Abstract
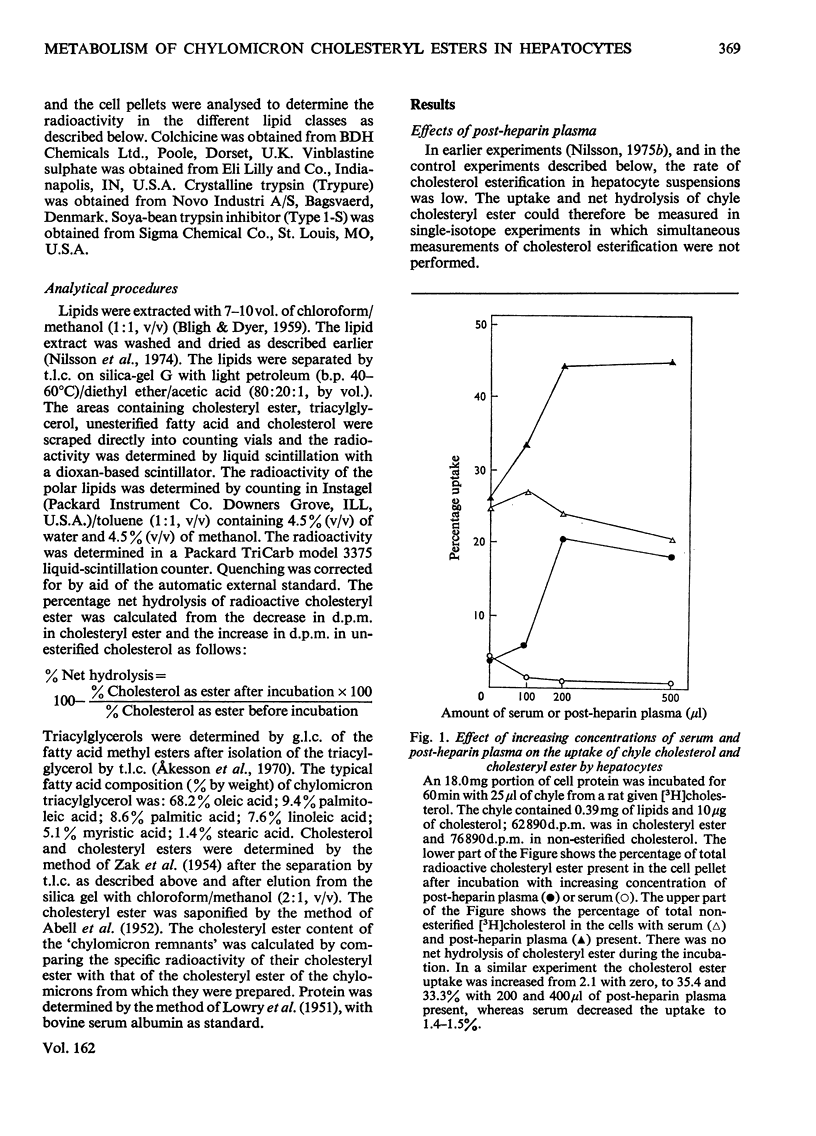
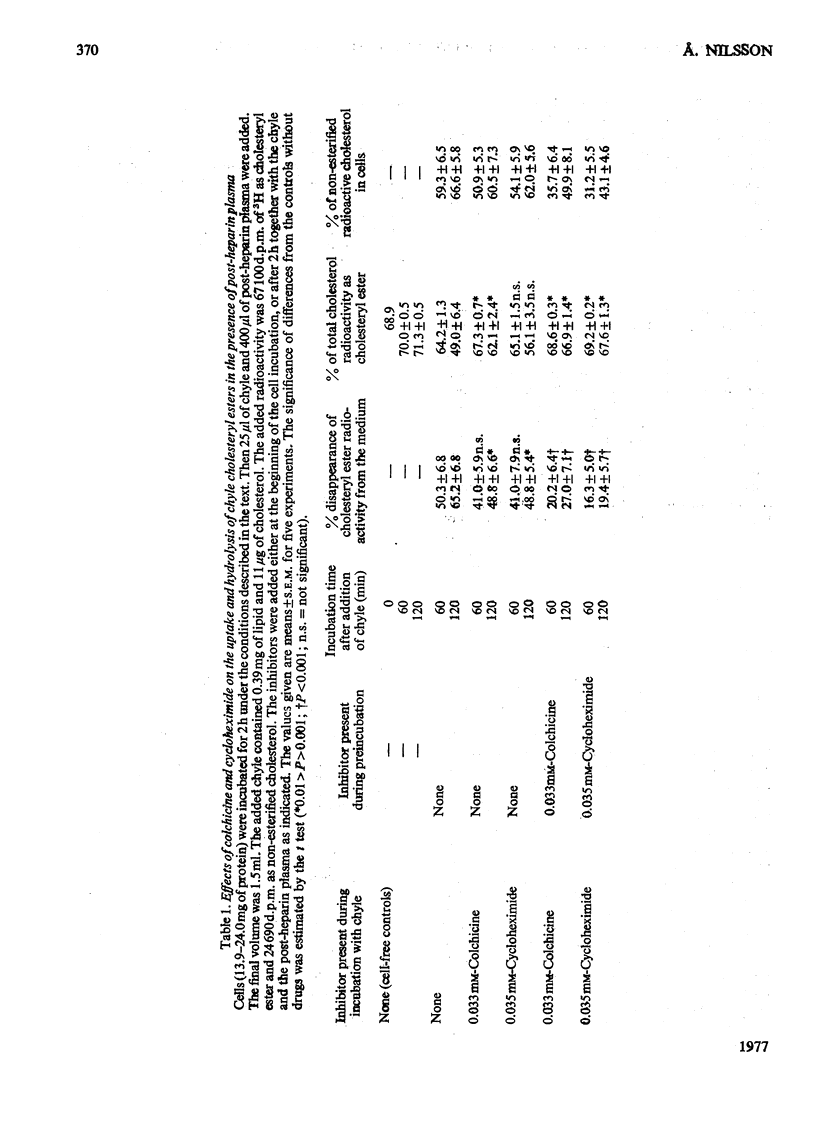
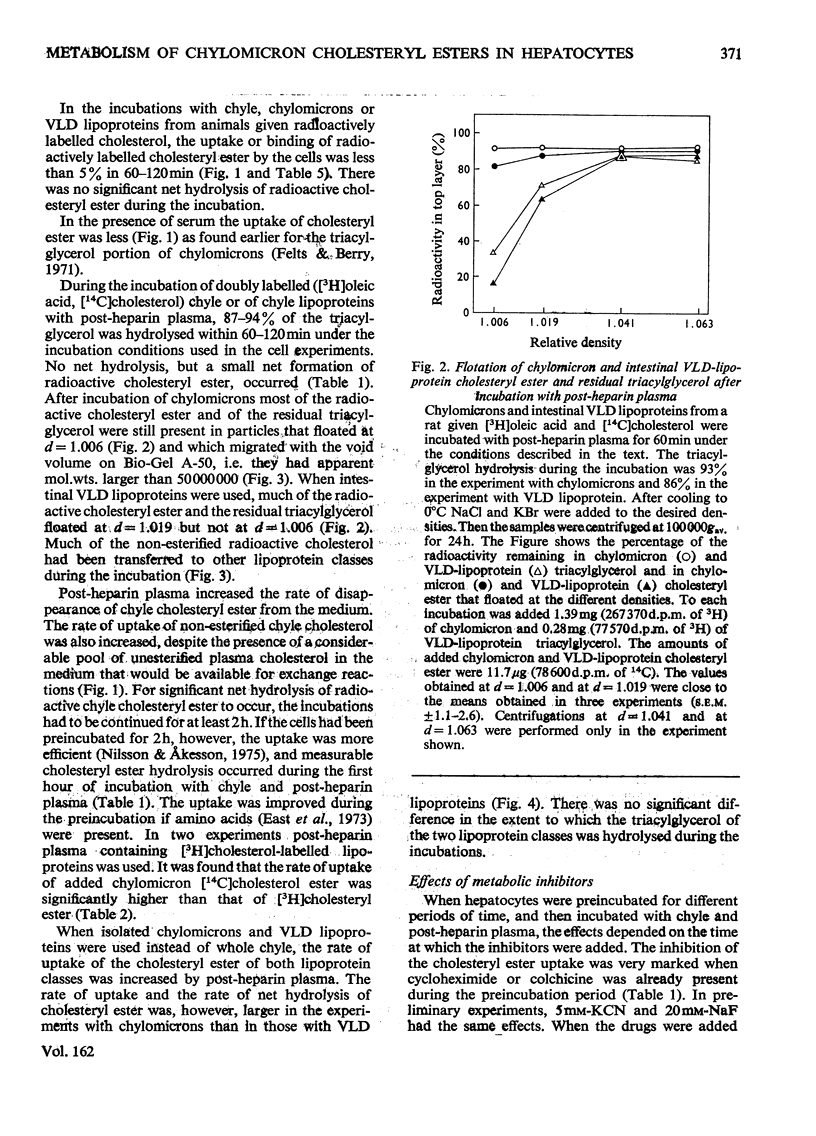
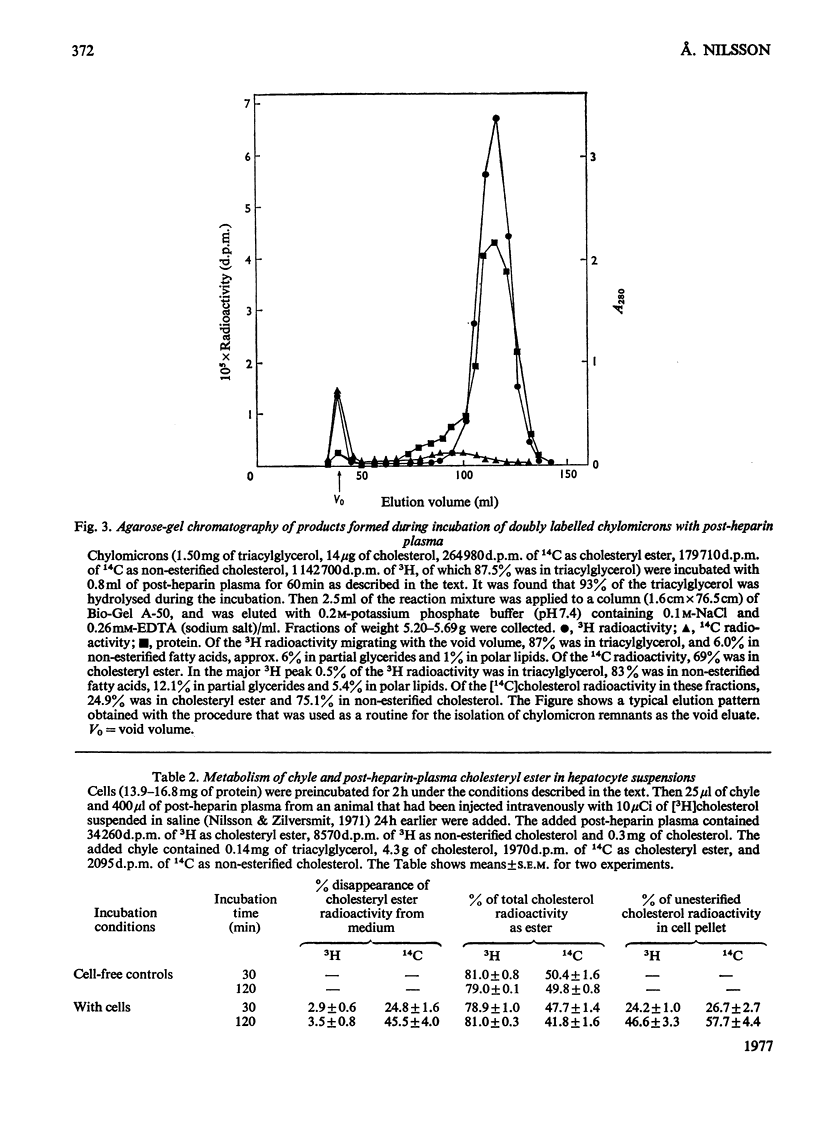
1. Post-heparin plasma that promoted rapid hydrolysis of about 90% of the triacylglycerol markedly stimulated the uptake or binding of chylomicron cholesteryl ester by suspended hepatocytes. The net hydrolysis of chyle cholesteryl ester after the uptake by the cells was, however, slower than in vivo. 2. The cholesteryl ester uptake in the presence of post-heparin plasma was larger if the cells had been preincubated for 2h. It was inhibited by the presence of colchicine, vinblastine or cycloheximide during the preincubation, and by mild trypsin treatment of the preincubated cells. 3. The results suggested that the anti-microtubular agents, but not cycloheximide, also inhibited the hydrolysis of chyle cholesteryl ester after uptake or binding to the cells. 4. The uptake of isolated chylomicron remnant particles was more efficient than that of native chyle lipoproteins. It was, however, still stimulated by heparin alone and by post-heparin plasma. The heparin-stimulated uptake was markedly decreased if cycloheximide was present during the preincubation period.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- Akesson B., Elovson J., Arvidson G. Initial incorporation into rat liver glycerolipids of intraportally injected (3H)glycerol. Biochim Biophys Acta. 1970 Jun 9;210(1):15–27. doi: 10.1016/0005-2760(70)90057-3. [DOI] [PubMed] [Google Scholar]
- Arnaud J., Boyer J. Lipolytic activity of whole isolated liver cells in aqueous suspension. Biochim Biophys Acta. 1976 Mar 26;424(3):460–468. doi: 10.1016/0005-2760(76)90035-7. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bergman E. N., Havel R. J., Wolfe B. M., Bohmer T. Quantitative studies of the metabolism of chylomicron triglycerides and cholesterol by liver and extrahepatic tissues of sheep and dogs. J Clin Invest. 1971 Sep;50(9):1831–1839. doi: 10.1172/JCI106674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell J. D., Hazzard W. R., Porte D., Jr, Bierman E. L. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973 Jul;52(7):1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- DEYKIN D., GOODMAN D. S. The hydrolysis of long-chain fatty acid esters of cholesterol with rat liver enzymes. J Biol Chem. 1962 Dec;237:3649–3656. [PubMed] [Google Scholar]
- East A. G., Louis L. N., Hoffenberg R. Albumin synthesis by isolated rat liver cells. Exp Cell Res. 1973 Jan;76(1):41–46. doi: 10.1016/0014-4827(73)90416-3. [DOI] [PubMed] [Google Scholar]
- Felts J. M., Berry M. N. The metabolism of free fatty acids and chylomicron triglyceride fatty acids by isolated rat liver cells. Biochim Biophys Acta. 1971 Feb 2;231(1):1–7. doi: 10.1016/0005-2760(71)90249-9. [DOI] [PubMed] [Google Scholar]
- Felts J. M., Itakura H., Crane R. T. The mechanism of assimilation of constituents of chylomicrons, very low density lipoproteins and remnants - a new theory. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1467–1475. doi: 10.1016/0006-291x(75)90524-0. [DOI] [PubMed] [Google Scholar]
- Filkins J. P., Di Luzio N. R. Effect of heparin and sulfated polysaccharides on in vitro hepatic phagocytosis. Proc Soc Exp Biol Med. 1966 Jun;122(2):548–551. doi: 10.3181/00379727-122-31187. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S. The metabolism of chylomicron cholesterol ester in the rat. J Clin Invest. 1962 Oct;41:1886–1896. doi: 10.1172/JCI104645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebretsen W. R., Jr, Wagle S. R. A rapid method for the isolation of large quantities of rat liver parenchymal cells with high anabolic rates. Biochem Biophys Res Commun. 1972 Apr 28;47(2):403–410. doi: 10.1016/0006-291x(72)90728-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MINARI O., ZILVERSMIT D. B. BEHAVIOR OF DOG LYMPH CHYLOMICRON LIPID CONSTITUENTS DURING INCUBATION WITH SERUM. J Lipid Res. 1963 Oct;4:424–436. [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A., Akesson B. Uptake of chyle cholesterol esters and intact triglycerides by suspended hepatocytes. FEBS Lett. 1975 Mar 1;51(1):219–224. doi: 10.1016/0014-5793(75)80891-x. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Antimicrotubular agents inhibit the degradation of chyle cholesterol ester in vivo. Biochem Biophys Res Commun. 1975 Sep 2;66(1):60–66. doi: 10.1016/s0006-291x(75)80294-4. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Increased cholesterol-ester formation during forced cholesterol synthesis in rat hepatocytes. Eur J Biochem. 1975 Feb 21;51(2):337–342. doi: 10.1111/j.1432-1033.1975.tb03933.x. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Nordén H., Wilhelmsson L. Hydrolysis and formation of cholesterol esters with rat liver lysosomes. Biochim Biophys Acta. 1973 Mar 8;296(3):593–603. [PubMed] [Google Scholar]
- Nilsson A., Sundler R., Akesson B. Effect of different albumin-bound fatty acids on fatty acid and cholesterol biosynthesis in rat hepatocytes. FEBS Lett. 1974 Sep 1;45(1):282–285. doi: 10.1016/0014-5793(74)80862-8. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Zilversmit D. B. Distribution of chylomicron cholesteryl ester between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta. 1971 Oct 5;248(1):137–142. doi: 10.1016/0005-2760(71)90085-3. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Zilversmit D. B. Fate of intravenously administered particulate and lipoprotein cholesterol in the rat. J Lipid Res. 1972 Jan;13(1):32–38. [PubMed] [Google Scholar]
- Noel S. P., Dolphin P. J., Rubinstein D. An in vitro model for the catabolism of rat chylomicrons. Biochem Biophys Res Commun. 1975 Apr 7;63(3):764–772. doi: 10.1016/s0006-291x(75)80449-9. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: role in triglyceride and cholesterol transport during fat absorption. J Clin Invest. 1969 Dec;48(12):2367–2373. doi: 10.1172/JCI106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarfordt S. H., Goodman D. S. Metabolism of doubly-labeled chylomicron cholesteryl esters in the rat. J Lipid Res. 1967 May;8(3):264–273. [PubMed] [Google Scholar]
- ROBINSON D. S. THE CLEARING FACTOR LIPASE AND ITS ACTION IN THE TRANSPORT OF FATTY ACIDS BETWEEN THE BLOOD AND TISSUES. Adv Lipid Res. 1963;1:133–182. doi: 10.1016/b978-1-4831-9937-5.50010-7. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970 Mar;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle M. C., Smuckler E. A., Glomset J. A. Cholesteryl ester hydrolytic acitivity of rat liver plasma membrane. Biochim Biophys Acta. 1975 Jun 23;388(3):339–348. doi: 10.1016/0005-2760(75)90092-2. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Lee J. A., Morris M. D., Felts J. M. Characterization of plasma lipoproteins separated and purified by agarose-column chromatography. Biochem J. 1974 Apr;139(1):89–95. doi: 10.1042/bj1390089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973 Dec;82(2):391–398. doi: 10.1016/0014-4827(73)90357-1. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y., Goodman D. S., Fidge N. H. The metabolism of chylomicron cholesteryl ester in rat liver. A combined radioautographic-electron microscopic and biochemical study. J Cell Biol. 1969 Dec;43(3):410–431. doi: 10.1083/jcb.43.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke K. T. Subcellular distribution and kinetics of the acid cholesterol esterase in liver. Biochim Biophys Acta. 1972 Oct 5;280(2):329–335. doi: 10.1016/0005-2760(72)90100-2. [DOI] [PubMed] [Google Scholar]
- Sundler R., Akesson B., Nilsson A. Triacylglycerol secretion in very low density lipoproteins by isolated rat liver parenchymal cells. Biochem Biophys Res Commun. 1973 Dec 10;55(3):961–968. doi: 10.1016/0006-291x(73)91236-9. [DOI] [PubMed] [Google Scholar]
- Wilson L., Bamburg J. R., Mizel S. B., Grisham L. M., Creswell K. M. Interaction of drugs with microtubule proteins. Fed Proc. 1974 Feb;33(2):158–166. [PubMed] [Google Scholar]
- Windmueller H. G., Lindgren F. T., Lossow W. J., Levy R. I. On the nature of circulating lipoproteins of intestinal origin in the rat. Biochim Biophys Acta. 1970 May 5;202(3):507–516. doi: 10.1016/0005-2760(70)90121-9. [DOI] [PubMed] [Google Scholar]


