Abstract
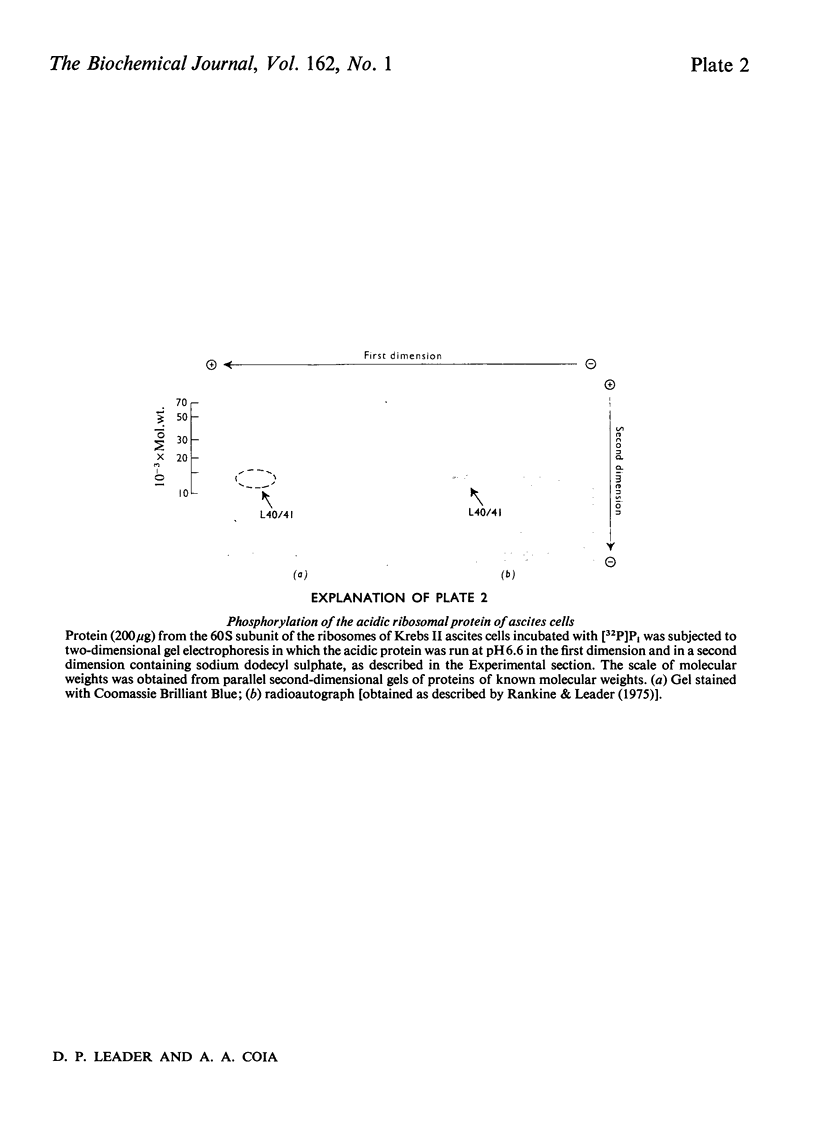
The ribosomes of Krebs II ascites cells contain an acidic protein, apparently analogous to proteins L7/12 of Escherichia coli. When ascites cells were incubated with [32P]Pi, this protein became labelled, indicating that it is a phosphoprotein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creusot F., Delaunay J., Schapira G. Eukaryotic ribosomal proteins. Molecular weights of proteins from rabbit liver subunits. Biochimie. 1975;57(2):167–173. doi: 10.1016/s0300-9084(75)80167-2. [DOI] [PubMed] [Google Scholar]
- Gordon J. Determination of an upper limit to the phosphorus content of polypeptide chain elongation factor and ribosomal proteins in Escherichia coli. Biochem Biophys Res Commun. 1971 Aug 6;44(3):579–586. doi: 10.1016/s0006-291x(71)80122-5. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Traugh J. A., Croser E. A., Traut R. R. Ribosomal proteins from rabbit reticulocytes: number and molecular weights of proteins from ribosomal subunits. J Mol Biol. 1975 Apr 15;93(3):391–404. doi: 10.1016/0022-2836(75)90285-5. [DOI] [PubMed] [Google Scholar]
- Issinger O. G., Traut R. R. Selective phosphorylation from GTP of proteins L7 and L12 of E. coli 50S ribosomes by a protein kinase from rabbit reticulocytes. Biochem Biophys Res Commun. 1974 Aug 5;59(3):829–836. doi: 10.1016/s0006-291x(74)80054-9. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Leader D. P. Enzymic iodination of eukaryotic ribosomal subunits. Characterization and analysis by two-dimensional gel electrophoresis. Biochem J. 1975 Nov;152(2):373–378. doi: 10.1042/bj1520373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Wool I. G. The molecular weights of rat liver ribosomal proteins determined by "three-dimensional" polyacrylamide gel electrophoresis. Mol Gen Genet. 1974;134(1):1–6. doi: 10.1007/BF00332807. [DOI] [PubMed] [Google Scholar]
- Martini O. H., Gould H. J. Enumeration of rabbit reticulocyte ribosomal proteins. J Mol Biol. 1971 Dec 14;62(2):403–405. doi: 10.1016/0022-2836(71)90435-9. [DOI] [PubMed] [Google Scholar]
- Möller W., Slobin L. I., Amons R., Richter D. Isolation and characterization of two acidic proteins of 60s ribosomes from Artemia salina cysts. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4744–4748. doi: 10.1073/pnas.72.12.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson I., Hardy S. J., Liljas A. The ribosomal protein L8 is a complex L7/L12 and L10. FEBS Lett. 1976 Apr 15;64(1):135–138. doi: 10.1016/0014-5793(76)80267-0. [DOI] [PubMed] [Google Scholar]
- Rankine A. D., Leader D. P. The phosphorylation of ascites cell ribosomes in vivo: identification of a phosphorylated protein of the small ribosomal subunit by two-dimensional gel electrophoresis. FEBS Lett. 1975 Apr 1;52(2):284–287. doi: 10.1016/0014-5793(75)80826-x. [DOI] [PubMed] [Google Scholar]
- Rankine A. D., Leader D. P. The variable location of phosphoproteins on eukaryotic ribosomal subunits. Mol Biol Rep. 1976 Jul;2(6):525–528. doi: 10.1007/BF00356942. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Sherton C. C., Wool I. G. A comparison of the proteins of rat skeletal muscle and liver ribosomes by two-dimensional polyacrylamide gel electrophoresis. Observations on the partition of proteins between ribosomal subunits and a description of two acidic proteins in the large subunit. J Biol Chem. 1974 Apr 10;249(7):2258–2267. [PubMed] [Google Scholar]
- Stöffler G., Wool I. G., Lin A., Rak K. H. The identification of the eukaryotic ribosomal proteins homologous with Escherichia coli proteins L7 and L12. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4723–4726. doi: 10.1073/pnas.71.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao K., Ogata K. Studies on structural proteins of the rat liver ribosomes. I. Molecular wights of the proteins of large and small subunits. Biochim Biophys Acta. 1975 Aug 21;402(2):214–229. doi: 10.1016/0005-2787(75)90041-6. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Möller W., Laursen R., Wittmann-Liebold B. The primary structure of an acidic protein from 50-S ribosomes of Escherichia coli which is involved in GTP hydrolysis dependent on elongation factors G and T. Eur J Biochem. 1973 Apr 2;34(1):138–152. doi: 10.1111/j.1432-1033.1973.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Zinker S., Warner J. R. The ribosomal proteins of Saccharomyces cerevisiae. Phosphorylated and exchangeable proteins. J Biol Chem. 1976 Mar 25;251(6):1799–1807. [PubMed] [Google Scholar]





