Abstract
Unpredicted responses to sedatives and analgesics are common in critically ill patients on mechanical ventilation (MV) and may be attributed to genetic variation. Our primary aim was to investigate the association between the pharmacogenomic (PGx) variation and sedation outcomes. The secondary aim was to capture intensive care unit (ICU) participants' perceptions of PGx. This was a prospective, observational PGx association study. Adult ICU patients receiving acute MV and sedatives/analgesics were enrolled. The number of altered PGx phenotypes in genes relevant to fentanyl, propofol, and midazolam (CYP2D6, CYP3A4/5, COMT, OPRM1, and CYP2B6) were tested with logistic regression for association with achieving ≥60% and ≥70% of time within Richmond Agitation‐Sedation Scale (RASS) target range (0 to −2) in the first 24 and 48 h of MV. Participants' perceptions of PGx testing and satisfaction with the return of PGx results were collected. Participants (n = 78) had a median of 2 altered PGx phenotypes. Fentanyl and propofol combination was the most frequently administered regimen. There were non‐significant associations of worse sedation outcomes with an increasing number of altered PGx phenotypes (i.e., adjusted odds ratio of achieving target RASS range = 0.46 to 0.96 for each altered phenotype increase at both 24 and 48 h). Individuals participating in the post‐discharge survey had positive perceptions toward PGx. There were no associations between sedation outcomes and PGx variants in the studied 6 genes. Larger studies are needed to investigate the impact of these genes and to evaluate additional genes. ICU participants had positive attitudes and perceptions toward PGx.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Sedatives and analgesics are used in critically ill patients to facilitate mechanical ventilation (MV). Highly variable and unpredicted responses to these medications are observed in practice increasing the risks of poor outcomes. Pharmacogenomic (PGx) variation in pharmacogenes associated with pharmacokinetics and/or pharmacodynamics of sedatives and analgesics can help in understanding sources of variability in response and improve outcomes.
WHAT QUESTION DID THIS STUDY ADDRESS?
Our primary aim was to investigate how the pharmacogenomic (PGx) variation impacts sedation outcomes in the first 24 and 48 h of MV.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We identified that it was feasible to obtain consent from patients in the intensive care unit (ICU) for PGx studies. There was a non‐significant association for lower odds of achieving desired sedation outcomes (achieving ≥60% and 70% of time within the target RASS range in the first 24 and 48 h of MV) with each increase in the number of altered PGx phenotypes. There was also a non‐significant association between number of altered phenotypes and time to target RASS. Individuals with >1 altered phenotypes had 3.5 times more ADRs than those with ≤1 altered phenotypes (14.8% vs. 4.16%) but was not significant.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The current study shows the feasibility and potential of PGx studies in ICU which will help in designing more robust studies with larger sample sizes to investigate the impact of PGx on sedation outcomes in critical care setting. If important PGx variants could be identified, it may allow for a personalized selection of sedative and analgesic drugs and doses at time of ICU admission.
INTRODUCTION
Precision medicine is a tool to improve health outcomes by tailoring disease prevention interventions and treatments. Pharmacogenomics (PGx) is one the most developed forms of precision medicine and matches medications and/or doses with each individual's genome to maximize effectiveness while minimizing side effects. Clinical practice guidelines have been systematically developed and are readily available from the NIH supported Clinical Pharmacogenomics Implementation Consortium (CPIC). 1 Currently, there are 26 published guidelines for more than 150 drugs and 25 genes, and they provide clinical recommendations for drug management in patients with PGx variants. Despite the growing utility of PGx, little is known about PGx in the intensive care unit (ICU). Use of PGx to guide therapy may reduce unwanted and poor outcomes.
Sedatives and analgesics are frequently administered to critically ill patients to relieve the discomfort, anxiety, and stress of mechanical ventilation (MV) and to prevent self‐injury. 2 , 3 The management of sedation is a major challenge in critically ill patients. Scientific evidence and practice guidelines support the goal of lighter levels of sedation to improve ICU outcomes and length of stay. 4 , 5 , 6 Deep sedation is associated with increased cognitive dysfunction, delirium, and increased mortality. 4 , 7 The Richmond Agitation‐Sedation Scale (RASS) is a common tool used to measure the level of sedation. 5 The scale takes into account the level of consciousness and motor activity and ranges from −5 (deep sedation) to +4 (agitated). 8 The current Society of Critical Care Medicine guideline‐driven sedation goals for most patients on MV is a RASS of 0 to −2, which corresponds to a lighter sedation. 5 Genetic variation in pharmacogenes, which regulate drug metabolism and pharmacodynamic effects of sedative and analgesic drugs, may contribute to poorly controlled sedation, adverse outcomes and failure to efficiently achieve the RASS target. The sedation depth and intensity at the early period (first 48 h) of MV have been shown to be risk factors for negative outcomes such as increased mortality and delirium and decreased in‐hospital survival. For some patients, multiple sedation and analgesic drug adjustments are required to determine the correct drug and dose that achieves optimal sedation which reduces their comfort and increases the complexity of care.
The primary aim of this study was to develop preliminary data on the association between the number of altered PGx phenotypes in genes relevant to sedatives and analgesics and sedation outcomes during MV. We hypothesized that patients with altered PGx phenotypes relevant to their sedation and analgesic regimen will spend less time in the target RASS during the initial 24 and 48 h on MV and achieve their first RASS in the target range later compared to patients with normal phenotypes. We also assessed patients' acceptability to be tested, perceptions and knowledge of PGx, and attitudes toward return of results through a PGx counseling session after discharge.
METHODS
Study design and participants selection
This was a prospective, observational, pragmatic PGx association study conducted between 2018 and 2021 at the University of Minnesota Medical Center. Participants were enrolled after obtaining written and informed consent from either the participant, if they were able to pass an IRB‐approved University of California, San Diego Brief Assessment of Capacity to Consent (UBACC) tool, or their legally authorized representative. Participants were included if admitted to the surgical ICU (SICU), medical ICU (MICU), or cardiovascular ICU (CVICU) services, receiving acute MV and sedatives and/or analgesics potentially associated with pharmacogenes, and had an order for a target RASS score of 0 to −2. Participants who were admitted to the ICU from surgery and were deeply sedated (e.g., RASS −4 to −5) were enrolled but their RASS scores were not included in the analysis until the sedation was lightened through a reduction in sedatives administration or dose (typically 2–6 h after ICU admission) and had a written order for a target RASS score between 0 to −2. Participants were excluded if admitted to the ICU with head trauma or other neurologic events that may reduce cognition, had a history of or active liver disease, or undergone liver transplantation, substance abuse within the past year, receiving a neuromuscular blocker, or moribund state with the planned withdrawal of support. This study was approved by the Institutional Review Board at our institution (IRB STUDY# 0002189).
Sedatives and analgesics administration
Sedative and analgesic choices, dosing, and duration were at the discretion of the ICU team. The administration, start and stop times, doses, and infusion rates of the nine sedative and analgesic medications (fentanyl, propofol, midazolam, dexmedetomidine, morphine, hydromorphone, ketamine, lorazepam, and haloperidol) observed in our study participants were recorded for the 48 h study period.
Data collection and pharmacogenomic testing
Demographic and clinical information was collected from the electronic health record. Genotyping was conducted on a CLIA‐certified assay using The RightMed® Comprehensive Test from OneOme® (Minneapolis, MN, USA). Six pharmacogenes (CYP2D6, CYP3A4, CYP3A5, CYP2B6, COMT, OPRM1) potentially associated (PharmGKB level of evidence of 3 or higher) with sedatives and/or analgesic medications that were administered to at least 10% of study patients (fentanyl, propofol and midazolam were the only agents in more than 10%) were taken from this panel and studied. Genes previously studied for dexmedetomidine (ADRA2A and PRKCB) were not contained on the panel and were not studied. The OneOme PGx test results were not shared with the ICU providers and were not used for clinical decision making.
More information on clinical data and DNA collection, and PGx testing are available in the Supplementary Material S1. A summary of pharmacogenes potentially associated with sedative/analgesics medications of interest and PharmGKB levels of evidence are shown the Supplementary Material (S1) Table S1. A list of the genetic variants taken from the OneOme panel and studied is presented in the Supplementary Material (S1) Table S2.
Altered phenotypes
Phenotypes were assigned for each gene using the OneOme genotype to phenotype algorithm. Normal phenotypes were defined as those with wildtype/typical function and are defined in the Supplementary Material (S1) Table S3. The CYP3A5 poor metabolizer phenotype (CYP3A5*3/*3) was considered wildtype because it is the most common phenotype in Caucasians and most doses are based on data derived from this population.
For each studied gene, a normal phenotype was assigned a score of zero and an altered phenotype (regardless of the diplotype) was assigned 1. Then the total number of altered phenotypes was calculated in each participant by summing the number of altered phenotypes with a maximum of 6 (representing the 6 genes of interest) based on what drugs they were receiving. For example, a patient receiving propofol (relevant gene CYP2B6) and midazolam (relevant genes CYP3A4, CYP3A5) with an altered CYP2B6 and CYP3A5 phenotype was assigned a total number of altered phenotypes of two. A patient receiving only fentanyl (relevant genes CYP3A4, CYP3A5, CYP2D6, COMT, and OPRM1) with an altered CYP2D6, COMT, and OPRM1 phenotype, and normal for CYP3A4 and CYP3A5 would have been assigned a total number of altered phenotypes of three.
Endpoints
The primary endpoint was achieving ≥60 and 70% of time within the target RASS range (0 to −2) in both the first 24 and 48 h of MV. RASS was clinically determined by the ICU nurses and documented in most all cases every 2 h. The linear interpolation method was used to estimate missing RASS scores. 9 For each participant, an overall percentage of time within the target RASS range in both the first 24 and 48 h of MV was calculated by dividing the number of RASS measurements in the target RASS range (0 to −2) by the total number of RASS measurements within that same period of time, and multiplied by 100. Because sedative and analgesic drugs and doses change over time, the primary PGx association analyses focused on % of time the RASS measurement was in target range but only during the periods of time when the patient was receiving propofol, fentanyl and/or midazolam (e.g., a patient may have received propofol and fentanyl for 20 h of the first 24‐h period and only the RASS measurements in those 20 h were analyzed representing the 24 h analysis). Supplementary Material (S1) Figure S1 represents a schematic diagram of the method used in calculating the percentage within the target range.
Other endpoints evaluated were time to first RASS in the target range defined as hours from the time of intubation to first RASS measurement in the target range, and adverse drug reactions (ADR) such as delirium or central nervous system changes associated with the sedative regimen and documented in nursing or provider notes which resulted in the drug(s) being discontinued.
Statistical analysis
Descriptive statistics such as mean and standard deviation (SD) were determined for continuous variables and frequency and percentages for categorical variables. To account for possible dose effects on the endpoints, total cumulative weight normalized doses for fentanyl, propofol, dexmedetomidine, and midazolam were calculated for each patient over 24 and 48 h and then stratified by the median into high dose, low dose, or none, if not receiving the agent. Given the rapid onset of action and short duration of action of the studied sedatives and analgesics, the cumulative doses in the first 24 and 48 h were used to represent the dose effect. Multivariate logistic regressions were performed to determine the association between the number of altered PGx phenotypes and achieving ≥60% and ≥70% of time within the target RASS range of 0 to −2 over both the first 24 and 48 h. Because the number of altered PGx phenotypes was normally distributed and equally spaced, it was tested first as a continuous independent variable in the logistic regression due to the ease of results interpretation when ordinal data is treated as continuous. We also tested the number of altered phenotypes as a categorical variable after grouping the number of altered phenotypes as following: reference group (0 to 1 altered phenotype), group 1 (2 to 3 altered phenotypes) and group 2 (4 to 5 altered phenotypes).
Kaplan–Meier plot and log‐rank test were used to assess the association of time to first RASS within the target range with a number of altered phenotypes as a categorical variable (≤1 and >1 altered phenotypes). COX proportional hazard (PH) model was used to test the association of number of altered phenotypes as a categorical variable (≤1 and >1 altered phenotypes) with the event of achieving the first RASS in the target range allowing adjustment for clinical factors and characteristics. Clinical characteristics and factors such as age, sex, creatinine clearance, ICU unit, baseline RASS score, and cumulative weight normalized doses (high, low, or none) of fentanyl, propofol, dexmedetomidine, and midazolam were considered a priori as covariates and adjusted for in the logistic regression and COX PH models. Type of ICU and entering the ICU post‐surgery were highly correlated (Chi‐square test, p < 0.01) therefore only ICU unit was included as a covariate to avoid collinearity. The adjusted odds ratio and adjusted hazard ratio (HR) with 95% confidence intervals (CI) were used to report the logistic regression and COX PH model associations. Fisher exact test was used to determine the association between ADRs and the number of altered phenotypes (categorized as ≤1 vs. >1). P‐value <0.05 was considered statistically significant. All analyses were conducted using R software (Vienna, Austria). R packages used in the analysis are reported in the Supplementary Material S1.
Return of PGx results and participants perceptions
Participants or their legally authorized representatives were asked, at the time of consent, if they would like to receive educational information about PGx, test results and to review them with one of the study pharmacists (DJS and JL) after discharge from the hospital. If yes, a phone call was scheduled and test results were mailed to the participant. The participants completed a 12‐question, IRB‐approved questionnaire to assess the participant's knowledge of PGx, satisfaction at the time of return of results, and plans to share their results with others (full questionnaire is available in Supplementary Material S2).
RESULTS
Participant characteristics
ICU admissions were screened (except during the COVID‐19 shutdown) and 86 participants were enrolled. Eight were excluded from the analysis (exclusion reasons are shown in Supplementary Material (S1) Table S4) leaving 78 patients. The demographic and baseline characteristics are presented in Table 1. The average age of the participants was 58 years, and the majority (92%) were Caucasian. Males and females were almost equally represented. The CVICU had the highest number of participants (41%) compared to MICU and SICU with 32.1% and 26.9%, respectively. Thirty‐seven participants (47%) were admitted to the ICU after a surgical procedure. The 78 patients had 1673 RASS measurements with 1447 measurements (86%) occurring when propofol, fentanyl, and/or midazolam were administered. Fentanyl and propofol combination was the most frequently administered regimen (Supplementary Material (S1) Figure S2).
TABLE 1.
Demographics and baseline characteristics (n = 78).
| Characteristics | Summary statistics | |
|---|---|---|
| Age, mean ± (SD) (years) | 57.78 (±13.9) | |
| Weight, mean ± (SD) (kg) | 84.39 (±21.8) | |
| CrCl at enrollment, mean ± (SD) (mL/min) | 90.88 (±51.4) | |
| Race, n (%) | White | 72 (92.3) |
| Other | 6 (7.7) | |
| Sex, n (%) | Female | 38 (48.7) |
| Male | 40 (51.3) | |
| ICU unit, n (%) | CVICU | 32 (41) |
| MICU | 25 (32.1) | |
| SICU | 21 (26.9) | |
| BMI, n (%) | Normal or Underweight a (BMI <25) | 24 (30.8) |
| Overweight (BMI between 25 to 30) | 23 (29.5) | |
| Obese (BMI ≥30) | 31 (39.7) | |
| Admitted to ICU after surgery, n (%) | 37 (47.4) | |
| Baseline RASS measurement, median [IQR] | −3.00 [−4, −2] | |
| Total number of RASS measurements | 1673 | |
| Total number of RASS measurements recorded while receiving fentanyl, propofol, and/or midazolam | 1447 | |
Abbreviations: BMI, body mass index; CrCl, creatinine clearance; CVICU, cardiovascular ICU; ICU, intensive care unit; IQR, interquartile range; MICU, medical ICU; PGx, pharmacogenomic; RASS, Richmond Agitation‐sedation scale; SD, standard deviation; SICU, surgical ICU.
One participant was underweight and was merged with the normal weight group.
The overall percentage of time in the target RASS range was highly variable (range 0 to 100%) among our population (Figure 1). The median percentage of time in the target RASS range was low; 25% in the first 24 h and 37% at 48 h. The number of individuals with an altered phenotype for COMT (n = 63, 80.1%), CYP2D6 (n = 47, 60.3%), and CYP2B6 (n = 42, 53.8%) was high (Figure 2).
FIGURE 1.
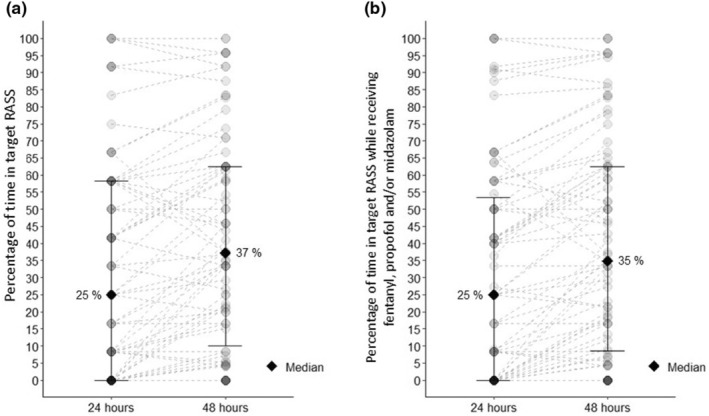
Percentage of time within the target RASS range in the first 24 and 48 h for each participant. Each point represents a patient, the solid diamond represents median, and bars represent 25 and 75 percentiles. The dashed line between the points at 24 and 48 h represents the change in RASS over time. For each participant, a percentage of time within the target RASS range was calculated for the first 24 and 48 h by dividing the number of measurements within the target range by the number of all available measurements then multiplied by 100. Plot a: represents the percentage of time in the target RASS range (0 to −2) including all RASS measurements and Plot b: represents the percentage of time in the target RASS range (0 to −2) including all RASS measurements when patients were administered fentanyl, propofol, and/or midazolam. RASS, Richmond Agitation‐Sedation Scale.
FIGURE 2.
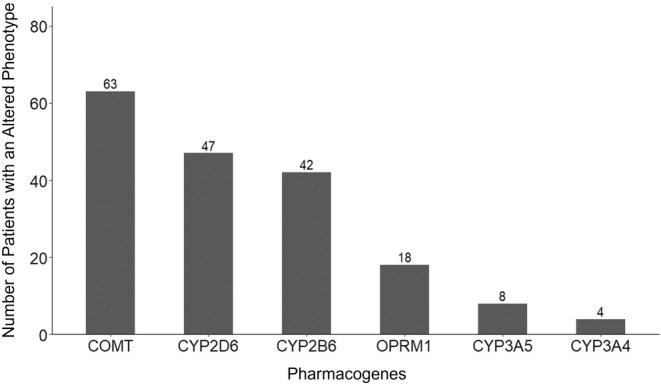
Number of participants with altered phenotypes in pharmacogenes associated with sedatives and analgesics. An altered phenotype was defined as any phenotype associated with genetic variants (i.e., not wild type) according to the OneOme genotype to phenotype algorithm. The normal/typical phenotype for CYP3A5 was defined as poor metabolizers carrying CYP3A5*3/*3 due to the high prevalence of this genetic variant in our study population of mostly Caucasians. A participant may be counted in more than one of the phenotypes.
The median time a patient was receiving one or more sedative/analgesics (fentanyl, propofol, and/or midazolam) with a potentially relevant PGx gene was 24 h [interquartile range (IQR) = 22, 24] and 40 h [IQR = 30, 46] in the first 24 and 48 h of MV, respectively. There was a median of 2 altered phenotypes per patient that were potentially relevant to propofol, fentanyl, and/or midazolam in the first 24 and 48 h. The distributions of the number of altered phenotypes are presented in Supplementary Material (S1) Figure S3. Propofol and fentanyl were administered in more than 80% of the patients in the 24‐ and 48‐h periods. In the first 24 and 48 h of acute MV, dexmedetomidine was only administered to approximately 45% and 50% of participants, respectively, and midazolam was used in 27% of participants. Time in target RASS range and the administered sedatives and analgesics are presented in Table 2 and Table 3 for the first 24 and 48 h, respectively.
TABLE 2.
RASS and medications during the first 24 h on mechanical ventilation a .
| Study period | Medication profile | Summary statistic | |
|---|---|---|---|
| First 24 h | Number of RASS measurements per participant, median [IQR] | 12 [12, 12] | |
| Time on sedatives/analgesics with a potentially relevant pharmacogene, hours median [IQR] | 24 [22, 24] | ||
| Number of altered phenotypes per patient, median [IQR] | 2 [1, 3] | ||
| Fentanyl weight normalized cumulative 24‐h dose, median [IQR] (mcg/h/kg) | 6.81 [3.8, 12.3] | ||
| Propofol weight normalized cumulative 24‐h dose, median [IQR] (mcg/min/kg) | 260 [162, 405] | ||
| Dexmedetomidine weight normalized cumulative 24‐h dose, median [IQR] (mcg/h/kg) | 3 [1.15, 4.6] | ||
| Midazolam weight normalized cumulative 24‐h dose, median [IQR] (mg/kg) | 0.198 [0.03, 0.34] | ||
| Fentanyl, n (%) | Did not receive medication | 11 (14.1) | |
| Low weight normalized cumulative dose | 33 (42.3) | ||
| High weight normalized cumulative dose | 34 (43.6) | ||
| Propofol, n (%) | Did not receive medication | 11 (14.1) | |
| Low weight normalized cumulative dose | 33 (42.3) | ||
| High weight normalized cumulative dose | 34 (43.6) | ||
| Dexmedetomidine, n (%) | Did not receive medication | 43 (55.1) | |
| Low weight normalized cumulative dose | 17 (21.8) | ||
| High weight normalized cumulative dose | 18 (23.1) | ||
| Midazolam, n (%) | Did not receive medication | 60 (76.9) | |
| Low weight normalized cumulative dose | 9 (11.5) | ||
| High weight normalized cumulative dose | 9 (11.5) | ||
Abbreviations: IQR, interquartile range; RASS, Richmond Agitation‐sedation scale.
Low cumulative dose is below the median cumulative dose and high is above the median cumulative dose in the first 24 h.
TABLE 3.
RASS and medications during the first 48 h on mechanical ventilation a .
| Period | Medication profile | Summary statistics | |
|---|---|---|---|
| First 48 h | Number of RASS measurements per participant, median [IQR] | 24 [20, 24] | |
| Time on sedatives/analgesics with pharmacogenes, hours median [IQR] | 40 [30, 46] | ||
| Number of altered phenotypes per patient, median [IQR] | 2 [1, 3] | ||
| Fentanyl weight normalized cumulative 48‐h dose, median [IQR] (mcg/kg/h) | 10.74 [6.35, 20] | ||
| Propofol weight normalized cumulative 48‐h dose, median [IQR] (mcg/kg/min) | 403.75 [218.75, 567.6] | ||
| Dexmedetomidine weight normalized cumulative 48‐h dose, median [IQR] (mcg/kg/h) | 4.4 [1.8, 8.28] | ||
| Midazolam weight normalized cumulative 48‐h dose, median [IQR] (mg/kg) | 0.32 [0.02, 0.49] | ||
| Fentanyl, n (%) | Did not receive medication | 8 (10.3) | |
| Low cumulative dose | 35 (44.9) | ||
| High cumulative dose | 35 (44.9) | ||
| Propofol, n (%) | Did not receive medication | 10 (12.8) | |
| Low cumulative dose | 34 (43.6) | ||
| High cumulative dose | 34 (43.6) | ||
| Dexmedetomidine, n (%) | Did not receive medication | 38 (48.7) | |
| Low cumulative dose | 20 (25.6) | ||
| High cumulative dose | 20 (25.6) | ||
| Midazolam, n (%) | Did not receive medication | 57 (73.1) | |
| Low cumulative dose | 10 (12.8) | ||
| High cumulative dose | 11 (14.1) | ||
Abbreviations: IQR, interquartile range; RASS, Richmond Agitation‐sedation scale.
Low cumulative dose is below the median cumulative dose and high is above the median cumulative dose in the first 48 h.
Association with outcomes
The odds of achieving ≥60% and ≥70% of time in the desired target range decreased (range of 4%–54%) with each one increase in the number of altered PGx phenotypes after adjusting for the important clinical factors, however, these associations were not statistically significant (Table 4). Similar non‐significant trends of decreasing the odds of achieving target ranges in groups with a higher number of altered phenotypes (group 1 [2‐3 altered phenotypes] and group 2 [4‐5 altered phenotypes]) compared to the reference group (0 to 1 altered phenotype) were observed when the number of altered phenotypes was treated as a categorical variable. The results of the logistic regression models with the number of altered phenotypes as a categorical variable are reported in the Supplementary Material (S1) Table S5.
TABLE 4.
Association between the number of altered PGx phenotypes and percentage of time in target RASS in the first 24 and 48 h.
| Endpoint | Percentage of time in target RASS range | Number of participants achieving target (total n = 78) | aOR of number of altered phenotypes (95% CI) a | p‐value |
|---|---|---|---|---|
| 24 h | ≥60% | 15 | 0.61 (0.24–1.37) | 0.25 |
| ≥70% | 9 | 0.46 (0.08–1.50) | 0.27 | |
| 48 h | ≥60% | 23 | 0.96 (0.48–1.89) | 0.91 |
| ≥70% | 15 | 0.52 (0.19–1.24) | 0.17 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; RASS, Richmond Agitation‐Sedation Scale.
Adjusted for age, sex, ICU unit, creatinine clearance, baseline RASS measurement, and medication dose groups (high, low, and not received) based on the cumulative weight‐adjusted dose for fentanyl, propofol, midazolam, and dexmedetomidine.
Participants with ≤1 altered phenotypes had a more rapid time to target RASS compared to those with >1 altered phenotypes however, this was not significant (log‐rank test, p = 0.3, Figure 3). The median time to first RASS in the target range in participants with ≤1 altered phenotypes (n = 24) was 5 h compared to 10 h in those with >1 altered phenotypes (n = 54). Similarly, in the COX PH model, the group with >1 altered phenotypes had 7.5% lower hazards of achieving the first RASS in the target range after adjusting for the important clinical factors, however, this association was not significant (HR = 0.93, 95%CI = 0.47–1.82, p‐value = 0.82).
FIGURE 3.
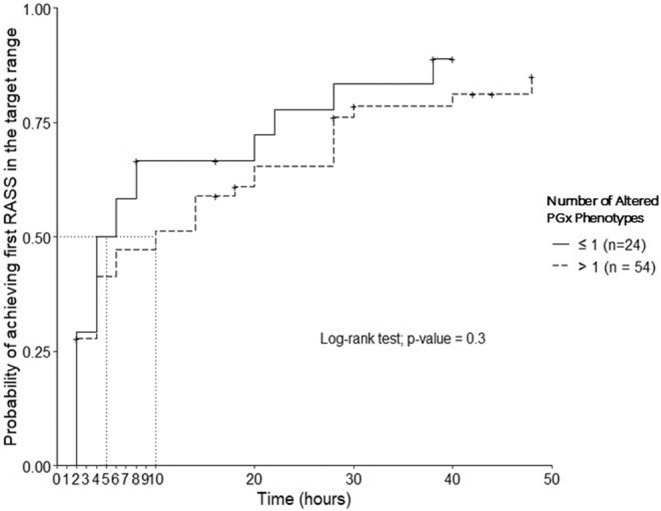
Kaplan–Meier plot for the time to the first RASS within the target range (0 to −2) in the first 48 h.
The number of altered phenotypes was not associated with ADRs (Fisher exact, p = 0.26). One (4.16%) and 8 (14.8%) participants developed ADRs in the ≤1 (n = 24) and >1 (n = 54) altered phenotype groups, respectively.
Return of PGx results and participants perceptions
Sixty‐nine patients requested to have results returned. Nine died before the return of results and five declined during follow‐up calls or emails. Eighteen participants did not respond to at least six telephone calls on three different days over at least 3 weeks or 2 encrypted email requests to reply to an investigator (DJS). Results were returned to 37 participants. Investigators spent an average time of 36 min (range 25–60 min) per patient on the return of results and answering their questions. Scores on the questionnaire after the return of results were high (95%) with only one participant missing more than 1 question. The most missed question (27%) was “If my test result says that I am a “poor metabolizer” it means that all medications given to me will stay in my body longer than other people.” All participants rated the return of the results session as extremely interesting and helpful (highly satisfactory) except one participant who ranked the counseling as satisfactory. This participant also did not plan to share his individualized PGx information with his doctor; all others said they plan to share results with their doctor (36/37, 97%). Thirty percent of study participants stated they would share their PGx test results with their pharmacist and 22% planned to share their results with their nurse. Plans to share their PGx information with their family were high with 75% of responses.
DISCUSSION
Managing sedation is difficult because individual responses to sedatives can be unpredictable and may be influenced by many factors. Pharmacogenomics may also impact the effectiveness of sedation and analgesia through modifying drug exposure and response. The application of PGx approaches could help in the management of critically ill. 10 Conducting PGx research in the ICU is challenging due to the unstable nature of critical illness, rapid changes in medications, and the high medication burden and potential for drug interactions. 11 In addition, many care teams are involved in patient management, and both patient and family are overwhelmed and unable to consider research participation. There are few studies that have investigated the association of PGx variation on outcomes in the ICU 12 and none to report attitudes and perceptions of ICU patients on PGx.
Achieving and maintaining a patient's sedation in the target RASS score is an important measure of the effectiveness of sedation management. An ideal sedative regimen reaches the target RASS quickly and maintains the RASS within the target range. The percentage of time in target RASS range is highly variable and is a main purpose of sedation protocols. Clinically, data show that the percentage of time in target RASS range varies depending on the population, the specific sedation protocol used, and other factors. A study reported a wide range of mean time within target RASS (10.7%–27.6%) in the first 48 h and varied based on the sedative agent selected and the body mass index. 13 In a study by DiCesare et al., 14 which investigated the predictors of response to dexmedetomidine in the first 48 h of MV, a sensitivity analysis was conducted on the optimal percentage of time and determined that ≥60% was the optimal cut‐off. Therefore, we studied two cut‐offs for the optimal percentage of time in the target RASS range for the first 24 and 48 h of MV of at least 60% and 70% within the target range.
Most critically ill patients have complex diseases and preexisting comorbidities and conditions that contribute to prolonged ICU stay and can impact outcomes and response to treatment. This complexity may obscure the PGx effect or the effect is only meaningful and observable in patients with the most severe phenotypes (i.e., ultrarapid or poor metabolizers). In addition, for efficient use of PGx results in the ICU setting testing must be rapidly available; this remains a major challenge as turnaround time is generally >48 h. The current evaluation and a previous study also conducted in an ICU showed the feasibility of obtaining consent and collecting genetic information from critically ill adults. 12 Most commonly used PGx variants are in drug metabolizing enzymes and may affect the pharmacokinetics of drugs. Previous studies in the non‐ICU settings have reported pharmacokinetic PGx associations of fentanyl with CYP3A4/5 15 , 16 , 17 , 18 , 19 and CY2D6 19 , midazolam with CYP3A4/5 20 , 21 , and propofol with CYP2B6 22 , but there is little data evaluating if pharmacokinetic changes translate into altered clinical outcomes. Therefore, most of these drug‐gene pairs have a PharmGKB level of evidence (LOE) of 3 except for fentanyl and CYP3A4 which has a LOE of 2. 23 , 24 , 25
In the current study, we found a non‐significant association of the number of altered phenotypes with the study endpoints. There was a non‐significant association for lower odds of achieving ≥60% and ≥70% RASS in the desired target with increasing number of altered phenotypes. Although numerically different, there was no significant difference between time to target RASS in individuals with ≤1 altered phenotypes (5 h) vs. those with >1 altered phenotypes (10 h). Individuals with >1 altered phenotypes had 3.5 times more ADRs than those with ≤1 altered phenotypes (14.8% vs. 4.16%). It may be possible that the presence of altered phenotypes that change the pharmacokinetics or pharmacodynamics of sedative and analgesics increases the risk of ADRs but we were not able to demonstrate a significant difference. This should be evaluated in a larger sample size. In the current study, all participants, who had their PGx results returned and received the individual counseling session, had satisfactory perceptions and positive attitudes toward PGx. Capturing patients' attitudes and perceptions toward PGx is essential for developing and implementing PGx in clinical settings. Future studies should evaluate the attitudes of the ICU medical and nursing providers.
The current study has limitations. Our PGx panel was limited to variants with known or probable PGx effects for fentanyl, propofol, midazolam, and other drugs but may have led to omitting other important pharmacogenes. We did not study the ADRA2A and PRKCB genes which have been studied for dexmedetomidine since they are rarely tested on PGx panels and were not available on the panel we used but have LOE of 3 on PharmGKB. 26 In our analysis, the primary PGx effect (percentage of time in target RASS range) is mainly related to fentanyl, propofol and/or midazolam which are the primary agents used in our ICUs. We did not account for concomitant medications which might have drug–drug interactions with the tested sedatives and analgesics because the study period was short (24 and 48 h) and we assumed the drug interaction effect would be small. The lack of biogeographical genetic diversity in our study participants resulted in only a few diplotypes that could be potentially important (i.e., CYP3A5*1/*1) for fentanyl and midazolam. Because of the many possible combinations of sedatives and analgesics that can be used, confounding effects are difficult to control. Future PGx studies should focus on a single agent and/or a single combination such as fentanyl and propofol. Another limitation is that the study was interrupted by the COVID‐19 pandemic and the enrollment process was halted for 7 months which affected the number of patients enrolled and the pandemic affected ICU practice. Future studies with larger sample sizes are needed.
CONCLUSION
An increase in the number of altered phenotypes in pharmacogenes relevant to propofol, fentanyl, and midazolam had a non‐significant association toward unfavorable sedation outcomes such as lower odds of achieving target RASS range and slower time to target RASS during the early period of acute MV. The positive attitudes and perceptions of ICU patients and their willingness to participate will help facilitate the advancement and acceptance of PGx testing in the critical care setting.
AUTHOR CONTRIBUTIONS
M.E.M., T.T.N, J.L., B.S., Z.R., G.B., D.S., and P.A.J. wrote the manuscript; T.T.N., J.L., B.S., Z.R., G.B. D.S., and P.A.J. designed the research; M.E.M., T.T.N, J.L., D.S., and P.A.J. performed the research; M.E.M., analyzed the data.
FUNDING INFORMATION
This research was funded by Enhance Comprehensive Pharmacist Services to Improve Patient Health Clinical Research Award and MM was supported by a student award by the National Institutes of Health's National Center for Advancing Translational Sciences, grant UM1TR004405. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health's National Center for Advancing Translational Sciences.
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Data S1.
Data S2.
ACKNOWLEDGMENTS
The authors would like to thank the participants and their families. We acknowledge the dedication and hard work of the nurses and pharmacists in the ICU.
Mohamed ME, Nguyen TT, Larson J, et al. Pharmacogenomic variation and sedation outcomes during early intensive care unit admission: A pragmatic study. Clin Transl Sci. 2024;17:e70107. doi: 10.1111/cts.70107
REFERENCES
- 1. The Clinical Pharmacogenetics Implementation Consortium (CPIC) Website. Accessed April 8, 2024. https://cpicpgx.org/
- 2. Grap MJ, Munro CL, Wetzel PA, et al. Sedation in adults receiving mechanical ventilation: physiological and comfort outcomes. Am J Crit Care. 2012;21(3):e53‐e63; quiz e64. doi: 10.4037/ajcc2012301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Su L, Liu C, Chang F, et al. Selection strategy for sedation depth in critically ill patients on mechanical ventilation. BMC Med Inform Decis Mak. 2021;21(S2):79. doi: 10.1186/s12911-021-01452-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearson SD, Patel BK. Evolving targets for sedation during mechanical ventilation. Curr Opin Crit Care. 2020;26(1):47‐52. doi: 10.1097/MCC.0000000000000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and Management of Pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):E825‐E873. doi: 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 6. Shehabi Y, Bellomo R, Kadiman S, et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180‐day mortality: a multinational prospective longitudinal cohort study. Crit Care Med. 2018;46(6):850‐859. doi: 10.1097/CCM.0000000000003071 [DOI] [PubMed] [Google Scholar]
- 7. Stephens RJ, Dettmer MR, Roberts BW, et al. Practice patterns and outcomes associated with early sedation depth in mechanically ventilated patients: a systematic review and meta‐analysis. Crit Care Med. 2018;46(3):471‐479. doi: 10.1097/CCM.0000000000002885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond agitation‐sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338‐1344. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 9. Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients a randomized trial. JAMA. 2009;301(5):489‐499. doi: 10.1001/jama.2009.56 [DOI] [PubMed] [Google Scholar]
- 10. MacKenzie M, Hall R. Pharmacogenomics and pharmacogenetics for the intensive care unit: a narrative review. Can J Anesth Can d'anesthésie. 2017;64(1):45‐64. doi: 10.1007/s12630-016-0748-1 [DOI] [PubMed] [Google Scholar]
- 11. Zhou S, Skaar DJ, Jacobson PA, Huang RS. Pharmacogenomics of medications commonly used in the intensive care unit. Front Pharmacol. 2018;9:1436. doi: 10.3389/fphar.2018.01436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Austin CA, Szeto A, Gupta A, Wiltshire T, Crona DJ, Kistler C. The Pharmacogenetics of opiates and its impact on delirium in mechanically ventilated adults: a pilot study. J Pharm Technol. 2022;38(4):195‐201. doi: 10.1177/87551225221085116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayes E, Esteves A. Adherence to sedation targets with weight‐based Propofol and Dexmedetomidine in patients with morbid obesity. Ann Pharmacother. 2022;57:240. doi: 10.1177/10600280221108429 [DOI] [PubMed] [Google Scholar]
- 14. DiCesare MA, Rech MA, DeMott JM. Predictors of a response to dexmedetomidine in intubated, critically ill adult patients. Pharmacotherapy. 2021;41(2):191‐197. doi: 10.1002/phar.2501 [DOI] [PubMed] [Google Scholar]
- 15. Tanaka N, Naito T, Yagi T, Doi M, Sato S, Kawakami J. Impact of CYP3A5*3 on plasma exposure and urinary excretion of fentanyl and norfentanyl in the early postsurgical period. Ther Drug Monit. 2014;36(3):345‐352. doi: 10.1097/FTD.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 16. Takashina Y, Naito T, Mino Y, Yagi T, Ohnishi K, Kawakami J. Impact of CYP3A5 and ABCB1 gene polymorphisms on fentanyl pharmacokinetics and clinical responses in cancer patients undergoing conversion to a transdermal system. Drug Metab Pharmacokinet. 2012;27(4):414‐421. doi: 10.2133/dmpk.dmpk-11-rg-134 [DOI] [PubMed] [Google Scholar]
- 17. Yuan R, Zhang X, Deng Q, Wu Y, Xiang G. Impact of CYP3A4*1G polymorphism on metabolism of fentanyl in Chinese patients undergoing lower abdominal surgery. Clin Chim Acta. 2011;412(9–10):755‐760. doi: 10.1016/j.cca.2010.12.038 [DOI] [PubMed] [Google Scholar]
- 18. Saiz‐Rodríguez M, Ochoa D, Herrador C, et al. Polymorphisms associated with fentanyl pharmacokinetics, pharmacodynamics and adverse effects. Basic Clin Pharmacol Toxicol. 2019;124(3):321‐329. doi: 10.1111/bcpt.13141 [DOI] [PubMed] [Google Scholar]
- 19. Grimsrud KN, Ivanova X, Sherwin CM, Palmieri TL, Tran NK. Identification of cytochrome P450 polymorphisms in burn patients and impact on fentanyl pharmacokinetics: a pilot study. J Burn Care Res. 2019;40(1):91‐96. doi: 10.1093/jbcr/iry053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seng KY, Hee KH, Soon GH, et al. CYP3A5*3 and bilirubin predict midazolam population pharmacokinetics in Asian cancer patients. J Clin Pharmacol. 2014;54(2):215‐224. doi: 10.1002/jcph.230 [DOI] [PubMed] [Google Scholar]
- 21. Elens L, Nieuweboer A, Clarke SJ, et al. CYP3A4 intron 6 C>T SNP (CYP3A4*22) encodes lower CYP3A4 activity in cancer patients, as measured with probes midazolam and erythromycin. Pharmacogenomics. 2013;14(2):137‐149. doi: 10.2217/pgs.12.202 [DOI] [PubMed] [Google Scholar]
- 22. Mourão AL, de Abreu FG, Fiegenbaum M. Impact of the cytochrome P450 2B6 (CYP2B6) gene polymorphism c.516G>T (rs3745274) on Propofol dose variability. Eur J Drug Metab Pharmacokinet. 2016;41(5):511‐515. doi: 10.1007/s13318-015-0289-y [DOI] [PubMed] [Google Scholar]
- 23. Fentanyl – PharmGKB. Accessed April 23, 2024. https://www.pharmgkb.org/chemical/PA449599/clinicalAnnotation
- 24. Midazolam – PharmGKB. Accessed April 23, 2024. https://www.pharmgkb.org/chemical/PA450496/clinicalAnnotation
- 25. Propofol – PharmGKB. Accessed April 23, 2024. https://www.pharmgkb.org/chemical/PA451141/clinicalAnnotation
- 26. Dexmedetomidine – PharmGKB. PharmGKB. Accessed April 8, 2024. https://www.pharmgkb.org/chemical/PA449256/clinicalAnnotation
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data S2.


