Abstract
Previous studies have shown that salicylic acid (SA) is an essential component of the plant resistance to pathogens. We now show that SA plays a role in the plant response to adverse environmental conditions, such as salt and osmotic stresses. We have studied the responses of wild-type Arabidopsis and an SA-deficient transgenic line expressing a salicylate hydroxylase (NahG) gene to different abiotic stress conditions. Wild-type plants germinated under moderate light conditions in media supplemented with 100 mm NaCl or 270 mm mannitol showed extensive necrosis in the shoot. In contrast, NahG plants germinated under the same conditions remained green and developed true leaves. The lack of necrosis observed in NahG seedlings under the same conditions suggests that SA potentiates the generation of reactive oxygen species in photosynthetic tissues during salt and osmotic stresses. This hypothesis is supported by the following observations. First, the herbicide methyl viologen, a generator of superoxide radical during photosynthesis, produced a necrotic phenotype only in wild-type plants. Second, the presence of reactive oxygen-scavenging compounds in the germination media reversed the wild-type necrotic phenotype seen under salt and osmotic stress. Third, a greater increase in the oxidized state of the glutathione pool under NaCl stress was observed in wild-type seedlings compared with NahG seedlings. Fourth, greater oxidative damage occurred in wild-type seedlings compared with NahG seedlings under NaCl stress as measured by lipid peroxidation. Our data support a model for SA potentiating the stress response of the germinating Arabidopsis seedling.
Environmental stresses such as salt (NaCl) and drought are among the factors most limiting to plant productivity (Greenway and Munns, 1980; Boyer, 1982; Bohnert et al., 1995). Such stresses are becoming even more prevalent as the intensity of agriculture increases. Therefore, elucidation of the mechanisms by which plants perceive and transduce these stresses is critical if we are to understand the plant response and introduce genetic or environmental improvement to stress tolerance. Previous studies in many plant species indicate that drought and salt tolerance are developmentally regulated, stage-specific phenomena because tolerance at one stage of plant development is not necessarily correlated with tolerance at other stages (Lauchli and Epstein, 1990; Johnson et al., 1992). Therefore, we must study the mechanisms of tolerance at specific stages of plant development, such as seed germination, if we are to understand the biochemical events that play an important role in the responses to salt or other abiotic stresses.
Salt stress can affect several physiological processes, from seed germination to plant development. The complexity of the plant response to salt stress can be partially explained by the fact that salinity imposes both an ionic and an osmotic stress (Pasternak, 1987). Photosynthesis, a key metabolic pathway in plants, is a target for salt stress. The abscisic acid produced in response to salt stress decreases turgor in guard cells and limits the CO2 available for photosynthesis (Leung et al., 1994). Moreover, during water stress brought about by salt stress, reduction of chloroplast stromal volume and generation of reactive oxygen species (ROS) are also thought to play important roles in inhibiting photosynthesis (Price and Hendry, 1991). ROS can be generated in the chloroplast by direct transfer of excitation energy from chlorophyll to produce singlet oxygen, or by univalent oxygen reduction at photosystem I, in the Mehler reaction (Foyer et al., 1994; Allen, 1995).
Salicylic acid (SA) plays an important role in the defense response in many plant species to pathogen attack. SA mediates the oxidative burst that leads to cell death in the hypersensitive response, and acts as a signal for the development of the systemic acquired resistance (Shirasu et al., 1997). Several studies also support a major role of SA in modulating the plant response to several abiotic stresses (Yalpani et al., 1994; Senaratna et al., 2000). A known effect of SA is to participate in the increase of the temperature in thermogenic plants (Raskin et al., 1987). Treating mustard seedlings with exogenous SA improved their thermotolerance and heat acclimation (Dat et al., 1998). In maize plants, pretreatment with SA induced antioxidant enzymes, which in turn increased chilling tolerance (Janda et al., 1999). Recent studies used an Arabidopsis transgenic line expressing the salicylate hydroxylase gene (NahG) to reduce levels of SA and to monitor its response to ozone (O3). This finding demonstrated that SA is required for O3 tolerance by maintaining the cellular redox state and allowing defense responses (Sharma et al., 1996). However, by using Cvi-0, an Arabidopsis genotype that accumulated high levels of SA, it was shown that SA activates an oxidative burst and a cell death pathway leading to O3 sensitivity (Rao and Davis, 1999).
Both salt and osmotic stress lead to oxidative stress and severe impairment of seedling survival. In this work, we show that SA is involved in the plant response to salt and osmotic stress by playing a role in the ROS-mediated damage caused by high salt and osmotic conditions. We conclude that SA greatly potentiates the effects of salt and osmotic stresses by enhancing ROS generation during photosynthesis and germination of Arabidopsis.
RESULTS
Lack of SA Enhances Arabidopsis Germination under Salt and Osmotic Stress in the Light
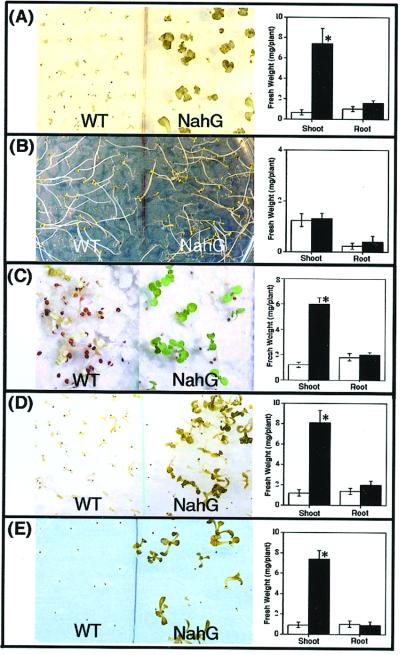
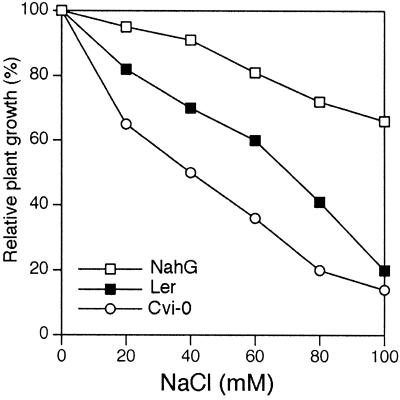
Previous studies indicated that SA plays an important role in the plant sensitivity to different types of abiotic stress (Dat et al., 1998; Rao and Davis, 1999). However, none of these studies has provided information about the possible role of SA during abiotic stress such as high NaCl or osmotic stress. It has been shown previously that salt stress sensitivity is increased in Arabidopsis by moderate light intensities (Tsugane et al., 1999). To investigate the possible role of SA in salt stress, seeds of wild-type Arabidopsis genotype Landsberg erecta (Ler) and the SA-deficient transgenic NahG Arabidopsis were germinated in several concentrations of NaCl at moderate light intensity. At 100 mm NaCl, wild-type seedlings were unable to expand and develop their cotyledons showing an extensive necrosis, whereas NahG seedlings germinated and developed expanded cotyledons and the first true leaves (Fig. 1A). A closer observation of the seedling's phenotype indicated that the parts most affected by salt stress were the photosynthetic tissues. This was confirmed by analyzing the fresh weight of the root and shoot of wild-type and NahG seedlings (Fig. 1A). The shoot of the NahG seedlings weighted around seven times more than the wild type after 15 d in 100 mm NaCl growing under light. However, no significant differences in terms of fresh weight were found in the root, though a different morphology was observed. When germinated in the dark either in the absence or the presence of NaCl in the medium, no differences were found between NahG and wild-type plants (Fig. 1B). We used a specific set of circumstances, as seedlings grown in agar plates and sterile conditions. Therefore, we determined whether a similar phenotype was reproducible in a medium more like soil, such as perlite and using nutrient solution with NaCl added. As shown in Figure 1C, a similar lethal phenotype only in wild type when germinated under high NaCl was observed. However, the NaCl concentration that mimicked such a phenotype was 250 mm NaCl. An Arabidopsis ecotype, Cvi-0, that hyperaccumulates SA upon oxidative stress has been described (Rao and Davis, 1999). We determined whether seedling growth was differentially affected in wild-type, NahG, and Cvi-0 seedlings. As shown in Figure 2, Cvi-0 seedlings were more sensitive to all NaCl concentrations tested.
Figure 1.
Phenotype of wild-type and NahG seedlings germinated under different stress conditions. Seedlings were germinated on plates and either grown under light (approximately 39 μmol m−2 s−1) or in the dark. Photos of plates after 15 d are shown on left. The seedlings then were collected and weighed (right). The photographs shown are representative of three independent trials and the fresh weight values are the means of three different experiments (n = 50) ±se. Asterisks indicate that mean values are significantly different between wild type and NahG (P < 0.05). A, 100 mm NaCl in the light in a petri dish. B, 100 mm NaCl in the dark in a petri dish. C, 250 mm NaCl in the light in perlite. D, 270 mm mannitol in the light in a petri dish. E, 5 nm methyl viologen (MV) in the light in a petri dish.
Figure 2.
The Arabidopsis ecotype Cvi-0 showed greater sensitivity to NaCl than Ler and NahG. Fresh weight of wild type, NahG, and Cvi-0 seedlings after growing in Murashige and Skoog media containing different NaCl concentrations. Seedlings were germinated and grown on plates under light (approximately 39 μmol m−2 s−1) and after 15 d the seedlings were collected and weighed. The experiment shown is representative of three independent trials (n = 50).
The response of wild-type and NahG seedlings to mannitol was analyzed to determine whether the observed differences were due to the osmotic or to the ionic component of the NaCl stress. Wild-type seed germination was more sensitive to 270 mm mannitol under light than NahG seeds (Fig. 1D). This suggests that the osmotic component generated by NaCl is responsible for the necrotic phenotype. Differences in fresh weight between wild-type and NahG seedlings for the mannitol treatment were found to be similar to the NaCl experiments, and these differences were also restricted to the photosynthetic tissues (Fig. 1D). Lithium is considered to be a more toxic analog for Na+, and has been used to create ionic toxicity without osmotic stress (Wu et al., 1996). No differences were found in germination between wild type and NahG at various Li+ concentrations (data not shown). This suggests that the ionic component of the NaCl stress by itself does not induce the observed necrosis.
ROS Mediate the SA Stress Response
The coupling of salt sensitivity to light exposure only in wild-type seedlings of Arabidopsis suggested that high NaCl enhanced the production of ROS, and that somehow SA could be involved in the increased ROS. This role of SA in the generation of ROS could explain the increased tolerance of NahG seedlings to NaCl. To test this hypothesis, we changed the levels of ROS in wild-type and NahG seedlings to see if they mimicked salt or osmotic stress. To increase ROS levels, we used the electron transfer uncoupler MV. The herbicide MV generates superoxide radicals during photosynthesis that cause damage to photosystems I and II (Dodge, 1994). Wild-type and NahG seedlings were germinated in the presence of several concentrations of MV. A similar phenotype to the one previously observed with NaCl or mannitol was obtained using 5 nm of MV (Fig. 1E). NahG seedlings were more tolerant during germination than wild type to the increased ROS generated by the presence of 5 nm of MV in the medium.
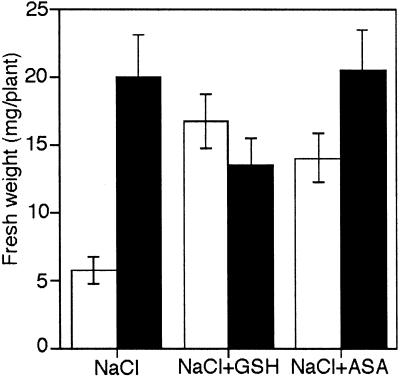
To reduce ROS levels, we used common quenching agents such as reduced glutathione (GSH) and ascorbic acid (ASA). Thus, the addition of 3 mm GSH to the germination medium reversed the toxic effect caused by NaCl to wild-type seedlings, suggesting that changes in the redox state take place under NaCl stress (Fig. 3). In contrast, the addition of oxidized glutathione (GSSG) increased the adverse effect of NaCl to both NahG and wild-type seedlings, both of them being unable to germinate in 100 mm NaCl (data not shown). The addition of 2 mm ASA also improved the germination and growth of wild-type seedlings in 100 mm NaCl (Fig. 3).
Figure 3.
Protection against NaCl stress is increased in wild-type seedlings by GSH and ASA. Fresh weight of wild-type seedlings (white bars) and NahG seedlings (black bars) after growing in Murashige and Skoog media containing 100 mm NaCl and supplemented with 3 mm of reduced glutathione (GSH) or 2 mm of ASA. Seedlings were collected and weighted after 15 d. The values shown are the means of three independent experiments. Error bars indicate se (n = 30).
Because we determined that increased GSH in the media could alleviate the NaCl phenotype during germination, we determined the levels of gluthatione in wild-type and NahG plants after NaCl stress (Table I). NaCl produced both a decrease in GSH and an increase in GSSG in wild-type seedlings. This resulted in a reduction of the GSH/GSSG ratio from 6.6 to 0.6. In NahG plants, the ratio GSH/GSSG declined from 7.2 to 2.8.
Table I.
GSH levels and redox state of GSH after NaCl stress are dependent on SA
| Treatment | Ler
|
NahG
|
||||
|---|---|---|---|---|---|---|
| GSH | GSSG | GSH/GSSG | GSH | GSSG | GSH/GSSG | |
| Control | 6.6 ± 2.5 | 1.0 ± 0.1 | 6.6 | 6.9 ± 2.1 | 1.0 ± 0.2 | 7.2 |
| 200 mm NaCl | 0.9a ± 1.1 | 1.4a ± 0.2 | 0.6a | 0.9a ± 0.6 | 0.3a ± 0.1 | 2.8a |
The GSH content is expressed as micromoles per gram fresh wt. Measurements are from control seedlings and seedlings treated with 200 mM NaCl as described in “Materials and Methods.” The mean values shown (±se) are the averages of two independent experiments.
Indicates values that are significantly different compared with their respective genotype controls (P > 0.05).
SA is transformed to catechol in NahG plants. Therefore, we wanted to determine that the observed differences were not due to the antioxidant properties of the catechol presumably accumulated in NahG seedlings. Experiments using various concentrations of catechol in the medium between wild-type or NahG plants under 100 mm NaCl failed to show significant differences (data not shown).
SA Increases Lipid Peroxidation Induced by NaCl
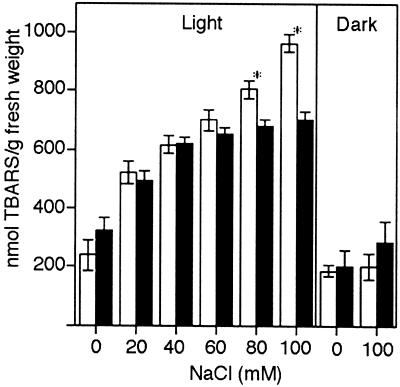
Oxidative damage can be assessed by monitoring changes in lipid peroxidation (Rao et al., 1997; Rao and Davis, 1999). To determine whether NaCl caused oxidative damage in wild-type and NahG seedlings, we monitored changes in lipid peroxidation by measuring the thiobarbituric acid-reactive substances (TBARS) at various NaCl concentrations under moderate light or under dark conditions (Fig. 4). Increasing NaCl concentrations increased the peroxidation of lipids in both wild-type and NahG plants under light. This increase was significantly higher in wild-type plants than in NahG plants at high NaCl concentrations. No significant differences were observed when the NaCl treatment was performed in the dark (Fig. 4).
Figure 4.
Lipid peroxidation is induced by NaCl only under light. TBARS content was determined as described in “Materials and Methods.” The values shown are the means of three independent experiments. Wild type, white bars; NahG, black bars. Asterisks indicate that mean values are significantly different between wild type and NahG (P < 0.05). Error bars indicate se (n = 50).
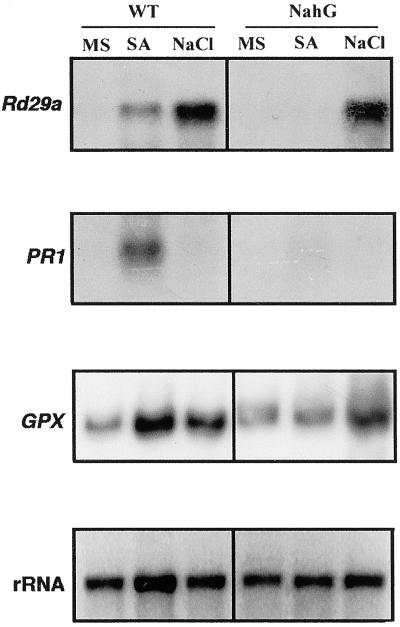
Expression Analysis of RD29A, PR1, and Glutathione Peroxidase (GPX)
To investigate the relationship between SA, NaCl stress, and oxidative stress, we analyzed the expression of the genes RD29A, PR1, and GPX, whose expression have been reported to increase after NaCl, SA, and oxidative stress, respectively. RD29A gene expression is induced by NaCl and osmotic stresses and encodes a protein with potential protective function during desiccation (Yamaguchi-Shinozaki and Shinozaki, 1993a, 1993b). The PR1 gene expression is induced by SA and pathogen attack (Hammond-Kosack and Jones, 1996). Therefore, it can be considered as a molecular marker for SA accumulation. Plants are capable of removing ROS using several antioxidant enzymes such as GPX (Rao and Davis, 1999). Therefore, GPX expression can be considered as a molecular marker for oxidative stress.
As shown in Figure 5, the expression of RD29A is induced by NaCl but also moderately by SA. In NahG plants, RD29A is induced by NaCl, which suggests that this induction is independent of SA. SA but not NaCl induces PR1 gene expression in wild-type plants. As expected, SA does not induce PR1 expression in NahG plants, because SA is actively degraded to catechol. Both SA and NaCl increased GPX expression in wild-type plants. It is interesting that GPX expression is also induced in NahG plants by NaCl, suggesting that NaCl produce an oxidative stress independent of SA. This is consistent with the increased lipid peroxidation in NahG plants caused by NaCl.
Figure 5.
Effect of NaCl and SA on the expression of RD29A and PR1 in wild-type and NahG. Ten micrograms of total RNA from the wild-type and NahG seedlings was loaded per lane. Plants were grown in Murashige and Skoog media as a control (MS), treated with 1 mm SA, or treated with 200 mm NaCl as described in “Materials and Methods.”
DISCUSSION
Changes in plant metabolism occur in response to the osmotic stress and the ionic imbalance caused by salinity (Bohnert et al., 1995; Bray, 1997). In addition, an oxidative stress has been reported to result from exposure of plants to osmotic stress that could also be responsible for the damage caused to the plants grown under high NaCl concentration (Smirnoff, 1993). However, the contribution and interaction among these components that may eventually end in plant death remains elusive. Our studies now show that SA is directly involved in the changes taking place in the plant under salt and osmotic stress. This interaction is further supported by recent data showing that osmotic stress can induce the activation of a SA-induced protein kinase (Mikolajczyk et al., 2000).
In Arabidopsis, SA has been proposed to have a dual role. First, SA is necessary for the induction of antioxidant defenses and maintaining the redox state of the gluthatione pool (Sharma et al., 1996). Thus, SA has been shown to be essential for the plant protection against the oxidative stress generated by O3 (Rao and Davis, 1999). Second, an excessive SA accumulation can induce a programmed cell death pathway, leading to a hypersensitive reaction in response to O3 (Rao and Davis, 1999). NaCl treatment decreased by approximately 91% the GSH/GSSG ratio in wild type, whereas in NahG seedlings the GSH/GSSG ratio decreased only approximately 71% after exposure to NaCl (Table I). This represents a final GSH/GSSG ratio of 0.6 in wild type versus 2.8 in NahG seedlings after NaCl stress. This result points to an SA-mediated effect of NaCl on the oxidized state in the glutathione pool that may explain the observed phenotype. This is supported by several reports showing that elevated levels of GSH are associated with increased oxidative stress tolerance. Thus, transgenic plants overexpressing glutathione reductase had both elevated levels of GSH and increased tolerance to oxidative stress in leaves (Broadbent et al., 1995). Here, we show that addition of chemical agents that reduce ROS levels also reduces the damaging effect of salt and osmotic stress, supporting the hypothesis that increased ROS is the primary cause of the seedling lethality under these stressing conditions. The Arabidopsis ecotype Cvi-0 has been shown to have high endogenous levels of SA (Rao and Davis, 1999). Our studies show that the Arabidopsis ecotype Cvi-0 is more sensitive to NaCl than the Arabidopsis ecotype Ler and NahG seedlings, supporting a role of SA in the increased Cvi-0 sensitivity.
NaCl treatment did not induce PR1 gene expression, a marker frequently used for SA accumulation, suggesting that if an increase of SA takes place under salt stress, it must be below the threshold required for PR1 induction. Thus, we propose for SA a similar role in stress response to the one proposed previously for plant-pathogen interaction; namely, that SA could be a signaling molecule forming a feedback amplification cycle in concert with ROS (Jabs, 1999). In this way, SA induction is not required but the endogenous SA present amplifies the effects of ROS initial levels. This is supported by our data showing that increased lipid peroxidation and GPX induction occurred in NahG seedlings at high NaCl. Moreover, the lack of SA in NahG Arabidopsis seedlings is not sufficient to protect these seedlings at very high levels of NaCl and mannitol (data not shown). This indicates that part of the oxidative stress generated during NaCl and mannitol exposure is independent of the presence of SA.
An Arabidopsis mutant with increased photoautotropic growth under salt stress recently has been isolated (Tsugane et al., 1999). This mutant showed enhanced ROS detoxification and was more tolerant to NaCl and MV than wild-type seedlings. The identification of this mutant suggests that the oxidative stress generated by NaCl can be critical for salt tolerance in certain environmental conditions and developmental stages.
In conclusion, this study contributes to define a role of SA during the NaCl or osmotic stresses. SA increases the oxidative damage generated by NaCl and osmotic stresses, which in turn is critical for seedling lethality under these conditions.
MATERIALS AND METHODS
Plant Culture
Seeds of Arabidopsis ecotype Landsberg erecta (Ler) and the transgenic NahG plants (ecotype Ler) were surface sterilized in 20% (v/v) commercial bleach for 20 min, followed by six washes with sterile distilled water. NahG seeds were provided by Jane Parker (John Innes Centre, Norwich, UK). The seeds were sown onto agar plates for germination. The basal agar medium contained Murashige and Skoog salts (Murashige and Skoog, 1962) with 2% (w/v) Suc and 0.7% (w/v) agar. The various agar plates used in this work were made by adding the appropriate amount of NaCl, mannitol, MV, GSH, GSSG, and ASA to the molten basal media. The plates with the seeds were placed at 4°C in the dark for 48 h to improve germination uniformity before transfer to growth chambers with 16 h of light (approximately 39 μmol m−2 s−1) at 22°C, 8 h of dark at 18°C, and 70% relative humidity for 15 d.
For GSH, GSSG determination, and gel-blot analysis, approximately 50 15-d-old seedlings were transferred from Murashige and Skoog plates to 1,000-mL flasks containing 500 mL of Murashige and Skoog solution and 2% (w/v) Suc. The Murashige and Skoog media was supplemented with the appropriate amount of NaCl to give a final concentration of 200 mm. The flasks were shaken at 120 rpm at 22°C with continuous cool fluorescent light illumination (approximately 80 μmol m−2 s−1). Eight hours later, the seedlings were collected from the flasks and frozen immediately in liquid nitrogen. The samples were ground in liquid nitrogen and kept at −80°C until use.
Lipid Peroxidation
Lipid peroxidation was estimated by measuring the TBARS as previously described with some modifications (Iturbe-Ormaetxe et al., 1998). Arabidopsis seedlings were harvested and ground using liquid nitrogen. Lipid peroxides were extracted from 0.5 g of powder using 2.5 mL of sodium phosphate buffer (0.2 m, pH 7.6), 1% (v/v) Triton X-100, and 1% (w/v) butylhydroxytoluene. The homogenate was centrifuged at 15,000g for 20 min at 4°C and 0.150 mL of the supernatant was mixed with 0.3 mL of 10% (w/v) trichloroacetic acid and boiled for 20 min. The mixture was centrifuged at 12,000g for 2 min. The supernatant was mixed with 0.15 mL 3% (w/v) SDS, 0.25 mL 3% (w/v) 2-thiobarbituric acid, and 0.25 mL 25% (v/v) HCl and vortexed. The mixture was heated at 80°C for 20 min and cooled in ice. The lipid peroxides were expressed as nanomoles of malonaldehyde, forming ε532 = 156 × 103 m−1 cm−1.
Determination of GSH and GSSG
Approximately 200 mg of the resulting powder described above was resuspended in 0.5 mL of 5% (w/v) sulfosalicylic acid and sonicated over 10 min. Extraction and determination of GSH and GSSG was as described previously (Law et al., 1983).
RNA Gel-Blot Analysis
RNA was extracted from the frozen tissue as described previously (Botella et al., 1994). Hybridizations were performed at 60°C in modified Church buffer (1 mm EDTA, 0.25 m Na2PO4, and 7% [w/v] SDS [pH 7.4]). Blots were washed twice at 60°C in 2× SSC and 0.1% (w/v) SDS for 20 min and once at 60°C in 0.2× SSC and 0.1% (w/v) SDS. The RD29A and GPX clones were supplied by the Arabidopsis Ohio Stock Center (Columbus) and corresponded to the expressed sequence tag accession nos. 31G2T7 and 139F9T7, respectively. The PR1 clone was supplied by Jane Parker (Sainsbury Laboratory, John Innes Centre).
ACKNOWLEDGMENTS
The authors acknowledge Des Bradley for critically reading the manuscript. We would like to thank Mary-Anne Newman and Carlitos Jiménez for technical assistance. Seed stocks were kindly provided by Jane Parker and Carlos Alonso-Blanco. We also thank the Arabidopsis Ohio Stock Center for providing the RD29A and GPX cDNA clones and Jane Parker for providing the PR1 cDNA clone used in this study.
Footnotes
This work was supported by the Universidad de Málaga, Junta de Andalucía (grant no. AGR–168) and by the European Union (return grant to M.A.B.).
LITERATURE CITED
- Allen R. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7:1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella MA, Quesada MA, Kononowicz A, Bressan RA, Hasegawa PM, Valpuesta V. Characterization and in situ localization of a salt induced tomato peroxidase gene. Plant Mol Biol. 1994;25:105–114. doi: 10.1007/BF00024202. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- Bray EA. Plant responses to water deficit. Trends Plant Sci. 1997;2:48–54. [Google Scholar]
- Broadbent P, Creissen GP, Kular B, Wellburn AR, Mullineaux P. Oxidative stress responses in transgenic tobacco containing altered levels of gluthatione reductase activity. Plant J. 1995;8:247–255. [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998;116:1351–1357. doi: 10.1104/pp.116.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge AD. Herbicide action and effects on detoxification process. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 219–236. [Google Scholar]
- Foyer CH, Descourvières P, Kunert KJ. Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. [Google Scholar]
- Greenway J, Munns R. Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol. 1980;31:149–190. [Google Scholar]
- Hammond-Kosack KE, Jones JD. Resistance gene-dependent plant defense response. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998;116:173–181. [Google Scholar]
- Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Janda T, Szalai G, Tari I, Páldi E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta. 1999;208:175–180. [Google Scholar]
- Johnson DW, Smith SE, Dobrenz AK. Genetic and phenotypic relationships in response to NaCl at different developmental stages in alfalfa. Theor Appl Genet. 1992;83:833–838. doi: 10.1007/BF00226705. [DOI] [PubMed] [Google Scholar]
- Lauchli A, Epstein E. Plant responses to saline and sodic conditions. In: Tanji KK, editor. Agricultural Salinity Assessment and Management. New York: American Society of Civil Engineering; 1990. pp. 113–137. [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) choloroplasts. Biochem J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chedfor F, Giraudat J. Arabidopsis ABA-response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Mikolajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell. 2000;12:165–178. [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Pasternak D. Salt tolerance and crop production: a comprehensive approach. Annu Rev Phytopathol. 1987;25:271–291. [Google Scholar]
- Price AH, Hendry GAF. Iron-catalyzed oxygen radical formation and its possible contribution to drought damage in nine native grasses and three cereals. Plant Cell Environ. 1991;14:477–484. [Google Scholar]
- Rao MV, Davis RD. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod D, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress and H2O2 metabolizing enzymes: salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Ehmann A, Melander WR, Meeuse BJD. Salicylic acid: a natural inducer of heat production of Arum lilies. Science. 1987;237:1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- Senaratna T, Touchell D, Bunn T, Dixon K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000;30:157–161. [Google Scholar]
- Sharma YK, Leon J, Raskin I, Davis KR. Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA. 1996;93:5099–5104. doi: 10.1073/pnas.93.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajashekar K, Dixon RA, Lamb C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signal in the activation of defense mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Tsugane K, Kobayashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell. 1999;11:1195–1206. doi: 10.1105/tpc.11.7.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S-J, Ding L, Zhu J-K. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell. 1996;8:617–627. doi: 10.1105/tpc.8.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Enyedi AJ, Leon J, Raskin I. Ultraviolet light and ozone stimulate accumulation of salicylic acid and pathogenesis-related proteins and virus resistance in tobacco. Planta. 1994;193:373–376. [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Arabidopsis DNA encoding two desiccation-responsive rd29 genes. Plant Physiol. 1993a;101:1119–1120. doi: 10.1104/pp.101.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet. 1993b;236:331–340. doi: 10.1007/BF00277130. [DOI] [PubMed] [Google Scholar]