Abstract
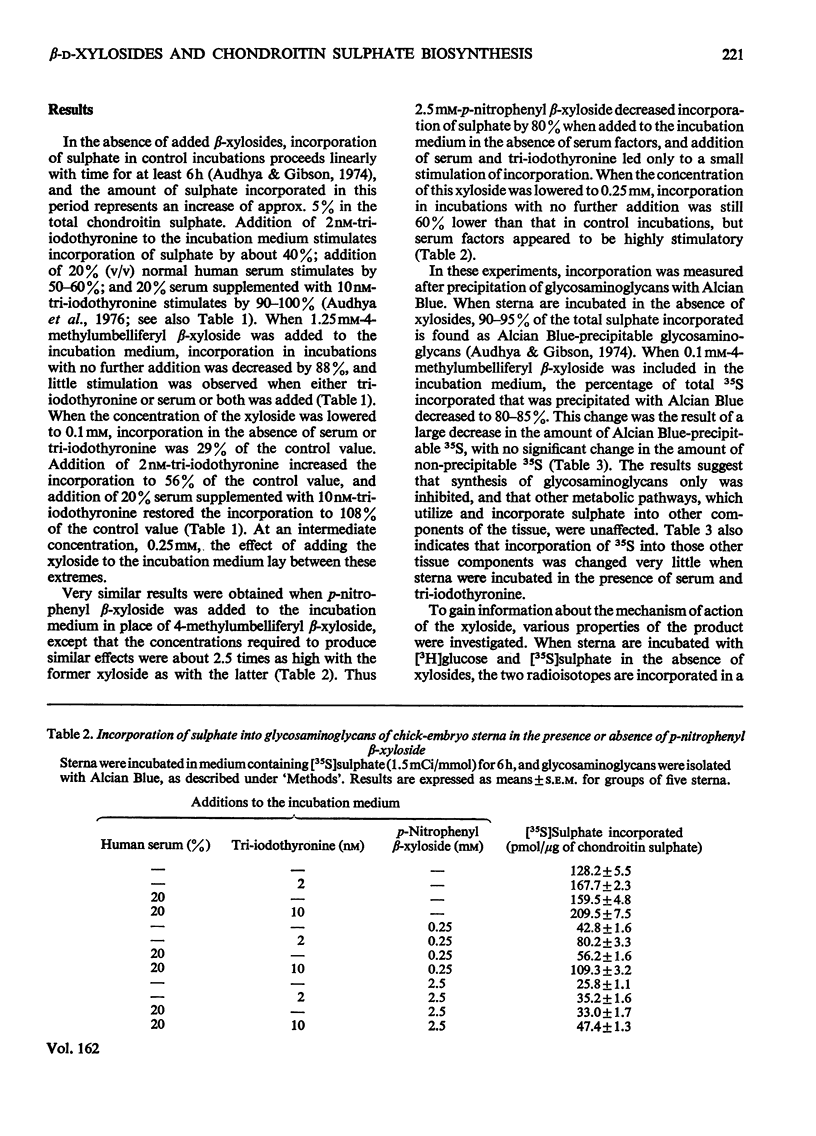
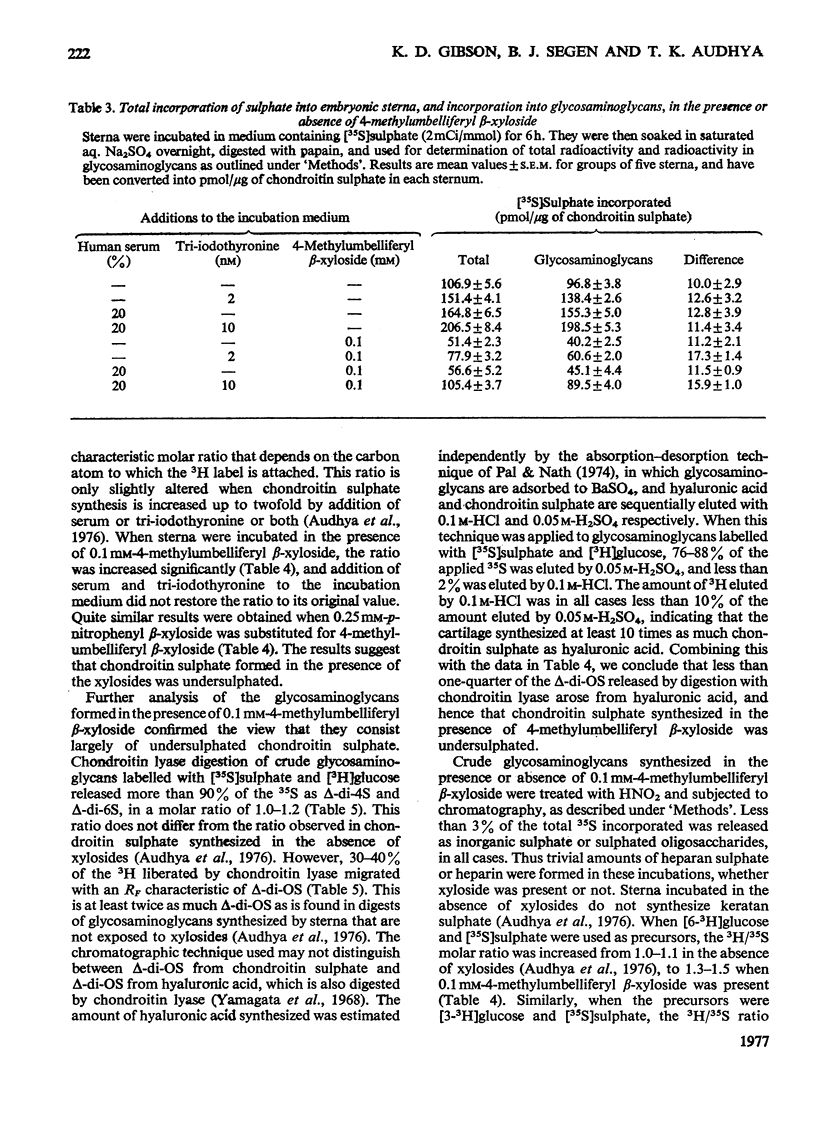
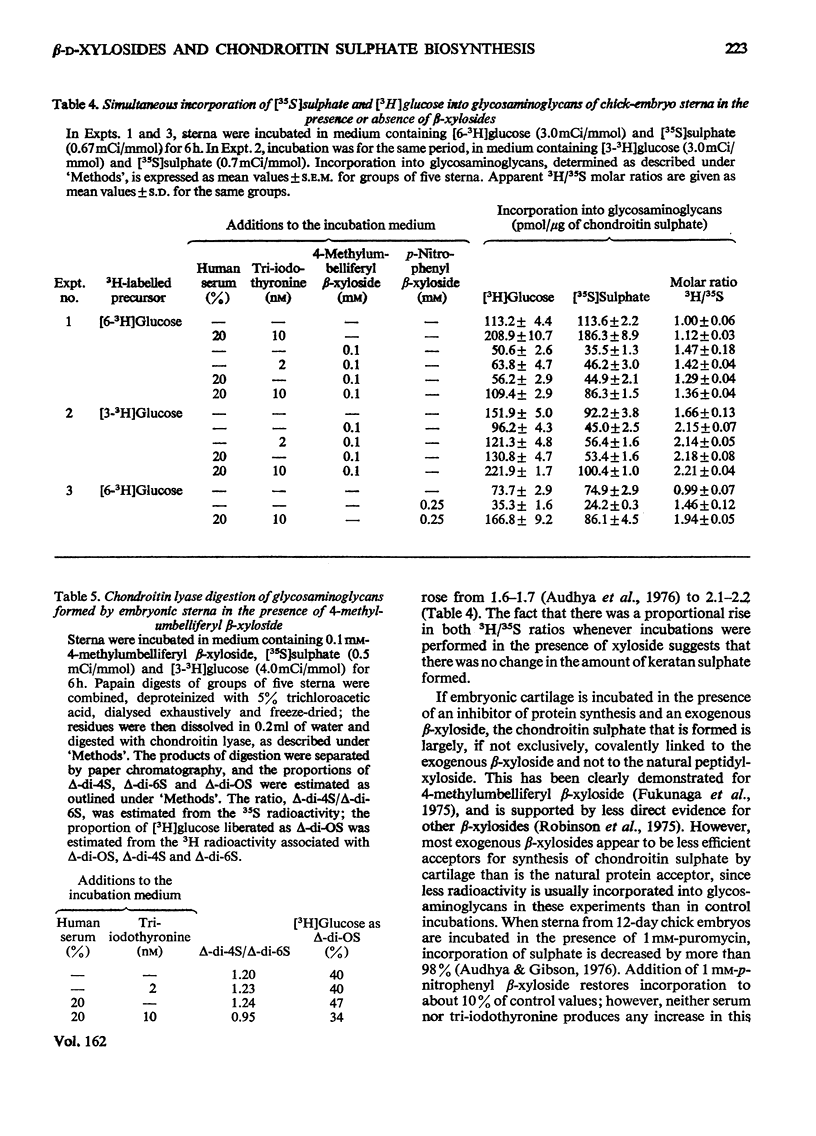
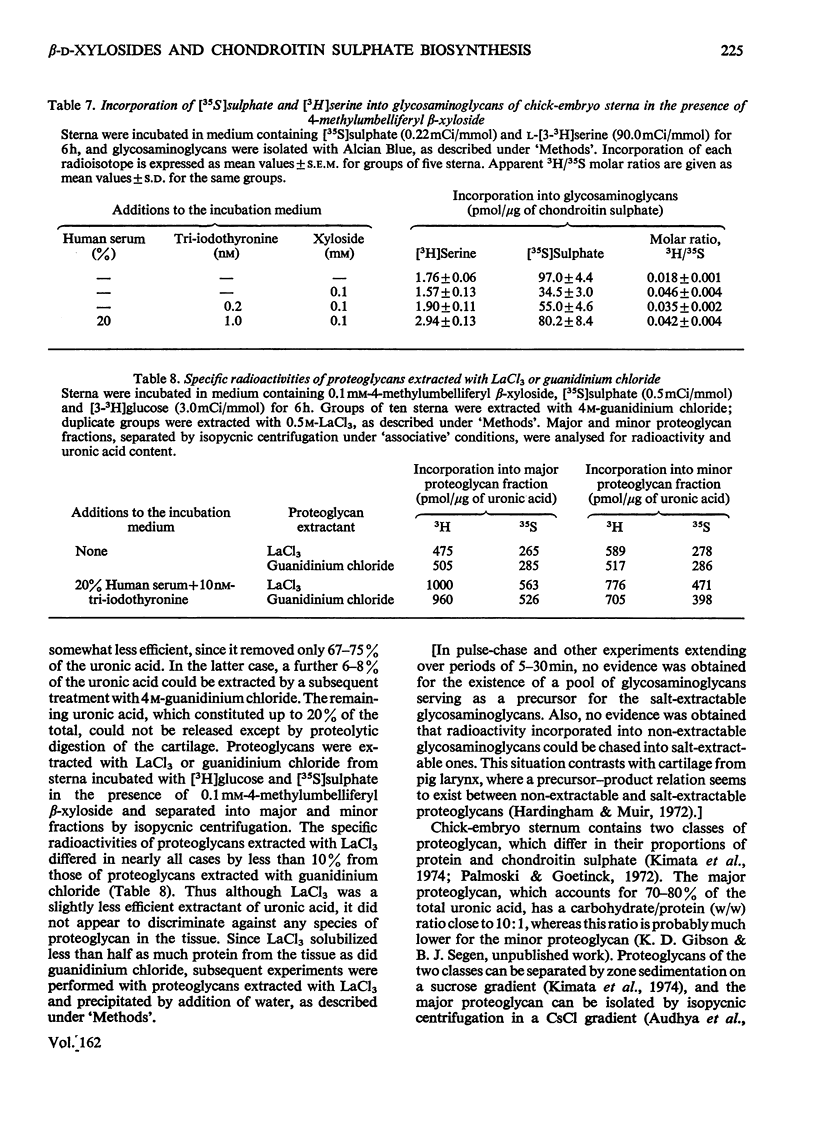
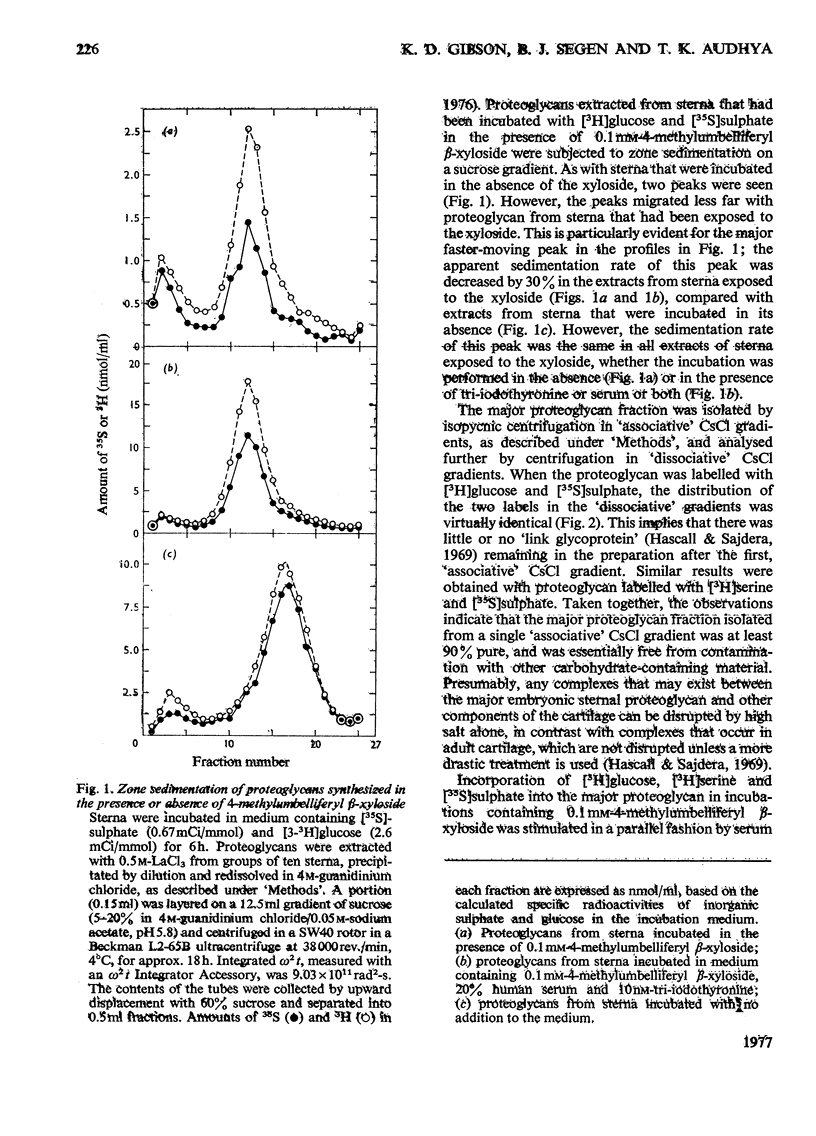
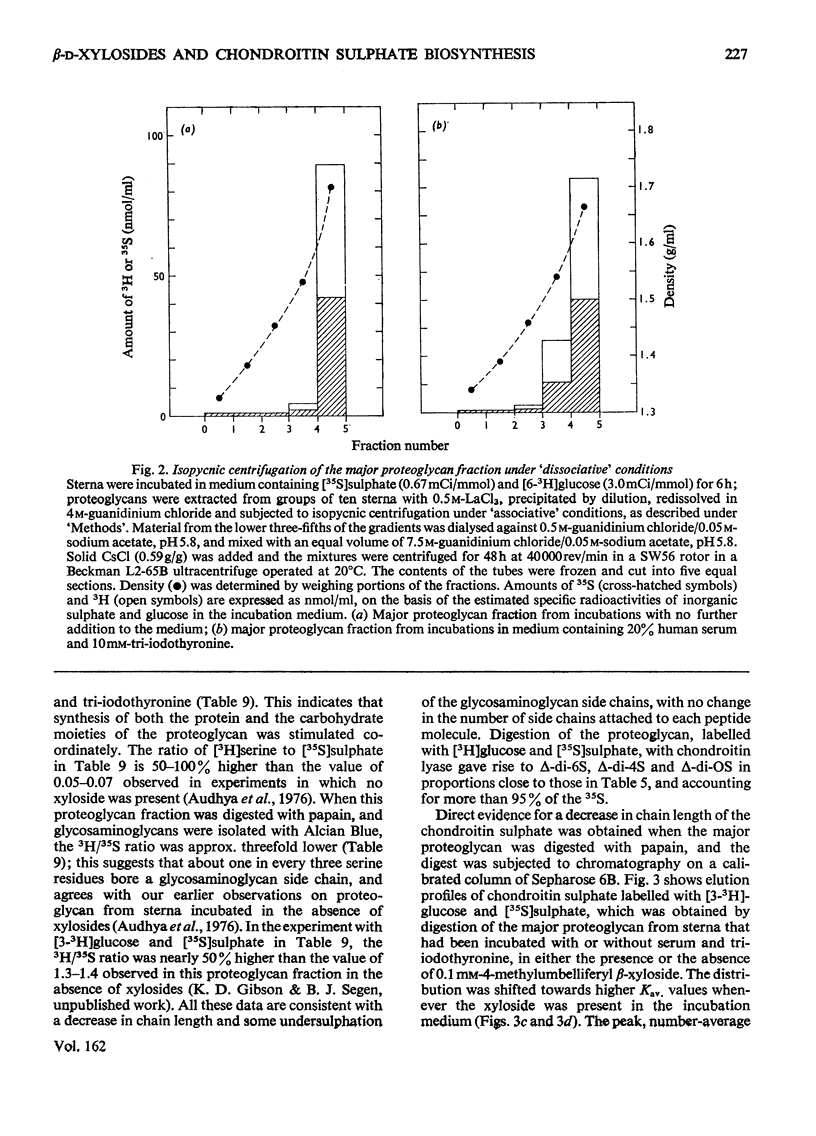
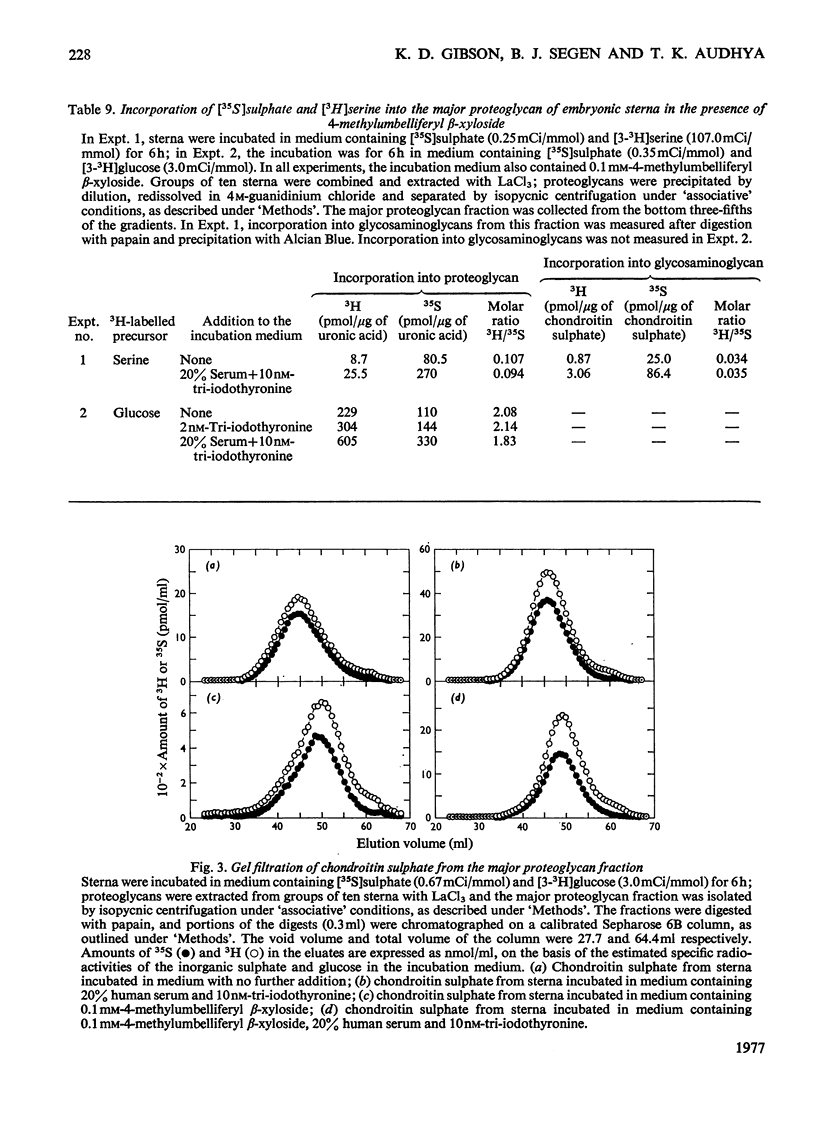
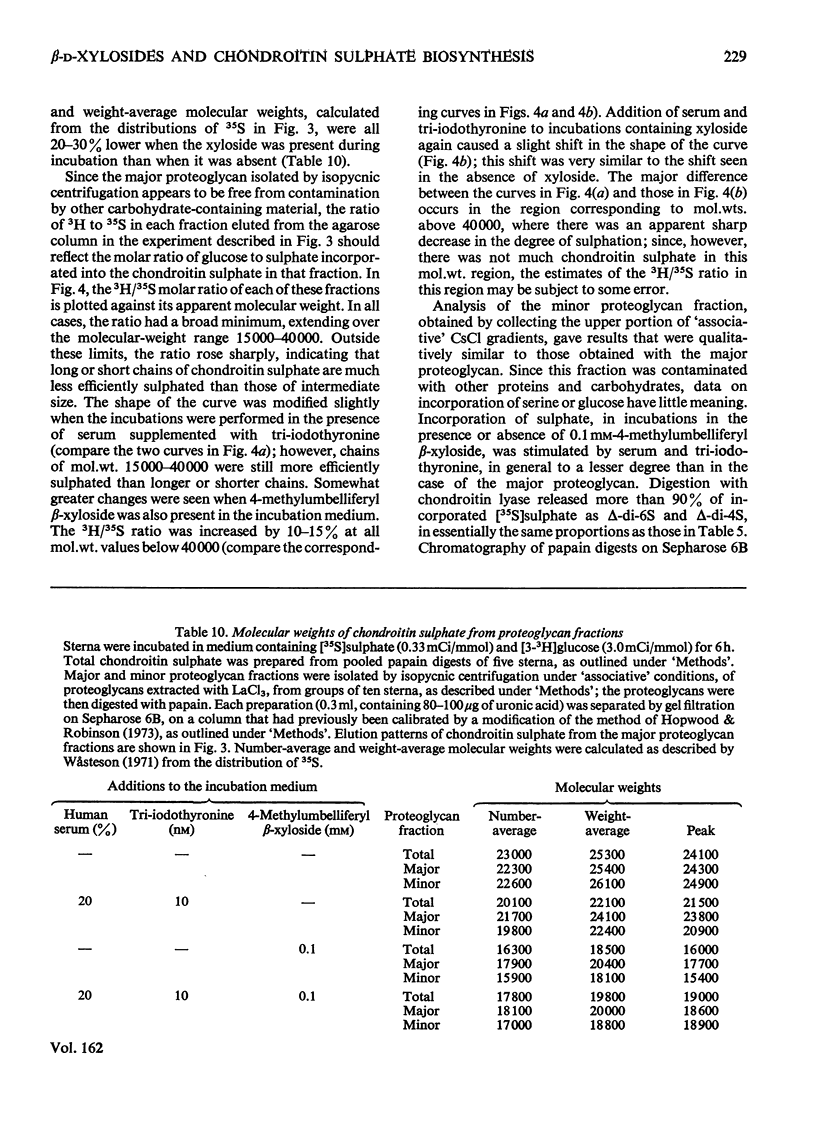
Incorporation of [35S]]sulphate, [3H]glucose and [3H]serine into glycosaminoglycans and proteoglycans of embryonic-chicken sternum was measured in vitro in incubation medium containing 4-methylumbelliferyl beta-D-xyloside or p-nitrophenyl beta-D-xyloside at low concentrations, and in the absence of inhibitors of protein synthesis. Incorporation of sulphate was decreased by 80% in incubations in which 1mM-4-methylumbelliferyl beta-xyloside or 2.5 mM-p-nitrophenyl beta-xyloside was present; under these conditions, serum factors stimulated incorporation to only a small extent. When the concentration of the xyloside was decreased tenfold, incorporation of sulphate was inhibited by 60-70%, but when normal human serum or L-3,3',5-tri-iodothyronine or both were also added to the incubation medium, incorporation was markedly stimulated. Experiments in which [35S]sulphate and [3H]glucose were incorporated simultaneously, and enzymic analysis of glycosaminoglycans formed in such experiments, indicated that chondroitin sulphate formed in the presence of 0.1 mM-4-methylumbelliferyl beta-xyloside contained 30-40% less sulphate than did chondrotin sulphate synthesized in the absence of xylosides. Similar experiments, with [3H]serine instead of [3H]glucose, suggested also a 20-30% decrease in chain length of the chondroitin sulphate; this was confirmed by direct gel filtration of labelled glycosaminoglycans on a calibrated column. Incorporation of [3H]glucose or [3H]serine was stimulated by serum and tri-iodothyronine in parallel with incorporation of sulphate. The changes seen in the total chondroitin sulphate were mirrored in the major proteoglycan fraction, purified by isopycnic centrifugation of salt-extracted proteoglycans. The labelling pattern of chondroitin sulphate from this proteoglycan indicated that decreased sulphation of chondroitin sulphate was largely due to the inferior ability of short polysaccharide chains to accept sulphate, with some direct interference with transfer of sulphate to all chains. The results also suggested that the action of serum factors on synthesis of proteochondroitin sulphate is exercised at the level of either protein synthesis or transport to the sites of initiation of polysaccharide synthesis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audhya T. K., Gibson K. D. Effects of medium composition and metabolic inhibitors on glycosaminoglycan synthesis in chick embryo cartilage and its stimulation by serum and triiodothyronine. Biochim Biophys Acta. 1976 Jul 21;437(2):364–376. doi: 10.1016/0304-4165(76)90006-4. [DOI] [PubMed] [Google Scholar]
- Audhya T. K., Gibson K. D. Enhancement of somatomedin titers of normal and hypopituitary sera by addition of L-triiodothyronone in vitro at physiological concentrations. Proc Natl Acad Sci U S A. 1975 Feb;72(2):604–608. doi: 10.1073/pnas.72.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya T. K., Gibson K. D. Serum inorganic sulfate and apparent somatomedin activity in an assay using chick embryo cartilage. Endocrinology. 1974 Dec;95(6):1614–1620. doi: 10.1210/endo-95-6-1614. [DOI] [PubMed] [Google Scholar]
- Audhya T. K., Segen B. J., Gibson K. D. Stimulation of proteoglycan synthesis in chick embryo sternum by serum and L-3,5,3'-triiodothyronine. J Biol Chem. 1976 Jun 25;251(12):3763–3767. [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca S., Richmond M. E., Silbert J. E. Biosynthesis of chondroitin sulfate. Sulfation of the polysaccharide chain. Biochemistry. 1973 Sep 25;12(20):3911–3915. doi: 10.1021/bi00744a019. [DOI] [PubMed] [Google Scholar]
- Freilich L. S., Lewis R. G., Reppucci A. C., Jr, Silbert J. E. Glycosaminoglycan-synthesizing activity of an isolated Golgi preparation from cultured mast cells. Biochem Biophys Res Commun. 1975 Apr 7;63(3):663–668. doi: 10.1016/s0006-291x(75)80435-9. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Zapf J., Audhya T. K., Ben-Porath E., Segen B. J., Gibson K. D. Nonsuppressible insulin-like activity and thyroid hormones: major pituitary-dependent sulfation factors for chick embryo cartilage. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2904–2908. doi: 10.1073/pnas.73.8.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga Y., Sobue M., Suzuki N., Kushida H., Suzuki S. Synthesis of a fluorogenic mucopolysaccharide by chondrocytes in cell culture with 4-methylumbelliferyl beta-D-xyloside. Biochim Biophys Acta. 1975 Feb 13;381(2):443–447. doi: 10.1016/0304-4165(75)90252-4. [DOI] [PubMed] [Google Scholar]
- GODMAN G. C., LANE N. ON THE SITE OF SULFATION IN THE CHONDROCYTE. J Cell Biol. 1964 Jun;21:353–366. doi: 10.1083/jcb.21.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos J. T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967 Apr;19(1):119–132. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- Galligani L., Hopwood J., Schwartz N. B., Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. J Biol Chem. 1975 Jul 25;250(14):5400–5406. [PubMed] [Google Scholar]
- Hall K. Quantative determination of the sulphation factor activity in human serum. Acta Endocrinol (Copenh) 1970 Feb;63(2):338–350. doi: 10.1530/acta.0.0630338. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Biosynthesis of proteoglycans in cartilage slices. Fractionation by gel chromatography and equilibrium density-gradient centrifugation. Biochem J. 1972 Feb;126(4):791–803. doi: 10.1042/bj1260791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Helting T., Rodén L. The carbohydrate-protein linkage region of chondroitin 6-sulfate. Biochim Biophys Acta. 1968 Dec 23;170(2):301–308. doi: 10.1016/0304-4165(68)90010-x. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The molecular-weight distribution of glycosaminoglycans. Biochem J. 1973 Dec;135(4):631–637. doi: 10.1042/bj1350631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K., Okayama M., Ooira A., Suzuki S. Heterogeneity of proteochondroitin sulfates produced by chondrocytes at different stages of cytodifferentiation. J Biol Chem. 1974 Mar 10;249(5):1646–1653. [PubMed] [Google Scholar]
- Kosher R. A., Lash J. W., Minor R. R. Environmental enhancement of in vitro chondrogenesis. IV. Stimulation of somite chondrogenesis by exogenous chondromucoprotein. Dev Biol. 1973 Dec;35(2):210–220. doi: 10.1016/0012-1606(73)90018-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mason R. M., Mayes R. W. Extraction of cartilage protein-polysaccharides with inorganic salt solutions. Biochem J. 1973 Mar;131(3):535–540. doi: 10.1042/bj1310535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama M., Kimata K., Suzuki S. The influence of p-nitrophenyl beta-d-xyloside on the synthesis of proteochondroitin sulfate by slices of embryonic chick cartilage. J Biochem. 1973 Nov;74(5):1069–1073. [PubMed] [Google Scholar]
- Pal M. K., Nath J. Separation of hyaluronate, chondroitin sulfate, and heparin by adsorption-desorption technique. Anal Biochem. 1974 Feb;57(2):395–402. doi: 10.1016/0003-2697(74)90094-3. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Goetinck P. F. Synthesis of proteochondroitin sulfate by normal, nanomelic, and 5-bromodeoxyuridine-treated chondrocytes in cell culture. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3385–3388. doi: 10.1073/pnas.69.11.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. C., Brett M. J., Tralaggan P. J., Lowther D. A., Okayama M. The effect of D-xylose, beta-D-xylosides and beta-D-galactosides on chondroitin sulphate biosynthesis in embryonic chicken cartilage. Biochem J. 1975 Apr;148(1):25–34. doi: 10.1042/bj1480025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schwartz N. B., Galligani L., Ho P. L., Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-D-xylosides in cultured cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4047–4051. doi: 10.1073/pnas.71.10.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz N. B., Rodén L. Biosynthesis of chondroitin sulfate. Purification of UDP-D-xylose:core protein beta-D-xylosyltransferase by affinity chromatography. Carbohydr Res. 1974 Oct;37(1):167–180. doi: 10.1016/s0008-6215(00)87072-x. [DOI] [PubMed] [Google Scholar]
- Schwartz N. B., Rodén L. Biosynthesis of chondroitin sulfate. Solubilization of chondroitin sulfate glycosyltransferases and partial purification of uridine diphosphate-D-galactose:D-xylose galactosyltrans. J Biol Chem. 1975 Jul 10;250(13):5200–5207. [PubMed] [Google Scholar]
- Shapiro S. S., Poon J. P. Effect of retinoic acid on chondrocyte glycosaminoglycan biosynthesis. Arch Biochem Biophys. 1976 May;174(1):74–81. doi: 10.1016/0003-9861(76)90325-8. [DOI] [PubMed] [Google Scholar]
- Stoolmiller A. C., Horwitz A. L., Dorfman A. Biosynthesis of the chondroitin sulfate proteoglycan. Purification and properties of xylosyltransferase. J Biol Chem. 1972 Jun 10;247(11):3525–3532. [PubMed] [Google Scholar]
- Telser A., Robinson H. C., Dorfman A. The biosynthesis of chondroitin-sulfate protein complex. Proc Natl Acad Sci U S A. 1965 Sep;54(3):912–919. doi: 10.1073/pnas.54.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971 Jul 8;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- Whiteman P. The quantitative determination of glycosaminoglycans in urine with Alcian Blue 8GX. Biochem J. 1973 Feb;131(2):351–357. doi: 10.1042/bj1310351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


