). Slides were viewed using a fluorescence microscope (Olympus BX53F; Olympus, Tokyo, Japan).
Western Blot Assay for Cell Survival Signals
All T4O-treated cells were lysed with an ice-cold radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and 0.1% sodium dodecyl sulfate) for 30 minutes. The debris was removed by centrifugation at 16,000 × g for 10 minutes. Equal amounts (20 µg) of total cell protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA, USA). After blocking with 3% BSA in TTBS buffer (10 mM Tris [pH 8.0], 150 mM NaCl, 0.1% Tween 20) for 1 hour at RT, membranes were incubated overnight at 4°C with the following primary antibodies: rabbit anti–mammalian target of rapamycin (mTOR) (1:1000; cat. 5536; Cell Signaling, Danvers, MA, USA), rabbit anti-phospho-mTOR (1:1000; cat. 2983; Cell Signaling), rabbit anti-Akt (1:1000; cat. 9272; Cell Signaling), rabbit anti-phospho-Akt (1:1000; cat. 4060; Cell Signaling), rabbit anti–Bcl-2-associated X protein (BAX) (1:1000; cat. 2772; Cell Signaling), rabbit anti–B-cell lymphoma (Bcl)/xL (1:1000; cat. 2764; Cell Signaling), and mouse anti–β-actin (1:50,000; cat. A5441; Sigma-Aldrich). Membranes were then incubated with horseradish peroxidase–conjugated secondary antibodies at RT for 1 hour. Blots were developed with a Pierce enhanced chemiluminescence substrate (cat. 32106; Thermo Fisher Scientific) and visualized with a Fusion Pulse 6 chemiluminescence (Vilber Lourmat, Marne-la-Vallee, France). Densitometric analysis was performed with a Multi Gauge V3.0 software (Fujifilm Life Science, Tokyo, Japan). Each experiment was performed in triplicate at a minimum.
Statistical Analysis
The data were presented as the mean ± standard error, and statistical significance was determined by analysis of variance and Dunnett's multiple comparison test. P values of less than 0.05 were regarded as significant. Analyses were performed using GraphPad Prism version 9.3.1 (GraphPad Software, La Jolla, CA, USA).
Results
CCK Assay of HCECs After T4O Exposure
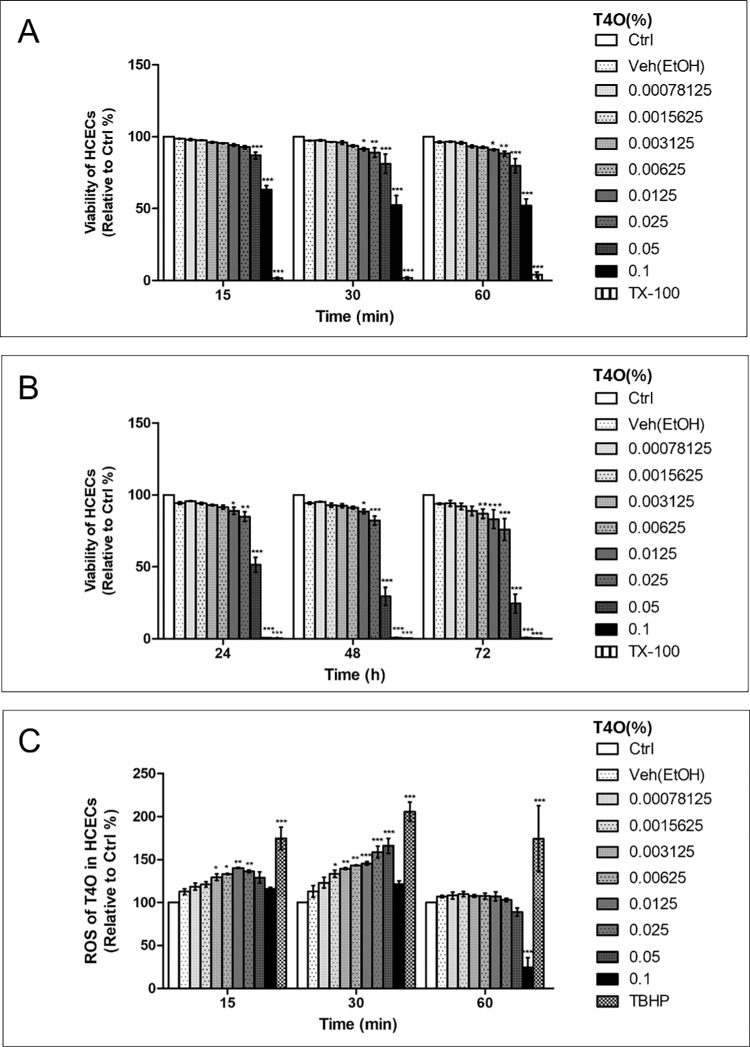
T4O treatment induced dose and time-dependent toxicity (Fig. 1; Supplementary Fig. 1). Exposure of HCECs to 0.05% of T4O for 15 minutes resulted in a significant decrease of viability. When exposed for 30 to 60 minutes, even lower concentrations (0.025% and 0.0125%) of T4O induced significant toxicity to HCECs (Fig. 1A). Prolonged exposure to T4O enhanced HCECs’ cytotoxicity. Exposure to 0.05% of T4O for 24 hours reduced HCECs’ viability by almost half, and exposure to 0.1% of T4O for more than 24 hours resulted in complete death of HCECs (Fig. 1B).
Figure 1.
Effect of T4O on HCEC viability. Cellular viability of HCECs after exposure to different concentrations of T4O. (A) T4O exposure for 15 to 60 minutes showed a dose-dependent cytotoxicity. Exposure to 0.05% of T4O for 15 minutes resulted in a significant decrease of viability. Exposure to 0.025% and 0.0125% of T4O for 15 minutes showed no significant toxicity, but increased exposure time for 30 to 60 minutes induced a significant toxicity to HCECs. (B) Prolonged exposure to T4O enhanced HCECs’ cytotoxicity. T4O concentrations less than 0.003125% induced no significant toxicity on HCECs for 72 hours. (C) Intracellular ROS generation increased with T4O exposure in HCECs. ROS increased in a dose-dependent manner up to the medium concentration of T4O tested in this experiment but decreased at high concentrations, especially at 0.1% of T4O. Values are presented as mean ± SEM and were obtained from three independent experiments; each independent experiment was performed in triplicate. Ctrl, control; TBHP, tert-butyl hydrogen peroxide (55 mM); TX-100, Triton X-100 (0.1%); Veh, vehicle. *P < 0.05, **P < 0.01, ***P < 0.001.
Intracellular ROS generation increased with T4O exposure in HCECs. This phenomenon was found to occur in the early phase after T4O exposure (15 and 30 minutes) and rapidly normalized at 60 minutes (Fig. 1C). However, in HCECs exposed to 0.1% T4O, ROS increase was not detected at 15 and 30 minutes after exposure. There was a significant reduction in intracellular ROS at 60 minutes with dramatically reduced cell numbers in the culture plate due to the high rate of cytotoxicity.
HCEC Morphology Change After T4O Exposure
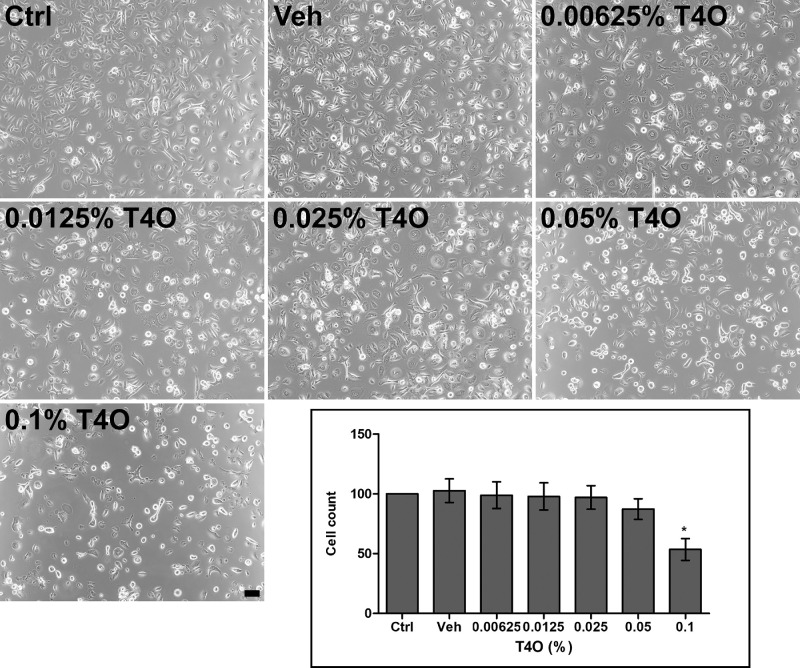
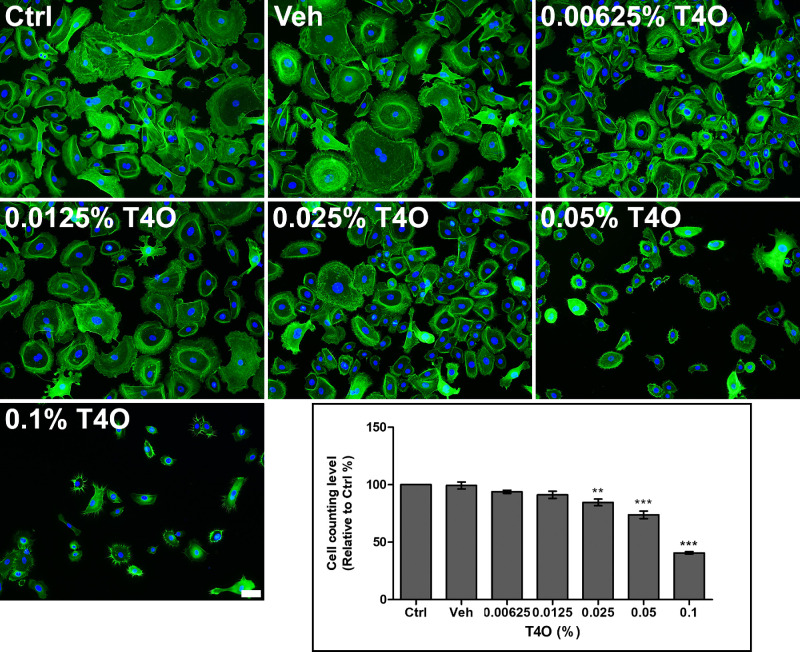
HCECs exposed to T4O showed decreased adhesion to the cell culture plate bottom. Cells that had lost adhesion had part of their cytoplasm separated from the bottom and often appeared round when observed with a phase contrast microscope. These changes became more pronounced as the concentration of exposed T4O increased, and in particular, some HCECs exposed to 0.1% T4O for 30 minutes completely detached from the bottom of the plate, resulting in a significant decrease in the number of cells observed by phase contrast microscopy (Fig. 2). These changes in cell adhesion could be observed more clearly through F-actin staining, and the actin filaments, which are important for cell adhesion, were not distributed homogeneously in the cytoplasm but were stained only around the nucleus, greatly damaging normal adhesion ability of cells. Compared to control and vehicle alone treatment, even 0.00625% T4O induced F-actin to localize to the perinuclear region with significant shrinkage of the cytoplasm. Exposure of HCECs to 0.025%, 0.05% and 0.1% T4O for 60 minutes resulted in a significant decrease in cell numbers (Fig. 34).
Figure 2.
Phase contrast microscopic images of HCECs after 30-minute exposure to various concentrations of T4O. HCECs exposed to higher concentrations of T4O showed a decrease in adhesion to the bottom of the plate, and more cells appeared rounded up. HCECs exposed to 0.1% T4O resulted in a significant decrease in cell number. Values in the graph are presented as mean ± SEM and were obtained from three independent experiments; each independent experiment was performed in triplicate. The number of cells in the control group (Ctrl) was set to 100, and the number of cells in the experimental group was compared to that of the control group. Scale bar: 100 µm. *P < 0.05.
Figure 3.
Cytoskeletal change of HCECs after 60-minute exposure to various concentrations of T4O. HCECs exposed to T4O showed a decrease in cell size, as shown by F-actin staining. Compared to the control and vehicle-only treatment, even 0.00625% T4O induced F-actin to localize to perinuclear area with severe shrinkage of cytoplasm. HCECs exposed to 0.05% and 0.1% T4O resulted in a significant decrease in cell number. F-actin, green. 4′,6-Diamidino-2′ phenylindole, blue. Values in the graph are presented as mean ± SEM and were obtained from three independent experiments; each independent experiment was performed in triplicate. The number of cells in the control group (Ctrl) was set to 100, and the number of cells in the experimental group was compared to that of the control group. Scale bar: 50 µm. *P < 0.05, **P < 0.01, ***P < 0.001.
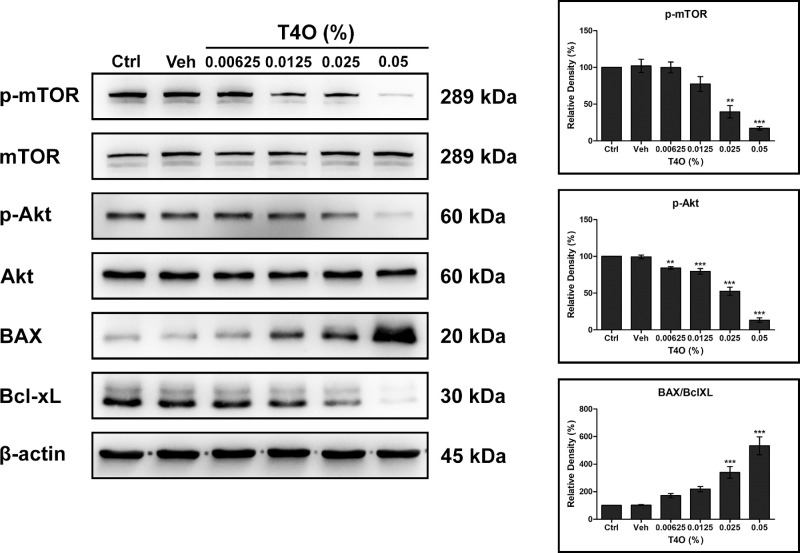
Figure 4.
Effect of T4O on the cell survival pathway of HCECs. The expression levels of phosphorylated mTOR, phosphorylated Akt, and Bcl-xL showed a dose-dependent decrease in HCECs after T4O exposure for 24 hours with the increased expression of BAX. Values are presented as mean ± SEM and were obtained from three independent experiments; each independent experiment was performed in triplicate. Ctrl, control; Veh, vehicle. **P < 0.01, ***P < 0.001.
Effect of T4O on HCECs’ Survival Pathway
T4O exposure inhibited the phosphorylation of Akt and mTOR, which were known to promote cell division and migration of HCECs. This phenomenon was more evident when HCECs were exposed to high concentrations of T4O. In addition, the expression of Bcl-xL, which suppresses cell apoptosis, was suppressed while the expression of BAX, which promotes apoptosis, increased in T4O-exposed HCECs (Fig. 4).
Discussion
In this study, we demonstrated that T4O exposure induced significant damages to HCECs. Notably, 0.1% T4O caused serious toxicity on HCECs after just 15 minutes of exposure. High concentrations of T4O generated significant ROS and inhibited cell survival pathways in HCECs.
Previous studies reported that tear film volume ranges from 3 to 10 µL with a secretion rate of 1 to 2 µL per minute under normal physiologic conditions.13 This means the total tear volume is replaced within 10 minutes, even in individuals with high tear volume (10 µL) and low secretion rate (1 µL per minute). When T4O is applied on the eyelash for demodex control, there is a risk of leakage into the tear film. However, considering normal tear film turnover, T4O is diluted more than 10 times every 10 minutes. Thus, an initial concentration of 100% T4O can be diluted to 1% after 20 minutes and to 0.1% after 30 minutes.
Clinically, T4O is primarily used to treat demodex infestation of the eyelid. In vitro studies have shown that demodex mites can be killed within 88 minutes with 1% T4O, within 32 minutes with 5% T4O, and within 40 minutes with 4% T4O.6,14 Therefore, high concentrations of T4O are needed for effective eradication of demodex. However, due to the irritation caused by high concentration of T4O, most commercial eyelid hygiene products for demodex treatment aim to balance ocular comfort and minimal demodex-killing activity by lowering T4O concentration.15,16 Most commercial tea tree oil preparations for eyelid scrub contain less than 1% of tea tree oil to avoid excessive irritation and discomfort. Cheung et al.16 measured T4O concentrations in commercial eyelid cleansing solutions using mass spectrometry analysis and found that T4O concentration was highest in Cliradex (4.61 mg/mL, 0.461%), followed by Oust Demodex (0.29 mg/mL, 0.029%), Blephadex (0.03 mg/mL, 0.003%), and TheraTears SteriLid (0.02 mg/mL, 0.002%). As T4O accounts for 30% to 40% of pure tee tree oil, these concentrations are far below the demodex-killing concentration, as mites can survive for 87.6 minutes in 1% of T4O. Moreover, our study and previous studies have shown that T4O is toxic to both HCECs and IHMGECs, making it challenging to increase the concentration of tea tree oil products to effectively kill demodex.
Because products containing T4O are intended for eyelid scrubs, previous research has investigated the safety of T4O on the meibomian glands, a major structure in the eyelids.12 In experiments using IHMGECs, exposure to 1% of T4O for 90 minutes resulted in almost total cell death, and even 0.01% T4O for 5 days decreased cell survival by more than 30%.12 This toxicity was also demonstrated in HCECs in our study. Incubation of HCECs in 0.1% T4O for 60 minutes resulted in a 50% decrease in cell viability, and 0.05% T4O for 24 hours also resulted in a 50% decrease in cell viability. Although our methods differed slightly from previous studies using IHMGECs, incubation of HCECs in 0.00125% of T4O for 72 hours showed a mild decrease in cell viability.
The discrepancies in cell toxicity between our HCECs and others’ IHMGECs might be due to the differences in cell metabolism and analysis methods. In the previous IHMGEC study, cell viability was assessed by counting live cells in the culture plate.12 However, in our study, we used both CCK8 assay and cell counting. The CCK8 assay uses a water-soluble salt (WST-8) that is reduced by dehydrogenases in viable cells to produce a formazan dye, which can be measured by a plate reader and is proportional to the number of living cells. However, the CCK8 assay may not accurately reflect cell viability due to its potential impact on cellular metabolic activity. Therefore, we verified cell viability through cell counting. As shown in Figures 2 and 3, there was a 48% and 58% decrease in cell numbers compared to the control when HCECs were exposed to 0.1% T4O for 30 and 60 minutes, respectively. These results are consistent with the CCK8 assay.
HCECs cover the ocular surface through proliferation and movement, making the arrangement of actin filaments crucial. The morphological changes of HCECs observed after T4O exposure, such as cell rounding, atrophy, poor adherence, and detachment, are consistent with previous findings in IHMGECs.12 High concentrations of T4O severely inhibited the AKT and mTOR pathways, which are important for cell proliferation and growth. Previous studies also reported significant inhibition of Akt phosphorylation in IHMGECs after T4O exposure.12 Additionally, T4O exposure in HCECs increased the proapoptotic protein BAX and decreased antiapoptotic protein Bcl-xL.
Our study has limitations. Although primary cultured cells were used, in vivo reactions cannot be perfectly replicated in cultured cell experiments. Clinically, the anticholinergic effect of T4O may contribute to corneal toxicity via corneal anticholinesterase inhibition. The corneal epithelium contains high concentrations of muscarinic receptors and acetylcholine, and disruption of the cholinergic system on the ocular surface can result in significant toxicity.17,18 However, demonstrating this in our study was challenging due to the limitations of experimental design. An animal model might be helpful, as the anticholinergic effect disrupts both microenvironmental homeostasis of corneal cells and nerve innervation of the ocular surface. Additionally, the potential for T4O leakage to the ocular surface and final tear content may vary depending on usage of eyelid scrubs. Therefore, predicting corneal epithelial toxicity caused by eyelid scrub products containing various concentrations of T4O based on this experiment alone is difficult.
In conclusion, our study demonstrated that T4O exposure causes significant toxicity in HCECs, depending on concentration and exposure time. These findings will be valuable for designing future eyelid scrub products containing T4O and for understanding potential pathological changes on the corneal surface with their use.
Supplementary Material
Acknowledgments
Supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI23C-0689), and National Research Foundation of Korea (NRF), funded by the Ministry of Education (Grant NRF-2021R1A2C1006087).
Disclosure: J.-H. Park, None; C.Y. Park, None
References
- 1. Elston CA, Elston DM.. Demodex mites. Clin Dermatol. 2014; 32: 739–743. [DOI] [PubMed] [Google Scholar]
- 2. Lemp MA, Nichols KK.. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009; 7: S1–S14. [DOI] [PubMed] [Google Scholar]
- 3. Yan Y, Yao Q, Lu Y, et al.. Association between demodex infestation and ocular surface microbiota in patients with demodex blepharitis. Front Med (Lausanne). 2020; 7: 592759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karakurt Y, Zeytun E.. Evaluation of the efficacy of tea tree oil on the density of Demodex mites (Acari: Demodicidae) and ocular symptoms in patients with demodectic blepharitis. J Parasitol. 2018; 104: 473–478. [DOI] [PubMed] [Google Scholar]
- 5. Savla K, Le JT, Pucker AD. Tea tree oil for Demodex blepharitis. Cochrane Database Syst Rev. 2020; 6: CD013333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tighe S, Gao YY, Tseng SC.. Terpinen-4-ol is the most active ingredient of tea tree oil to kill Demodex mites. Transl Vis Sci Technol. 2013; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bezabh SA, Tesfaye W, Christenson JK, Carson CF, Thomas J.. Antiparasitic activity of tea tree oil (TTO) and its components against medically important ectoparasites: a systematic review. Pharmaceutics. 2022; 14: 1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao YY, Di Pascuale MA, Li W, et al.. In vitro and in vivo killing of ocular Demodex by tea tree oil. Br J Ophthalmol. 2005; 89: 1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mills C, Cleary BJ, Gilmer JF, Walsh JJ.. Inhibition of acetylcholinesterase by tea tree oil. J Pharm Pharmacol. 2004; 56: 375–379. [DOI] [PubMed] [Google Scholar]
- 10. Gao YY, Di Pascuale MA, Elizondo A, Tseng SC.. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007; 26: 136–143. [DOI] [PubMed] [Google Scholar]
- 11. Tharmarajah B, Coroneo MT.. Corneal effects of tea tree oil. Cornea. 2021; 40: 1363–1364. [DOI] [PubMed] [Google Scholar]
- 12. Chen D, Wang J, Sullivan DA, Kam WR, Liu Y.. Effects of terpinen-4-ol on meibomian gland epithelial cells in vitro. Cornea. 2020; 39: 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dartt DA, Willcox MD.. Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res. 2013; 117: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kabat AG. In vitro demodicidal activity of commercial lid hygiene products. Clin Ophthalmol. 2019; 13: 1493–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ergun SB, Saribas GS, Yarayici S, et al.. Comparison of efficacy and safety of two tea tree oil-based formulations in patients with chronic blepharitis: a double-blinded randomized clinical trial. Ocul Immunol Inflamm. 2020; 28: 888–897. [DOI] [PubMed] [Google Scholar]
- 16. Cheung IMY, Xue AL, Kim A, Ammundsen K, Wang MTM, Craig JP.. In vitro anti-demodectic effects and terpinen-4-ol content of commercial eyelid cleansers. Cont Lens Anterior Eye. 2018; 41: 513–517. [DOI] [PubMed] [Google Scholar]
- 17. Sloniecka M, Danielson P.. Acetylcholine decreases formation of myofibroblasts and excessive extracellular matrix production in an in vitro human corneal fibrosis model. J Cell Mol Med. 2020; 24: 4850–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kempuraj D, Zhang E, Gupta S, Gupta RC, Sinha NR, Mohan RR.. Carbofuran pesticide toxicity to the eye. Exp Eye Res. 2023; 227: 109355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.