Abstract
Background:
Molecular tests have contributed to reducing the mortality rate through early and accurate diagnosis of tuberculosis (TB). This is due to their low processing complexity and diagnostic accuracy superior to conventional methods.
Objective:
To evaluate the diagnostic performance of Cobas MTB and Logix Smart MTB compared to Xpert MTB/RIF Ultra for pulmonary tuberculosis (PTB) and extrapulmonary tuberculosis (EPTB).
Design:
A cross-sectional study of diagnostic tests was carried out in a clinical laboratory in Lima, Peru.
Methods:
All pulmonary and extrapulmonary samples from patients with presumptive TB who had been subjected to smear microscopy, Xpert MTB/RIF Ultra, Cobas MTB, Logix Smart MTB, and mycobacteria culture were included to determine their diagnostic performance.
Results:
A total of 175 samples were included, 102 (58.3%) of pulmonary origin and 73 (41.7%) of extrapulmonary origin. Among the total samples, 19 (10.9%) had positive cultures (all were pulmonary samples), 48 (27.4%) had positive Xpert MTB/RIF Ultra results, 45 (25.7%) had positive Cobas MTB results, and 36 (20.6%) had positive Logix Smart MTB results. The agreement between Cobas MTB and Logix Smart with the Xpert MTB/RIF Ultra was 97.1% and 93.8%, respectively. Compared to Xpert MTB/RIF Ultra, the area under the curve/receiver operating characteristic and sensitivity of the Cobas MTB and Logix Smart methods were 0.95 and 91.7%, and 0.90 and 81.0%, respectively.
Conclusion:
Cobas MTB and Logix Smart presented adequate performance for diagnosing pulmonary and extrapulmonary tuberculosis comparable to the Xpert MTB/RIF Ultra.
Keywords: extrapulmonary, molecular diagnostic techniques, Mycobacterium tuberculosis, pulmonary, tuberculosis
Introduction
At the level of the Americas, Peru is the second country with the highest number of estimated tuberculosis (TB) cases. 1 The use of molecular tests to diagnose TB has contributed to a 3.4% reduction in the global mortality rate per year and a 1.9% reduction in the incidence of new cases per year. 2 TB control requires early and timely diagnosis. Currently, the World Health Organization (WHO) recommends molecular tests such as Xpert MTB/RIF Ultra and Truenat MTB. 3 This is due to its low processing complexity and sensitivity and specificity superior to smear microscopy and culture for the rapid diagnosis of TB. 4
Molecular tests have good diagnostic performance for the detection of TB. Xpert MTB/RIF Ultra has the highest sensitivity (91%) for pulmonary tuberculosis (PTB). 5 However, its diagnostic performance is variable in extrapulmonary tuberculosis (EPTB), with a sensitivity of 50%–95.8% for lymph node samples,6,7 71.4%–90.9% for cerebrospinal fluid,8,9 and 47.6%–84.2% for pleural fluid.10–12
It is necessary to identify new diagnostic methods that allow infections to be identified earlier than conventional methods, mainly in groups that are difficult to diagnose (such as patients with EPTB and HIV coinfections), enabling early diagnosis of resistance to anti-TB drugs and with lower costs, for universal implementation.
It is unknown whether there are differences in the molecular diagnosis platforms for TB, specifically the three molecular methods marketed in our country: Xpert MTB/Rif Ultra, Cobas MTB, and Logix Smart MTB. Therefore, the objective of the present study was to determine the diagnostic performance of Cobas MTB and Logix Smart MTB compared to Xpert MTB/RIF Ultra for the rapid diagnosis of PTB and EPTB.
Materials and methods
Research setting and design
A cross-sectional study of diagnostic tests was carried out. To write the manuscript, we followed the guidelines of the Standards for Reporting of Diagnostic Accuracy Studies (STARD) for diagnostic tests (Supplemental File). 13 The study was carried out in the private clinical laboratory ROE, at its locations in the metropolitan city of Lima, Peru.
Population and eligibility criteria
All pulmonary samples (sputum, bronchial aspirate, and bronchoalveolar lavage) and extrapulmonary samples (pleural fluid, urine, cerebrospinal fluid, breast biopsy, soft tissue abscess, ascitic fluid, pericardial fluid, serum, bone biopsy, and bone marrow) were collected from patients with presumptive TB who underwent smear microscopy, Xpert MTB/RIF Ultra and mycobacterial culture in Löwenstein–Jensen media, which were stored in a biobank at −70°C and subsequently and simultaneously subjected to Cobas MTB and Logix Smart MTB to determine their diagnostic performance.
Reference standard
For this study, we used the Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, CA, USA) as the reference standard. This automated, nested polymerase chain reaction (PCR) test runs on the GeneXpert platform, detecting Mycobacterium tuberculosis (MTB) and rifampicin resistance. The process begins with sample collection (e.g., sputum), followed by mixing with a reagent that liquefies and inactivates the bacteria. The sample is loaded into the Xpert cartridge, which is inserted into the GeneXpert system for automated DNA extraction, amplification, and detection. The test targets the IS6110 and IS1081 multicopy sequences and mutations in the rpoB gene for rifampicin resistance. With a detection limit of 15.6 colony-forming units (CFU)/mL, results available in approximately 90 minutes, and a sensitivity and specificity in patients with positive smear microscopy of 90% and 96%, respectively. 14
Index test
The first index test was the Cobas MTB test (Roche Diagnostics, Mannheim, Germany) is performed on the Cobas 5800/6800/8800 systems and is designed to detect M. tuberculosis complex DNA in respiratory samples. Initially, samples are liquefied and inactivated using Cobas Microbial Inactivation Solution (MIS). After incubation at room temperature, the samples undergo sonication to ensure proper liquefaction. The system then automates nucleic acid extraction using magnetic glass particles, where bacterial DNA is bound and purified. This is followed by real-time PCR amplification using dual-target primers (16S rRNA and esx genes: esxJ, esxK, esxM, esxP, esxW), ensuring accurate detection. The results are automatically interpreted by the system’s software, with a detection limit of 7.6–8.8 CFU/mL. The test is highly specific and requires the use of additional modules, such as the Cobas MTB-RIF/INH, for detecting rifampicin and isoniazid resistance by identifying mutations in the RRDRs and inhA promoter regions, respectively .3,15
The second index test, Logix Smart MTB (Co-Diagnostics, Inc., South Salt Lake, UT, USA), is a qualitative real-time PCR test that detects M. tuberculosis by targeting the IS6110 and MPB64 genes from respiratory samples. With a detection limit of 2–8 copies/mL and a sensitivity and specificity of 98% and 99%, respectively, it has been validated for use with the QIAamp DNA Mini Kit and can be run on the CoDx Box thermal cycler or other compatible platforms. The test processes respiratory fluid samples, undergoing nucleic acid extraction followed by real-time PCR amplification to identify the M. tuberculosis complex. Fluorescent dyes monitor the amplification of these genetic markers, ensuring precise detection. The Logix Smart MTB kit includes ready-to-use reagents like a master mix, positive, and negative controls. The entire process, from extraction to result interpretation, takes 40–60 min depending on the equipment, with results typically available in 2 h. Designed for streamlined processing, the test ensures reliable detection of both pulmonary and extrapulmonary tuberculosis with minimal manual intervention. 16
The details of the analytical process used for the detection of Logix Smart MTB, Cobas MTB, and Xpert MTB/RIF Ultra are described in Table 1.
Table 1.
Characteristics of three molecular methods (Logix Smart MTB, Cobas MTB, and Xpert MTB/RIF Ultra) and their processing for the diagnosis of tuberculosis.
| Characteristics | Logix Smart MTB | Cobas MTB a | Xpert MTB/RIF Ultra |
|---|---|---|---|
| Number of samples per processing | 32 concentrated samples | 20 concentrated samples (maximum capacity of 90 samples) | 16 concentrated samples (by GeneXpert® XVI platform) |
| Type of conventional samples | Sputum, airway fluids | Sputum, sputum sediments, or BAL with NALC-NaOH | Sputum or Sputum sediments |
| Detection target | Presence of IS6110 and MPB64 genes | Presence of esx genes and 16S rARN gene/Mutation in rpoB, katG and inhA genes | Presence of IS6110 and IS1081 genes/rpoB gene mutation |
| Detection limit | 8 copies/μL | 7.6 UFC/mL | 15.6 UFC/mL |
| Minimum sample quantity | 200 µL | 1000 µL | 500 µL |
| Sample pretreatment | Liquefaction with reagents inside the plate + proteinase K | Inactivaction + liquefaction with MIS and sonication | Liquefaction with sample reagent (composed of sodium hydroxide and isopropanolol) |
| Extraction type | Magnétic beads in HollySys Extraction Kit b | Magnetic beads in Cobas 6800 | Sonication |
| Treatment and extraction time | 30 min | 150 min | 20 min |
| Conservation temperature | HollySys between 4–30°C. For Logix Smart, below −15°C | 2–8°C | 2–28°C |
| Preparation time for amplification | 40 min | 20 min | 1 min |
| Type of PCR | qPCR | qPCR | semi-nested qPCR |
| Amplification time | 80 min | 180 min | 90 min |
| Results information | Qualitative presence of mycobacteria | Qualitative presence of mycobacteria. Determines resistance to R and H | Qualitative presence of mycobacteria. Determines resistance to R |
If positive samples exist another detection kit for rifampicin and isoniazid that meets the same parameters would be run.
Components on the HollySys plate are Guanidine Hydrochloride, Sodium Chloride, SDS, Tris-HCl, Isopropanolol, and absolute ethanol.
BAL, bronchoalveolar lavage; H, isoniazid; MIS, Cobas® Microbial Inactivating Solution; MTB, Mycobacterium tuberculosis; NALC-NaOH, N-acetyl-L-cysteine-sodium hydroxide; qPCR, Real-time polymerase chain reaction; R, rifampin; rRNA, ribosomal ribonucleic acid.
Statistical analysis
The data were entered into a Microsoft Excel v.2016 spreadsheet and subsequently exported for analysis in Stata v17 software (StataCorp, College Station, TX, USA) and R studio v2023.03.1 (Posi Software, PBC, MA, USA).
The performance of the reference tests for diagnosing pulmonary and extrapulmonary TB was calculated using the area under the curve (AUC) of the receiver operating characteristic (ROC), sensitivity, specificity, positive and negative likelihood ratios, and positive and negative predictive values. Finally, we measured the proportion of absolute agreement using Cohen’s Kappa coefficient.
Ethics
The protocol was approved by the research ethics committee of the Faculty of Health Sciences at the Private University of Tacna (code: 94/FAC). The ethics committee waived the requirement for informed consent due to the nature of the study, which prevented contacting patients from thawed samples stored for laboratory quality control purposes. Similarly, confidentiality of the database was ensured, which was used exclusively for research purposes.
Results
One hundred seventy-five samples from patients with presumptive TB were included, of which 58.3% (n = 102) were pulmonary samples, and 41.7% (n = 73) were extrapulmonary samples. The median age was 57.8 years (IQR: 39.3–71.0). MTB was identified by Xpert MTB/RIF Ultra in up to 27.4% (n = 48) of the samples studied, the majority of which were pulmonary samples (85.4%). Regarding the extrapulmonary samples, the majority were pleural fluid (32.9%), urine (28.8%), cerebrospinal fluid (9.6%), and breast biopsy (8.2%) (Table 2). We observed that 27.4% were positive for Xpert MTB/RIF Ultra, with a median cycle threshold (Ct) of 17.4 (IQR: 16.2–20.4), 25.7% were positive for Cobas MTB, with a median Ct of 29.8 (IQR: 25.6–31.9); and 20.6% (n = 36) were positive for Logix Smart MTB, with a median Ct of 29.4 (IQR: 26.8–36.8). In contrast, only 7.9% and 10.9% of samples were positive for Ziehl-Neelsen staining and mycobacterial culture, respectively. Only 1/48 patients with a positive result of the Xpert MTB/RIF Ultra presented resistance to rifampin, which was also detected by Cobas MTB, who presented resistance to rifampicin and isoniazid simultaneously. Additionally, one sample was resistant to isoniazid but not to rifampicin according to Cobas MTB, which was not detected using Xpert MTB/RIF Ultra (Table 3).
Table 2.
Main characteristics of the sample origin according to type and result of Xpert MTB/RIF Ultra.
| Variable | Total (n = 175) | Positive (n = 48) | Negative (n = 127) |
|---|---|---|---|
| Age, years (n = 99) a | 57.8 (39.3–71.0) | 52.7 (31.8–65.7) | 59.7 (40.2–72.0) |
| Sample origin | |||
| Pulmonary (%) | 102 (58.3) | 41 (85.4) | 61 (48.0) |
| Bronchoalveolar lavage | 33 (32.4) | 13 (31.7) | 20 (32.8) |
| Bronchial aspirate | 33 (32.4) | 16 (39.0) | 17 (27.9) |
| Sputum | 36 (35.3) | 12 (29.3) | 24 (39.3) |
| Extrapulmonary (%) | 73 (41.7) | 7 (14.6) | 66 (52.0) |
| Cerebrospinal fluid | 7 (9.6) | 0 (0.0) | 7 (10.6) |
| Urine | 21 (28.8) | 3 (42.8) | 18 (27.3) |
| Pleural fluid | 24 (32.9) | 1 (14.3) | 23 (34.8) |
| Ascitic fluid | 5 (6.8) | 0 (0.0) | 5 (7.6) |
| Serum | 1 (1.4) | 0 (0.0) | 1 (1.5) |
| Pericardial fluid | 2 (2.7) | 0 (0.0) | 2 (3.0) |
| Bone biopsy | 1 (1.4) | 1 (14.3) | 0 (0.0) |
| Breast biopsy | 6 (8.2) | 0 (0.0) | 6 (9.1) |
| Bone marrow biopsy | 1 (1.4) | 0 (0.0) | 1 (1.5) |
| Soft tissue abscess | 4 (5.4) | 1 (14.3) | 3 (4.6) |
| Intrathoracic abscess | 1 (1.4) | 1 (14.3) | 0 (0.0) |
Median and interquartile range.
Table 3.
Laboratory results of conventional and molecular tests for the diagnosis of pulmonary and extrapulmonary tuberculosis.
| Diagnostic method | Total | Positive (%) | Negative (%) |
|---|---|---|---|
| Smear (%) | 126 | 10 (7.9) | 116 (92.1) |
| Mycobacteria culture (%) | 175 | 19 (10.9) | 156 (89.1) |
| Xpert MTB/RIF Ultra (%) | 175 | 48 (27.4) | 127 (72.6) |
| Threshold cycle (Ct) a | – | 17.4 (16.2–20.4) | – |
| Rifampicin resistance (%) | 48 | 1 (2.0) | – |
| Cobas MTB (%) | 175 | 45 (25.7) | 130 (74.3) |
| Threshold cycle (Ct) a | – | 29.8 (25.6–31.9) | – |
| Isoniazid resistance (%) | 45 | 2 (4.4) | – |
| Rifampicin resistance (%) | 45 | 1 (2.2) | – |
| Logix Smart MTB (%) b | 175 | 36 (20.6) | 124 (70.9) |
| Threshold cycle (Ct) a | – | 29.4 (26.8–36.8) | – |
Median and interquartile range.
Logix Smart presented 15 samples with indeterminate results, which were excluded for this analysis.
Ct, cycle threshold; MTB, Mycobacterium tuberculosis.
Accuracy of Cobas MTB and Logix Smart for TB diagnosis compared to positive molecular result of Xpert MTB/RIF Ultra
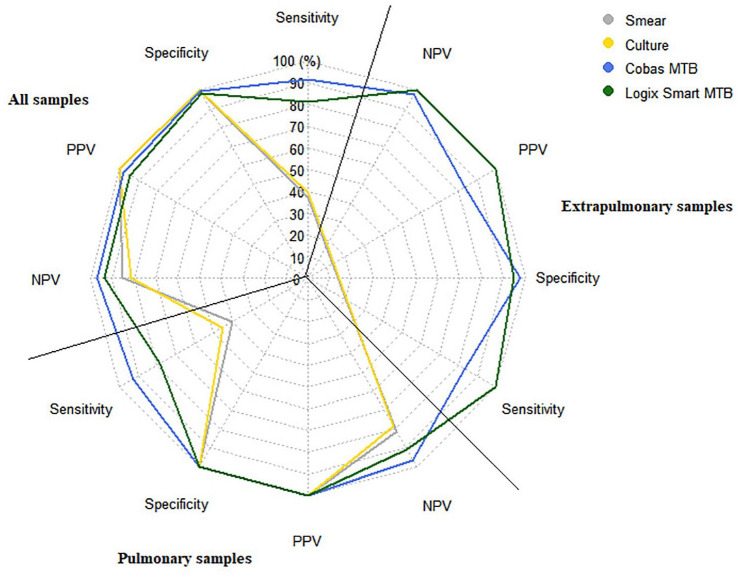
In pulmonary samples, smear microscopy presented an AUC/ROC of 0.70, with a sensitivity of 40.0% and specificity of 100.0% for the diagnosis of TB. Mycobacterial culture presented an AUC/ROC of 0.72, with a sensitivity of 45.2% and specificity of 100%. In comparison, the Cobas MTB molecular test showed better performance with an AUC/ROC of 0.96, presenting a sensitivity and specificity of 92.9% and 100.0%, respectively. In contrast, the Logix Smart MTB showed an AUC/ROC of 0.89, with a sensitivity of 78.4% and a specificity of 100.0%. Regarding the performance of molecular tests in extrapulmonary samples, Logix Smart MTB showed the best performance with a sensitivity and specificity of 100.0%, while Cobas MTB presented an AUC/ROC of 0.91, with a sensitivity and specificity of 83.3% and 97.7%, respectively (Table 4; Figure 1). Of the seven extrapulmonary samples that tested positive by Xpert MTB/RIF Ultra (three urine, one ascitic fluid, one bone biopsy, one soft tissue abscess, and one intrathoracic abscess), Logix Smart MTB was positive in 100% of them, while Cobas MTB was positive in 83.3% (failing to detect one positive urine sample), while none of them were positive in culture or smear (Table 4; Figure 1).
Table 4.
The area under the ROC curve and cut-off points of conventional and molecular tests compared to Xpert MTB/RIF Ultra.
| Variable | AUC/ROC (CI 95%) | Sensibility | Especificity | LHR+ | LHR− | VPP | VPN | Prevalence |
|---|---|---|---|---|---|---|---|---|
| All samples | ||||||||
| Smear | 0.685 (0.592–0.777) | 37.04 | 100.0 | – | 0.629 | 100.0 | 85.30 | 21.0 |
| Mycobacteria culture | 0.697 (0.628–0.767) | 39.58 | 100.0 | – | 0.604 | 100.0 | 81.40 | 27.0 |
| Cobas MTB | 0.954 (0.914–0.994) | 91.67 | 99.21 | 116.4 | 0.084 | 97.80 | 96.90 | 27.0 |
| Logix Smart MTB | 0.896 (0.835–0.957) | 80.95 | 98.31 | 47.76 | 0.193 | 94.40 | 93.50 | 26.0 |
| Pulmonary samples | ||||||||
| Smear | 0.700 (0.602–0.798) | 40.00 | 100.0 | – | 0.600 | 100.0 | 81.30 | 28.0 |
| Mycobacteria culture | 0.726 (0.650–0.802) | 45.24 | 100.0 | – | 0.547 | 100.0 | 78.50 | 33.0 |
| Cobas MTB | 0.964 (0.924–0.999) | 92.86 | 100.0 | – | 0.071 | 100.0 | 96.60 | 33.0 |
| Logix Smart MTB | 0.891 (0.824–0.959) | 78.38 | 100.0 | – | 0.216 | 100.0 | 90.80 | 32.0 |
| Extrapulmonary samples | ||||||||
| Smear | – | – | – | – | – | – | – | – |
| Mycobacteria culture | – | – | – | – | – | – | – | – |
| Cobas MTB | 0.905 (0.740–0.999) | 83.33 | 97.67 | 35.83 | 0.170 | 83.30 | 97.70 | 12.0 |
| Logix Smart MTB | 0.974 (0.939–0.999) | 100.0 | 94.87 | 19.5 | 0.000 | 100.0 | 100.0 | 16.0 |
AUC, area under the curve; MTB, Mycobacterium tuberculosis; ROC, receiver operating characteristic; LHR, Likelihood ratio; 95% CI, 95% confidence interval.
Figure 1.
Radar graph summarizing the diagnostic performance of conventional and molecular tests in pulmonary and extrapulmonary samples compared to Xpert MTB/RIF Ultra.
Concordance of molecular tests for the diagnosis of pulmonary and extrapulmonary TB
The percentage of agreement between the Cobas MTB and the Xpert MTB/RIF Ultra was 97.6% for pulmonary samples and 95.9% for extrapulmonary samples, with Cohen’s Kappa index of 0.95 and 0.81, respectively. On the other hand, the Logix Smart MTB presented agreement of 93.1% for pulmonary samples and 95.5% for extrapulmonary samples, with Cohen’s Kappa index of 0.83 and 0.81, respectively (Table 5).
Table 5.
Percentage of agreement of the Cobas MTB and Logix Smart MTB test with the Xpert MTB/RIF Ultra.
| Comparator test | Sample origin | Agreement (95% CI) | Cohen’s Kappa index (95% CI) |
|---|---|---|---|
| Cobas MTB vs Xpert MTB/RIF Ultra | Total | 97.14 (94.65–99.64) | 0.926 (0.86–0.99) |
| Pulmonary | 97.62 (94.9–100) | 0.945 (88.3–100) | |
| Extrapulmonary | 95.92 (90.1–100) | 0.810 (0.54–100) | |
| Logix Smart MTB vs Xpert MTB/RIF Ultra | Total | 93.75 (88.02–99.48) | 0.830 (0.72–0.94) |
| Pulmonary | 93.10 (86.3–99.8) | 0.831 (0.71–0.95) | |
| Extrapulmonary | 95.45 (84.1–100) | 0.807 (0.54–100) | |
| Cobas MTB vs Logix Smart MTB | Total | 96.25 (90.93–100) | 0.897 (0.80–0.98) |
| Pulmonary | 95.69 (89.4–100) | 0.893 (0.79–0.99) | |
| Extrapulmonary | 97.73 (87.1–100) | 0.906 (0.69–100) |
95% CI, 95% confidence interval; MTB, Mycobacterium tuberculosis.
We also analyzed the positive molecular results, in which the mycobacterial culture was negative (Table 6). Thirty-one samples were positive according to molecular methods but negative according to mycobacterial culture, of which 23/31 were pulmonary, and 8/31 were extrapulmonary. Among these 23 pulmonary samples, 23/23 were detected by Xpert MTB/RIF Ultra (Ct range: 16–26), while Cobas MTB detected 20/23 samples (Ct range: 19–35) and Logix Smart 19/31 samples (Ct range: 24–37) with five samples with indeterminate results. Among the eight extrapulmonary samples, 7/8 were detected by Logix Smart MTB (Ct range: 25–37), 6/8 were positive by Cobas MTB (Ct range: 24–38), and 6/8 were positive by Xpert MTB/ RIF Ultra (Ct range: 16–26) (Table 6).
Table 6.
List of patients in whom MTB was identified by some molecular method, but not by culture.
| Patient | Sample origin | Xpert MTB/RIF Ultra (Ct) | Cobas MTB (Ct) | Logix Smart MTB (Ct) |
|---|---|---|---|---|
| 1 | Pulmonary | 17 | 31 | Indeterminate |
| 2 | Pulmonary | 16 | 23 | Indeterminate |
| 3 | Extrapulmonary | 26 | Negative | Indeterminate |
| 4 | Pulmonary | 16 | 19 | Indeterminate |
| 5 | Pulmonary | 17 | 31 | Indeterminate |
| 6 | Pulmonary | 20 | 30 | Indeterminate |
| 7 | Extrapulmonary | 17 | 32 | 27 |
| 8 | Extrapulmonary | 18 | 34 | 29 |
| 9 | Pulmonary | 21 | 34 | Negative |
| 10 | Pulmonary | 24 | 32 | 36 |
| 11 | Pulmonary | 26 | 35 | Negative |
| 12 | Pulmonary | 16 | 22 | 28 |
| 13 | Pulmonary | 23 | 36 | Negative |
| 14 | Extrapulmonary | 16 | 25 | 28 |
| 15 | Pulmonary | 16 | 20 | 27 |
| 16 | Extrapulmonary | 20 | 30 | 34 |
| 17 | Pulmonary | 16 | 27 | 27 |
| 18 | Pulmonary | 16 | 28 | 29 |
| 19 | Pulmonary | 25 | 35 | Negative |
| 20 | Pulmonary | 27 | Negative | Negative |
| 21 | Pulmonary | 16 | 25 | 24 |
| 22 | Pulmonary | 24 | Negative | Negative |
| 23 | Pulmonary | 21 | 32 | 35 |
| 24 | Extrapulmonary | 16 | 24 | 25 |
| 25 | Pulmonary | 19 | 32 | 32 |
| 26 | Pulmonary | 17 | 27 | 37 |
| 27 | Pulmonary | 19 | 32 | 34 |
| 28 | Pulmonary | 20 | Negative | Negative |
| 29 | Pulmonary | 20 | 30 | 34 |
| 30 | Extrapulmonary | Negative | 38 | 34 |
| 31 | Extrapulmonary | Negative | Negative | 37 |
Ct, cycle threshold; MTB, Mycobacterium tuberculosis.
Finally, 100% of samples positive by smear microscopy were also positive by Xpert MTB/RIF Ultra, Cobas MTB, and Logix Smart MTB methods. While 100%, 100%, and 94.7% of samples were positive by mycobacterial culture, they were also positive by Xpert MTB/RIF Ultra, Cobas MTB, and Logix Smart MTB, respectively.
Discussion
The study evaluated the diagnostic performance of Cobas MTB and Logix Smart MTB for the diagnosis of PTB and EPTB. We observed that both molecular tests had adequate diagnostic performance on total samples when compared to the Xpert MTB/RIF Ultra. We also observed that the performance of Logix Smart was superior (AUC/ROC: 0.97) to Cobas MTB for the diagnosis of EPTB, although it was inferior for the diagnosis of PTB (AUC/ROC: 0.91). These findings demonstrate that both tests meet the minimum diagnostic performance requirements for PTB and EPTB proposed by the WHO. 17
The use of molecular tests such as Xpert MTB/RIF for the initial diagnosis of PTB has been shown to reduce mortality compared with smear microscopy, although not statistically significant. 4 Currently, this test is recommended by the WHO because it has the best diagnostic performance, so we decided to compare its performance with the Cobas MTB and the Logix Smart MTB. It should also be noted that according to the WHO, a defined case of TB is through the identification of MTB either by culture or by some molecular method. 18
Xpert MTB/RIF Ultra has the advantage over Xpert MTB/RIF of slightly higher sensitivity (88% vs 83%), especially when smear microscopy is negative (63% vs 43%).18,19 It also has a lower detection limit of 16 CFU per milliliter (compared to 114 CFU/mL with Xpert MTB/RIF), and it offers better precision in detecting rifampicin resistance due to the incorporation of a melting temperature analysis using four probes that identify mutations in the rpoB gene. 19
Cobas MTB showed a sensitivity of 89.2% (95% CI: 81.7%–93.9%) and specificity of 98.6%, compared to respiratory samples with positive cultures. 20 At the same time, the reported sensitivity of Cobas MTB in smear-negative samples was 63.0%–81.8%.
Unlike Xpert MTB-RIF Ultra, Cobas MTB-RIF/INH has the advantage of being able to confirm true MDR-TB since Xpert MTB-RIF Ultra only estimates MDR based on rifampicin resistance. A study evaluated its performance for this objective, showing a sensitivity of 77.8% and 90% for detecting resistance to isoniazid and rifampicin, respectively. 21 The cases of false susceptibility to isoniazid in the Cobas MTB-RIF/INH were due to nontarget cobas mutations. Nevertheless, in terms of performance, Cobas MTB has shown greater analytical sensitivity than Xpert MTB/RIF and is similar to Genotype MTBDR plus for resistance to rifampicin and isoniazid. 22
According to the manufacturer’s instructions, Cobas MTB-RIF/INH is only validated for respiratory samples; however, in our study, we observed adequate performance for extrapulmonary samples, similar to the Xpert, although inferior to the Logix Smart MTB.
Compared to other medium-complexity molecular methods supported by the WHO, the Cobas MTB-RIF/INH assay requires 3.7 h to process 94 samples and has the advantage of being able to simultaneously analyze 384 samples on the Cobas 6800 and 960 samples on the Cobas 8000. In addition, PCR can be used for MTB, hepatitis B, hepatitis C, and HIV simultaneously. One of its main limitations in sample preparation with Cobas MTB was sonication, which was the reason why it received a lower score regarding sample preparation by users (86% approval).23,24 Another disadvantage is that DNA extraction must be repeated if resistance testing is required, which can lead to increased costs and time, mainly in settings with high incidences of DR-TB. In addition, this technique requires more space and has special electrical requirements than the Xpert MTB/RIF Ultra.
In our study, we observed that Logix Smart MTB presented the best diagnostic performance for extrapulmonary samples, although according to the supplier, this method has only validated for pulmonary samples. 16 To our knowledge, there are no studies that have evaluated the diagnostic performance of Logix Smart MTB in this context; however, in pulmonary samples, it presented a lower performance, with a significant proportion of indeterminate or false negative results, most of which were detected using Xpert MTB/RIF Ultra or Cobas MTB, possibly due to a lower mycobacterial load, since some of these discordant results presented high Ct in the Cobas MTB. As a limitation, Logix Smart has only been validated with the QIAGEN and with the CoDx Box thermal cycler. Although it can provide results in less than 2 h, it is a method of moderate complexity and is not completely automated as is the case for the Xpert MTB/RIF Ultra. Furthermore, this test does not detect resistance to anti-TB drugs, which may be a major limitation.
This research is part of the third pillar of “The End TB strategy” 25 since although Xpert MTB/RIF Ultra is a test with high sensitivity and specificity for the diagnosis of TB, its implementation has not yet been universal, probably due to associated costs; Cobas MTB and Logix Smart MTB could be approximately 30–50% less expensive, and have the advantage of being able to be mass-processed, and with comparable performance. Likewise, although conventional tests like smear microscopy are more economical, they have low sensitivity in identifying TB cases. 26
This study has some strengths. It is the only study that compares the performance of the Xpert MTB/RIF Ultra, Cobas MTB, and Logix Smart MTB for diagnosing PTB and EPTB, even though some of these methods are out of license. In addition to being developed in a high-performance reference laboratory in a country with high TB endemicity, such as Peru, the generalizability of the results is further supported by the inclusion of a diverse range of samples (both pulmonary and extrapulmonary) and the use of different molecular methods. These factors provide a broader context that enhances the applicability of the results to different clinical and geographical settings.
Some of the limitations of this study are the following. Sample processing was carried out after freezing and thawing a limited volume of samples, and it is unknown whether this process could have affected the performance of the tests. It was also not possible to know relevant clinical information such as sex, the presence of symptoms, clinical evolution, history of TB, and HIV infection or treatment with antiretrovirals because we did not have access to medical records and were unable to interview the patients to collect this information. Furthermore, the limited number of samples, due to the limited budget available, could lead to inaccuracy in the reported results. More studies with a greater number of samples analyzed are needed, especially in specific scenarios and with clinical information at the time of diagnosis and its outcomes. Additionally, a sample size calculation was not performed, which could also affect the precision of the results. Future studies should ensure proper sample size calculations to strengthen statistical validity and the detection of significant diagnostic differences.
Conclusion
We found that the Cobas MTB and Logix Smart MTB tests demonstrated adequate performance for tuberculosis diagnosis when compared to the Xpert MTB/RIF Ultra. For pulmonary samples, Cobas MTB showed a higher diagnostic yield, while for extrapulmonary samples, the Logix Smart MTB test was the most suitable. Based on our results, we conclude that molecular tests complement each other, and their selection should be guided by the type of sample being analyzed rather than a one-size-fits-all approach.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361241304516 for Diagnostic performance of the Cobas MTB and Logix Smart MTB for diagnosing pulmonary and extrapulmonary tuberculosis: a cross-sectional study of diagnostic tests by Miguel Hueda-Zavaleta, Juan Carlos Gomez de la Torre, Diana Minchón-Vizconde, Claudia Barletta-Carrillo, Cesar Copaja-Corzo, Gustavo Tapia-Sequeiros, Cinthya Flores, Cristian Piscoche, Cecilia Miranda, Ada Mendoza and Vicente A. Benites-Zapata in Therapeutic Advances in Infectious Disease
Acknowledgments
None.
Footnotes
ORCID iDs: Miguel Angel Hueda Zavaleta  https://orcid.org/0000-0002-8049-7787
https://orcid.org/0000-0002-8049-7787
Juan Carlos Gomez de la Torre  https://orcid.org/0000-0003-4566-2027
https://orcid.org/0000-0003-4566-2027
Diana Julissa Minchon Vizconde  https://orcid.org/0000-0003-2256-7609
https://orcid.org/0000-0003-2256-7609
Claudia Barletta-Carrillo  https://orcid.org/0000-0001-9982-5368
https://orcid.org/0000-0001-9982-5368
Cesar Copaja-Corzo  https://orcid.org/0000-0002-3497-0158
https://orcid.org/0000-0002-3497-0158
Gustavo Tapia Sequeiros  https://orcid.org/0000-0002-9277-2731
https://orcid.org/0000-0002-9277-2731
Cinthya Gisele Flores Flores  https://orcid.org/0009-0009-3452-3276
https://orcid.org/0009-0009-3452-3276
Nilver Cristian Piscoche Botello  https://orcid.org/0000-0002-9907-8013
https://orcid.org/0000-0002-9907-8013
Cecilia Miranda  https://orcid.org/0009-0000-7904-5735
https://orcid.org/0009-0000-7904-5735
Ada Patricia Mendoza Farro  https://orcid.org/0009-0008-7202-856X
https://orcid.org/0009-0008-7202-856X
Vicente A. Benites-Zapata  https://orcid.org/0000-0002-9158-1108
https://orcid.org/0000-0002-9158-1108
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Miguel Hueda-Zavaleta, Diagnóstico, tratamiento e investigación de enfermedades infecciosas y tropicales, Universidad Privada de Tacna, Tacna, Peru.
Juan Carlos Gomez de la Torre, Laboratorio clínico Roe, Lima, Peru; Facultad de Medicina Humana, Universidad de Piura, Lima, Peru.
Diana Minchón-Vizconde, Diagnóstico, tratamiento e investigación de enfermedades infecciosas y tropicales, Universidad Privada de Tacna, Tacna, Peru.
Claudia Barletta-Carrillo, Sequence Reference Lab, Lima, Peru.
Cesar Copaja-Corzo, Unidad de Investigación para la Generación y Síntesis de Evidencias en Salud, Universidad San Ignacio de Loyola, Lima, Peru; Servicio de Infectología, Hospital Nacional Edgardo Rebagliati Martins, EsSalud. Lima, Peru.
Gustavo Tapia-Sequeiros, Diagnóstico, tratamiento e investigación de enfermedades infecciosas y tropicales, Universidad Privada de Tacna, Tacna, Peru.
Cinthya Flores, Sequence Reference Lab, Lima, Peru.
Cristian Piscoche, Sequence Reference Lab, Lima, Peru.
Cecilia Miranda, Sequence Reference Lab, Lima, Peru.
Ada Mendoza, Sequence Reference Lab, Lima, Peru.
Vicente A. Benites-Zapata, Unidad de Investigación para la Generación y Síntesis de Evidencias en Salud, Universidad San Ignacio de Loyola, Lima, Campus 2, avenida La Fontana 750, La Molina, Lima, Peru.
Declarations
Ethics approval and consent to participate: The protocol was approved by the research ethics committee of the Faculty of Health Sciences at the Private University of Tacna (code: 94/FAC). The ethics committee waived the requirement for informed consent due to the nature of the study, which prevented contacting patients from thawed samples stored for laboratory quality control purposes. Similarly, confidentiality of the database was ensured, which was used exclusively for research purposes.
Consent for publication: Not applicable.
Author contributions: Miguel Hueda-Zavaleta: Conceptualization; Formal analysis; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Juan Carlos Gomez de la Torre: Conceptualization; Investigation; Resources; Supervision; Writing – review & editing.
Diana Minchón-Vizconde: Data curation; Writing – original draft.
Claudia Barletta-Carrillo: Investigation; Writing – original draft.
Cesar Copaja-Corzo: Data curation; Methodology; Writing – original draft.
Gustavo Tapia-Sequeiros: Data curation; Formal analysis; Writing – original draft.
Cinthya Flores: Investigation; Writing – original draft.
Cristian Piscoche: Investigation; Writing – original draft.
Cecilia Miranda: Investigation; Writing – original draft.
Ada Mendoza: Investigation; Writing – original draft.
Vicente A. Benites-Zapata: Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding through the Private University of Tacna, Tacna, Peru (323-2022-UPT-CU).
Competing interests: JCGdlT is a speaker at Cepheid, manufacturer of the GeneXpert platforms. The other authors declare that they have no competing interests.
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Pan American Health Organization. Tuberculosis in the Americas, 2018. Washington, DC: PAHO, https://iris.paho.org/handle/10665.2/49510 (2018, accessed 24 September 2023). [Google Scholar]
- 2. Floyd K, Glaziou P, Zumla A, et al. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med 2018; 6(4): 299–314. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Module 3: Diagnosis—Rapid Diagnostics for Tuberculosis Diagnosis, 2021 Update. Geneva, Switzerland: WHO, https://www.who.int/publications/i/item/9789240029415 (2021, accessed 31 March 2023). [Google Scholar]
- 4. Di Tanna GL, Miotto P, Migliori GB, et al. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Glob Health 2019; 7(2): e191–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zifodya JS, Kreniske JS, House PS, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev 2021; 2: CD009593. [DOI] [PubMed] [Google Scholar]
- 6. Bisognin F, Lombardi G, Lombardo D, et al. Improvement of Mycobacterium tuberculosis detection by Xpert MTB/RIF Ultra: a head-to-head comparison on Xpert-negative samples. PLoS One 2018; 13(8): e0201934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mekkaoui L, Vandersmissen G, Courcol R, et al. Performance of Xpert MTB/RIF Ultra for diagnosis of pulmonary and extra-pulmonary tuberculosis, one year of use in a multi-centric hospital laboratory in Brussels, Belgium. PLoS One 2021; 16(4): e0249734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donovan J, Phu Huong Lan N, Dinh Ple T, et al. Xpert MTB/RIF Ultra versus Xpert MTB/RIF for the diagnosis of tuberculous meningitis: a prospective, randomised, diagnostic accuracy study. Lancet Infect Dis 2020; 20(3): 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang M, Gao Y, Wang X, et al. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in a clinical practice setting of China. Diagn Microbiol Infect Dis 2021; 100(1): 115306. [DOI] [PubMed] [Google Scholar]
- 10. Wang G, Chen L, Wu X, et al. Xpert MTB/RIF Ultra improved the diagnosis of paucibacillary tuberculosis: a prospective cohort study. J Infect 2019; 78(4): 311–316. [DOI] [PubMed] [Google Scholar]
- 11. Perez-Risco D, Montuenga LM, Saiz-Montero M, et al. Evaluation of the Xpert MTB/RIF Ultra assay for direct detection of Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples. J Clin Microbiol 2018; 56(9): e00659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rindi L. Rapid molecular diagnosis of extra-pulmonary tuberculosis by Xpert/RIF Ultra. Front Microbiol 2022; 13: 817661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016; 6(11): e012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cepheid. Xpert MTB RIF Ultra Brochure. Sunnyvale, United States, https://cepheid.widen.net/view/pdf/s6lwefaviz/Cepheid-Xpert-MTB-RIF-Ultra-Brochure-CE-IVD-3098-Spanish.pdf?t.download=true&u=escyfu (2020, accessed 25 September 2023). [Google Scholar]
- 15. Roche Diagnostics. Cobas MTB. Basel, Switzerland, https://diagnostics.roche.com/global/en/products/params/cobas-mtb.html#productSpecs (2022, accessed 31 March 2022). [Google Scholar]
- 16. Co-Diagnostics. Logix Smart TM MTB. United States: Co-Dx, www.codiagnostics.com (2022, accessed 31 March 2022). [Google Scholar]
- 17. World Health Organization. High priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. Geneva, Switzerland: WHO, https://www.who.int/publications/i/item/WHO-HTM-TB-2014.18 (2014, accessed 23 September 2023). [Google Scholar]
- 18. World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneva: WHO, https://www.ncbi.nlm.nih.gov/books/NBK138748/ (2010, accessed 31 March 2023). [PubMed] [Google Scholar]
- 19. Zhang M, Xue M, He J. Diagnostic accuracy of the new Xpert MTB/RIF Ultra for tuberculosis disease: a preliminary systematic review and meta-analysis. Int J Infect Dis 2020; 90: 35–45. [DOI] [PubMed] [Google Scholar]
- 20. Horita N, Miyazawa N, Yoshiyama T, et al. Sensitivity and specificity of Cobas TaqMan MTB real-time polymerase chain reaction for culture-proven Mycobacterium tuberculosis: meta-analysis of 26999 specimens from 17 studies. Sci Rep 2015; 5: 18113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nadarajan D, Schlager D, Clark AE, et al. Evaluation of the Roche Cobas MTB and MTB-RIF/INH assays in samples from Germany and Sierra Leone. J Clin Microbiol 2021; 59(5): e02983-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aono A, Yano S, Yoshimura A, et al. Clinical evaluation of the Cobas® MTB-RIF/INH reagent and the cobas® 6800 for the detection of isoniazid and rifampicin resistance. Tuberculosis 2022; 134: 102199. [DOI] [PubMed] [Google Scholar]
- 23. De Vos M, Collins S, Frahm N, et al. Comparative analytical evaluation of four centralized platforms for the detection of Mycobacterium tuberculosis complex and resistance to rifampicin and isoniazid. J Clin Microbiol 2021; 59(3): e02168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. David A, Shetty S, Anthony RM, et al. Feasibility, ease-of-use, and operational characteristics of World Health Organization–recommended moderate-complexity automated nucleic acid amplification tests for the detection of tuberculosis and resistance to rifampicin and isoniazid. J Mol Diagn 2023; 25(1): 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization. The End TB Strategy. Geneva, Switzerland: WHO, https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19 (2015, accessed 24 September 2023). [Google Scholar]
- 26. Figueredo LJA, Miranda SS, Santos LBD, et al. Cost analysis of smear microscopy and the Xpert assay for tuberculosis diagnosis: average turnaround time. Rev Soc Bras Med Trop 2020; 53: e20200314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361241304516 for Diagnostic performance of the Cobas MTB and Logix Smart MTB for diagnosing pulmonary and extrapulmonary tuberculosis: a cross-sectional study of diagnostic tests by Miguel Hueda-Zavaleta, Juan Carlos Gomez de la Torre, Diana Minchón-Vizconde, Claudia Barletta-Carrillo, Cesar Copaja-Corzo, Gustavo Tapia-Sequeiros, Cinthya Flores, Cristian Piscoche, Cecilia Miranda, Ada Mendoza and Vicente A. Benites-Zapata in Therapeutic Advances in Infectious Disease