ABSTRACT
Prognostic factors for the long‐term evolution of chronic hepatitis B e antigen (HBeAg)‐negative hepatitis B virus (HBV) infection may vary depending on local epidemiology. We aimed to identify these factors in France, where the epidemiology is influenced by diverse immigration. Hepatitis B surface antigen (HBsAg)‐positive, HBeAg‐negative adults with normal transaminase levels and viral loads < 20,000 IU/mL for 1 year, without viral co‐infection or advanced liver disease, were enrolled for a 5‐year follow‐up. A total of 564 patients were recruited from 23 centres (54.4% women, mean age 42.3 ± 12 years, 47.7% from sub‐Saharan Africa). HBV DNA was detectable but < 2000 IU/mL for most (71.3%). Genotypes E (27.8%) and A (20.0%) were predominant. The mean HBsAg titre was 3.8 ± 3.4 log IU/mL, > 1000 IU/mL in 60% of cases, and higher in genotype E (p < 0.0001). During follow‐up, 18 patients received antiviral treatment, 9 for viral reactivation (0.3% per year) and 9 preemptively. HBsAg loss occurred in 39 patients (1.4% per year). These patients were older (p < 0.0001), more frequently treated for dyslipidemia, hypertension or diabetes (p < 0.05), and had lower baseline HBV DNA (p = 0.0112) and HBsAg (p < 0.0001), but similar levels of HBcrAg compared to those who did not clear HBsAg. Baseline HBsAg was the only independent predictor of HBsAg loss (p = 0.009). In this cohort, HBsAg < 153 IU/mL predicted clearance with 87% sensitivity and specificity. In conclusion, baseline HBsAg accurately predicted seroclearance at 5 years in patients with chronic HBeAg‐negative infection, regardless of genotype, sex, or geographical origin, indicating that this marker is widely applicable for reducing the frequency of patient monitoring.
Keywords: HBcrAg, HBeAg‐negative chronic HBV infection, HBsAg, HBsAg loss prediction, HBV‐DNA, HBV genotype, HBV reactivation
Abbreviations
- ALT
alanine aminotransferase
- ANGH
Association Nationale des Hépato‐Gastroentérologues des Hôpitaux Généraux
- BMI
body mass index
- cccDNA
covalently closed and circular DNA
- EASL
European Association for the Study of the Liver
- HBcrAg
hepatitis B core‐related antigen
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- ROC curve
receiver operating characteristic curve
Hepatitis B remains a public health problem in France, despite availability of effective vaccines and treatments, which currently only allow for a functional cure [1, 2]. The natural history of chronic hepatitis B virus (HBV) infection has been divided into five phases, according to the presence of hepatitis B e antigen (HBeAg), HBV DNA levels and alanine aminotransferase (ALT) values. In most cases, multiple assessments of ALT levels and viral markers are required to define the infection phase. HBeAg‐negative chronic HBV infection, formerly ‘inactive carrier’ phase, is characterised by the presence of serum antibodies to HBeAg (anti‐HBe), undetectable or low (< 2000 IU/mL) HBV DNA levels and persistently normal ALT in patients without viral coinfection and mild or absent liver fibrosis. Some patients in this phase may have HBV DNA levels > 2000 IU/mL, and < 20,000 IU/mL [3]. Patients with HBeAg‐negative infection have a low risk of progression to cirrhosis (< 0.1 per 100 persons/year) or hepatocellular carcinoma (0.05 per 100 persons/year), and little change in life expectancy (mortality 0.03 per 100 persons/year). Hepatitis B surface antigen (HBsAg) loss and/or seroconversion may occur spontaneously in 0.7%–2.3% per year, but progression to chronic hepatitis B may also occur in 0%–10% of cases, depending on the cohort [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. Hence, long‐term surveillance is recommended for this heterogeneous population. The 2017 guidelines of the European Association for the Study of the Liver (EASL) advised to monitor ALT every 6–12 months in patients with B viral load < 2000 IU/mL, with HBV DNA and liver fibrosis assessment every 2–3 years, depending on HBsAg quantification. Those with HBV DNA > 2000 UI/mL need closer monitoring with ALT determination every 3 months for the first year and every 6 months thereafter, with yearly HBV DNA and fibrosis assessment. If no treatment indication arises during the first 3 years of surveillance, the follow‐up is then the same as for patients with HBV DNA < 2000 IU/mL [3].
The correct and early identification of the stage of HBV disease has been the subject of much research. Indeed, the early identification of HBeAg‐negative infection would avoid the need for close monitoring, particularly during the first year. In addition, identifying among patients with HBeAg‐negative infection those who will lose their HBsAg would further simplify follow‐up, given the very low risk of disease progression [15]. Typically, such patients have > 15 years of chronic HBV infection [16], and low HBsAg levels. Threshold values predicting HBsAg loss have been proposed, but may differ according to HBV genotype. In Asian populations infected with genotypes B and C, HBsAg level < 100 IU/mL was associated with HBsAg loss during follow‐up, while in European populations infected with genotype D, the threshold was < 1000 IU/mL [17]. The magnitude of HBsAg titre reduction over time, and low or undetectable HBV RNA and Hepatitis B core‐related antigen (HBcrAg) levels were also predictive of HBsAg loss, but accurate cut‐offs have not yet been proposed [18].
The aim of our study was to investigate the 5‐year outcome of patients with HBeAg‐negative chronic HBV infection to identify factors predictive of HBsAg loss. The analysis of a French cohort seems valuable because French epidemiology differs from that of Asia, Italy, or the United States, where similar studies have been conducted.
1. Patients and Methods
PIBAC is a multicentre cohort study involving 23 French and 1 Belgian centres, in general and university hospitals, and in private practice. HBsAg positive and HBeAg negative adults with normal aminotransferases levels (ALT ≤ 40 IU/L) and HBV DNA < 20,000 IU/mL, repeated every 3 or 4 months for at least 1 year were prospectively included. Other inclusion criteria were the absence of Hepatitis C, Hepatitis D or Human Immunodeficiency Virus infection and the absence of signs of advanced liver disease, whether clinical, biological, ultrasonographic or elastographic. Transient elastography has been validated to distinguish HBV patients with significant liver fibrosis from those with minimal or absent fibrosis [19].
Data collected included sex, age, geographical origin, body mass index (BMI) and use of antihypertensive, antidiabetic or lipid‐lowering medications. Alcohol, tobacco and cannabis consumption were collected at inclusion using an open‐ended questionnaire. Patients were followed for 5 years, with annual collection of clinical, biological, ultrasound and elastographic data.
1.1. Virological Analyses
HBsAg titre was determined using Elecsys* HBsAg II quant II reagent on a Cobas 6000 system (Roche Diagnostics), with a measurement range of 0.05 to > 52,000 IU/mL. The threshold for HBsAg negativity was < 0.05 IU/mL. HBcrAg titre was determined using Lumipulse* G HBcrAg reagent (Fujirebio Inc., Japan, Research use only) on a Lumipulse G600II automated immunoassay system. The result was given in log10 arbitrary units (U)/mL: < 2 = negative, between 2 and 3 = detectable, non‐quantifiable, from 3 to 7 = quantification range. HBV genotype was determined by sequencing and phylogenetic analysis of 600 nucleotides encompassing the polymerase and surface genes. These analyses were carried out centrally at the virology laboratory of the Paul Brousse Hospital in Villejuif. The viral load required for patient management was carried out on an ongoing basis at each centre.
1.2. Statistical Analysis
Three evolutionary profiles were defined: (1) Unfavourable: diagnosis of cirrhosis or liver cancer, HBV‐related death, or HBV reactivation; (2) Favourable: HBsAg clearance; (3) Stable: absence of any of the events listed above. To investigate factors associated with HBsAg loss, a univariate analysis was performed using chi‐2 or Fisher tests for categorical variables, and Student's t‐test for continuous variables. After interaction analysis of the model variables and imputation of missing data by the MICE package, a multivariate logistic regression analysis was conducted to identify independent predictors of HBsAg loss. Receiver operating characteristic (ROC) curves were constructed to compare the respective diagnostic performance of baseline HBV DNA and HBsAg titres in predicting HBsAg loss. The area under the curve (AUC) and 95% confidence intervals were calculated. Statistical analysis was performed using RStudio 2023.09 and Analyse‐it for Microsoft Excel 6.15.4.
2. Results
From September 2014 to June 2016, 566 patients were pre‐selected as ‘inactive carriers’. Of them, 2 were excluded due to elastography value > 10 kPa, indicating significant fibrosis, and 564, 1–100 per centre, were included.
2.1. Baseline Characteristics
Fifty‐four percent were women, with a mean age of 42.3 ± 12 years (Table 1). Sub‐Saharan Africa was the most frequent area of origin (47.7%). The mean BMI was 25.7 ± 4.5 kg/m2, 1.2% of men reported alcohol consumption of over 40 g/day, 1% of women reported alcohol consumption of over 20 g/day, 8.7% were current smokers and 1.2% reported cannabis use.
TABLE 1.
Patient's characteristics at baseline, by outcome.
| All patients, N = 564 | HBsAg clearance during follow‐up, N = 39 | HBsAg persistence at 5 years w/o treatment, N = 300 | p | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 307 (54.4) | 19 (48.7) | 166 (55.3) | 0.4351 |
| Male | 257 (45.6) | 20 (51.3) | 134 (44.7) | |
| Age mean (SD) | 42.3 (11.9) | 52 (11.6) | 41.9 (11.2) | < 0.0001 |
| Origin, n (%) | ||||
| Sub‐Saharian Africa | 269 (47.7) | 11 (28.2) | 144 (48.0) | 0.3330 |
| France | 87 (15.4) | 8 (20.5) | 45 (15.0) | |
| North Africa | 74 (13.1) | 7 (17.9) | 45 (15.0) | |
| Asia/Oceania | 68 (12.1) | 7 (17.9) | 34 (11.3) | |
| Western Europe/North America | 39 (6.9) | 4 (10.3) | 18 (6.0) | |
| Eastern Europe | 19 (3.4) | 2 (5.1) | 10 (3.3) | |
| Other | 8 (1.4) | 0 | 4 (1.3) | |
| BMI mean (SD) | 25.7 (4.5) | 25.3 (3.8) | 25.8 (4.5) | 0.5424 |
| Hypertension therapy, n (%) | (10.1) | 9 (23.1%) | 27 (9.0%) | 0.0056 |
| Diabetes therapy, n (%) | (4.4) | 4 (10.3%) | 11 (3.7%) | 0.0372 |
| Hypolipidemic therapy, n (%) | (5.0) | 6 (15.4%) | 9 (3.0%) | 0.0004 |
| HBV genotype, n (%) | ||||
| A | 115 (20.0) | 5 (12.8) | 64 (21.3) | 0.2187 |
| B | 26 (4.4) | 3 (7.7) | 15 (5.0) | |
| C | 22 (3.9) | 2 (5.1) | 9 (3.0) | |
| D | 105 (18.3) | 5 (12.8) | 57 (19.0) | |
| E | 158 (27.8) | 8 (20.5) | 85 (28.3) | |
| F | 2 (0.4) | 0 | 1 (0.3) | |
| ND | 145 (25.2) | 16 (41.1) | 69 (23.0) | |
| HBV DNA mean log IU/mL (SD) | 3.16 (3.44) | 2.59 (3.02) | 3.15 (3.4) | 0.0112 |
| HBVDNA class | ||||
| Not detected, n (%) | (10.1) | 14 (35.9) | 20 (0.7) | < 0.0001 |
| Detected < 2000, n (%) | (71.3) | 23 (59) | 220 (66.7) | |
| 2000–20,000, n (%) | (18.6) | 2 (5.1) | 60 (32.6) | |
| HBsAg mean log IU/mL (SD) | 3.77 (3.91) | 2.12 (2.69) | 3.78 (3.90) | < 0.0001 |
| HBsAg class | ||||
| < 100, n (%) | (16.5) | 32 (82) | 33 (11) | < 0.0001 |
| 100–999, n (%) | (23.8) | 6 (15.4) | 78 (26) | |
| ≥ 1000, n (%) | (59.8) | 1 (2.6) | 189 (63) | |
| HBcrAg class | ||||
| Not detected, n (%) | 234 (41.5) | 21 (53.8) | 125 (41.7) | 0.0720 |
| Detected, n (%) | 237 (42.0) | 16 (41.1) | 120 (40.0) | |
| Quantifiable, n (%) | 68 (12.1) | 1 (2.6) | 41 (13.7) | |
| NA, n (%) | 25 (4.4) | 1 (2.6) | 14 (4.7) | |
Most patients had detected but < 2000 IU/mL viral load (71.3%), HBsAg titre > 1000 IU/mL (59.8%) and low (< 3 log U/mL) or undetected HBcrAg (85.5%). Viral genotyping was successful in 74.8% of subjects with a sufficient viral load. Genotype E was predominant (27.8%), followed by A (20.0%), D (18.3%), B (4.4%), C (3.9%) and F (0.4%). Higher HBsAg titres were observed for patients infected by genotype E, compared with all other genotypes except F, which was detected in only 2 patients (Figure 1a). Patients infected by genotypes B and C presented higher HBcrAg titres, which were quantifiable in 42 and 30% of cases respectively, compared with 7%–13% for other genotypes (Figure 1b). HBV genotype had no impact on viral load.
FIGURE 1.
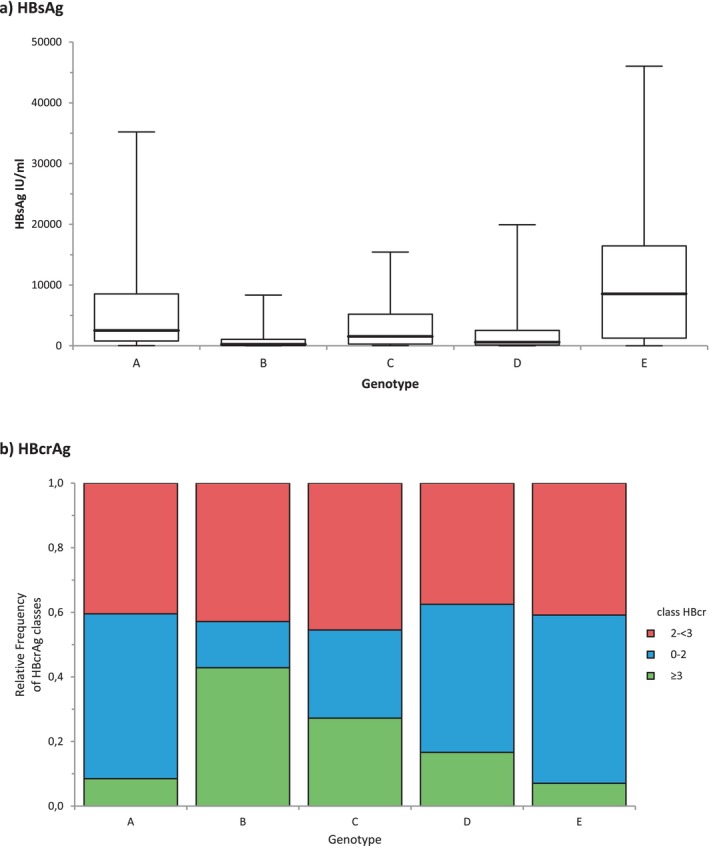
Baseline HBsAg and HBcrAg levels, by genotype. (a) HBsAg titres (mean ± SD log IU/mL). Genotype A: 3.33 ± 0.8; B: 2.36 ± 0.9; C: 3.0 ± 0.2; D: 2.62 ± 1.1; E: 3.57 ± 0.9. HBsAg titres in genotype E were higher than titres in all other genotypes (p < 0.0001). (b) HBcrAg levels. The frequency of quantifiable HBcrAg levels was higher in genotypes B and C compared to genotypes A, D and E (p < 0.05).
2.2. Five‐Year Follow‐Up
The attrition rate at 5 years was 39% (Table S1). During follow‐up, two deaths occurred, unrelated to HBV (accidental fall, breast cancer). No cases of acute liver failure, cirrhosis or hepatocarcinoma were reported.
Antiviral treatment was given to 18 patients, with a pre‐emptive objective in nine cases: progressive cancer (n = 3), initiation of immunosuppressive therapy (n = 5) and occupational precaution in a healthcare worker (n = 1). In the nine other patients, the reason for antiviral treatment was HBV reactivation, defined as viral load > 20,000 IU/mL and/or ALT > N. The reactivation rate was 0.3% per year and 1.6% at 5 years. Reactivation occurred in 7 women and 2 men, mean age 39 ± 9 years, mean HBsAg titre at inclusion 4.06 + 4.15 log IU/mL, > 1000 IU/mL in all cases, and mean viral load 3.6 ± 3.4 log IU/mL, > 2000 IU/mL in 6/9 patients. Genotype E was identified in 6/9 patients. HBcrAg was tested in eight patients and undetected in 4/8 cases.
Spontaneous HBsAg clearance was observed in 39 patients, that is 1.4%/year, 6.9% at 5 years. Among these 39 patients, 21 had available anti‐HBs results: 15/21 (71.5%) seroconverted to anti‐HBs.
Among patients followed up to 5 years, 300 had no need for antiviral treatment, nor HBsAg clearance. Year‐5 HBsAg titre, available for 218 of these patients, was significantly lower than at baseline, 3.8 ± 3.9 log IU/mL vs. 3.6 ± 3.8 log IU/mL (p < 0.0001), which is a mean decrease of 0.2 log at 5 years.
2.3. Factors Associated With HBsAg Clearance
Table 1 compares the baseline characteristics of the 39 patients with HBsAg clearance with those of the 300 patients who remained HBsAg‐positive without initiation of antiviral therapy. Patients with HBsAg loss were 10 years older (p < 0.0001), their HBsAg level was > 10 times lower (p < 0.0001), and their viral load was > 2 times lower (p = 0.0112). They were also more often treated for dyslipidaemia (p = 0.0004), hypertension (p = 0.0056), or diabetes (p = 0. 0372). Viral genotype, HBcrAg level, geographic origin, sex, BMI or recruiting centre were not associated with HBsAg loss.
In multivariate logistic regression, only baseline HBsAg titre (p = 0.009) was associated with HBsAg clearance. Approximately 25% of patients with HBsAg < 1000 and half of patients with HBsAg < 100 have lost their HBsAg at 5 years, while only 13.8% of those with HBV DNA < 2000 and 41.2% of those with undetectable HBV DNA had this outcome.
The performance of baseline HBsAg and HBV DNA quantification to identify patients who will clear HBsAg during follow‐up is shown in Figure 2, with areas under the ROC curve of 0.927 (CI 95% [0.889–0.965]), p < 0.0001, and 0.771 (CI 95% [0.689–0.853]), p < 0.0001, respectively. An HBsAg < 153 UI/mL predicted HBsAg loss at 5 years, with the highest sensitivity and specificity, which is 87%. However, performance at this threshold varied according to genotype, with sensitivity and specificity of 100% and 97.6% for the E genotype, compared with 84% and 83% for the non‐E genotype.
FIGURE 2.
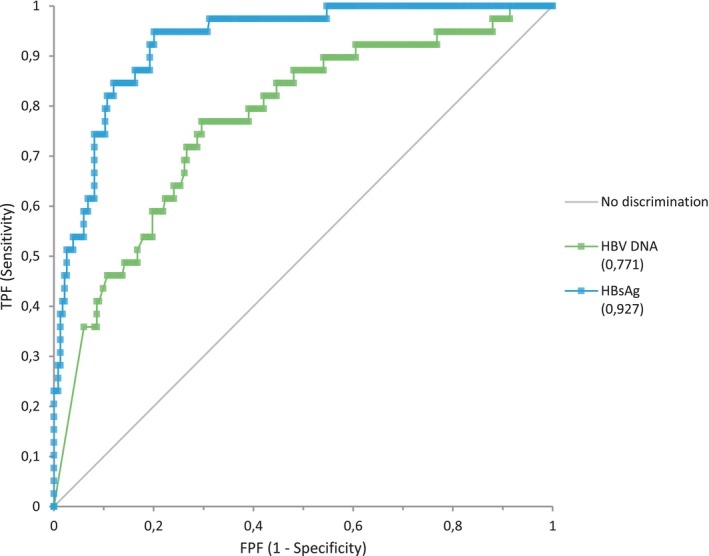
Performance of baseline HBsAg and HBV DNA quantification to identify patients who will lose HBsAg during 5‐years follow‐up. Receiver operating characteristic curve (ROC) for prediction of HBsAg loss based on baseline serum levels of HBsAg and HBV DNA. Areas under the curve were 0.927 for HBsAg levels, and 0.771 for HBV DNA levels.
3. Discussion
Prospective follow‐up of a large cohort of patients with chronic HBeAg‐negative HBV infection in France confirms the favourable prognosis of this population, with rare viral reactivations, occurring in 0.3% of cases per year. The other patients saw their HBsAg levels decrease over time, with a very slow decline of 0.2 log on average over the 5 years of follow‐up; functional cure, that is loss of HBsAg, was a rare event, occurring in 1.4% of cases per year, similar to previously reported rates [3, 20, 21, 22], and was accurately predicted over 5 years by a low baseline HBsAg titre. This simple tool can be used to tailor the care of patients with chronic HBeAg‐negative HBV infection.
During the natural history of chronic HBV infection, the formerly ‘inactive carrier’ stage is reached after many years of evolution and has a favourable long‐term outcome [3]. At this stage, the decrease in HBsAg levels over time is expected [16, 23], with infrequent loss of HBsAg, but when it does occur, seroclearance has been shown to be sustained in most cases [20, 21]. Among the factors predictive of HBsAg loss in these patients, a low baseline HBsAg titre and/or the dynamics of the decline in the titre are highly predictive of seroclearance in many studies, reflecting the decline in cccDNA levels. In Asian cohorts, an annual decrease > 0.5 log IU/mL had a positive predictive value of HBsAg clearance of 89% when the initial HBsAg was < 200 IU/mL [23, 24]. In a large and diverse North American and Asian Pacific population, the annual seroclearance rate reached 7% in patients aged over 55 years with HBsAg < 100 UI/mL [25]. HBsAg < 100 UI/mL was also the best predictor of spontaneous seroclearance in an Italian cohort [22]. Combinations of factors have been proposed to improve the prediction of HBsAg loss in patients with chronic HBeAg‐negative HBV infection: a low HBsAg (< 100 IU/mL) with low anti‐HBc levels [26] or change in HBsAg levels and fibronectin level [27] for seroclearance at 1 year; a low HBsAg with a low level of the IP‐10 cytokine [28] or a score combining sex, change in HBsAg, age and for the prediction of HBsAg loss at 3 years [21].
In our study, where patients were enrolled with a strict definition of inactive HBeAg‐negative infection, baseline HBsAg titre was the only independent predictor of functional cure: a value of 153 IU/mL was identified, with the best possible sensitivity and specificity (87%), patients who were HBsAg‐negative within 5 years, performance was even better for genotype E‐infected patients.
One of the original features of our cohort is the great genotypic diversity of our patients, with a majority of E and A, endemic in sub‐Saharan Africa. We show higher HBsAg titres in patients infected with genotypes F, E and A than in those infected with genotypes B, C or D (Figure 1, Table S2). A Spanish study also reported that HBsAg levels varied across genotypes in HBeAg‐negative patients with higher titres for genotypes F, H, E and A than for genotype D [29]. To date, EASL guidelines for the identification of HBeAg‐negative chronic infections have been based on results from Asian or Caucasian cohorts where B and C, or D genotypes predominate [3, 17, 30, 31]. Previously defined thresholds may not be transposable to France due to the difference in genotype distribution. Indeed, most of our patients (60%) had baseline HBsAg over 1000 UI/mL, the threshold proposed by Brunetto et al. [30] to define inactive infection among genotype D‐infected patients, but the absence of liver events in most of our patients suggests that they were true ‘inactive carriers’.
In univariate analysis, older age and lower baseline viral load were associated with HBsAg seroclearance, a result consistent with previous studies [18]. The association of HBsAg loss with age and pathologies whose frequency increases with age (dyslipidaemia, hypertension and diabetes) are consistent with a decrease in titre with the duration of infection [16]. However, most of the migrants included in this study were diagnosed with B virus infection when they were screened on arrival in France, so the age of the infection, although probably neonatal in most cases, is not known precisely. We therefore did not examine the reported age of infection in our analysis. Similarly, we did not record which lipid‐lowering treatment was used (statin or fibrate), which made it impossible to study whether there was a specific effect of statins.
In a large retrospective Taiwanese study, while HBsAg level was the best predictor of HBsAg clearance in treatment naïve patients at 5 years of follow‐up, it was the HBcrAg level that was the best long‐term predictor, at 10, 15 or 20 years [32]. However, inactive carriers have typically low or undetected HBcrAg levels reflecting the low transcriptional activity of cccDNA at this stage [18], and this was the case for most of our patients (83.5%). Accordingly, HBcrAg level was not a relevant predictor of spontaneous HBsAg seroclearance at this late stage of the natural history. In addition, we observed a genotype‐dependent bias of HBcrAg quantification that deserves further investigation.
Viral reactivation was infrequent and well controlled by the available nucleos(t)ide analogues (tenofovir and entecavir), which were initiated as soon as reactivation was diagnosed, probably contributing to the absence of hepatic complications in this cohort. The small number of reactivations makes it impossible to define reliable risk thresholds. However, these patients all had baseline HBsAg > 1000 IU/mL, and most were infected with genotype E, with viral load > 2000 IU/mL. It would be interesting to explore the factors associated with reactivation in a cohort with a larger number of patients who have HBV DNA levels between 2000 and 20,000 IU/mL. For these patients, it is important to emphasise the need for long‐term follow‐up, especially since treatment does not lead to HBV eradication.
One might question the representativeness of our cohort, particularly given the high attrition rate. This high rate can be explained by the absence of therapeutic indications. This is especially true since many participants, being migrants, had other immediate concerns (housing with a change of address during the study, access to employment, administrative situation, etc.). In addition, the last monitoring period (2020–2021) was severely disrupted by the COVID‐19 epidemic, which prevented or delayed many consultations. Nevertheless, despite high attrition rate, our cohort is one of the largest prospective cohorts reported to date [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. This cohort is also representative of the population treated in mainland France in several respects: (i) the majority of patients were of African origin [1]; (ii) baseline BMI was comparable to that of the general population in the OBEPI‐ROCHE 2020 study (Figure S1) [33]; (iii) Liver elastography data are consistent with previous reports [7, 12, 13, 34]. Alcohol, tobacco, and cannabis consumption were however lower than in the general population. Although self‐reporting may lead to an underestimate, this reduced consumption could be linked to the different lifestyles of the patients included, many of whom are not of French origin. In our study, the main recruiting centres were associated with large maternity wards. As screening for hepatitis B is systematic during pregnancy, this probably contributed to the predominance of women in our cohort.
In Europe, the prevalence and the diversity of HBV are strongly affected by immigration. France, and Europe in general, have seen high rates of immigration from countries with intermediate or high HBV seroprevalence, including Asian and African countries [35]. HBV genotypes, which show distinct geographical distributions, or patient's genetics may impact disease progression. The results of our cohort, which is characterised by great genotypic diversity and, by extension, great ethnic diversity, could therefore be extrapolated to most European countries, and probably also to countries of emigration: Once inactive carrier status has been carefully characterised, a low HBsAg level reliably identifies patients who will lose the HBsAg and who could therefore safely benefit from reduced monitoring.
4. Conclusion
This study of a large and diverse French cohort of patients with strict diagnostic criteria for HBeAg‐negative chronic HBV infection confirms the rarity of clinical events and the low frequency of functional cure after 5 years of follow‐up. In this population, with predominantly E and A genotypes, HBsAg levels slowly declined over time and a low initial titre < 153 UI/mL was the best predictor of functional cure at 5 years. Patient compliance with the monitoring protocol was limited, as confirmed by the high attrition rate observed. This surveillance could be reduced, at low risk, for people whose HBsAg level is below this threshold, once it has been confirmed in a validation cohort. On the other hand, surveillance should not be relaxed for those with a viral load > 2000 IU/mL and HBsAg > 1000 IU/mL. Only the availability of more potent antivirals, capable of eradicating the virus, seems likely to change this strategy.
Author Contributions
X.C., P.P. and A.‐M.R.‐A. analysed, interpreted the data, and wrote the manuscript. A.V. contributed to data analysis A.‐M.R.‐A. and L.M. performed HBcrAg and HBsAg quantification, and HBV genotyping. The PIBAC study group (X.C., H.L., G.M., J.‐F.C., T.F.) recruited patients, performed clinical monitoring, and provided clinical expertise. All approved the manuscript.
Ethics Statement
The ethics of the study was approved by the Comité de Protection des Personnes (CPP) of Tours with number n° 2014‐s10.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1.
Acknowledgements
Roche Diagnostics kindly provided part of the reagents for HBsAg quantification. We thank Barbara de Dieuleveult for database management, all the patients who participated in this study, and all the doctors in the PIBAC study group: H. Salloum, M. Picon‐Coste, S Hommel, J. Henrion, J.P. Arpurt, I. Rosa, I. Ollivier, S Bresson‐Hadni, D. Zanditenas, M. Schnee, F. Heluwaert, S Cosconea, B. Lambare, A. Garioud, P. Delasalle, C. Renou, B. Hanslik, N. Boyer, and C. Castelnau.
Funding: The study was supported by the Association Nationale des Hépato‐Gastroentérologues des Hôpitaux Généraux (ANGH).
Contributor Information
Anne‐Marie Roque‐Afonso, Email: anne-marie.roque@aphp.fr.
the PIBAC Study Group of Association Nationale des Hépato‐Gastroentérologues des Hôpitaux Généraux (ANGH):
H. Salloum, M. Picon‐Coste, S Hommel, J. Henrion, J. P. Arpurt, I. Rosa, I. Ollivier, S Bresson‐Hadni, D. Zanditenas, M. Schnee, F. Heluwaert, S Cosconea, B. Lambare, A. Garioud, P. Delasalle, C. Renou, B. Hanslik, N. Boyer, and C. Castelnau
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Tamandjou C., Laporal S., Lot F., and Brouard C., “Données épidémiologiques récentes sur les hépatites C, B et Delta,” Bulletin Epidémiologique Hebdomadaire 15–16 (2023): 311–317. [Google Scholar]
- 2. Hadey C. and Mélin P., “«Vivre avec une hépatite B»: une enquête des Etats généraux de l'hépatite B en France métropolitaine et d'outre‐mer,” Bulletin Epidémiologique Hebdomadaire 3–4 (2022): 57–63. [Google Scholar]
- 3. European Association for the Study of the Liver , “EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection,” Journal of Hepatology 67, no. 2 (2017): 370–398. [DOI] [PubMed] [Google Scholar]
- 4. de Franchis R., Meucci G., Vecchi M., et al., “The Natural History of Asymptomatic Hepatitis B Surface Antigen Carriers,” Annals of Internal Medicine 118, no. 3 (1993): 191–194. [DOI] [PubMed] [Google Scholar]
- 5. Villeneuve J. P., Desrochers M., Infante‐Rivard C., et al., “A Long‐Term Follow‐Up Study of Asymptomatic Hepatitis B Surface Antigen‐Positive Carriers in Montreal,” Gastroenterology 106, no. 4 (1994): 1000–1005. [DOI] [PubMed] [Google Scholar]
- 6. Martinot‐Peignoux M., Boyer N., Colombat M., et al., “Serum Hepatitis B Virus DNA Levels and Liver Histology in Inactive HBsAg Carriers,” Journal of Hepatology 36, no. 4 (2002): 543–546. [DOI] [PubMed] [Google Scholar]
- 7. Hsu Y. S., Chien R. N., Yeh C. T., et al., “Long‐Term Outcome After Spontaneous HBeAg Seroconversion in Patients With Chronic Hepatitis B,” Hepatology 35, no. 6 (2002): 1522–1527. [DOI] [PubMed] [Google Scholar]
- 8. Manno M., Camma C., Schepis F., et al., “Natural History of Chronic HBV Carriers in Northern Italy: Morbidity and Mortality After 30 Years,” Gastroenterology 127, no. 3 (2004): 756–763. [DOI] [PubMed] [Google Scholar]
- 9. Fattovich G., Olivari N., Pasino M., D'Onofrio M., Martone E., and Donato F., “Long‐Term Outcome of Chronic Hepatitis B in Caucasian Patients: Mortality After 25 Years,” Gut 57, no. 1 (2008): 84–90. [DOI] [PubMed] [Google Scholar]
- 10. Habersetzer F., Moenne‐Loccoz R., Meyer N., et al., “Loss of Hepatitis B Surface Antigen in a Real‐Life Clinical Cohort of Patients With Chronic Hepatitis B Virus Infection,” Liver International 35, no. 1 (2015): 130–139. [DOI] [PubMed] [Google Scholar]
- 11. Brouwer W. P., Chan H. L., Brunetto M. R., et al., “Repeated Measurements of Hepatitis B Surface Antigen Identify Carriers of Inactive HBV During Long‐Term Follow‐Up,” Clinical Gastroenterology and Hepatology 14, no. 10 (2016): 1481–1489 e1485. [DOI] [PubMed] [Google Scholar]
- 12. Castera L., Bernard P. H., Le Bail B., et al., “Transient Elastography and Biomarkers for Liver Fibrosis Assessment and Follow‐Up of Inactive Hepatitis B Carriers,” Alimentary Pharmacology & Therapeutics 33, no. 4 (2011): 455–465. [DOI] [PubMed] [Google Scholar]
- 13. Wong G. L., Chan H. L., Yu Z., Chan H. Y., Tse C. H., and Wong V. W., “Liver Fibrosis Progression Is Uncommon in Patients With Inactive Chronic Hepatitis B: A Prospective Cohort Study With Paired Transient Elastography Examination,” Journal of Gastroenterology and Hepatology 28, no. 12 (2013): 1842–1848. [DOI] [PubMed] [Google Scholar]
- 14. Ngo Y., Benhamou Y., Thibault V., et al., “An Accurate Definition of the Status of Inactive Hepatitis B Virus Carrier by a Combination of Biomarkers (FibroTest‐ActiTest) and Viral Load,” PLoS One 3, no. 7 (2008): e2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okada K., Nakayama Y., Xu J., Cheng Y., and Tanaka J., “A Nation‐Wide Medical Record Database Study: Value of Hepatitis B Surface Antigen Loss in Chronic Hepatitis B Patients in Japan,” Hepatology Research 54 (2024): 1004–1015. [DOI] [PubMed] [Google Scholar]
- 16. Chu C. M. and Liaw Y. F., “Hepatitis B Surface Antigen Seroclearance During Chronic HBV Infection,” Antiviral Therapy 15, no. 2 (2010): 133–143. [DOI] [PubMed] [Google Scholar]
- 17. Chan H. L., Thompson A., Martinot‐Peignoux M., et al., “Hepatitis B Surface Antigen Quantification: Why and How to Use It in 2011—A Core Group Report,” Journal of Hepatology 55, no. 5 (2011): 1121–1131. [DOI] [PubMed] [Google Scholar]
- 18. Riveiro‐Barciela M., Pericas J. M., and Buti M., “How to Interpret Viral Markers in the Management of Chronic Hepatitis B Infection,” Clinical Microbiology and Infection 28, no. 3 (2022): 355–361. [DOI] [PubMed] [Google Scholar]
- 19. Li Y., Huang Y. S., Wang Z. Z., et al., “Systematic Review With Meta‐Analysis: The Diagnostic Accuracy of Transient Elastography for the Staging of Liver Fibrosis in Patients With Chronic Hepatitis B,” Alimentary Pharmacology & Therapeutics 43, no. 4 (2016): 458–469. [DOI] [PubMed] [Google Scholar]
- 20. Bruden D., McMahon B. J., Snowball M., et al., “Rate and Durability of the Clearance of HBsAg in Alaska Native Persons With Long‐Term HBV Infection: 1982–2019,” Hepatology 79, no. 6 (2024): 1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terrault N. A., Wahed A. S., Feld J. J., et al., “Incidence and Prediction of HBsAg Seroclearance in a Prospective Multi‐Ethnic HBeAg‐Negative Chronic Hepatitis B Cohort,” Hepatology 75, no. 3 (2022): 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barone M., Iannone A., Mezzapesa M., et al., “Natural History and Hepatitis B Virus Surface Antigen (HBsAg) Spontaneous Seroclearance in Hepatitis B Virus e‐Antigen (HBeAg)‐Negative Patients With Inactive Chronic Infection: A Multicenter Regional Study From South Italy,” Pathogens 12, no. 10 (2023): 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seto W. K., Wong D. K., Fung J., et al., “A Large Case‐Control Study on the Predictability of Hepatitis B Surface Antigen Levels Three Years Before Hepatitis B Surface Antigen Seroclearance,” Hepatology 56, no. 3 (2012): 812–819. [DOI] [PubMed] [Google Scholar]
- 24. Lin H. C., Liu J., Pan M. H., et al., “Rapid Decline Rather Than Absolute Level of HBsAg Predicts Its Seroclearance in Untreated Chronic Hepatitis B Patients From Taiwanese Communities,” Clinical and Translational Gastroenterology 14, no. 8 (2023): e00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeo Y. H., Tseng T. C., Hosaka T., et al., “Incidence, Factors, and Patient‐Level Data for Spontaneous HBsAg Seroclearance: A Cohort Study of 11,264 Patients,” Clinical and Translational Gastroenterology 11, no. 9 (2020): e00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kan K., Wong D. K., Hui R. W., Seto W. K., Yuen M. F., and Mak L. Y., “Anti‐HBc: A Significant Host Predictor of Spontaneous HBsAg Seroclearance in Chronic Hepatitis B Patients—A Retrospective Longitudinal Study,” BMC Gastroenterology 23, no. 1 (2023): 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu F., Seto W. K., Wong D. K., et al., “Plasma Fibronectin Levels Identified via Quantitative Proteomics Profiling Predicts Hepatitis B Surface Antigen Seroclearance in Chronic Hepatitis B,” Journal of Infectious Diseases 220, no. 6 (2019): 940–950. [DOI] [PubMed] [Google Scholar]
- 28. Kan K., Wong D. K., Hui R. W., Seto W. K., Yuen M. F., and Mak L. Y., “Plasma Interferon‐Gamma‐Inducible‐Protein 10 Level as a Predictive Factor of Spontaneous Hepatitis B Surface Antigen Seroclearance in Chronic Hepatitis B Patients,” Journal of Gastroenterology and Hepatology 39, no. 1 (2024): 202–209. [DOI] [PubMed] [Google Scholar]
- 29. Riveiro‐Barciela M., Bes M., Rodriguez‐Frias F., et al., “Serum Hepatitis B Core‐Related Antigen Is More Accurate Than Hepatitis B Surface Antigen to Identify Inactive Carriers, Regardless of Hepatitis B Virus Genotype,” Clinical Microbiology and Infection 23, no. 11 (2017): 860–867. [DOI] [PubMed] [Google Scholar]
- 30. Brunetto M. R., Oliveri F., Colombatto P., et al., “Hepatitis B Surface Antigen Serum Levels Help to Distinguish Active From Inactive Hepatitis B Virus Genotype D Carriers,” Gastroenterology 139, no. 2 (2010): 483–490. [DOI] [PubMed] [Google Scholar]
- 31. Brunetto M. R., Carey I., Maasoumy B., et al., “Incremental Value of HBcrAg to Classify 1582 HBeAg‐Negative Individuals in Chronic Infection Without Liver Disease or Hepatitis,” Alimentary Pharmacology & Therapeutics 53, no. 6 (2021): 733–744. [DOI] [PubMed] [Google Scholar]
- 32. Tseng T. C., Chiang C., Liu C. J., et al., “Low Hepatitis B Core‐Related Antigen Levels Correlate Higher Spontaneous Seroclearance of Hepatitis B Surface Antigen in Chronic Hepatitis B Patients With High Hepatitis B Surface Antigen Levels,” Gastroenterology 164, no. 4 (2023): 669–679 e666. [DOI] [PubMed] [Google Scholar]
- 33. Ligue Contre l'Obésité , “Enquête épidémiologique nationale sur le surpoids et l'obésité,” accessed June, 2024, https://liguecontrelobesite.org/enquete‐epidemiologique‐nationale‐sur‐le‐surpoids‐et‐lobesite/ (2021).
- 34. Cornberg M., Wong V. W., Locarnini S., Brunetto M., Janssen H. L. A., and Chan H. L., “The Role of Quantitative Hepatitis B Surface Antigen Revisited,” Journal of Hepatology 66, no. 2 (2017): 398–411. [DOI] [PubMed] [Google Scholar]
- 35. Sharma S., Carballo M., Feld J. J., and Janssen H. L., “Immigration and Viral Hepatitis,” Journal of Hepatology 63, no. 2 (2015): 515–522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


