Abstract
We have studied the mechanism of UV protection in two duckweed species (Lemnaceae) by exploiting the UV sensitivity of photosystem II as an in situ sensor for radiation stress. A UV-tolerant Spirodela punctata G.F.W. Meyer ecotype had significantly higher indole-3-acetic acid (IAA) levels than a UV-sensitive ecotype. Parallel work on Lemna gibba mutants suggested that UV tolerance is linked to IAA degradation rather than to levels of free or conjugated IAA. This linkage is consistent with a role for class III phenolic peroxidases, which have been implicated both in the degradation of IAA and the cross-linking of various UV-absorbing phenolics. Biochemical analysis revealed increased activity of a specific peroxidase isozyme in both UV-tolerant duckweed lines. The hypothesis that peroxidases play a role in UV protection was tested in a direct manner using genetically modified tobacco (Nicotiana sylvestris). It was found that increased activity of the anionic peroxidase correlated with increased tolerance to UV radiation as well as decreased levels of free auxin. We conclude that phenol-oxidizing peroxidases concurrently contribute to UV protection as well as the control of leaf and plant architecture.
UV-B (280–315 nm) radiation is a minor component of the solar spectrum, yet it has the potential to disproportionately affect metabolic processes in humans, animals, plants, and microorganisms. In plants, UV-B can potentially interfere with growth, development, photosynthesis, flowering, pollination, and transpiration (Rozema et al., 1997; Jansen et al., 1998). The molecular targets of UV-driven photomodification and photosensitisation reactions (Greenberg et al., 1997) include nucleotides, aminoacids, lipids, and pigments (Jordan, 1996). The UV-driven inactivation of photosystem II (PSII) has attracted considerable attention. PSII is a protein pigment complex, the core of which is formed by the D1 and D2 proteins (Barber et al., 1997). Degradation of the D1 and D2 reaction center proteins is driven by UV-B fluence rates as low as 1 μmol m−2 s−1 (Jansen et al., 1996a). Many UV effects are abated in the presence of a background of visible radiation (Cen and Bornman, 1990). However, UV-driven D1-D2 degradation is strongly accelerated in the presence of visible radiation (Jansen et al., 1996a). The acceleration is likely to reflect increased UV absorbance of a photosensitizer charged during photosynthetic electron flow, vis-à-vis the uncharged species (Babu et al., 1999). The increase in inactivated PSII centers can be measured as a decrease in oxygen evolution or variable chlorophyll fluorescence (Greenberg et al., 1997). In this study, we have exploited the UV sensitivity of PSII to study the processes that underlie tolerance to broadband UV radiation.
Many of the detrimental UV-B effects on PSII, as well as other targets, are readily observed under laboratory conditions but are difficult to detect in field experiments (Fiscus and Booker, 1995; Rozema et al., 1997; Jansen et al., 1998). A failure to take into consideration naturally occurring tolerance mechanisms is likely to contribute to the discrepancy between laboratory and field studies (Jansen et al., 1998). Repair and acclimation responses are readily induced in response to UV exposure in many species. A typical repair mechanism is the light-dependent photoreactivation by photolyases, resulting in the restoration of UV-damaged DNA to its native form (Britt, 1996). Acclimation responses include increased oxygen radical scavenging activity (Strid et al., 1994; Rao et al., 1996), and the accumulation of soluble UV-screening flavonoids (Cen and Bornman, 1990; Olsson et al., 1999). In addition, polyamines, waxes, and specific alkaloids may contribute to UV tolerance (Jansen et al., 1998). Levels of UV tolerance differ considerably between genera, species, and even closely related cultivars. Efficient protection from UV radiation effects is particularly found among plants that thrive in areas of high UV-B-like lower latitudes or higher altitudes (Sullivan et al., 1992).
Long-term UV acclimation involves increased UV tolerance as well as changes in plant architecture and secondary metabolism. UV-induced changes in plant morphology include increased leaf thickness, altered leaf shape, increased axillary branching, smaller internodes, and decreased plant height (Ziska et al., 1993; Teramura and Sullivan, 1994; Greenberg et al., 1997; Jansen et al., 1998). Some of these responses could involve a specific UV photoreceptor (Ballaré et al., 1995; Greenberg et al., 1997) and may be of greater importance for plant productivity than direct UV effects on photosynthesis (Barnes et al., 1990; Teramura and Sullivan, 1994). UV effects on secondary metabolism include accumulation of phenolic compounds like flavonoids, cinnamate esters, lignin, and tannin (Rozema et al., 1997). A particular difficulty in the analysis of long-term UV acclimation phenomena is distinguishing adaptive responses from UV-B-induced damage. Despite the ecophysiological importance of UV acclimation, little is known about the molecular-physiological mechanisms underlying this response.
We previously demonstrated differences in UV tolerance among a collection of Spirodela punctata G.F.W. Meyer ecotypes (duckweed family; Lemnaceae; Jansen et al., 1999). A constitutively UV-tolerant ecotype (760) was found to be able to sustain PSII activity and biomass accumulation if exposed to UV-B radiation, relative to a UV-sensitive ecotype (203). Protection was found not to be particularly wavelength specific, but rather it covered the broad wavelength area of the UV-A, UV-B, and UV-C bands (Jansen et al., 1999). However, UV-tolerant plants were not protected against other abiotic stresses, including excessive fluences of photosynthetically active radiation (PAR), heat, or chilling. Tolerance in S. punctata could not be correlated with well-characterized UV adaptation responses like increased accumulation of bulk, soluble UV-screening pigments in the epidermis, or increased oxygen radical scavenging activity (Jansen et al., 1999). In this paper, we show that a UV-tolerant S. punctata ecotype (760) contains significantly more free indole-3-acetic acid (IAA) than a UV-sensitive ecotype (203). Parallel work on Lemna gibba mutants indicated that UV tolerance is related to IAA catabolism, rather than to IAA levels. Class III phenolic peroxidases have been implicated in the degradation of the major endogenous auxin, IAA, as well as the cross-linking of various UV-B-absorbing phenolics. The hypothesis that the activity of phenolic peroxidases can, simultaneously, contribute to UV tolerance as well as auxin catabolism was tested in a direct manner using transgenic tobacco (Nicotiana sylvestris) plants altered in their peroxidase levels. Increased peroxidase activity was found to be correlated with increased UV tolerance and decreased IAA levels. Thus, we conclude that peroxidases play a role in UV protection.
RESULTS
Differences in Auxin Metabolism between S. punctata Ecotypes
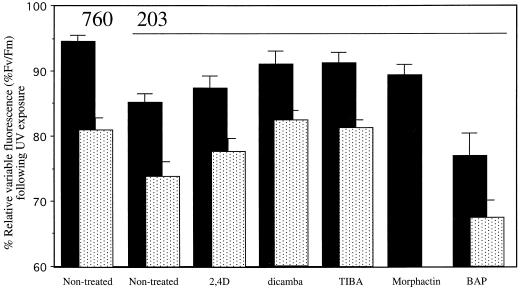
S. punctata ecotypes 203 and 760 were raised under laboratory conditions, in the absence of UV radiation. Exposure of the fronds to UV led to a decrease of the relative variable chlorophyll fluorescence (Fv/Fm), reflecting a decrease in the photochemical yield of open PSII reaction centers (Fig. 1). The two ecotypes were differentially affected. A 24-h exposure to 4.4 W m−2 UV resulted in a significant decrease in variable fluorescence in ecotype 203 (Fig. 1). Yet, this high dose of UV radiation caused only a minor UV effect in ecotype 760. These data extend those of Jansen et al. (1999) who showed that ecotypes 203 and 760 differ in their capability to protect PSII reaction centers and in plant biomass production under low fluences of UV.
Figure 1.
Effects of UV radiation on the relative variable fluorescence of S. punctata ecotypes 203 and 760. Plants were raised phototrophically on standard Hutner's medium (203 and 760 non-treated) or on medium supplemented with 10 μm dicamba, 0.1 μm 2,4 dichlorophenoxyacetic acid (2,4D), 1 μm tri-iodobenzoic acid (TIBA), 0.1 μm morphactin, or 1 μm 6-benzylaminopurine (6-BAP). Intact fronds (▪) were exposed to 4.4 W m−2 UV for 24 h. Cells (░⃞) isolated from fronds that had been raised on supplemented medium were exposed to 4.4-W m−2 UV for 1 h. Following the UV treatment the minimal fluorescence (Fo) and the maximal fluorescence (Fm) were measured on dark-adapted samples. The relative variable fluorescence (Fv/Fm) was normalized to that of the non-treated control (intact fronds 100% = 0.80 ± 0.02 or isolated cells 100% = 0.76 ± 0.02). Values represent averages of nine to 10 (intact fronds) or seven (isolated cells) measurements. ses of the mean are given. Statistical analysis (Student's t test) reveals differences between intact fronds of ecotypes 203 and 760 (P < 0.01) and between fronds of non-treated 203 and 203-dicamba (P < 0.05), 203-TIBA (P < 0.05), and 203-BAP (P < 0.10). Differences between cells isolated from ecotypes 203 and 760, and between non-treated 203 and 203-TIBA and 203-dicamba were all significant (P < 0.05). The UV effect on isolated morphactin cells was not determined.
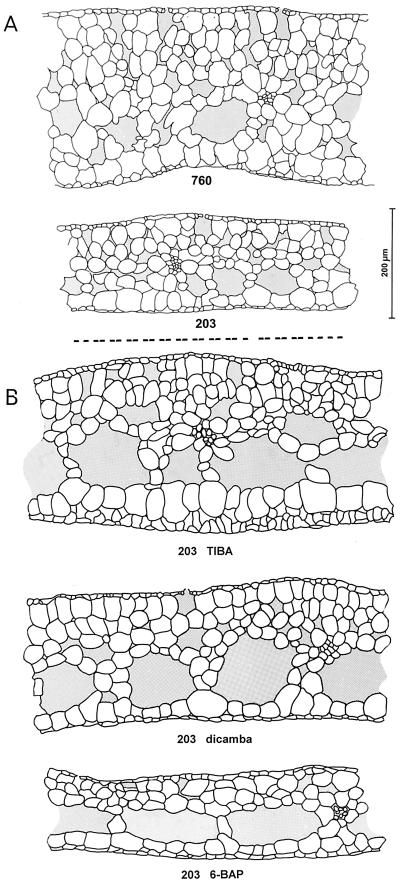
Ecotypes 203 and 760 appeared very similar macroscopically. However, a more detailed microscopic analysis revealed significant differences in the architecture of the colonies. Fronds of the UV-tolerant ecotype 760 were about 40% thicker than those of the UV-sensitive ecotype 203 (Fig. 2A). The difference in leaf thickness was reflected in the number of cell layers. Ecotype 203 typically consisted of five cell layers, and ecotype 760 of eight layers. A second significant difference between the two duckweed ecotypes was the branching pattern of the vascular bundles. Fronds of the UV-tolerant ecotype 760 normally contained five main vascular bundles, whereas those of 203 contained three, rarely four, bundles. Intercellular spaces were somewhat larger in the thick fronds of ecotype 760. However, the contribution of intercellular air pockets to the total leaf volume appeared unchanged.
Figure 2.
Frond architecture of S. punctata ecotypes. Plants were raised phototrophically on Hutner's medium (A; 203 and 760) or on Hutner's medium (B) supplemented with 1 μm TIBA (203 TIBA), 10 μm dicamba (203 dicamba), or 1 μm 6-BAP (203 BAP). Fresh fronds were dissected with a microtome and 20- to 40-μm-thick cross sections were studied using a light microscope. Sections reveal the upper epidermis with stomata, spongy photosynthetic tissue containing large intercellular spaces, and vascular bundles and a thin lower epidermis. The average frond thickness (±se of the mean) was 254 ± 9 μm (760), 181 ± 9 μm (203), 288 ± 30 (203 TIBA), 241 ± 3 (203 dicamba), and 161 ± 8 (203 BAP). Statistical analysis (Student's t test) reveals the significance of the differences between 203 and 760 (P < 0.01) and between 203 and 203-dicamba and 203-TIBA (P < 0.01). Values reflect the analysis of three to 14 leaves, with five measurements per leaf. Pictures are representative cross sections. The black bar represents a length of 200 μm.
The phytohormone auxin has been reported to control duckweed frond architecture, including leaf thickness and the branching pattern of the vascular bundles (Landolt and Kandeler, 1987). Thus, morphological differences between ecotypes 203 and 760 may reflect differences in auxin metabolism. To test this hypothesis, we perturbed auxin homeostasis and studied effects on frond morphology. UV-sensitive ecotype 203 was raised on medium containing low concentrations of the synthetic auxin analog dicamba, the polar auxin transport inhibitor TIBA, or the cytokinin 6-BAP. Dicamba and TIBA induced increases in leaf thickness of 33% and 59%, respectively (Fig. 2B). This correlated with an increase in the number of cell layers. Untreated fronds of ecotype 203 typically consisted of five cell layers, whereas TIBA- and dicamba-treated fronds of the same ecotype were made up of seven to eight and eight layers, respectively (Fig. 2B). The number of main vascular bundles was also increased. Untreated fronds of ecotype 203 typically contained three main vascular bundles, whereas TIBA- and dicamba-treated fronds of this ecotype had six to nine and five to six bundles, respectively. Thus, the differences in frond architecture between UV-sensitive ecotype 203 and UV-tolerant ecotype 760 could be significantly diminished by treating fronds with auxin-like compounds. In contrast, exposure of ecotype 203 to the cytokinin 6-BAP exacerbated the difference between the two ecotypes (Fig. 2B). BAP-treated fronds were relatively thin. The average thickness was significantly decreased compared with untreated fronds of ecotype 203. The fronds consisted of five cell layers and contained four to five main vascular bundles (Fig. 2B). The effect of 6-BAP on leaf thickness was the opposite of that induced by TIBA and dicamba, thus resembling a classical auxin cytokinin antagonism.
Ecotypes 203 and 760 differed in frond architecture and these differences could be diminished by treating fronds with dicamba and TIBA. This suggests that the two ecotypes differed in aspects of their IAA homeostasis. To further study this point, we determined the dose sensitivity of ecotypes 203 and 760 toward the externally added synthetic auxin analogs dicamba and 2,4D. Both compounds were strongly herbicidal and micromolar concentrations arrested the growth of duckweed cultures (Fig. 3). The dicamba concentration that impeded growth by 50% (I50) was 55 and 190 μm for ecotypes 760 and 203, respectively. 2,4D impeded growth with I50 values of 2 and 8 μm for ecotypes 760 and 203, respectively (Fig. 3). Thus, the UV-tolerant 760 ecotype was found to be significantly more susceptible to synthetic auxin analogs than the UV-sensitive 203 ecotype.
Figure 3.
Inhibition of duckweed growth by dicamba or 2,4D. Plants were grown phototrophically on medium supplemented with dicamba or 2,4D. Experiments were started by inoculating flasks with an equal amount of fresh weight (10 mg). Values represent the average accumulated dry weight at d 10. n = 3. ses are given. Statistical analysis (two-factor ANOVA) shows that the two ecotypes differ significantly (P < 0.05) in their responses to both 2,4D and dicamba.
Levels of free IAA and IAA conjugates were measured in full-grown, non-senescent cultures, using gas chromatography (GC)-mass spectrometry (MS). We found that levels of free IAA were significantly higher in the UV-tolerant ecotype 760, as compared with the UV-sensitive ecotype 203 (Table I). Levels of auxin conjugates were also higher in the UV-tolerant ecotype as compared with the sensitive type (Table I). These differences cannot reflect UV-driven photodegradation of IAA because the plants were raised in the absence of UV. We conclude that ecotypes 203 and 760 differed in aspects of their auxin homeostasis and that this was reflected as auxin-dependent changes in leaf structure (Fig. 2), differential sensitivity to externally added synthetic auxins (Fig. 3), and altered levels of free and conjugated auxin (Table I).
Table I.
Levels of free and conjugated IAA in S. punctata ecotypes 203 and 760
| Plant Materiala | Free IAA | IAA Conjugates |
|---|---|---|
| pmol mg−1 dry wt | ||
| 203 | 88 | 0.76 |
| 760 | 1,591 | 27 |
Plants were grown phototropically on Hutner’s medium. Mature, but non-senescent, cultures of UV-sensitive 203 and UV-tolerant 760 S. punctata were analyzed for IAA levels using GC-MS (Prinsen et al., 1995). The error on measurement is ≤10%.
A Link between Auxin Metabolism and UV Tolerance
Changes in auxin metabolism might be, but are not necessarily, related to the observed differences in UV tolerance. The existence of a potential link between the two parameters was investigated by manipulating auxin metabolism while monitoring UV tolerance in parallel. UV-sensitive ecotype 203 was raised on medium supplemented with a low concentration of a synthetic auxin (2,4D or dicamba) or an inhibitor of polar auxin transport (TIBA or morphactin). As shown in Figure 2, the addition of these compounds to the growth medium resulted in significant effects on frond architecture, including frond thickness, vascular branching, and root length. We studied the effects of UV on the efficiency of PSII and found that TIBA- or dicamba-treated fronds were significantly more UV tolerant than untreated fronds of the same ecotype (Fig. 1). Growth on medium supplemented with the cytokinin 6-BAP appeared to increase the UV sensitivity of the fronds (Fig. 1), resembling classic auxin-cytokinin antagonism. Thus, our experiments showed that interference with auxin metabolism affected the ability of fronds to tolerate UV-B radiation.
The increase in UV tolerance of TIBA- or dicamba-treated fronds may be due to changes in leaf thickness (Figs. 1 and 2). UV-B radiation, unlike PAR, does only penetrate the top cell layers of a leaf (Olsson et al., 1999); therefore, leaf thickening may constitute an effective damage limitation strategy. To investigate this, we studied the UV susceptibility of suspensions of isolated mesophyll cells. Such cells were extremely UV sensitive (Fig. 1), presumably due to the absence of the protecting epidermal layer. The UV sensitivity of the isolated cells was quantitatively comparable to that of duckweed fronds from which the epidermis had been removed (Jansen et al., 1999). However, notwithstanding the overall UV susceptibility, cells isolated from fronds raised on dicamba or TIBA were significantly more UV tolerant than cells from fronds of untreated ecotype 203 (Fig. 1). We conclude that interference with auxin metabolism induced UV tolerance, which was, at least partially, localized at the cellular level.
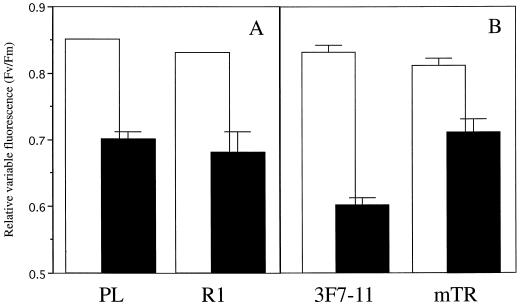
The link between auxin metabolism and UV tolerance was further studied in an independent manner using mutant duckweed lines altered in auxin metabolism. L. gibba line R1 has a giant phenotype and was originally obtained by regeneration from primary callus cultures (Slovin and Cohen, 1988). Endogenous levels of free IAA are, depending on the stage of the plant culture cycle, up to 100-fold higher in R1 as compared with the parental line (PL; Slovin and Cohen, 1988). We determined the UV tolerance of R1 relative to PL and found that exposure of these lines to UV resulted in similar depressions of the photochemical efficiency of PSII (Fig. 4). This suggests that auxin levels per se are not relevant for the control of UV-B tolerance.
Figure 4.
Effects of UV radiation on the relative variable fluorescence of L. gibba mutants. Fronds were raised phototrophically on Hutner's medium and exposed for 24 h to 4.4-W m−2 UV. Before (▪) and after (□) UV treatment, the minimal fluorescence (Fo) and the maximal fluorescence (Fm) were measured on dark-adapted samples. Fronds of mutant R1 (A) and the corresponding PL fronds (B) of mutant mTR and the corresponding PL 3F7-11. Values represent averages of eight measurements. ses of the mean are given. Statistical analysis (Student's t test) shows a significant difference between mTR and 3F7-11 (P < 0.01) following UV treatment.
L. gibba line mTR is an α-methyl-Trp-resistant line generated by nitrosomethylurea treatment of inbred line 3F7-11, and selected for its resistance to methyl Trp (Tam et al., 1995). The removal of the feedback inhibition of anthranilate synthase results in a line characterized by a somewhat increased level of free IAA, normal levels of total IAA, and a high rate of IAA turnover. We compared the UV tolerance of the mTR mutant and the parental 3F7-11 line and found that the efficiency of PSII was significantly less affected in the mutant (Fig. 4). It was investigated whether UV tolerance of the mTR mutant is specific for UV radiation stress, as has previously been noted for ecotype 760 (Jansen et al., 1999). We exposed fronds to excessive intensities of visible light (photoinhibition), heat, or cold and found that tolerance against UV radiation was not coupled to any other stress tolerance (Table II).
Table II.
Effects of heat, chilling, and excessive PAR on the variable fluorescence of duckweed ecotypes and mutants and tobacco transgenics
| Species | Relative Variable Fluorescence
of Plants Exposed to:
|
|||
|---|---|---|---|---|
| Control | Heat | Chilling | Excessive PAR | |
| Fv/Fm | ||||
| S. punctata ecotypes | ||||
| 760 | 0.79 ± 0.02 | 0.56 ± 0.05 | 0.70 ± 0.03 | 0.65 ± 0.02 |
| 203 | 0.79 ± 0.02 | 0.56 ± 0.04 | 0.67 ± 0.02 | 0.61 ± 0.02 |
| L. gibba mutants | ||||
| 3F7-11 | 0.79 ± 0.01 | 0.59 ± 0.03 | 0.56 ± 0.02 | 0.61 ± 0.02 |
| mTR | 0.77 ± 0.01 | 0.56 ± 0.04 | 0.52 ± 0.03 | 0.61 ± 0.01 |
| PL | 0.78 ± 0.01 | 0.54 ± 0.03 | 0.54 ± 0.04 | 0.58 ± 0.03 |
| R1 | 0.77 ± 0.01 | 0.59 ± 0.04 | 0.61 ± 0.02 | 0.61 ± 0.04 |
| Tobacco transgenics | ||||
| WT | 0.79 ± 0.01 | 0.53 ± 0.02 | 0.64 ± 0.02 | 0.54 ± 0.04 |
| Overexpressor | 0.78 ± 0.01 | 0.55 ± 0.03 | 0.63 ± 0.02 | 0.54 ± 0.04 |
| Antisense | 0.79 ± 0.01 | 0.55 ± 0.03 | 0.68 ± 0.02 | 0.54 ± 0.04 |
A Role for Peroxidases in Coupling Auxin Turnover and UV Tolerance
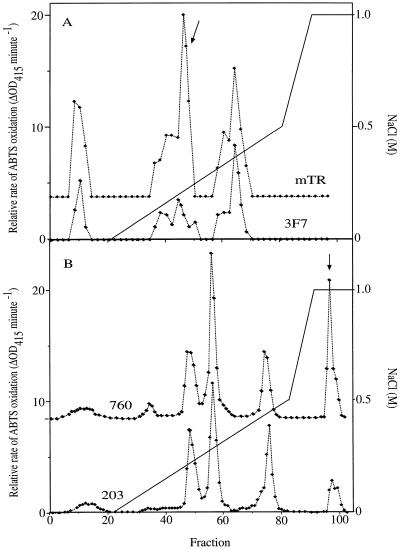
Our data on the UV sensitivities of auxin-treated fronds (Fig. 1) and duckweed mutants (Fig. 4) appear to link UV tolerance and auxin metabolism. The data also suggest that it is not auxin levels per se, but rather auxin turnover that is related to the acquisition of UV tolerance (Fig. 4). Class III phenolic peroxidases have long been implicated in the degradation of auxins (Krylov and Dunford, 1996; Normanly, 1997; Gazaryan et al., 1998). It is the dual functionality of class III peroxidases as auxin- and phenol-oxidases that potentially links auxin catabolism to the metabolism of the UV-absorbing phenolics. We compared peroxidase isozyme patterns of ecotypes 203 and 760, and mutant lines 3F7-11 and mTR. Mutant lines 3F7-11 and mTR were found to contain at least three major isozymes activities (Fig. 5A). The same isozymes were identified for both lines, which is consistent with them being genetically closely related. However, there was a major difference in the relative activity of one particular isozyme (Fig. 5A). This isozyme, eluted by 0.2 m NaCl, was boosted severalfold in the UV-tolerant mTR mutant relative to 3F7-11. Differences in the relative activity of the other two isozymes were either absent or much smaller. Ecotypes 203 and 760 were found to contain at least four major isozymes activities (Fig. 5B). Again, the similarity of the isozyme pattern underlined that the two ecotypes are very closely related. However, there was a particularly strong difference in the relative activity of one major isozyme (Fig. 5B) that was boosted severalfold in UV-tolerant ecotype 760 relative to 203. Differences in peroxidase isozyme expression patterns of the 203 and 760 ecotypes and the mTR and 3F7-11 mutant lines are consistent with a role for peroxidases in linking UV tolerance and auxin catabolism.
Figure 5.
Separation of duckweed peroxidase isozymes. Plants were homogenized in extraction buffer and the resulting extract was dialysed. Isozymes were eluted from a Hi Load SP-Sepharose High Performance 26/10 column using a two-stage gradient of NaCl in 50 mm malonate (pH 5.0), and assayed using 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS). A, Isozymes of the UV-tolerant L. gibba mutant mTR and the UV-sensitive PL 3F7-11. B, Isozymes of the UV-sensitive S. punctata ecotype 203 and the UV-tolerant ecotype 760. Major differences in activity are indicated with an arrow. Each elution profile reflects an average activity, obtained by combining the biomass of five separate duckweed cultures. In three independent repeats, the ratio of activity of mTR relative to 3F7-11 was on average 1.3 for peak I (0 m NaCl), 3.2 for peak II (0.2 m NaCl), and 1.0 for peak III (0.4 m NaCl).
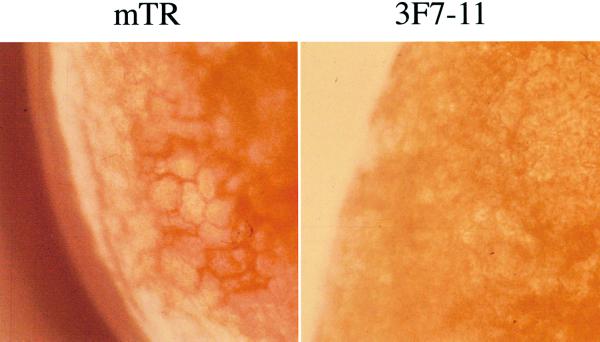
In situ, differential peroxidase activity might be, but is not necessarily, reflected in altered biochemical composition of the plants. Class III peroxidases are involved in the deposition of lignin and other phenolic polymers in cell walls (Lagrimini, 1991). Most duckweed species contain only very low levels of lignin (Landolt and Kandeler, 1987). However, it is possible that more substantial levels of lignin and/or other phenolic polymers accumulate in duckweed lines that contain elevated peroxidase activity. The phloroglucinol/HCl assay revealed lignin deposition in the walls of mesophyll cells of the UV-tolerant mutant mTR but not 3F7-11 (Fig. 6). The observed difference in lignin deposition in leaf tissue complemented the observed differential peroxidase activity in duckweed extracts (Fig. 5). No lignin deposition could be visualized in ecotype 760, although the plants are characterized by increased peroxidase activity. This does not necessarily exclude an effect of peroxidases on phenolic metabolism in S. punctata ecotype 760. A range of different phenolic compounds can be cross-linked with cell wall components. At present, we cannot exclude that cell walls of ecotype 760 contain relatively high levels of non-lignin-type phenolics.
Figure 6.
Lignin-specific staining of leaf sections of the UV-tolerant duckweed mutant lines mTR and the UV-sensitive PL 3F7-11. Fronds were infiltrated with phloroglucinol/HCl and studied using a light microscope. Walls of mesophyll cells are colored orange-red. A representative picture shows the edge of a leaf.
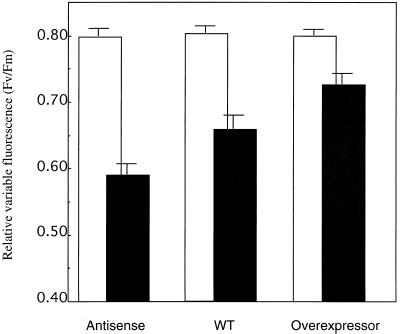
To provide direct evidence that peroxidases play a role in UV protection, we have overexpressed or repressed peroxidase activity and studied the effect on UV tolerance. Difficulties in duckweed transformation forced us to use another organism. The generation of tobacco plants antisense or overexpressing the major anionic tobacco peroxidase has been described in detail (Lagrimini et al., 1997). Differential expression of the anionic peroxidase is reflected in alterations in plant and leaf architecture. Plants overexpressing the anionic peroxidase are characterized by thin leaves, suppressed growth of axillary shoots, decreased stem elongation, and increased lignin deposition (Lagrimini et al., 1997). The localized increases in peroxidase activity are matched by local decreases in auxin levels (Lagrimini, 1999). We have investigated whether the tobacco transgenics are similar to the duckweed lines with regard to their UV susceptibility. Leaf discs from these tobacco plants were exposed to UV radiation. The UV treatment diminished the relative variable chlorophyll fluorescence (Fv/Fm) by 16% in the non-transformed controls and by just 9% in the line overexpressing the anionic peroxidase (Fig. 7). In contrast, the antisense line was severely affected by UV-B (Fig. 7), the decrease of Fv/Fm being 28%. Differential UV tolerance was also reflected in macroscopic symptoms. Severe bronzing was noted for the antisense line, minor or no bronzing for the control and the peroxidase-overexpressing line. Thus, we conclude that the activity of an anionic peroxidase conferred both UV tolerance as well as altered auxin catabolism in transgenic tobacco plants.
Figure 7.
Effects of UV radiation on the relative variable fluorescence of tobacco transgenics altered in the expression of the anionic peroxidase. Plants were raised under long-day conditions in the greenhouse. Leaf discs were exposed for 24 h to 4.4-W m−2 UV. Before (□) and after (▪) UV treatment, the minimal fluorescence (Fo) and the maximal fluorescence (Fm) were measured on dark-adapted samples. Values represent averages of 15 to 21 measurements. ses of the mean are given. Statistical analysis (Student's t test) shows that the antisense line differs from both WT (P < 0.01) and overexpressor (P < 0.01), and that the WT differs from the overexpressor (P < 0.10), following UV treatment.
Non-physiological changes in peroxidase activity may induce a general stress tolerance pathway(s) in transgenic tobacco plants. Therefore, it was important to demonstrate that the observed stress tolerance was specific against UV radiation stress (as observed for duckweed ecotypes and mutants; Table II). We exposed tobacco leaf discs to excessive intensities of visible light, heat, and cold and found that tolerance against UV radiation was not coupled to any other stress tolerance (Table II).
DISCUSSION
Peroxidases (EC 1.11.1.7) are monomeric hemoproteins that catalyze the oxidation of a range of substrates by hydrogen peroxide. These enzymes have been implicated in physiological processes like phenol-oxidation (Kobayashi et al., 1994); cross-linking of phenolic compounds to proteins and polysaccharides; and/or the deposition of polyphenols and lignin (Lagrimini, 1991); suberization (Bernards and Lewis, 1998); pathogen resistance (Bestwick et al., 1998); and the oxidative degradation of the major endogenous auxin, IAA (Krylov and Dunford, 1996; Normanly, 1997; Gazaryan et al., 1998).
Physiological evidence indicates a role for phenol-oxidizing class III peroxidases in UV protection in natural S. punctata ecotypes as well as in L. gibba mutants (Figs. 1, 4, and 5). Using transgenic tobacco plants altered in the expression of an anionic peroxidase, we have now demonstrated, for the first time, that phenol-oxidizing class III peroxidases are able to contribute to the protection of PSII from UV radiation stress.
Peroxidases and Tolerance to UV Radiation
We observed a tight link between peroxidase activity and UV tolerance in transgenic tobacco plants as well as duckweed ecotypes and mutants. It is likely that the mechanism underlying this link is a peroxidase mediated change in leaf phenolic content and/or composition because: (a) Tolerance against UV radiation stress does not result in cotolerance to other stresses, neither in the tobacco transgenics (Table II) nor in duckweed ecotypes and mutants (Table II), which suggests a screening-based mechanism; (b) phenolics absorb strongly in the UV region of the spectrum, making them excellent screening compounds (Landry et al., 1995); (c) many phenolics are substrates for peroxidases; and (d) cross-linked, cell wall-bound polyphenols could complement the screening by soluble flavonoids that are located in the vacuoles. We have observed increased lignification in the UV-tolerant duckweed mutant mTR, although this was not visible in ecotype 760 (Fig. 6). Levels of soluble phenolics were similar in S. punctata ecotypes 203 and 760 (Jansen et al., 1999), as well as in L. gibba mutants mTR and 3F7-11 (data not shown). In the tobacco transgenics, levels of lignin increased in reponse to the overexpressing of the anionic peroxidase (Lagrimini, 1991). However, in these transgenics lines, the levels of soluble phenolics increased in parallel (Lagrimini, 1991). Future work will need to address the relative contributions of soluble phenolics and leaf lignin to UV screening in tobacco.
It could be speculated that class III peroxidases contribute to UV tolerance by removing hydrogen peroxide. Efficient scavenging of active oxygen species has been shown to alleviate UV radiation stress (Jansen et al., 1996b) and commonly results in cotolerance against a range of different stresses (Gressel and Galun, 1994). Yet, we note that neither the duckweed ecotypes and mutants nor the tobacco transgenics possess cotolerance against excessive fluences of PAR, heat, or cold (Table II). Thus, we consider it unlikely that class III peroxidases contribute to UV tolerance as a result of their oxygen radical scavenging activity.
Peroxidases, Auxin Catabolism, and Plant Architecture
Increased activity of the anionic peroxidase is not only linked to an increase in UV tolerance, but also to a decrease in auxin levels in tobacco transgenics (Lagrimini, 1999). Increased peroxidase activity similarly is correlated with increased UV tolerance as well as accelerated auxin turnover in the duckweed mTR mutant (Fig. 5). Both observations are consistent with the well-documented role of peroxidases in IAA catabolism (Krylov and Dunford, 1996; Normanly, 1997; Gazaryan et al., 1998). Although IAA catabolism is driven by peroxidases, there appears to be no direct relationship between peroxidase activity and IAA levels. This apparent paradox can be rationalized because in the overexpressing tobacco transgenic, the increase in peroxidase activity results in the local depletion of IAA (Lagrimini, 1999). However, in the UV-tolerant duckweed mTR mutant IAA levels are increased severalfold because high rates of IAA synthesis are insensitive to feedback inhibition (Tam et al., 1995). The increase in peroxidase activity is a secondary effect that prevents the accumulation of high levels of IAA in the mTR mutant.
The local decrease in auxin levels in the overexpressing tobacco plants is correlated with thin leaves and decreased axillary branching (Lagrimini et al., 1997). Low levels of auxin in duckweed ecotype 203 similarly are reflected in a decrease in leaf thickness, decreased vascular branching, as well as other changes in frond architecture (Fig. 2). Such morphological changes resemble known auxin effects. In S. punctata, these morphological alterations can be induced by treating the UV-sensitive 203 ecotype with auxin-type compounds like dicamba and TIBA (Fig. 2). However, it cannot be excluded that other peroxidase-catalyzed reactions contribute to the changes in plant architecture in transgenic tobacco. Lignification in particular may hamper cell elongation, resulting in a reduction in internode length.
We have demonstrated relationships between UV tolerance and peroxidase activity and auxin catabolism. However, it appears that IAA levels per se are not directly related to the control of UV tolerance. Increased IAA levels correlated with increased UV sensitivity in the tobacco peroxidase antisense transgenic. In contrast, increased IAA levels correlated with increased UV tolerance in duckweed ecotype 760 and mutant mTR. The strong elevation of IAA levels in the duckweed R1 line had no effect on UV tolerance. Leaf architecture, which reflects auxin levels, similarly does not appear to be a critical factor in UV tolerance under the experimental conditions. UV tolerance correlated with thin leaves in the anionic peroxidase-overexpressing tobacco (Lagrimini et al., 1997) but with relatively thick leaves in duckweed ecotype 760. Moreover, differential UV tolerance was retained in suspensions of isolated cells (Fig. 1), indicating that leaf thickness was, at best, only a minor determinant of UV tolerance in our system.
Peroxidases are capable of oxidizing a range of substrates, including many phenolics and IAA. The tobacco anionic peroxidase is associated with cell walls, thereby optimizing phenolic and IAA oxidation activities. It is the co-oxidation of phenolics and IAA that underlies the observed linkage between UV tolerance and IAA catabolism. The functional significance of this linkage is unclear. The relationship between peroxidase activity and auxin catabolism and phenol metabolism may be part of a more extensive regulatory system in the plant. The peroxidase-catalyzed oxidation of IAA is inhibited by physiological concentrations of quercetin, but promoted by the closely related flavonoid, kaempferol. Quercetin and kaempferol also act as endogenous auxin transport inhibitors, affecting IAA distribution in the plant (Jacobs and Rubery, 1988). It is interesting that the quercetin to kaempferol ratio in the plant is not constant, but increases upon UV exposure (Olsson et al., 1999).
The Environmental Relevance of Peroxidase-Mediated UV Protection
Plants raised under natural radiation conditions are generally well protected from the potentially detrimental effects of UV-B radiation. Our laboratory study has demonstrated that peroxidases can contribute to the protection of PSII from UV radiation stress. A pertinent question is whether peroxidase-mediated UV tolerance contributes to the UV acclimation of field-grown plants. S. punctata duckweed ecotypes were collected in the wild, clearly indicating differences in peroxidase activity and peroxidase-mediated UV tolerance among natural populations. However, it is not known how effective peroxidases are in protecting plants in their natural light environment. It is also not known what the relative functional contributions are of peroxidase-mediated UV tolerance and of other UV tolerance mechanisms that operate under field conditions. However, a survey of the literature shows UV-induced alterations in peroxidase activity in a number of plant species (Panagopoulos et al., 1989; Rao et al., 1996; Ambasht and Agrawal, 1997; Huang et al., 1997; Tekchandani and Guruprasad, 1998). A UV-responsive cis element has been identified in the promotor region of a rice peroxidase (Ito et al., 2000), whereas other studies have indicated UV-induced NADPH oxidase activity, leading to hydrogen peroxide production (Rao et al., 1996) and altered auxin metabolism (Ros and Tevini, 1995; Huang et al., 1997). We are led to conclude that peroxidase-mediated UV tolerance is not confined to the family of the Lemnaceae, but constitutes a widespread UV protection strategy with potential environmental relevance.
MATERIALS AND METHODS
Biological System
Spirodela punctata G.F.W. Meyer (often referred to as Spirodela oligorrhiza) ecotypes were obtained courtesy of Prof. Elias Landolt (Eidgenössische Technische Hochschule, Zurich). Ecotype 203 (referred to as NSW in Jansen et al., 1999) originates in Minto, New South Wales, Australia. Ecotype 760 (referred to as Sau in Jansen et al., 1999) has its origin at Mt. Gambier, South Australia. These ecotypes were collected about 25 to 30 years ago, and have since been grown indoors, in the absence of UV-B. Lemna gibba is a closely related member of the Lemnaceae family (Landolt and Kandeler, 1987). Four different mutant lines were obtained through the courtesy of Dr. Janet P. Slovin (U.S. Department of Agriculture, Beltsville, MD). Line R1 is a tissue culture mutant characterized by high levels of free IAA relative to its PL (Slovin and Cohen, 1988). Line mTR is generated by nitrosomethylurea treatment, and is characterized by decreased feedback inhibition of the enzyme anthranilate synthase, as well as rapid IAA turnover relative to the corresponding inbred line 3F7-11 (Tam et al., 1995). Duckweed ecotypes and mutants were grown under sterile conditions, in flasks containing one-half-strength Hutner's medium (Jansen et al., 1996a). Phototrophic growth was sustained by the continuous irradiation with 25 μmol m−2 s−1 PAR (cool-white fluorescent lamps). Plants acclimated to grow on auxin-like compounds were raised for at least 6 weeks on medium supplemented with such chemicals. For determinations of the toxicity of 2,4D and dicamba, plants were raised in open petri dishes containing medium supplemented with these compounds. Statistical analysis of biomass accumulation was based on two-factor ANOVA (Excel, Microsoft, Redmond, WA).
Tobacco (Nicotiana sylvestris) plants were grown from seed under a long-day regime (daylength > 14 h) in greenhouses. Young but fully expanded leaves from bolting plants were used for experiments. The generation of the transgenic lines that overproduce the anionic peroxidase (cauliflower mosaic virus 35S promoter) or silence this peroxidase using antisense RNA has been described in detail (Lagrimini et al., 1997). Differential activity of the anionic peroxidase was confirmed for each individual plant. A small patch of stem epidermis was isolated, homogenized using phosphate-buffered saline buffer, centrifuged (10,000g for 3 min), and the peroxidase activity of the supernatant was determined by following the oxidation of ABTS at 415 nm in potassium acetate buffer (pH 4.5) and in the presence of 2 mm H2O2 (Klotz et al., 1998).
Experimental Treatments
UV treatments consisted of a 24-h exposure of duckweed fronds or tobacco leaf discs to UV radiation, generated by Philips TL12 fluorescent tubes (λmax 315 nm; for a spectrum, see Nogués and Baker, 1995). The output of the bulb was filtered through either a UV-blocking 233 filter (50% transmission [T] at 378 nm; 10% T at 367 nm; 5% T at 364 nm) or a UV-transmitting 2458 filter covered with a single layer of cellulose acetate (50% T at 299 nm; 10% T at 293 nm, 5% T at 292 nm; Wientjes, Nieuw Vennep, The Netherlands). Irradiance at the plant level, and under the 2458 filter, was 4.4 W m−2 and this represents emission in the spectral range between 295 nm and 345 nm. Exposure times were set to result in a significant inhibition of the photosynthetic activity, as indicated in figure legends. This decrease in photosynthetic activity is largely attributed to the UV-B wavelengths because accompanying UV-A was found to be relatively ineffective in impeding PSII activity (Jansen et al., 1999). No additional PAR was applied during any of the UV treatments. UV levels were measured using an optometer (United Detector Technology Inc., Hawthorn, Baltimore) equipped with a probe specific for UV wavelengths and/or a quantum meter (LiCor, Lincoln, NE) calibrated versus the aforementioned optometer. Each experiment consisted of exposing nine to 10 (S. punctata) or eight (L. gibba) individual fronds, or 15 to 21 leaf discs (tobacco) to UV radiation.
Photoinhibitory treatment consisted of a 1-h exposure of duckweed fronds or tobacco leaf discs to a fluence rate of 1,400 μmol m−2 s−1 PAR. Radiation was generated by a projector containing a 1,000-W tungsten-halogen bulb (Hanimax, Tokyo), the output of which was filtered through 1 cm of saturated CuSO4 and a KG-4 heat-absorbing filter. Leaf material was dark adapted for at least 40 min prior to the measurement of photoinhibitory damage. Each experiment consisted of exposing four (S. punctata) or 14 (L. gibba) individual fronds, or 11 leaf discs (tobacco) to photoinhibitory light.
Heat shock treatment consisted of floating duckweed fronds for 6 min on water kept at a temperature of 42°C and tobacco discs for 8 min at 46.6°C. Plant material was kept in the dark during the treatment. Damage was assessed after 30 min of recovery in the dark. Each experiment consisted of exposing four (S. punctata) or 16 (L. gibba) individual fronds, or 17 leaf discs (tobacco) to a heat shock.
Chilling treatment consisted of a 16-h exposure of duckweed fronds or tobacco leaf discs to temperatures of −1°C and −2°C, respectively. The cold treatment was followed by a slow thawing process (4 h) and damage was measured at room temperature. Plant material was kept in the dark during the treatment. Each experiment consisted of exposing three (S. punctata) or 16 (L. gibba) individual fronds, or 17 leaf discs (tobacco) to chilling stress.
In all the experiments, ses of the mean, and Student's t tests were calculated using Excel.
Assay of Photosynthetic Performance
The photosynthetic efficiency of PSII was determined by the saturating pulse fluorescence technique (Schreiber et al., 1986), using a pulse amplitude modulation fluorimeter (Walz, Effeltrich, Germany) and/or a plant efficiency analyser (Hansatech, King's Lynn, UK). The minimal fluorescence (Fo), maximal fluorescence (Fm), and the variable fluorescence (Fv = Fm − Fo) were all measured according to vanKooten and Snel (1990). The photochemical yield of open PSII reaction centers, commonly known as the relative variable fluorescence, is calculated as Fv/Fm. It reflects the maximal efficiency of PSII that is measured in dark-adapted tissue.
Photosynthetic measurements were on intact fronds or, where indicated, suspensions of isolated mesophyll cells. Fronds were sliced using a new, sharp razor blade, vacuum infiltrated with macerozyme R10 (Serva, Heidelberg rhizopus polygalacturonase), and incubated for 60 min at room temperature in the dark. Cells were filtered through a 50-μm nylon mesh, washed, diluted to equal chlorophyll content, and exposed to UV. The suspension consisted largely of mesophyll cells. Epidermal cells and vascular strands solubilized poorly, and such non-dissolved tissue was filtered out. For each of seven repeats, cells were isolated from a new S. punctata culture and exposed to UV radiation. ses of the mean and Student's t tests were calculated using Excel.
Analysis of Endogenous IAA and Its Conjugates
IAA and abscisic acid (ABA) were analyzed using a combined solid phase extraction procedure (Prinsen et al., 1995). One hundred nanograms of 13C6-IAA (Cambridge Isotope Laboratories Inc., Andover, MA) and 50 ng 18O-ABA were used for isotope dilution purposes. Following pentafluorobenzylation, the pentafluorobenzyl esters of IAA (PFB-IAA) and ABA (PFB-ABA) were analyzed by GC-MS SIM CI− (HP5890 series II coupled to a TRIO 2000 quadrupole mass spectrometer; VG-Micromass, Winsford, Cheshire, UK) as previously described (Prinsen et al., 1995). IAA conjugates (total pool of IAA-amino esters and IAA sugar esters) analyzed from a one-third fraction of the initial sample extract were hydrolyzed in 7 n NaOH at 100°C for 3 h under a water-saturated nitrogen stream and subsequently analyzed like IAA. Data for the total IAA fraction are corrected for possible degradation of indoleacetonitrile into IAA. All data were processed using Labbase software (VG-Micromass). Each datapoint reflects an average activity, obtained by combining biomass from five separate duckweed cultures.
Analysis of Duckweed Peroxidase Activity and Lignin Deposition
Duckweed fronds were homogenized with ice-cold extraction buffer containing 50 mm [MES 2-(N-morpholino)ethanesulfonic acid, pH 5.5], 30 mm ascorbic acid, 1% (v/v) Triton X-100, 1 m NaCl, and 2% (w/v) polyvinylpyrrolidone (40,000) using a pestle and mortar. After centrifugation of the homogenate at 9,000 rpm for 30 min, the supernatant was dialyzed for 24 h against 25 mm malonate buffer (pH 5.5). The pellet formed during dialysis was removed by centrifugation (30 min, 9,000 rpm). The supernatant was loaded onto a Hi Load SP-Sepharose High Performance 26/10 FPLC column (Pharmacia, Uppsala), previously equilibrated with 25 mm Malonate (pH 5.5). A two-stage gradient of 0 to 500 mm NaCl (60 mL) and 500 to 1,000 mm NaCl (10 mL) was used to separate the peroxidase isozymes. Fractions (1 mL) were assayed for peroxidase activity by following the oxidation of ABTS at 415 nm, in potassium acetate buffer (pH 4.5), and in the presence of 2 mm H2O2. Each elution profile reflects an average activity, obtained by combining the biomass of five separate duckweed cultures. In the case of the 3F7-11 and mTR lines, we performed two further independent separations, each involving plant material from five separate cultures.
Lignin was visualized by decolorizing fronds overnight in 70% (v/v) ethanol containing 1% (w/v) phloroglucinol, and infiltrating the fronds with concentrated HCl. The orange-red stain was viewed using a light microscope.
Microscopy of Duckweed Fronds
Duckweed fronds were fixed in 5% formaldehyde, 5% acetic acid, and 90% ethanol, stepwise dehydrated using a series of ethanol solutions of increasing concentrations, and embedded in Technovit 7100 (Kulzer Hist-Tec). Cross-sections of 3- to 7-μm thickness were prepared using a rotary microtome, stained with toluidine-blue, and studied using a light microscope (Zeiss, Jena, Germany). The dehydration procedure may cause the fronds to shrink. To obtain accurate measurements of leaf thickness, 20- to 40-μm-thick sections of fresh duckweed fronds were prepared on a microtome and studied under a light microscope in the absence of ethanol.
ACKNOWLEDGMENTS
The authors thank Prof. E. Landolt for kindly providing S. punctata ecotypes, Dr. J.P. Slovin for generously providing us with L. gibba mutants, and Dr. Phil M. Mullineaux (Department of Disease and Stress Biology, John Innes Centre, Norwich, UK) for comments on the manuscript.
Footnotes
M.A.K.J. was supported by the Royal Netherlands Academy of Arts and Sciences and by the European Community (Training, Mobility, and Research Network “Peroxidases in Agriculture, the Environment, and Industry,” contract no. ERB–FMRXCT–980200).
LITERATURE CITED
- Ambasht NK, Agrawal M. Influence of supplemental UV-B radiation on photosynthetic characteristics of rice plants. Photosynthetica. 1997;34:401–408. [Google Scholar]
- Babu TS, Jansen MAK, Greenberg BM, Gaba V, Malkin S, Mattoo AK, Edelman M. Amplified degradation of photosystem II D1 and D2 proteins under a mixture of photosynthetically active radiation and UV-B radiation: dependence on redox status of photosystem II. Photochem Photobiol. 1999;69:553–559. [Google Scholar]
- Ballaré CL, Barnes PW, Flint SD. Inhibition of hypocotyl elongation by ultraviolet-B radiation in de-etiolating tomato seedlings: I. The photoreceptor. Physiol Plant. 1995;93:584–592. [Google Scholar]
- Barber J, Nield J, Morris EP, Zheleva D, Hankamer B. The structure, function and dynamics of photosystem 2. Physiol Plant. 1997;100:817–827. [Google Scholar]
- Barnes PW, Flint SD, Caldwell MM. Morphological responses of crop and weed species of different growth forms to ultraviolet-B radiation. Am J Bot. 1990;77:1354–1360. [Google Scholar]
- Bernards MA, Lewis NG. The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry. 1998;47:915–933. doi: 10.1016/s0031-9422(98)80052-6. [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Mansfield JW. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol. 1998;118:1067–1078. doi: 10.1104/pp.118.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt AB. DNA damage and repair in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:75–100. doi: 10.1146/annurev.arplant.47.1.75. [DOI] [PubMed] [Google Scholar]
- Cen Y-P, Bornman JF. The response of bean plants to UV-B radiation under different irradiances of background visible light. J Exp Bot. 1990;41:1489–1495. [Google Scholar]
- Fiscus EL, Booker FL. Is increased UV-B a threat to crop photosynthesis and productivity? Photosynth Res. 1995;43:81–92. doi: 10.1007/BF00042965. [DOI] [PubMed] [Google Scholar]
- Gazaryan IG, Lagrimini LM, Mellon FA, Naldrett MJ, Ashby GA, Thorneley RNF. Identification of the skatolyl hydroperoxide and its role in the peroxidase-catalyzed oxidation of indol-3-yl acetic acid. Biochem J. 1998;333:223–232. doi: 10.1042/bj3330223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg BM, Wilson MI, Huang X-D, Duxbury CL, Gerhardt KA, Gensemer RW. The effects of ultraviolet-B radiation on higher plants. In: Wang W, Gorsuch J, Hughes JS, editors. Plants for Environmental Studies. Boca Raton, FL: CRC Press; 1997. pp. 1–35. [Google Scholar]
- Gressel J, Galun E. Genetic controls of photooxidant tolerance. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 237–273. [Google Scholar]
- Huang S, Dai Q, Peng S, Chavez AQ, Miranda MLL, Visperas RM, Vergara BS. Influence of supplemental ultraviolet-B on indoleacetic acid and calmodulin in the leaves of rice (Oryza sativaL.) Plant Growth Regul. 1997;21:59–64. [Google Scholar]
- Ito H, Hiraga S, Tsugawa H, Matsui H, Honma M, Otsuki Y, Murakami T, Ohashi Y. Xylem-specific expression of wound-inducible rice peroxidase genes in transgenic plants. Plant Sci. 2000;155:85–100. doi: 10.1016/s0168-9452(00)00209-0. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH. Naturally occuring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- Jansen MAK, Babu TS, Heller D, Gaba V, Mattoo AK, Edelman M. Ultraviolet-B effects on Spirodela oligorrhiza: induction of different protection mechanisms. Plant Sci. 1996b;115:217–223. [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;3:131–135. [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM, Mattoo AK, Edelman M. Low threshold levels of ultraviolet-B in a background of photosynthetically active radiation trigger rapid degradation of the D2 protein of photosystem II. Plant J. 1996a;9:693–699. [Google Scholar]
- Jansen MAK, vandenNoort RE, Boeke SJ, Huggers SAM, deHaan JH. Differences in UV-B tolerance among Spirodela punctataecotypes. J Photochem Photobiol B Biol. 1999;48:194–199. [Google Scholar]
- Jordan BR. The effect of ultraviolet-B radiation on plants: a molecular perspective. Adv Bot Res. 1996;22:97–161. [Google Scholar]
- Klotz KL, Liu TTY, Liu L, Lagrimini LM. Expression of the tobacco anion peroxidase gene is tissue specific and developmentally regulated. Plant Mol Biol. 1998;36:509–520. doi: 10.1023/a:1005939600344. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Koguchi Y, Kanzaki H, Kajiyama S, Kawazu K. A new type of antimicrobial phenolics produced by plant peroxidase and its possible role in the chemical defense systems against plant pathogens. Z Naturforsch. 1994;49:411–414. doi: 10.1515/znc-1994-7-804. [DOI] [PubMed] [Google Scholar]
- Krylov SN, Dunford HB. Detailed model of the peroxidase-catalyzed oxidation of indole-3-acetic acid at neutral pH. J Phys Chem. 1996;100:913–920. [Google Scholar]
- Lagrimini LM. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 1991;96:577–583. doi: 10.1104/pp.96.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini LM. The role of peroxidase in auxin metabolism. In: Lagrimini LM, editor. Abstracts Peroxidase ‘99. International Symposium for Plant Peroxidases. Ohio State University, Columbus. 1999. p. 27. [Google Scholar]
- Lagrimini LM, Gingas V, Finger F, Rothstein S, Liu T-TY. Characterization of antisense transformed plants deficient in the tobacco anionic peroxidase. Plant Physiol. 1997;114:1187–1196. doi: 10.1104/pp.114.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt E, Kandeler R. The Family of Lemnaceae, a Monographic Study. Vol. 2. 1987. (heft 95), Biosystematic Investigations in the Family of Duckweeds (Lemnaceae) Vol 4. Veröffentlichungen des Geobotanischen Institutes der Eidgenössisch Technische Hochschule, Stiftung Rübel, Zurich. [Google Scholar]
- Landry LG, Chapple CCS, Last RL. Arabidopsismutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995;109:1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogués S, Baker NR. Evaluation of the role of damage to photosystem II in the inhibition of CO2assimilation in pea leaves on exposure to UV-B radiation. Plant Cell Environ. 1995;18:781–787. [Google Scholar]
- Normanly J. Auxin metabolism. Physiol Plant. 1997;100:431–442. [Google Scholar]
- Olsson LC, Veit M, Bornman JF. Epidermal transmittance and phenolic composition in leaves of atrazine-tolerant and atrazine-sensitive cultivars of Brassica napusgrown under enhanced UV-B radiation. Physiol Plant. 1999;107:259–266. [Google Scholar]
- Panagopoulos I, Bornman JF, Björn LO. The effect of UV-B and UV-C radiation on Hibiscusleaves determined by ultraweak luminescence and fluorescence induction. Physiol Plant. 1989;76:461–465. [Google Scholar]
- Prinsen E, Redig P, Strnad M, Galis I, van Dongen W, van Onckelen H. Quantifying phytohormones in transformed plants. In: Gartland K, Davey M, editors. Methods in Molecular Biology. 44, Agrobacterium Protocols. Totowa, NJ: Humana Press Inc.; 1995. pp. 245–262. [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–136. doi: 10.1104/pp.110.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros J, Tevini M. Interaction of UV-radiation and IAA during growth of seedlings and hypocotyl segments of sunflower. J Plant Physiol. 1995;146:295–302. [Google Scholar]
- Rozema J, vandeStaaij J, Björn LO, Caldwell M. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol. 1997;12:22–28. doi: 10.1016/s0169-5347(96)10062-8. [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- Slovin JS, Cohen JD. Levels of indole-3-acetic acid in Lemna gibba G-3 and in a large Lemnamutant regenerated from tissue culture. Plant Physiol. 1988;86:522–526. doi: 10.1104/pp.86.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strid A, Chow WS, Anderson JM. UV-B damage and protection at the molecular level in plants. Photosynth Res. 1994;39:475–489. doi: 10.1007/BF00014600. [DOI] [PubMed] [Google Scholar]
- Sullivan JH, Teramura AH, Ziska LH. Variation in UV-B sensitivity in plants from a 3,000-m elevational gradient in Hawaii. Am J Bot. 1992;79:737–743. [Google Scholar]
- Tam YY, Slovin JP, Cohen JD. Selection and characterization of α-methyltryptophan resistant lines of Lemna gibbashowing a rapid rate of indole-3-acetic acid turnover. Plant Physiol. 1995;107:77–85. doi: 10.1104/pp.107.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekchandani S, Guruprasad KN. Modulation of a guaiacol peroxidase inhibitor by UV-B in cucumber cotyledons. Plant Sci. 1998;136:131–137. [Google Scholar]
- Teramura AH, Sullivan JH. Effects of UV-B radiation on photosynthesis and growth of terrestrial plants. Photosynth Res. 1994;39:463–473. doi: 10.1007/BF00014599. [DOI] [PubMed] [Google Scholar]
- vanKooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Ziska LH, Teramura AH, Sullivan JH, McCoy A. Influence of ultraviolet-B (UV-B) radiation on photosynthetic and growth characteristics in field-grown cassava (Manihot esculentumCrantz) Plant Cell Environ. 1993;16:73–79. [Google Scholar]