ABSTRACT
Background and Hypothesis
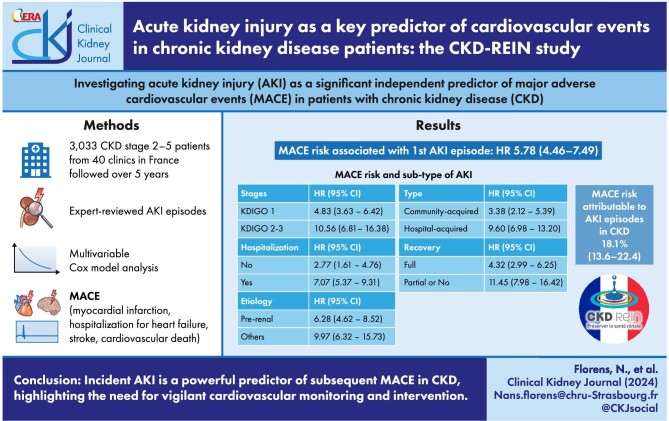
Cardiovascular diseases are a leading cause of morbidity and mortality in patients with chronic kidney disease (CKD). Acute kidney injury (AKI) has been increasingly recognized as a potential exacerbating factor for cardiovascular events in these patients. The CKD-REIN study aims to explore the relationship between AKI and the risk of major adverse cardiovascular events (MACE) in a cohort of CKD patients. We hypothesize that AKI is a significant and independent predictor of MACE in patients with CKD, and that the severity of AKI correlates with the risk of subsequent cardiovascular events.
Methods
This prospective cohort study included 3033 adult CKD patients from 40 outpatient nephrology clinics in France. Patients were followed for a median of 5.2 years. AKI episodes were identified and staged based on the KDIGO-AKI criteria. Cardiovascular events, including myocardial infarction, stroke, heart failure hospitalization, and cardiovascular death, were systematically recorded. The association between AKI and MACE was analyzed using a multivariable Cox model, adjusting for confounders such as demographic characteristics, medical history, and baseline kidney function.
Results
During the follow-up, 530 patients experienced at least one episode of AKI. The cumulative incidence of MACE at 1 year post-AKI was 8.1%. Patients with AKI had a significantly increased risk of MACE, with an adjusted hazard ratio (HR) of 5.78 (P < .001). The risk was consistent across different MACE components and was independent of age, sex, CKD stage, or comorbidities. The risk of MACE was higher for more severe AKI stages and for AKI events requiring hospitalization or associated with incomplete renal recovery.
Conclusion
The findings of this study confirm that AKI is a significant independent predictor of MACE in CKD patients, demonstrating a strong severity–response relationship. These results underscore the importance of vigilant cardiovascular monitoring and preventive strategies in CKD patients following AKI episodes. Understanding the mechanisms linking AKI to cardiovascular outcomes is crucial for developing targeted interventions to mitigate these risks.
Keywords: acute kidney injury, cardiovascular events, chronic kidney disease, CKD-REIN, risk factors
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
Acute kidney injury (AKI) has been associated with increased cardiovascular risk, but its role as an independent predictor of major adverse cardiovascular events (MACE) in chronic kidney disease (CKD) patients was not fully established.
This study adds:
This study demonstrates that AKI is a significant independent predictor of MACE in CKD patients, with a strong severity–response relationship based on AKI severity.
Potential impact:
The findings highlight the need for enhanced cardiovascular monitoring and preventative strategies in CKD patients following AKI episodes, potentially improving patient outcomes and reducing cardiovascular events.
INTRODUCTION
Cardiovascular mortality and morbidity represent significant health challenges in contemporary society [1]. Chronic kidney disease (CKD) is identified as a crucial, non-traditional cardiovascular risk factor [2]. A robust association exists between reduced glomerular filtration rate (GFR), elevated albuminuria and the increased incidence of cardiovascular events [3–5]. Furthermore, recent findings from an extensive Mendelian randomization study have associated mild to moderate CKD with a heightened incidence of cardiovascular events [6], suggesting a pathophysiological interconnection between the heart and kidneys [7], which is also corroborated by experimental studies [8–10].
When considering acute kidney injury (AKI) events, only a limited number of studies have established a link with long-term cardiovascular outcomes [11–14]. In the meta-analysis by Odutayo et al., AKI related to heightened risks of major adverse cardiovascular events (MACE), such as acute myocardial infarction, stroke, heart failure, and cardiovascular death [13]. However, as recognized by the authors, these conclusions are limited by the diverse definitions of AKI used, the variability in follow-up periods, the restricted set of confounding factors examined, and the focus on highly selected populations, particularly in scenarios involving ischemia-reperfusion injury. Currently, the potential association between AKI and MACE within the CKD population is even less well explored [15]. Given the shared cardiovascular risk factors between AKI and CKD, the relationship between kidney diseases (acute or chronic) and the risk of subsequent MACE continues to be contentious [12, 14].
We hypothesize that AKI is a significant and independent predictor of cardiovascular events in the CKD population. Using data of a prospective national cohort study of patients with moderate-to-severe CKD, we here investigate the connection between incident AKI episodes and the subsequent occurrence of MACE, including acute myocardial infarction, stroke, hospitalization for heart failure, and cardiovascular death.
MATERIALS AND METHODS
The results of this cohort study are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [16].
Study design and participants
CKD-REIN is a prospective cohort study conducted in 40 nationally representative outpatient nephrology centers in France. Eligible adult patients had a confirmed diagnosis of stage 2–5 CKD based on two values of estimated GFR (eGFR) at 3-month intervals during the screening period; they were not on dialysis and had not undergone kidney transplantation. Between July 2013 and March 2016, the CKD-REIN investigators enrolled 3033 patients, all of whom gave their written, informed consent. Details of the study protocol and study flow chart have been published previously [17, 18]. The study was approved by the institutional review board at the French National Institute of Health and Medical Research (INSERM; reference IRB00003888) and was registered at ClinicalTrials.gov (NCT03381950).
Study data
Data were collected at baseline and then annually by trained clinical research associates (CRAs) from patient interviews and medical records. The study data included baseline socio-demographic characteristics and medical background including any history of diabetes, obesity, cirrhosis, cancer, low birth weight, dyslipidemia, tobacco use, cardiovascular disease, or AKI. Participants underwent blood and urine tests, including measurements of serum creatinine, hemoglobin, uric acid, albumin, and urinary albumin-to-creatinine ratio. The 2009 creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation was used to estimate the baseline GFR [19]. Additionally, to account for the decline in kidney function, the individual slope of GFR decline was estimated prior to inclusion for all patients through mixed effects modeling [11]. This estimation was derived from an average of five values per patient, spread over 1.2 years before inclusion.
Patients were asked to bring all their drug prescriptions for the preceding 3 months (for the enrollment visit) or all the year's prescriptions (for each annual follow-up visit). Accordingly, drug prescriptions were continuously recorded from 3 months preceding study inclusion to the end of the follow-up period. We used the Anatomical Therapeutic and Chemical thesaurus to code medications, and we recorded the drug start and stop dates [20]. Medication adherence was assessed with the validated, questionnaire-based Girerd score [21]. Polypharmacy was defined as the prescription of >10 different therapeutic classes per day. Longitudinal data (appointments with the nephrologist, hospital admissions, laboratory tests: whether prescribed annually as per the study protocol or routinely by the nephrologist or by any other physician) were recorded at 1-year intervals.
Exposure definition: identification and staging of AKI events
During the study follow-up, all AKI episodes in outpatients or inpatients were identified. Several sources were used to identify AKI episodes: (i) the study's CRAs were trained to identify any mention of AKI in hospital reports, and (ii) a study physician reviewed all the hospital discharge reports for any mention of elevated serum creatinine levels, regardless of whether AKI was specified. Additional data on the AKI adjudication process in the CKD-REIN cohort have been published elsewhere [22]. The AKI classification was based on the 2012 KDIGO-AKI criteria, using the baseline and peak serum creatinine levels as follows: Stage 1: 1.5–1.9 × baseline serum creatinine or ≥0.3 mg/dl increase in serum creatinine; Stage 2: 2.0–2.9 × baseline serum creatinine; Stage 3: 3 × baseline serum creatinine or ≥4.0 mg/dl increase, or dialysis initiation [23]. The baseline serum creatinine was set as the average of the values measured in the year preceding the AKI [24]. Hospital-acquired AKI refers to AKI that occurred during a hospital stay, whereas community-acquired AKI refers to events that did not involve a hospitalization or were reported as a cause of hospital admission. Only the occurrence of the first incident episode of AKI during the follow-up period was used to define exposure, considering its time-dependent nature.
Outcome definitions
Hospitalizations occurring during study follow-up were identified from medical reports, hospital records, and/or patient interviews; deaths were ascertained from death certificates, hospital records, reports by family members, and by linkage with the French national death registry [25]. A physician reviewed and coded all events using the International Classification of Diseases version 10 (ICD-10). In addition, this physician classified all cardiovascular events according to the Cardiovascular and Stroke Endpoint Definitions for Clinical Trials and a cardiologist adjudicated all CV deaths [26]. The definition of a MACE thus encompasses the occurrence of one of the following events: acute myocardial infarction, stroke, hospitalization for heart failure, or cardiovascular-related death.
Kidney failure with replacement therapy (KFRT) events were defined as initiation of maintenance dialysis or pre-emptive kidney transplantation, identified from medical records, patient interviews, or by linkage with the national REIN (Renal Epidemiology and Information Network) registry [27].
Statistical analyses
Baseline characteristics were described for the participants as a whole and according to a previous history of cardiovascular events before inclusion. Data were expressed as the median [Q1, Q3], or the number (percentage).
For the primary analysis examining the relationship between AKI and MACE, observations were followed from cohort inclusion until the first event among MACE, kidney failure (dialysis or transplantation), non-cardiovascular death, or the date of last follow-up, whichever occurred first. Cumulative incidences of MACE after AKI events were estimated using the Aalen–Johansen method, considering competitive risks of kidney failure and non-cardiovascular death. These cumulative incidences were compared according to the in-/outpatient and community-/hospital-acquired status of the AKI event, using the Gray method. The relation of incident AKI on the risk of subsequent MACE was assessed using a multivariable cause-specific Cox model, with AKI modeled as a time-dependent variable and stratified according to various characteristics (AKI stage, in-/outpatient, pre-renal etiology, hospital-/community-acquired, and renal recovery at hospital discharge). Results were expressed as hazard ratios and 95% confidence intervals. Adjustment variables were selected a priori based on their clinical relevance and a literature review, and the final set was retrieved using a directed acyclic graph [28]. In consequence, all the multivariable analyses included: socio-demographic characteristics (age, sex, education, tobacco use), personal medical history (hypertension, coronary artery disease, cerebrovascular disease, heart failure, peripheral artery disease, diabetes, obesity, dyslipidemia, cirrhosis, cancer, low birth weight), history of past-AKI before inclusion, markers of CKD severity (eGFR at baseline and estimated annual slope before inclusion, urinary albumin-to-creatinine ratio), other laboratory data (serum hemoglobin, uric acid and albumin levels at baseline), and drug prescriptions [proton pump inhibitors, diuretics, beta-blockers, anti-inflammatory drugs, renin-angiotensin-aldosterone system (RAAS) inhibitors, polypharmacy, and treatment adherence]. Considering the expected risk of discontinuation of RAAS inhibitors associated with the primary exposure (AKI), their prescription was also treated as a time-dependent variable in the models [29]. Secondary analyses were conducted to examine the association between AKI and each of the events involved in the definition of MACE, individually. The fraction of MACE risk attributable to AKI events (and its 95% confidence interval) was derived using the effect size obtained from the primary model.
We investigated the potential modifying effect of sex, age (<75 versus ≥75 years), diabetes, CKD stage (stage 2–3 versus 4–5), baseline history of AKI, or cardiovascular disease by testing interactions between these characteristics and AKI in the association with MACE. Since some patients experienced an AKI and MACE event concurrently (i.e. within the same 24 hours), a sensitivity analysis was also conducted by censoring these observations, assuming that AKI occurred after MACE. Finally, the primary analysis was also conducted after exclusion of patients with a history of AKI and/or cardiovascular event(s) before inclusion.
The proportional hazards assumption was checked with the Schoenfeld residuals test. Continuous variables were included in the different models without transformation after we verified the log-linearity assumption. Missing data were managed by multiple imputation with chained equations (20 iterations, 20 datasets), including all covariates in the Cox models. We used Rubin's rules to combine estimates from each regression model across the imputed datasets [30].
The significance threshold for all statistical tests was set at 5%. The analyses were performed using R software, version 3.6.2.
RESULTS
Baseline characteristics
Among the 3033 patients included in the cohort (median age of 69 years, 65.4% male, median eGFR of 32.8 ml/min/1.73 m²), 758 of them (25%) had a cardiovascular history at baseline, corresponding to a prevalence of coronary artery disease, stroke, or heart failure of 24.8%, 10.0%, and 13.0%, respectively (Table 1). Patients with a history of major cardiovascular events were significantly older, more often male, had lower education levels, and more cardiovascular risk factors (diabetes, obesity, hypertension, and smoking). It is noteworthy that they also more frequently had a history of AKI and markers of poorer kidney function (lower eGFR, higher albuminuria levels). Finally, these patients were also more often treated with diuretics, beta-blockers, lipid-lowering agents, and proton pump inhibitors.
Table 1:
Characteristics of the study population, according to a history of major cardiovascular event (i.e. myocardial infarction, stroke, or heart failure) at baseline.
| History of major cardiovascular event | |||||
|---|---|---|---|---|---|
| All | No | Yes | Missing data | ||
| (N = 3033) | (N = 2275) | (N = 758) | P value | (%) | |
| Socio-demographic characteristics | |||||
| Age (years) | 69.0 [60.0,76.0] | 67.0 [58.0,75.0] | 72.0 [66.0,78.0] | <.001 | |
| Male sex | 1983 (65.4%) | 1405 (61.8%) | 578 (76.3%) | <.001 | |
| Education | |||||
| <9 years | 443 (14.8%) | 314 (13.9%) | 129 (17.4%) | .002 | 37 (1.2%) |
| 9–11 years | 1471 (49.1%) | 1088 (48.3%) | 383 (51.5%) | ||
| ≥12 years | 1082 (36.1%) | 851 (37.8%) | 231 (31.1%) | ||
| Occupational status | |||||
| Unemployed or retired | 2199 (82.9%) | 1599 (79.5%) | 600 (93.5%) | <.001 | 380 (12.5%) |
| Employed | 454 (17.1%) | 412 (20.5%) | 42 (6.5%) | ||
| Kidney disease history | |||||
| CKD vintage (years) | 5.11 [2.45,10.0] | 5.41 [2.56,10.8] | 4.52 [2.30,8.17] | <.001 | 142 (4.7%) |
| Primary kidney disease | |||||
| Diabetic nephropathy | 611 (21.4%) | 404 (18.9%) | 207 (29.1%) | <.001 | 181 (6.0%) |
| Hypertensive/vascular nephropathy | 849 (29.8%) | 566 (26.4%) | 283 (39.7%) | ||
| Other | 1392 (48.8%) | 1170 (54.7%) | 222 (31.2%) | ||
| AKI history | 658 (23.6%) | 452 (21.7%) | 206 (29.2%) | <.001 | 244 (8.0%) |
| CKD stage | |||||
| 2–3 | 1725 (56.9%) | 1332 (58.5%) | 393 (51.8%) | .001 | . |
| 4–5 | 1308 (43.1%) | 943 (41.5%) | 365 (48.2%) | ||
| Personal medical history | |||||
| Heart failure | 392 (13.0%) | 28 (1.2%) | 364 (48.0%) | <.001 | 8 (0.3%) |
| Coronary artery disease | 737 (24.8%) | 242 (10.9%) | 495 (65.6%) | <.001 | 63 (2.1%) |
| Cardiac rhythm disorder | 674 (22.3%) | 374 (16.5%) | 300 (39.6%) | <.001 | 8 (0.3%) |
| Stroke | 297 (10.0%) | 61 (2.8%) | 236 (31.4%) | <.001 | 75 (2.5%) |
| Peripheral artery disease | 574 (19.4%) | 316 (14.3%) | 258 (34.4%) | <.001 | 67 (2.2%) |
| Obesity (body mass index >30 kg/m²) | 1050 (35.4%) | 743 (33.3%) | 307 (41.5%) | <.001 | 65 (2.1%) |
| Diabetes | 1307 (43.2%) | 876 (38.6%) | 431 (57.0%) | <.001 | 7 (0.2%) |
| Hypertension | 2747 (90.8%) | 2042 (90.0%) | 705 (93.0%) | .017 | 7 (0.2%) |
| Smoking status | |||||
| Current smoker | 358 (11.9%) | 271 (12.0%) | 87 (11.5%) | <.001 | 23 (0.8%) |
| Non-smoker | 1242 (41.3%) | 999 (44.3%) | 243 (32.2%) | ||
| Former smoker | 1410 (46.8%) | 985 (43.7%) | 425 (56.3%) | ||
| Cirrhosis | 50 (1.7%) | 40 (1.9%) | 10 (1.4%) | .495 | 174 (5.7%) |
| Respiratory disease | 708 (23.9%) | 463 (20.9%) | 245 (32.7%) | <.001 | 68 (2.2%) |
| Cognitive disorder | 19 (0.6%) | 10 (0.5%) | 9 (1.2%) | .034 | 78 (2.6%) |
| Cancer | 621 (21.2%) | 478 (21.8%) | 143 (19.4%) | .193 | 103 (3.4%) |
| Dyslipidemia | 2223 (73.6%) | 1558 (68.9%) | 665 (87.7%) | <.001 | 14 (0.5%) |
| Low birth weight (<2500 g) | 215 (9.8%) | 158 (9.7%) | 57 (10.4%) | .687 | 846 (27.9%) |
| Laboratory data (at baseline) | |||||
| eGFR (ml/min/1.73 m²) | 32.8 [23.6,43.1] | 33.3 [23.9,43.5] | 30.9 [22.9,41.4] | .001 | . |
| eGFR slope (ml/min/1.73 m²/year) | −1.53 [−5.35,2.02] | −1.54 [−5.32,1.81] | −1.50 [−5.43,2.42] | .31 | . |
| Urinary albumin-creatinine ratio category | |||||
| Normal or low (A1) | 834 (30.2%) | 639 (30.8%) | 195 (28.3%) | ||
| Moderately increased (A2) | 969 (35.0%) | 718 (34.6%) | 251 (36.5%) | .453 | 268 (8.8%) |
| Severely increased (A3) | 962 (34.8%) | 720 (34.7%) | 242 (35.2%) | ||
| Sodium (mmol/l) | 140 [139 142] | 140 [139 142] | 140 [139 142] | .593 | 16 (0.5%) |
| Potassium (mmol/l) | 4.50 [4.20,4.90] | 4.50 [4.20,4.90] | 4.50 [4.20,4.90] | .897 | 13 (0.4%) |
| Chlorine (mmol/l) | 104 [102 107] | 104 [102 107] | 103 [101 106] | <.001 | 195 (6.4%) |
| Uric acid (µmol/l) | 426 [348 505] | 416 [345 494] | 452 [369 541] | <.001 | 250 (8.2%) |
| HbA1c (%) | 6.00 [5.50,7.00] | 5.90 [5.50,6.80] | 6.30 [5.72,7.30] | <.001 | 600 (19.8%) |
| Hemoglobin (g/dl) | 12.9 [11.8,14.1] | 13.0 [11.8,14.1] | 12.9 [11.8,14.1] | 0.454 | 23 (0.8%) |
| Albumin (g/l) | 40.3 [38.0,43.0] | 40.7 [38.0,43.0] | 40.0 [37.6,42.0] | .003 | 489 (16.1%) |
| Ferritin (ng/ml) | 128 [71.0 228] | 134 [75.0 233] | 113 [62.2 220] | .001 | 459 (15.1%) |
| CRP (mg/l) | 3.90 [1.80,7.80] | 3.40 [1.60,7.00] | 4.40 [2.20,10.0] | <.001 | 1473 (48.6%) |
| Total cholesterol (g/l) | 4.71 [3.96,5.61] | 4.87 [4.11,5.75] | 4.25 [3.62,5.15] | <.001 | 334 (11.0%) |
| HDL (g/l) | 1.24 [1.01,1.53] | 1.26 [1.03,1.60] | 1.16 [0.948,1.42] | <.001 | 355 (11.7%) |
| LDL (g/l) | 2.56 [1.90,3.35] | 2.69 [2.01,3.45] | 2.23 [1.70,2.92] | <.001 | 414 (13.6%) |
| Triglycerides (g/l) | 1.55 [1.11,2.25] | 1.52 [1.10,2.25] | 1.60 [1.15,2.28] | .036 | 343 (11.3%) |
| Cardiac ultrasound | |||||
| Available cardiac ultrasound data | 1254 (48.4%) | 815 (42.0%) | 439 (67.6%) | <.001 | 444 (14.6%) |
| Left ventricular ejection fraction (%) | 61.0 [55.0,68.0] | 65.0 [60.0,70.0] | 58.5 [45.0,65.0] | <.001 | 301 (24.0%) |
| Left ventricular hypertrophy | 234 (18.7%) | 132 (16.2%) | 102 (23.2%) | .001 | 421 (33.6%) |
| Drug prescriptions at baseline | |||||
| Diuretics | 1650 (54.6%) | 1106 (48.8%) | 544 (71.8%) | <.001 | 9 (0.3%) |
| RAAS inhibitors | 2294 (75.9%) | 1739 (76.7%) | 555 (73.2%) | .056 | 9 (0.3%) |
| Beta-blockers | 1269 (41.8%) | 779 (34.2%) | 490 (64.6%) | <.001 | 9 (0.3%) |
| Lipid-lowering agents | 1908 (63.1%) | 1312 (57.9%) | 596 (78.6%) | <.001 | 9 (0.3%) |
| Proton pump inhibitors | 991 (32.8%) | 638 (28.2%) | 353 (46.6%) | <.001 | 9 (0.3%) |
| Antigout drugs | 1025 (33.9%) | 740 (32.7%) | 285 (37.6%) | .015 | 9 (0.3%) |
| Anti-inflammatory | 43 (1.4%) | 38 (1.7%) | 5 (0.7%) | .061 | 9 (0.3%) |
| Polypharmacy | 1006 (33.3%) | 615 (27.1%) | 391 (51.6%) | <.001 | 9 (0.3%) |
| Adherence to medication | |||||
| Good | 1129 (37.6%) | 853 (37.9%) | 276 (36.6%) | .801 | 31 (1.0%) |
| Moderate | 1651 (55.0%) | 1229 (54.7%) | 422 (56.0%) | ||
| Poor | 222 (7.4%) | 166 (7.4%) | 56 (7.4%) | ||
Quantitative values are presented by median [Q1, Q3], qualitative values are presented by frequency and percentage
Incidence of AKI and MACE
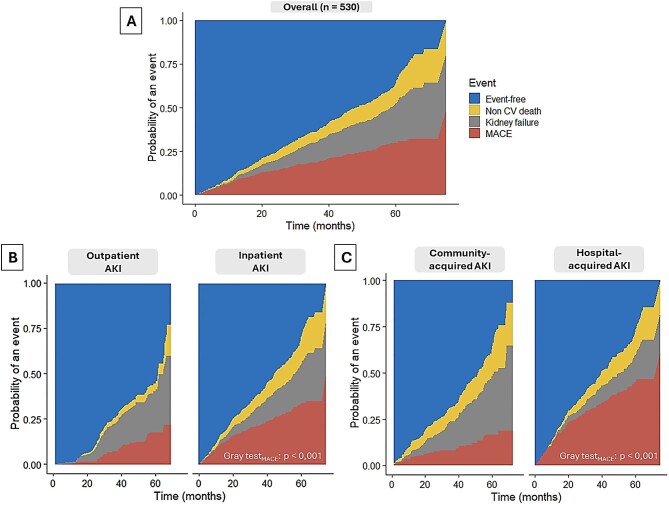
Over a median follow-up of 5.2 years [3.2–5.9], a total of 530 patients experienced at least one episode of AKI in a median delay of 1.7 years. These events were predominantly of mild severity (71.7% stage 1), of pre-renal etiology (72.5%), and required hospitalization (78.1%). Concurrently, there were 498 major cardiovascular events, 628 KFRT, and 316 non-cardiovascular deaths.
Following an AKI episode, the estimated cumulative incidences of MACE, KFRT, and non-cardiovascular death were 8.1% (6.1–10.8), 1.3% (0.6–2.8), and 2.3% (1.3–4.0), respectively, at 12 months; 18.5% (15.5–22.1), 11.5% (9.1–14.6), and 6.0 (4.3–8.4), respectively, at 36 months; and 30.1% (26.4–34.3), 22.1% (18.8–26.0), and 11.2% (8.8–14.3), respectively, at 60 months (Fig. 1A). The cumulative incidence of MACE after an AKI episode appeared significantly higher for inpatient (vs outpatient) and hospital-acquired (vs community-acquired) events (P < .001) (Fig. 1B and C). In total, subsequent MACE occurred at a median of 6.7 months (1.8–16.6) after the AKI episode.
Figure 1:
Cumulative incidences of MACE, kidney failure, and non-cardiovascular death after a first incident AKI episode during follow-up (N = 530): (A) overall, (B) according to out-/inpatient status of the AKI episode, and (C) according to community-/hospital-acquired status of the AKI episode.
Association between incident AKI events and subsequent MACE
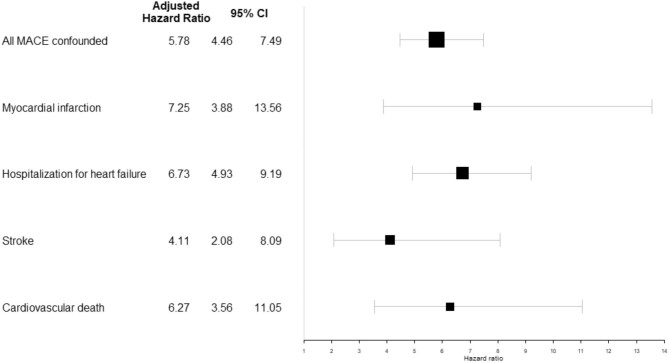
In the entire cohort, the adjusted HR (95%CI) for subsequent MACE was more than five times higher in patients with, versus without, an AKI event during follow-up (HR: 5.78 (4.46–7.49), P < .001) (Fig. 2).
Figure 2:
Adjusted hazard ratios (95% confidence interval) of subsequent MACE associated with incident AKI event within the entire cohort (N = 3033, number of AKI events = 530, number of MACE = 498).
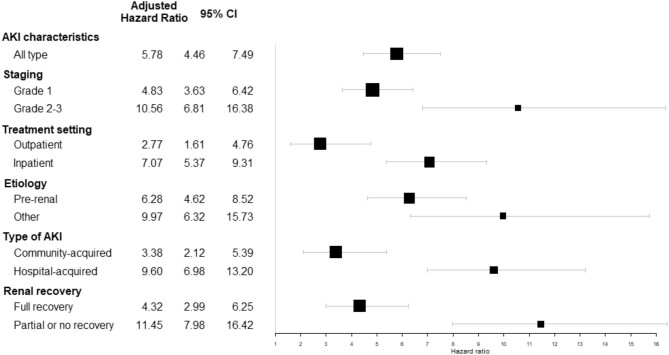
This association was also confirmed for each of the different events defining MACE, with a similar magnitude for acute myocardial infarction, hospitalization for heart failure, and cardiovascular death. It appeared slightly less pronounced concerning strokes (HR: 4.11 (2.08–8.09), P < .001) (Fig. 2). The excess risk of subsequent MACE appeared higher for stage 2–3 AKI (vs stage 1), those requiring or complicating hospitalization (vs outpatient or community-acquired), and those for which complete renal recovery was not observed on hospital discharge (Fig. 3).
Figure 3:
Adjusted hazard ratios (95% confidence interval) of subsequent MACE associated with incident AKI event within the entire cohort (N = 3033), stratified by AKI characteristics.
Overall, the fraction of MACE risk attributable to AKI episodes within this population was estimated at 18.1% (13.6–22.4).
Interaction and sensitivity analyses
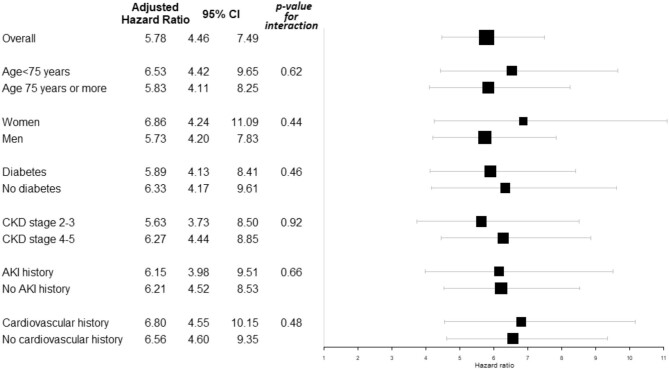
No interaction with age, sex, history of diabetes, CKD stage, AKI, or cardiovascular event was observed; the association between incident AKI and subsequent MACE was significant across all subgroups with a comparable effect size (Fig. 4). Although still highly significant, the associations were less pronounced when concurrent AKI–MACE events were censored (Supplementary Table S1) and globally similar when patients with a previous history of AKI and/or cardiovascular events before inclusion were excluded (Supplementary Table S2).
Figure 4:
Interaction analyses: adjusted hazard ratios (95% confidence interval) of subsequent MACE associated with incident AKI event within the entire cohort (N = 3033), according to different subgroups of patients.
DISCUSSION
This study identifies AKI as an independent risk factor for MACE within the CKD patient population, attributing to it an estimated contribution of nearly 20%. It encompasses all types of MACE, with a 5-fold increase in the risk of subsequent hospitalization due to heart failure, acute myocardial infarction, or cardiovascular death following an AKI episode in this population. The elevated risk of stroke, although notable, is less pronounced by comparison. On average, MACE manifests ∼6 months post-AKI, underscoring the critical need for vigilant monitoring following such an event.
While the heightened risk of mortality in both the short and long term following AKI is well-documented, the potential mediation of this risk through increased cardiovascular vulnerability remains uncertain [12, 31]. Currently, data rigorously examining the connection between AKI and the subsequent risk of MACE is sparse, predominantly originating from studies involving highly specific populations such as those undergoing cardiac surgery, coronary revascularization, experiencing contrast-induced AKI, or patients in intensive care units [32–34, 35]. A meta-analysis by Odutayo et al., which encompassed >250 000 patients from 25 studies, primarily reflecting populations with significant cardiovascular risks, acknowledged an association between AKI and an elevated risk of MACE. However, it also pointed out considerable limitations, including substantial heterogeneity in exposure definitions and follow-up durations. [13] Conversely, research by Go et al., based on nearly 150 000 hospitalized patients within the Kaiser Permanente Northern California system with or without AKI, identified an increased risk of heart failure only within the first year of follow-up [12]. The discrepancies observed in comparison to our study might be attributable to a shorter follow-up period and the relatively preserved kidney function in most patients. In recent findings from a study of 2177 adults in the Chronic Renal Insufficiency Cohort, McCoy et al. revealed a correlation between AKI episodes and the risk of heart failure and atherosclerotic cardiovascular events over an average follow-up time of 3 years [11]. When juxtaposed with existing literature, the effect sizes noted in our study are particularly pronounced, potentially indicative of an additive impact of CKD and AKI, where the combined influence of each may manifest in a multiplicative manner [7, 36].
A significant strength of our investigation is the detailed collection, verification, and granularity of event-related information throughout patient follow-up [18, 37]. Each AKI episode underwent rigorous validation by a panel of experts, enabling the inclusion of specific details on severity, context, and the adjudicated etiology in our analyses [22]. This research thus reaffirms the potentially detrimental effect of even moderately severe AKI episodes (i.e. grade 1), including those not necessitating hospitalization or followed by rapid renal recovery. As previously established, even modest increases in serum creatinine levels should be regarded as non-trivial events [11, 38, 39]. Moreover, we observed a severity–response correlation, with a stronger association between the severity markers of AKI episodes and subsequent MACE risk, aligning with previous literature findings [12, 14]. Another distinctive aspect of this study is the consideration of often overlooked confounding factors; unlike prior studies, we accounted for potential withdrawals of ACE inhibitors or ARBs following AKI episodes when estimating MACE risk. Similarly, in alignment with McCoy et al.’s recent study, our analyses adjusted for eGFR level and slope, acknowledging their potential influence on the association between AKI and subsequent MACE risk [11].
Our findings call for further exploration of what we describe as the “butterfly effect” of AKI episodes on the cardiovascular system [7]. This concept pertains to the acute alterations occurring during an AKI episode that initiate pathophysiological responses within the cardiac muscle, potentially culminating in cardiovascular events over time. Previous research has demonstrated that the early and substantial release of interleukin-33 (IL-33) following ischemic-reperfusion AKI is associated with long-term cardiac hypertrophy and dysfunction [40]. Interestingly, animal models have shown that inhibiting IL-33 release during AKI episodes can protect against cardiac remodeling, suggesting a viable therapeutic approach for CKD patients. Another study highlighted the significance of Galectin-3, noting its specific upregulation post-AKI as a precursor to enduring cardiac remodeling in a mouse model of renal ischemia-reperfusion, warranting further investigation [41]. Recent research on IL-11 further highlights its role in inducing heart failure, hypertrophy, and fibrosis. Studies in mouse models have shown that anti-IL-11 treatments can prevent renal fibrosis in both AKI and CKD, which may represent a novel therapeutic avenue. Given the emerging relevance of IL-11, as discussed by Widjaja et al. [42], its potential impact on cardiovascular and renal outcomes should be further explored in future studies. Therefore, it is critical to consider targeting these pathways during the course of AKI or introducing cardioprotective drugs such as iSGLT2 inhibitors and ACE inhibitors in the immediate aftermath of AKI. The latter is now being evaluated in an ongoing trial (NCT05272878). It is also important to acknowledge that SGLT-2 inhibitors and GLP-1 analogs, which were not included in our analysis due to the cohort being established before the completion of relevant RCTs, could potentially influence the incidence of AKI and its association with MACE. While it is plausible that the inclusion of these medications might only numerically alter our findings without affecting the overall conclusions, this remains speculative. Future studies should consider the potential impact of these therapies on the AKI–MACE relationship to ensure a more comprehensive understanding. While the connection between AKI and stroke risk is less emphasized, as corroborated by prior studies, recent research has unveiled several potential pathophysiological mechanisms, including disruptions to the blood–brain barrier, systemic inflammation/neuroinflammation, and impaired coagulation and thrombosis balance [43].
Our research findings compellingly indicate that episodes of AKI, including those not requiring hospitalization or deemed less severe, are associated with substantial increased risk of cardiovascular complications in patients with CKD. This situation demands a 2-fold approach in patient management. First, there is a critical need for the prevention of AKI episodes, underscored by the importance of enhanced patient education regarding their medication regimen, including diuretics, renin-angiotensin system blockers, and mineralocorticoid receptor antagonists. This recommendation is particularly relevant given the proportion of pre-renal causes of AKI identified in our study, coupled with the high incidence of severe adverse drug reactions in this population [44, 45]. Moreover, there is an urgent need for improved communication among various healthcare providers, acknowledging that AKI episodes are frequently underreported in medical records, especially among CKD patients, where such incidents may be minimized [22, 46]. Special emphasis should be placed on meticulous monitoring of these patients following an AKI episode, considering the significantly elevated risk of cardiovascular incidents, even in the short term [47, 48]. Previous research demonstrated that the initial consultation with the referring nephrologist post-AKI could be delayed by nearly 3 months [22]. Based on our findings, healthcare providers should consider implementing: (i) early post-AKI consultations to monitor renal recovery and assess cardiovascular risk; (ii) regular cardiovascular assessments to detect early signs of potential complications; (iii) enhanced patient education regarding the importance of medication adherence and lifestyle modifications to reduce cardiovascular risk; and (iv) consideration of cardioprotective therapies, such as iSGLT2 inhibitors and ACE inhibitors, in the immediate aftermath of AKI. These measures can help mitigate the heightened cardiovascular risk observed in CKD patients following an AKI episode, potentially improving long-term outcomes.
Nonetheless, our study acknowledges several limitations. The observational nature of this study precludes definitive conclusions about the causality of the observed relationships, especially because the identification of events relied on medical records and patient interviews, which could lead to misclassification. While our data indicate that a portion of the cohort had a reduced ejection fraction (left ventricle ejection fraction <45%) in the subgroup with a history of AKI at baseline, it is important to acknowledge that heart failure with preserved ejection fraction is more commonly associated with CKD. Given that heart failure with preserved ejection fraction has only recently been clearly defined as a distinct clinical entity, it is likely that its incidence in our cohort was similar, even if not formally identified. This limitation should be considered when interpreting the cardiovascular outcomes in this study. However, the prospective design, along with the careful identification and classification of AKI and MACE events, lends substantial validity to our investigation. While cohort studies inherently face the challenges of attrition bias and residual confounding, the low lost to follow-up rate during active monitoring (<5%) and the triangulation of data from various sources, including national registries, stand as strengths of our research. The a priori selection of potential confounders using a directed acyclic graph and the robustness of the observed association minimized the risk for confounding bias. Although our study accurately reflects nephrological care in France, the inclusion of patients under nephrological follow-up may introduce a selection bias. Nevertheless, our findings imply that the association could be underestimated for patients with less frequent medical follow-up. A limitation could stem from the analysis being confined to the first AKI episode during the follow-up period, not considering the potential prognostic significance of recurrent events. However, further analyses revealed no interaction with a history of past-AKI, suggesting that the adverse impact of AKI is predominantly not influenced by its recurrence. Another limitation is that the diagnosis of AKI is made through laboratory workup, and patients may be asymptomatic. This could lead to an underestimation of the number of patients who experienced AKI, particularly in cases of community-acquired AKI. Another limitation of our study is the potential misclassification of some stage 1 AKI cases, particularly among outpatients who achieve full recovery without hospitalization. It is possible that these cases reflect a response to ACE inhibitor/ARB/MRA overdosing or up-titration, especially given the expected presence of renal artery stenosis or diabetic nephropathy in this cohort. As with our earlier CKD-REIN papers, our approach does not allow for precise differentiation in such instances.
In summary, our study highlights a pronounced severity–response relationship between the severity of AKI events and the increased risk of subsequent MACE in a cohort of CKD patients. These results propose that AKI serves not only as an indicator of increased cardiovascular risk but may also play a role as a mediator between CKD and this risk. Heightened awareness within the medical community, coupled with the adoption of preventive strategies and therapeutic interventions, holds the potential to significantly enhance patient outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the CKD-REIN study coordination staff for their efforts in setting up the cohort: Elodie Speyer, Madonna Salib, Reine Ketchemin, Marie Metzger, Oriane Lambert, and all the CRAs.
Contributor Information
Nans Florens, Nephrology, Dialysis & Transplantation Department, Nouvel Hôpital Civil, Hôpitaux Universitaires de Strasbourg, 1 Place de l'hôpital, Strasbourg, France; UMR1109 Molecular Immuno-Rhumatology, FHU TARGET, Translational Medicine Federation of Strasbourg (FMTS), Faculty of Medicine, University of Strasbourg, Strasbourg, France; INI-CRCT (Cardiovascular and Renal Trialists), F-CRIN Network, Vandoeuvre-les-Nancy, France.
Estelle Aymes, Public Health Department, Epidémiologie – Maison régionale de la recherche clinique, CHU Lille, Lille, France; UMR1167 RIDAGE, Institut Pasteur de Lille, INSERM, Univ Lille, Lille, France.
Victoria Gauthier, Public Health Department, Epidémiologie – Maison régionale de la recherche clinique, CHU Lille, Lille, France; UMR1167 RIDAGE, Institut Pasteur de Lille, INSERM, Univ Lille, Lille, France.
Luc Frimat, Nephrology Department, CHRU de Nancy, Vandoeuvre-lès-Nancy; Lorraine University, APEMAC, Vandoeuvre-lès-Nancy, France.
Maurice Laville, Université de Lyon, Carmen INSERM, Lyon.
Dimitri Bedo, Nephrology, Dialysis & Transplantation Department, Nouvel Hôpital Civil, Hôpitaux Universitaires de Strasbourg, 1 Place de l'hôpital, Strasbourg, France; UMR1109 Molecular Immuno-Rhumatology, FHU TARGET, Translational Medicine Federation of Strasbourg (FMTS), Faculty of Medicine, University of Strasbourg, Strasbourg, France.
Thomas Beaudrey, Nephrology, Dialysis & Transplantation Department, Nouvel Hôpital Civil, Hôpitaux Universitaires de Strasbourg, 1 Place de l'hôpital, Strasbourg, France; UMR1109 Molecular Immuno-Rhumatology, FHU TARGET, Translational Medicine Federation of Strasbourg (FMTS), Faculty of Medicine, University of Strasbourg, Strasbourg, France.
Philippe Amouyel, Public Health Department, Epidémiologie – Maison régionale de la recherche clinique, CHU Lille, Lille, France; UMR1167 RIDAGE, Institut Pasteur de Lille, INSERM, Univ Lille, Lille, France.
Nicolas Mansencal, Cardiology Department, Centre de référence des cardiomyopathies et des troubles du rythme cardiaque héréditaires ou rares, AP-HP, Ambroise Paré Hospital, Université de Versailles-Saint Quentin (UVSQ), Boulogne-Billancourt, France; Centre for Research in Epidemiology and Population Health (CESP), Paris-Saclay University, INSERM U1018, Versailles-Saint-Quentin University, Clinical Epidemiology Team, Villejuif, France.
Céline Lange, Centre for Research in Epidemiology and Population Health (CESP), Paris-Saclay University, INSERM U1018, Versailles-Saint-Quentin University, Clinical Epidemiology Team, Villejuif, France; Agence de la Biomédecine, La Plaine Saint-Denis, France.
Sophie Liabeuf, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, MP3CV Laboratory, Jules Verne University of Picardie, Amiens, France.
Ziad A Massy, INI-CRCT (Cardiovascular and Renal Trialists), F-CRIN Network, Vandoeuvre-les-Nancy, France; Centre for Research in Epidemiology and Population Health (CESP), Paris-Saclay University, INSERM U1018, Versailles-Saint-Quentin University, Clinical Epidemiology Team, Villejuif, France; AURA Paris - Association pour l'Utilisation du Rein Artificiel en région Parisienne, and Department of Nephrology, Ambroise Paré University Hospital, APHP, Boulogne-Billancourt, Paris, France.
Benedicte Stengel, Centre for Research in Epidemiology and Population Health (CESP), Paris-Saclay University, INSERM U1018, Versailles-Saint-Quentin University, Clinical Epidemiology Team, Villejuif, France.
Natalia Alencar de Pinho, Centre for Research in Epidemiology and Population Health (CESP), Paris-Saclay University, INSERM U1018, Versailles-Saint-Quentin University, Clinical Epidemiology Team, Villejuif, France.
Aghiles Hamroun, Public Health Department, Epidémiologie – Maison régionale de la recherche clinique, CHU Lille, Lille, France; UMR1167 RIDAGE, Institut Pasteur de Lille, INSERM, Univ Lille, Lille, France.
FUNDING
CKD-REIN is funded by the Agence Nationale de la Recherche through the 2010 «Cohortes-Investissements d'Avenir » program (ANR-IA-COH-2012/3731) and by the 2010 national Programme Hospitalier de Recherche Clinique. CKD-REIN is also supported through a public–private partnership with Fresenius Medical Care and GlaxoSmithKline (GSK) since 2012, Sanofi Genzyme from 2012 to 2015, Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017, Amgen from 2012 to 2020, Lilly France from 2013 to 2018, Otsuka Pharmaceutical from 2015 to 2020, AstraZeneca from 2018 to 2021, Vifor France from 2018 to 2022, and Boeringher Ingelheim since 2022. The funding sources had no role in the study design, conduct, and reporting.
AUTHORS’ CONTRIBUTIONS
N.F., E.A., D.B., T.B., and A.H. contributed to the conception and design of the study. C.L., B.S., and N.A.d.P. were involved in the acquisition and validation of data. E.A., V.G., P.A., and A.H. performed the statistical analysis. N.M., L.F., M.L., S.L., and Z.A.M. were part of the expert committee for AKI and MACE adjudication. N.F., E.A., and A.H. drafted the initial manuscript. All authors contributed to the interpretation of the results, critically revised the manuscript for important intellectual content, and approved the final version to be published.
DATA AVAILABILITY STATEMENT
Data set is available upon request to the CKD-REIN Consortium. As data are not available on an open science platform, access to the data should be requested to Dr Natalia Alencar de Pinho (CKD-REIN cohort coordinator).
CONFLICT OF INTEREST STATEMENT
N.F. has received grants from Theradial as well as speaker fees from Boehringer Ingelheim, Astellas, Theradial, and CSL Vifor, which are unrelated to the content of this manuscript. Z.A.M. reports having received grants for CKD-REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp & Dohme-Chibret, Sanofi- Genzyme, Lilly, Otsuka, AstraZeneca, Vifor, Boehringer Ingelheim, and the French government, as well as fees and grants to charities from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. The sponsors had no role in the design, execution, interpretation, or writing of the study. B.S. and A.d.P. have received grants for the CKD-REIN cohort study from Amgen, Baxter, Boeringher Ingelheim, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp, and Dohme-Chibret, Sanofi Genzyme, Lilly, Otsuka, and Vifor Fresenius. The other authors declare that they have no relevant financial interests.
REFERENCES
- 1. Roth GA, Mensah GA, Johnson CO et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019 update from the GBD 2019 Study. J Am Coll Cardiol 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsushita K, Ballew SH, Wang AY-M et al. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol 2022;18:696–707. 10.1038/s41581-022-00616-6 [DOI] [PubMed] [Google Scholar]
- 3. Matsushita K, Coresh J, Sang Y et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3:514–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoccali C, Mallamaci F, Adamczak M et al. Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc Res 2023;119:2017–32. 10.1093/cvr/cvad083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 6. Gaziano L, Sun L, Arnold M et al. Mild-to-moderate kidney dysfunction and cardiovascular disease: observational and mendelian randomization analyses. Circulation 2022;146:1507–17. 10.1161/CIRCULATIONAHA.122.060700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bedo D, Beaudrey T, Florens N. Unraveling chronic cardiovascular and kidney disorder through the butterfly effect. Diagnostics 2024;14:463. 10.3390/diagnostics14050463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin FL, McKie PM, Cataliotti A et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol-Regul Integr Comp Physiol 2012;302:R292–R9. 10.1152/ajpregu.00194.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Y, Wang S, Zhou J et al. IRF1-mediated downregulation of PGC1α contributes to cardiorenal syndrome type 4. Nat Commun 2020;11:4664. 10.1038/s41467-020-18519-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshida Y, Matsunaga N, Nakao T et al. Alteration of circadian machinery in monocytes underlies chronic kidney disease-associated cardiac inflammation and fibrosis. Nat Commun 2021;12:2783. 10.1038/s41467-021-23050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCoy IE, Hsu JY, Zhang X et al. Probing the association between acute kidney injury and cardiovascular outcomes. Clin J Am Soc Nephrol 2023;18:850–7. 10.2215/CJN.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Go AS, Hsu C, Yang J et al. Acute kidney injury and risk of heart failure and atherosclerotic events. Clin J Am Soc Nephrol 2018;13:833–41. 10.2215/CJN.12591117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odutayo A, Wong CX, Farkouh M et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017;28:377–87. 10.1681/ASN.2016010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chawla LS, Amdur RL, Shaw AD et al. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol 2014;9:448–56. 10.2215/CJN.02440213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu RK, Hsu C. The role of acute kidney injury in chronic kidney disease. Semin Nephrol 2016;36:283–92. 10.1016/j.semnephrol.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stengel B, Metzger M, Combe C et al. Risk profile, quality of life and care of patients with moderate and advanced CKD: the French CKD-REIN Cohort Study. Nephrol Dial Transplant 2019;34:277–86. 10.1093/ndt/gfy058 [DOI] [PubMed] [Google Scholar]
- 18. Stengel B, Combe C, Jacquelinet C et al. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol Dial Transplant 2014;29:1500–7. 10.1093/ndt/gft388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. https://atcddd.fhi.no/atc_ddd_index_and_guidelines/guidelines/, 2024.
- 21. Girerd X, Radauceanu A, Achard JM et al. [Evaluation of patient compliance among hypertensive patients treated by specialists]. Arch Mal Coeur Vaiss 2001;94:839–42. [PubMed] [Google Scholar]
- 22. Hamroun A, Frimat L, Laville M et al. New insights into acute-on-chronic kidney disease in nephrology patients: the CKD-REIN study. Nephrol Dial Transplant 2022;37:1700–9. 10.1093/ndt/gfab249 [DOI] [PubMed] [Google Scholar]
- 23. Khwaja AKDIGO. Clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–c84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 24. Siew ED, Ikizler TA, Matheny ME et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 2012;7:712–9. 10.2215/CJN.10821011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guardiolle V, Bazoge A, Morin E et al. Linking biomedical data warehouse records with the national mortality database in France: large-scale matching algorithm. JMIR Med Inform 2022;10:e36711. 10.2196/36711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hicks KA, Mahaffey KW, Mehran R et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018;137:961–72. 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 27. Couchoud C, Stengel B, Landais P et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant 2006;21:411–8. 10.1093/ndt/gfi198 [DOI] [PubMed] [Google Scholar]
- 28. Digitale JC, Martin JN, Glymour MM. Tutorial on directed acyclic graphs. J Clin Epidemiol 2022;142:264–7. 10.1016/j.jclinepi.2021.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Menez S, Parikh CR. Renin-angiotensin system blockade after acute kidney injury: the plot thickens. Clin J Am Soc Nephrol 2020;15:2–4. 10.2215/CJN.13801119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buuren S. Flexible Imputation of Missing Data. New York, USA: Chapman & Hall/CRC Interdisciplinary Statistics Series; 2012;20125245. [Google Scholar]
- 31. Chertow GM, Burdick E, Honour M et al. Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. J Am Soc Nephrol 2005;16:3365–70. 10.1681/ASN.2004090740 [DOI] [PubMed] [Google Scholar]
- 32. Hansen MK, Gammelager H, Jacobsen C-J et al. Acute kidney injury and long-term risk of cardiovascular events after cardiac surgery: a population-based cohort study. J Cardiothorac Vasc Anesth 2015;29:617–25. 10.1053/j.jvca.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 33. Parikh CR, Puthumana J, Shlipak MG et al. Relationship of kidney injury biomarkers with long-term cardiovascular outcomes after cardiac surgery. J Am Soc Nephrol 2017;28:3699–707. 10.1681/ASN.2017010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James MT, Samuel SM, Manning MA et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv 2013;6:37–43. 10.1161/CIRCINTERVENTIONS.112.974493 [DOI] [PubMed] [Google Scholar]
- 35. Parikh CR, Coca SG, Wang Y et al. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 2008;168:987–95. 10.1001/archinte.168.9.987 [DOI] [PubMed] [Google Scholar]
- 36. Chawla LS, Eggers PW, Star RA et al. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66. 10.1056/NEJMra1214243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinho NAd, Metzger M, Hamroun A et al. Chronic kidney disease and nephrological practices in France: lessons from the CKD-REIN cohort, 2013-2023. Nephrol Ther 2023;19:233–50. [DOI] [PubMed] [Google Scholar]
- 38. Bucaloiu ID, Kirchner HL, Norfolk ER et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012;81:477–85. 10.1038/ki.2011.405 [DOI] [PubMed] [Google Scholar]
- 39. Jones J, Holmen J, Graauw JD et al. Association of complete recovery from acute kidney injury with incident CKD Stage 3 and all-cause mortality. Am J Kidney Dis 2012;60:402–8. 10.1053/j.ajkd.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Florens N, Kasam RK, Rudman-Melnick V et al. Interleukin-33 mediates cardiomyopathy after acute kidney injury by signaling to cardiomyocytes. Circulation 2023;147:746–58. 10.1161/CIRCULATIONAHA.122.063014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prud'homme M, Coutrot M, Michel T et al. Acute kidney injury induces remote cardiac damage and dysfunction through the galectin-3 pathway. JACC Basic Transl Sci 2019;4:717–32. 10.1016/j.jacbts.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Widjaja AA, Lim W-W, Viswanathan S et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature 2024;632:157–65. 10.1038/s41586-024-07701-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bobot M, Suissa L, Hak J-F et al. Kidney disease and stroke: epidemiology and potential mechanisms of susceptibility. Nephrol Dial Transplant 2023;38:1940–51. 10.1093/ndt/gfad029 [DOI] [PubMed] [Google Scholar]
- 44. Laville SM, Gras-Champel V, Moragny J et al. Adverse drug reactions in patients with CKD. Clin J Am Soc Nephrol 2020;15:1090–102. 10.2215/CJN.01030120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Laville SM, Gras-Champel V, Hamroun A et al. Kidney function decline and serious adverse drug reactions in patients with CKD. Am J Kidney Dis 2023;83:601–14. 10.1053/j.ajkd.2023.09.012 [DOI] [PubMed] [Google Scholar]
- 46. Sautenet B, Caille A, Giraudeau B et al. Deficits in information transfer between hospital-based and primary-care physicians, the case of kidney disease: a cross-sectional study. J Nephrol 2015;28:563–70. 10.1007/s40620-015-0175-3 [DOI] [PubMed] [Google Scholar]
- 47. Harel Z, Wald R, Bargman JM et al. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 2013;83:901–8. 10.1038/ki.2012.451 [DOI] [PubMed] [Google Scholar]
- 48. Siew ED, Peterson JF, Eden SK et al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol 2012;23:305–12. 10.1681/ASN.2011030315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data set is available upon request to the CKD-REIN Consortium. As data are not available on an open science platform, access to the data should be requested to Dr Natalia Alencar de Pinho (CKD-REIN cohort coordinator).