Abstract
The very high energy demand of the heart is primarily met by adenosine triphosphate (ATP) production from mitochondrial oxidative phosphorylation, with glycolysis providing a smaller amount of ATP production. This ATP production is markedly altered in heart failure, primarily due to a decrease in mitochondrial oxidative metabolism. Although an increase in glycolytic ATP production partly compensates for the decrease in mitochondrial ATP production, the failing heart faces an energy deficit that contributes to the severity of contractile dysfunction. The relative contribution of the different fuels for mitochondrial ATP production dramatically changes in the failing heart, which depends to a large extent on the type of heart failure. A common metabolic defect in all forms of heart failure [including heart failure with reduced ejection fraction (HFrEF), heart failure with preserved EF (HFpEF), and diabetic cardiomyopathies] is a decrease in mitochondrial oxidation of pyruvate originating from glucose (i.e. glucose oxidation). This decrease in glucose oxidation occurs regardless of whether glycolysis is increased, resulting in an uncoupling of glycolysis from glucose oxidation that can decrease cardiac efficiency. The mitochondrial oxidation of fatty acids by the heart increases or decreases, depending on the type of heart failure. For instance, in HFpEF and diabetic cardiomyopathies myocardial fatty acid oxidation increases, while in HFrEF myocardial fatty acid oxidation either decreases or remains unchanged. The oxidation of ketones (which provides the failing heart with an important energy source) also differs depending on the type of heart failure, being increased in HFrEF, and decreased in HFpEF and diabetic cardiomyopathies. The alterations in mitochondrial oxidative metabolism and glycolysis in the failing heart are due to transcriptional changes in key enzymes involved in the metabolic pathways, as well as alterations in redox state, metabolic signalling and post-translational epigenetic changes in energy metabolic enzymes. Of importance, targeting the mitochondrial energy metabolic pathways has emerged as a novel therapeutic approach to improving cardiac function and cardiac efficiency in the failing heart.
Keywords: HFpEF, HFrEF, Fatty acid oxidation, Glucose oxidation, Ketone oxidation
1. Introduction
Heart failure is a serious clinical syndrome that results in the inability of the heart to adequately pump enough blood to meet the body's needs for nutrients and oxygen. This results in heart failure patients having significant disabilities and a high mortality rate.1 Heart failure is often classified by the degree by which alterations in ejection fraction (EF) occur, including heart failure with reduced EF (HFrEF), heart failure with mildly reduced EF (HFmrEF), and heart failure with preserved EF (HFpEF).2–4 Heart failure is associated with multiple different co-morbidities, with coronary artery disease, hypertension, diabetes, obesity, valvular disease, arrhythmias, aging, and chronic kidney disease being prominent contributors to heart failure development. It is also becoming clear that alterations in energy metabolism also characterize the failing heart, resulting in an ‘energy deficit’ that contributes to heart failure severity.5 This compromised energy production results from a number of factors, which include impaired mitochondrial metabolism, alterations in energy substrate preference by the heart, and a decrease in cardiac efficiency.6
To sustain contractile function the heart must produce a large quantity of energy, in the form of adenosine triphosphate (ATP). There are no significant ATP reserves in the heart, and if not continually replaced, the heart would run out of ATP in 2–10 s, resulting in contractile failure.6 As a result, the continuous production of ATP must occur to maintain cardiac function. The heart normally achieves this by metabolizing a variety of fuels, primarily by mitochondrial oxidative phosphorylation (OXPHOS) and, to a lesser extent, from glycolysis. Any disruptions in these metabolic pathways that produce ATP can have catastrophic consequences on heart function. As a result, compromised cardiac energy production is an important contributor to heart failure severity.7–11
Despite the critical importance of energy production in maintaining heart function, what energy metabolic changes occur in heart failure it is still not completely understood. This is in part due to the many co-morbidities associated with heart failure. For instance, heart failure associated with diabetes or obesity can have very different effects on the use of fatty acids as an energy substrate by the heart, compared to heart failure associated with hypertension or coronary artery disease.12–16 The goal of this review is to overview what cardiac energetic changes occur in the various forms of heart failure. We will also discuss how optimizing energy metabolism may be used as an approach to prevent or treat heart failure.
2. Energy metabolism in the normal heart
In a healthy state, the heart can utilize a variety of energy substrates including carbohydrates (glucose and lactate), fatty acids, ketones, and amino acids to generate ATP.6,17 This omnivorous characteristic is crucial for supporting the high ATP demand of the heart for contractile function and for maintaining the action of various ion pumps. Importantly, the healthy heart is metabolically flexible and can switch its preferences towards using different energy substrates depending on its environment, including changes in energy substrate availability, hormonal control, inotropic state, and the workload of the heart. For example, the heart can rapidly increase the rate of ATP synthesis by up-regulating both glucose and fatty acid metabolism during intense exercise to accommodate the increase in energy demand.17–20 Alternatively, during fasting or low-carbohydrate supply, the heart can rewire its metabolism to increase its reliance on fatty acid oxidation for ATP production.21,22 The majority of myocardial ATP production (∼95%) is derived from mitochondrial OXPHOS, with glycolysis generating the remaining ATP (∼5%).23,24 Fatty acid oxidation contributes approximately 40–60% of the reduced equivalents for OXPHOS), carbohydrate metabolism (glucose and lactate) contributes 20–40%, ketone oxidation 10–15%, and amino acid oxidation the remainder (<2%) (Figure 1).
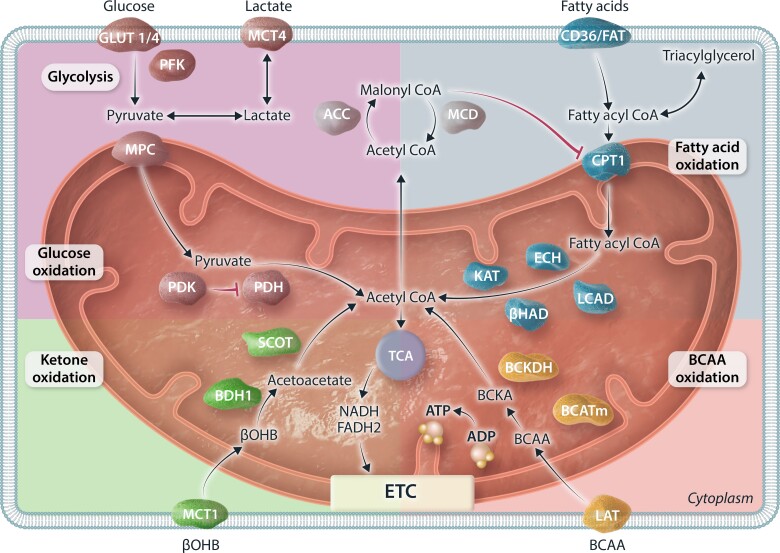
Figure 1.
Depiction of major metabolic pathways and its associated key enzymes involved in metabolism of glucose, fatty acids, ketones, and BCAA in the heart. Glucose metabolism can be divided as glycolysis and downstream pyruvate metabolism/glucose oxidation. Fatty acid oxidation can be regulated by the endogenous inhibitor malonyl-CoA. Ketone oxidation is depicted using the major form of ketone bodies, βOHB. BCAA oxidation includes oxidation of leucine, isoleucine, and valine. All four metabolic substrates are converted to acetyl-CoA for entering the TCA cycle to generate reduced equivalent NADH and FADH2, which will enter the ETC and generate a proton gradient across the mitochondrial membrane to drive the action of ATP synthase for ATP genesis. GLUT1/4, glucose transporter 1/4; MCT4, monocarboxylate transporter 4; PFK, phosphofructokinase; MPC, mitochondrial pyruvate carrier; PDH, pyruvate dehydrogenase; PDK, PDH kinase; ACC, acetyl-CoA carboxylase; MCD, malonyl-CoA decarboxylase; CD36/FAT, fatty acid transporter; CPT1, carnitine palmitoyltransferase 1; LCAD, long-chain acyl-CoA dehydrogenase; ECH, enoyl CoA hydratase; βHAD, 3-OH-acyl-CoA dehydrogenase; KAT, 3-ketoacyl-CoA thiolase; BCAA, branched-chain amino acids; BCATm, mitochondrial BCAA amimotransferase; BCKDH, branched-chain α-ketoacid dehydrogenase; LAT, L-type amino acid transporters; βOHB, β-hydroxybutyrate; BDH1, βOHB dehydrogenase 1; SCOT, succinyl-CoA:3-ketoacid CoA transferase; TCA, tricarboxylic acid; NADH, nicotinamide adenine dinucleotide; FADH2, flavin adenine dinucleotide; ETC, electron transport chain; ADP, adenosine diphosphate; ATP, adenosine triphosphate.
2.1. Fatty acid metabolism
Fatty acids are the major fuel for the heart, with oxidation of fatty acids contributing 40–60% of cardiac ATP production. Fatty acids are supplied to the heart from the circulation either as free fatty acids bound to albumin, or as fatty acids hydrolyzed by lipoprotein lipase from chylomicron and very-LDL (VLDL) triacylglycerols (TAG).25–27 The heart can also derive fatty acids from internal TAG stores, which continually cycle fatty acids through these TAG stores.28 Circulating free fatty acids enter the cardiomyocyte via facilitated diffusion or by fatty acid transport protein (FATP) or fatty acid translocase (FAT/CD36).25,29,30 Around 90% the fatty acids within the cytosol are shuttled into mitochondria for further oxidation, while the remaining 10% can be stored as TAG as an energy source backup. Fatty acid oxidation itself occurs within the mitochondria, with several steps required to first ‘activate’ the fatty acids and transport them into the mitochondria.31–33 Cytosolic fatty acids are first esterified to fatty acyl-CoA (a process consuming two high-energy phosphate bonds as ATP is converted to AMP), followed by the transfer of the fatty acid moiety to carnitine via the action of carnitine palmitoyltransferase 1 (CPT1) to form fatty acyl carnitine. CPT1 residing on the outer mitochondrial membrane works collaboratively with carnitine acyltranslocase and carnitine palmitoyltransferase 2 (CPT2), residing on the inner mitochondrial member, to transfer fatty acylcarnitine into the mitochondrial matrix, where it is converted back to fatty acyl-CoA. These acyl-CoAs then undergo ß-oxidation to produce reduced equivalents (NADH and FADH2) for the electron transport chain (ETC), as well as acetyl-CoA for the tricarboxylic acid (TCA) cycle. The passage of acetyl-CoA through the TCA cycle produces further reduced equivalents for the ETC, as well as some ATP via guanosine triphosphate (GTP). The ETC then uses the reduced equivalents in the presence of oxygen to phosphorylate adenosine diphosphate to ATP (OXPHOS).
A key step regulating fatty acid oxidation in the heart occurs at the level of mitochondrial CPT1. Malonyl-CoA is an endogenous inhibitor of CPT1.34–36 Malonyl-CoA can be generated by acetyl-CoA carboxylase (ACC) from cytosolic acetyl-CoA.37,38 Alternatively, malonyl-CoA can be converted back to acetyl-CoA by malonyl-CoA decarboxylase (MCD).39,40 When the demand for ATP is low, acetyl groups can be transferred out of the mitochondria to increase cytoplasmic acetyl-CoA supply for ACC, leading to increased malonyl-CoA production.41 This creates a negative feedback loop that inhibits fatty acid uptake into the mitochondria for further oxidation. In contrast, a decrease in malonyl-CoA levels, due to activation of AMP-kinase (which phosphorylates and inhibits ACC) or to increased MCD activity, accelerates fatty acid oxidation.28,39,42
2.2. Glucose metabolism
Glucose is an important source of fuel for the heart.6 Cardiomyocytes primarily taken up glucose via both insulin-dependent glucose transporter 4 (GLUT4) and insulin-independent glucose transporter 1 (GLUT1).43 Upon entry, glucose can have many fates, including being directed towards, glycogen synthesis, the polyol pathway, the hexosamine biosynthetic pathway (HBP), galactosamine and mannose synthesis, one carbon metabolism, or the pentose phosphate pathway (PPP), although the majority glucose passes through glycolysis to be converted to pyruvate.6 The conversion of glucose to pyruvate produces two ATP, which importantly does not require oxygen, and as such can be up-regulated dramatically when mitochondrial OXPHOS decreases, such as during oxygen-deficient conditions like ischaemia. Most of the pyruvate from glycolysis is then either taken up by the mitochondria and oxidized or converted to lactate and transported out the cardiomyocyte.
In addition to glycolysis, a second important source of pyruvate originates from lactate, which, once entered the cardiomyocyte, is converted to pyruvate by lactate dehydrogenase. To undergo mitochondrial metabolism, this pyruvate is transported into the mitochondria via a mitochondrial pyruvate carrier (MPC).44 The majority of pyruvate is then oxidized to acetyl-CoA via pyruvate dehydrogenase (PDH). If this pyruvate originates from glucose, the process is termed ‘glucose oxidation’, and if the pyruvate originates from lactate it is termed ‘lactate oxidation’. Pyruvate can also be converted to oxaloacetate and malate via pyruvate carboxylase to replenish the TCA cycle as an anaplerotic substrate. Acetyl-CoA produced from glucose and lactate oxidation can feed into the TCA cycle, where it has a similar fate as acetyl-CoA originating from fatty acid oxidation.
Like fatty acid oxidation, glucose oxidation can be regulated at many stages. First and foremost, the action of PDH, the rate-limiting enzyme for glucose oxidation, is regulated by phosphorylation. PDH kinase (PDK) phosphorylates and inhibits PDH, whereas PDH phosphatase removes the phosphate group and activates PDH.45 For example, PDK is stimulated in response to increased acetyl-CoA/CoA and NADH/NAD+ ratios, which suppresses PDH, and therefore glucose oxidation.
2.3. Ketone metabolism
Ketones, which can be an important source of fuel for the heart,46,47 are normally produced by the liver during fasting and starvation when blood glucose level drops. Liver ketogenesis produces three forms of ketones: β-hydroxybutyrate (βOHB), acetoacetate, and acetone. βOHB is the predominant ketone used by the heart. Circulating ketones are transported into the cardiomyocyte through a monocarboxylate transporter (MCT1) and then shuttled into the mitochondria for further oxidation. βOHB is first oxidized to acetoacetate by βOHB dehydrogenase (BDH1), followed by conversion to acetoacetyl-CoA by succinyl-CoA:3 oxoacid-CoA transferase (SCOT). The end-product of ketone oxidation is acetyl-CoA, which has a similar fate as acetyl-CoA produced from fatty acid or glucose oxidation. In contrast to glucose and fatty acid oxidation, metabolism of ketones is not under much regulatory control. Therefore, the rate of myocardial ketone oxidation is highly dependent on its availability, which is directly linked with blood ketone levels,48 and to the transcriptional regulation of ketolytic enzymes. Although ketone oxidation is not a major source of ATP production in the heart of healthy non-fasting individuals (around 10–15% of total ATP produced), it can become a more important source of ATP production during fasting, or in some forms of heart failure (as will be discussed in the following section).
2.4. Amino acid metabolism
Besides being crucial building blocks of proteins, amino acids can also be oxidized as a source of ATP production for the heart. Oxidation of branched-chain amino acids (BCAAs), namely leucine, isoleucine, and valine, has received the most attention in the heart.49 In a healthy heart, BCAA oxidation has a minor contribution to overall myocardial ATP production, (~2%).50 However, BCAAs and metabolites of BCAA oxidation, particularly branched-chain ketoacids (BCKAs), do have an important role as signalling molecules that regulate the balance of fatty acid and glucose oxidation,51,52 and the hypertrophic process.53,54 For example, BCAAs can stimulate the mammalian target of rapamycin (mTOR) signalling pathway,53–55 while BCKAs can inhibit insulin stimulation of glucose oxidation.51,52 Increased systemic BCAA can also promote the development of insulin resistance.56 As such, even though BCAA oxidation is not a major source of fuel for the heart, it is critically involved in regulating the overall metabolic profile of the heart.
Two key enzymes regulating BCAA oxidation are mitochondrial branched-chain amino-transaminase (BCATm) and branched-chain α-keto acid dehydrogenase (BCKDH).57 BCATm converts BCAA to BCKA through transamination, followed by oxidative decarboxylation by BKCDH. Similar to PDH, BCKDH is inhibited by its kinase, BCKDH kinase (BDK). On the other hand, dephosphorylation by protein phosphatase 2Cm (PP2Cm) can reactivate BCKDH.
3. Energy metabolism in the failing heart
The failing heart undergoes dramatic alterations in energy substrate utilization and overall metabolic profile, which can not only lead to a disrupted balance of cardiac energy metabolism but also to an overall diminished ATP production (Figure 2). The concept of the ‘energy-starved’ failing heart was first proposed in 1939 by Herrmann and Decherd,58 and has since been demonstrated in a number of studies.5,59–64 However, it should be clarified that the energy starvation concept can be misleading to think there is a lack of energy source, but in reality, the delivery of both oxygen and substrates to the heart is not limited. Therefore, another concept promoted by the Taegtmeyer group summarized it with the words ‘failure in the midst of plenty’.65,66 This energy deficiency/failure in midst of plenty occurs primarily due to a compromised mitochondrial oxidative metabolism and alterations in the source of ATP production.50,62,67–69 The failing heart has a decreased cardiac efficiency (cardiac work/O2 consumed).69–72 This decrease in cardiac efficiency is due, in part, to alterations in the source of fuel used by the heart. However, the precise alteration of each metabolic pathway in heart failure has yet to be universally established. This is particularly true for potential alterations in fatty acid oxidation in the failing heart, where many studies propose that cardiac fatty acid oxidation is decreased,73–77 while others suggest that fatty acid oxidation is either increased,78–80 or not changed.81–83 As will be discussed, these discrepant results may, at least in part, be explained by differences in disease aetiology, co-morbidities involved with heart failure, differences in transcriptional and post-translational control of energy metabolisms, and/or the severity of the disease.84
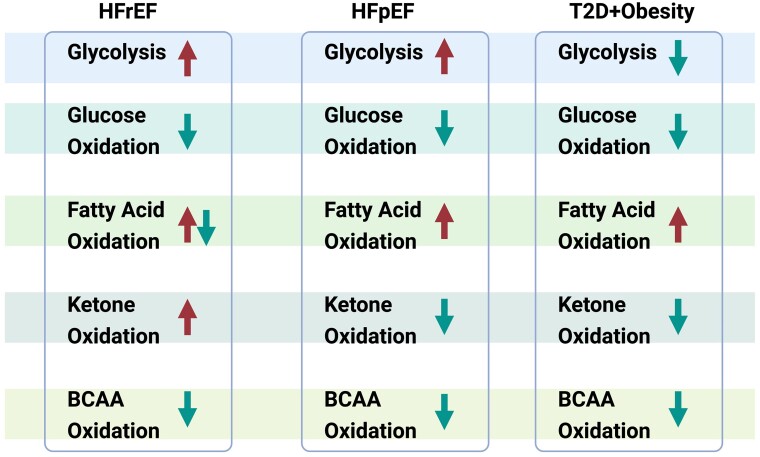
Figure 2.
Alterations of myocardial energy metabolism in heart failure. Summary chart showing the alterations of five major energy metabolic pathways, including glycolysis, glucose oxidation, fatty acid oxidation, ketone oxidation, branched-chain amino acid (BCAA) oxidation in three different types of HFrEF, HFpEF, heart failure associated with obesity, and T2D. Arrow pointing upward indicates the corresponding pathway is stimulated, and arrow pointing downward indicates the corresponding pathway is suppressed.
Fuel source in the failing heart is an important consideration, as the efficiency of ATP production can vary dramatically depending on the energy substrate used. This is important in the failing heart, which not only may have an ‘energy deficit’ but may also be oxygen limited. Although the oxidation of fatty acid produces a large amount of ATP per each molecule oxidized, it is the least efficient fuel in terms of ATP produced per molecule of oxygen consumed.6 Glucose is the most efficient energy substrate, with each molecule of glucose passing through glycolysis producing two ATP without the expense of oxygen. However, if glycolysis is uncoupled from glucose oxidation (i.e. the pyruvate from glycolysis is not taken up and oxidized by the mitochondria) it produces lactate and H+'s as metabolic by-products that can also contribute to a decrease in cardiac efficiency. Ketones have been proposed to be a ‘thrifty’ or ‘super fuel’ for the failing heart,85,86 but while they are a more efficient energy substrate than fatty acids, they are less efficient than glucose.87
3.1. Cardiac energy metabolism in HFrEF
HFrEF is characterized by a reduced EF (<40%), with ischaemic heart disease and hypertension being two important causes of HFrEF. HFrEF is associated with marked alterations in the overall capacity for cardiac energy production, as well as in alterations of cardiac fuel source.6 Although there is still a lack of consensus on the precise alterations of each metabolic pathway, it is well-accepted that the failing heart in HFrEF has impaired overall oxidative capacity and lowered myocardial ATP production (Figure 2).5 As discussed earlier, proper mitochondria function is pivotal for energy production, as OXPHOS occurs within mitochondria and provides around 95% of the total ATP produced. Both clinical and pre-clinical studies have shown that mitochondrial function is disrupted in HFrEF, which can explain the overall impaired cardiac energy production.
3.2. Alterations of glucose metabolism (glycolysis and glucose oxidation) in HFrEF
There is a widely held concept that the failing heart ‘switches from fatty acid to glucose metabolism’.73,76,88,89 However, we propose that this concept would be more fittingly re-worded as the failing heart ‘switches from mitochondrial OXPHOS to glycolysis to produce ATP’. An overall decrease in mitochondrial OXPHOS occurs in HFrEF, accompanied by an increase in glycolysis.81,90–92 However, cardiac glucose oxidation decreases in HFrEF in parallel to the decrease in mitochondrial OXPHOS.83,93 Therefore, it would be inaccurate to suggest that the failing heart is switching to glucose metabolism, given that glucose oxidation (which produces the majority of ATP originating from glucose), is markedly impaired.
Increased glycolytic ATP production in HFrEF may be an attempted compensatory response to a decrease in mitochondrial ATP production. Increased rates of cardiac glycolysis have been demonstrated in HFrEF and hypertrophic animal studies induced with transverse aortic constriction (TAC),93 or abdominal aortic constriction (ACC).83,91 This is further supported by human studies using metabolomics with heart samples collected from patients with HFrEF receiving left ventricular assist device.92 Diakos et al.78,92 found that glucose uptake and glycolytic rates were up-regulated in myocardial samples from patients with HFrEF compared to those of non-failing control. This increase in cardiac glycolytic rates, is also seen in animal models of HFrEF, such as in swine94 and rats.91,95–97
While glycolysis is up-regulated in the failing heart, the subsequent mitochondrial oxidation of glycolytically derived pyruvate (glucose oxidation) is actually decreased. This is supported by evidence from both preclinical studies using different animal models of HFrEF,81,90,94–96 and from human data collected from patients with HFrEF.98 While most studies support impaired cardiac glucose oxidation in the failing heart, opposing results showing that in canine model of pacing-induced heart failure, glucose oxidation is actually stimulated instead of being suppressed.99 This may have occurred due to the marked increase in glucose uptake and glycolysis in these hearts, as glucose uptake rates were 4 × higher in hearts from dogs with heart failure than that of control dogs, providing a large amount of pyruvate available for oxidation.
The decrease of glucose oxidation in HFrEF is tightly linked with the suppressed activity of PDH. This is evidenced by a study using hyperpolarized carbon-13 (13C) magnetic resonance spectroscopy to show that the activity of PDH diminished accordingly with the progression of cardiac dysfunction after myocardial infarction (MI) surgery in rats.96 Similar results were also shown in rapid pacing-induced heart failure in pigs, where the flux of PDH was reduced as evidenced by a 67% reduction in the [13C] bicarbonate/[1-13C] pyruvate ratio.94 Of importance, this suppression of cardiac glucose oxidation rates can, at least in part, contribute to the pathogenesis of HFrEF. Cardiac-specific knockout of PDH results in accelerated development of left ventricular hypertrophy and a marked impairment of systolic function, suggesting the imperative role of cardiac glucose oxidation in maintaining proper cardiac structure and function.100
Prior to PDH, another regulatory step of glucose oxidation is mitochondrial pyruvate uptake by MPC. MPC is located on the inner mitochondrial membrane and controls for pyruvate transfer into the mitochondria. In mice with a cardiac-specific MPC deletion, an age-dependent pathologic cardiac hypertrophy and LV dilation develop, ultimately leading to premature death.101 As expected, MPC deletion decreases cardiac glucose oxidation rates without any changing glycolytic rates.101 Importantly, this suppression of glucose oxidation occurs with increased fatty acid oxidation rates.101 This suggests that maintaining myocardial glucose oxidation is central to cardiac energetics and overall heart function. Another study shows that overexpression of MPC in cultured H9c2 cardiomyocytes attenuates drug induced cellular hypertrophy, suggesting that stimulating cardiac glucose oxidation is beneficial in ameliorating adverse remoulding.102 In the clinical setting, MPC expression is down-regulated in heart samples collected from patients with chronic heart failure.102 Therefore, this suggests that impaired myocardial glucose oxidation with MPC deficiency can, at least in part, contribute to the development of cardiac hypertrophy and the progression into heart failure.101
A recent study by Murashige et al.50 assessed myocardial uptake of major energy substrates in hearts from patients with HFrEF and HFpEF compared to healthy control participants. The authors found that around 85% of myocardial ATP is attributed to fatty acid sources, 6.4% from ketone bodies, 4.6% from amino acids, and 2.8% from lactate in hearts from patients with HFpEF.50 On the contrary, these relative substrate contributions to overall cardiac ATP production differs greatly in the hearts of patients with HFrEF, where fatty acids accounted for 71%, ketones for 16.4%, amino acids for 6.7%, and lactate for 5%.50 Curiously, the study did not report any net glucose uptake by the heart. This contrasts many earlier studies, including the original study from 1954 by Richard Bing et al.103 which showed significant glucose extraction by the heart. Other studies used metabolomic assessment with myocardial biopsies from patient with end-stage heart failure also found an accumulation of pyruvate and glycolytic intermediates,92,102,104,105 suggesting overall impaired pyruvate metabolism/glucose oxidation by the heart, yet the heart is actively using glucose as an energy substrate, but with the pyruvate metabolism step being suppressed.
In summary, the dysregulated glucose metabolism observed in HFrEF is manifested as an increase of glycolysis coupled with a suppression of glucose oxidation. This uncoupling between the two processes can lead to the accumulation of lactate and protons, contributing to cellular acidosis. These protons can impair cardiac function by interfering with calcium binding to contractile proteins and inhibiting slow calcium currents.41,106,107 Disrupted ionic balance within the cardiomyocytes also requires ATP to support the action of various ionic pumps to re-establish ionic homeostasis, which can decrease cardiac efficiency, as ATP is directed towards to regain ionic balance instead of supporting contractile function. Interestingly, this uncoupling between glycolysis and glucose oxidation has been recognized as a potential therapeutic target. In support of this, pharmacological stimulation of cardiac glucose oxidation has been shown to improve cardiac function and lower lactate production in a rat model of congestive heart failure.75
3.3. Alterations of fatty acid oxidation in HFrEF
Cardiac fatty acid oxidation also undergoes significant alterations in the setting of HFrEF. However, the precise pattern of these changes remains controversial, with studies proposing increased,78 decreased,88,99,108 or no change81,82 in cardiac fatty acid oxidation in HFrEF. While the idea that fatty acid oxidations are impaired in HFrEF has been proposed and accepted in general,64,109 most evidence is based on a decreased transcription of enzymes involved in fatty acid oxidation,88,110 as opposed to direct flux measurements of fatty acid oxidation. In the following section, we will critically discuss the major findings from both pre-clinical and clinical studies.
In the clinical setting, it has been repeatedly shown that circulating fatty acid levels are elevated in patients with heart failure.111,112 Related to this, the rate of cardiac fatty acid oxidation and fatty acid uptake is negatively correlated with the degree of insulin resistance and left ventricular function.113 Increasing the availability of circulating free fatty acids results in greater fatty acid uptake and oxidation by the heart in depressed cardiac function and an intense insulin resistant state.114 Additionally, this result is further supported by a study using positron emission tomography (PET) imaging techniques, showing that patients with severe depression of cardiac function (% EF around 25%) had a significant increase in fatty acid uptake rates compared to healthy controls.78 However, there have been some opposing results that found decreased fatty acid uptake in patients with non-ischaemic heart disease,77 and with idiopathic dilated cardiomyopathy.76 These divergent results might be partially due to the differences in disease aetiology, where patients assessed across various studies might have different degree of EF and/or involvement of other risk factors, such as obesity and/or metabolic syndromes.
The findings from preclinical studies also provide conflicting results. Using TAC models with isolated working heart perfusions, several studies have shown that the absolute rates of fatty acid oxidation are not significantly altered.115,116 However, it should be recognized that cardiac work is an important determinant of oxidative rates, and cardiac work is decreased in the TAC hearts.90,115,116 This would significantly increase fatty acid oxidation rates when normalized to per unit work.90 On the contrary, other studies have shown that cardiac fatty acid oxidation is impaired in rats,108 and canines117 with heart failure. Although the absolute rates of cardiac fatty acid oxidation were shown to be unchanged or decreased in these studies, the relative percentages of fatty acid contributing to overall myocardial ATP production are, in fact, elevated.118
Fatty acid metabolism is complicated in nature, as it is regulated at multiple levels.118 One of which is through the action of insulin. Insulin can not only positively regulate glucose metabolism but also negatively regulate fatty acid metabolism.119 In a healthy heart, insulin can readily inhibit the rate of fatty acid oxidation.120,121 However, the failing heart becomes insulin resistant, as evidenced by the lack of fatty acid oxidation inhibition with insulin.90,120,121 Importantly, a recent study by Watson et al.122 found that in patients with non-ischemic HFrEF, the heart was preserved with metabolic flexibility. Metabolic rates of glucose, fatty acids, and ketones were dependent on availability of substrates infused, being whether glucose or intralipids. Therefore, it is important to note that metabolism of fatty acids can be varied with respect alterations of the circulating levels.
3.4. Alterations of ketone oxidation in HFrEF
Recent interest has focused on ketones as a fuel source for the failing heart. Since the failing heart is energy-starved/failure in midst of plenty, ketone oxidation may provide the heart with an additional energy source. In patients with heart failure123 and in mice with heart failure,124 blood ketone levels are elevated. Two important studies by Bedi et al.125 and Aubert et al.124 examining metabolomics and ketolytic protein expression in humans and mice suggested that cardiac ketone oxidation is increased in HFrEF. Direct flux measurements by us in isolated working hearts from HFrEF mice also showed that ketone oxidation rates increase in the failing heart.90 A study by Voros et al.80 also showed increased myocardial uptake of βOHB and acetoacetate in patients with HFrEF compared to healthy controls. This increase in ketone oxidation appears to represent an adaptive mechanism to protect the failing heart.126 Support for ketone oxidation in the failing heart being adaptive also originates from studies in which genetic overexpression127 or knockout (KO)126 of genes involved in ketone oxidation were performed. Cardiac-specific deletion of BDH1 worsens cardiac remodelling and further impairs cardiac function in mice subjected to TAC/MI-induced heart failure.126 In contrast, overexpressing BDH1 decreases cardiac fibrosis and improves contractile function in mice subjected to TAC-induced heart failure.127 While it is has been proposed that ketones can act as a thrifty fuel for the failing heart,85 we demonstrated that increasing cardiac ketone oxidation does not increase cardiac efficiency.48 Similarly, in HFrEF patients with decreased cardiac efficiency, acutely increasing ketone supply to the heart improves heart function but does not improve cardiac efficiency.70 We propose that the major benefit of increasing cardiac ketone oxidation is to provide the heart with an additional source of fuel to increase ATP production to the energy-starved/failure in midst of plenty failing heart.128
3.5. Alterations of BCAA oxidation in HFrEF
BCAA, namely leucine, isoleucine, and valine, are important amino acids for both systemic and cardiac energy metabolism, as well as for assisting protein synthesis.129 Interestingly, recent studies have proposed that impaired BCAA metabolism correlates with the degree of insulin resistance and cardiac dysfunction in HFrEF, as evidenced by elevated circulating levels of BCAAs and its metabolites BCKAs.52,130,131 While elevated plasma levels of BCAA can potentially translate to an increased metabolism of BCAA for myocardial ATP production,129 it is important to recognize that BCAA oxidation accounts for less than 2% of overall ATP production.132 It is also apparent that cardiac BCAA oxidation is impaired in HFrEF,130,131,133 although not all studies support this.134 This appears to be due to phosphorylation and inhibition of BCKDH, resulting in an increase in both cardiac BCAAs and BCKAs.131,135,136 Despite having a minor contribution to cardiac ATP production, BCAAs and BCKAs may be important signalling molecules that adversely affect cardiac structure and energy metabolism in HFrEF.132 Genetic deletion of cardiac BCATm, the first enzyme of BCAA oxidation that converts BCAA to BCKA, leads to a build-up of BCAAs in the heart, which can activate mTOR and promote hypertrophy.132 Interestingly, deletion of BCATm deceases BCKA levels, resulting in an improved cardiac insulin signalling and enhanced insulin-stimulated glucose oxidation.132 However, the beneficial effects of BCATm deletion on glucose oxidation rates in HFrEF hearts, are offset by adverse remodelling due to increased BCAA levels.132
4. Cardiac energy metabolism in metabolic phenotype of HFpEF
HFpEF is a specific type of heart failure characterized by diastolic dysfunction and heterogeneous pathophysiology.137 Patients with HFpEF are typically older and associated with diseases such as type 2 diabetes (T2D), obesity, and hypertension.138 The prevalence of HFpEF is increasing at an alarming rate,139 and is predicted to reach 8.5 million by 2030 for the US population alone, and a great proportion of that population (∼ 6 million) will be attributed to elderly people with aged over 65 years.140 Even more alarming is that there are still few clinically effective treatments for HFpEF. Most effective medications for HFrEF fail to show any benefits in HFpEF.141 As outlined by Sanjv et al.,142 such lack of promising results from clinical trials in HFpEF may be explained by the high phenotypic heterogeneity. Specifically for the metabolic phenotype of HFpEF, one potential therapeutic approach is to optimize cardiac energy metabolism. However, this first requires a better understanding of what energy metabolic changes occur in HFpEF hearts.
In a recent study by Murashige et al.,50 the authors systematically assessed the gradient differences of metabolites between the radial artery and coronary sinus to quantify the uptake of various metabolites of the hearts in patients with HFpEF and HFrEF. The study found that the contribution of ketones to overall myocardial ATP was 6.4% in patients with HFpEF, whereas this number almost tripled (16.4%) in patients with HFrEF. In line with this, another metabolomic study from Hahn et al.143 showed that both plasma and myocardial levels of ketones are higher in patients with HFrEF but not HFpEF.
Clarifying the energy metabolic changes in HFpEF is hindered by the lack of animal models that can fully characterize the complex pathophysiology of HFpEF. However, recent studies have introduced several animal models for studying HFpEF, such as the ‘2-Hit’,144 ‘3-Hit’,145 and many others.146–148 These animal models aim to recapitulate the human HFpEF phenotype with multiple co-morbidities. Although they might not be the ‘perfect’ models hitting all multitude of syndromes observed in patients,149 they are useful to investigate the underlying pathology of HFpEF. That being said, very few studies using these models have actually directly assessed cardiac energy metabolism in HFpEF (Figure 2).
A ‘2-Hit’ model of HFpEF has been described where mice received both a metabolic insult from a 60% high-fat diet (HFD) and a haemodynamic insult from the endothelial nitric oxide synthase (eNOS) inhibitor L-NAME to induce hypertension.144 This 2-Hit mouse model results in diastolic dysfunction, as evidenced by the elevated E/E′ ratio, with preserved EF.144 Importantly, a study from Tong et al.150 employed this HFpEF protocol in mice and found an impaired cardiac fatty acid oxidation associated with hyperacetylation of enzymes in the fatty acid oxidation pathway. The rate of fatty acid oxidation was measured using permeabilized mitochondria with 25 μM palmitoyl-carnitine to assess the oxygen consumption rates (OCR).150 Although not the focus of the study, these mitochondrial OCR studies also showed impaired pyruvate metabolic rates, suggesting an impaired cardiac glucose oxidation in HFpEF.150 This suppression of glucose oxidation was also observed in our recent study where we employed the ‘2-Hit’ HFpEF mouse model and directly measured cardiac energy metabolism in isolated working hearts. Of importance, insulin-stimulated glucose oxidation rates are significantly decreased in HFpEF hearts.121 Contrary to Tong et al.,121 however, we found that cardiac fatty acid oxidation is not decreased but rather increased in HFpEF hearts. Because the loss of glucose oxidation is compensated by fatty acid oxidation, this results in overall preserved ATP production rates.121 As such, the heart in HFpEF is energetically preserved, instead of being energetically starved/failure in midst of plenty as is seen in HFrEF.90 We believe, this is largely because the heart is switching from oxidation of glucose to fatty acid in the setting of HFpEF, which is attributed to the impaired regulation of insulin on energy metabolism.121 At the molecular level, we observed an increased expression of PDK4 and associated phosphorylation of PDH, the rate-limiting enzyme of glucose oxidation. As a result, one possible avenue to restore the energy balance in the HFpEF heart is to stimulate cardiac glucose oxidation by inhibiting the action of PDK4. In support of this, we recently showed that in aged female mice subjected to the 2-Hit protocol, pharmacological inhibition of PDK4 restored glucose oxidation rates and improved cardiac function.151
In a ‘3-Hit’ model, Deng et al.145 induced HFpEF with long-term (13 months) high-fat feeding combined with deoxycorticosterone pivalate challenge. Interestingly, Deng et al. found that fatty acid oxidation decreased in these hearts, but unfortunately no assessment of glucose oxidation was made. Interestingly, ketone (ßOHB) oxidation was decreased by around 50% in HFpEF mice hearts assessed using carbon 13 nuclear magnetic resonance isotopomer analysis. This was associated with a lower expression of BDH1 enzymes. Similarly, genes for fatty acid uptake were also found to be down-regulated in ‘3-Hit’ HFpEF mouse hearts, but with only modest changes in the expression of β-oxidation genes. The decrease in ketone oxidation observed in the preclinical ‘3-Hit’ mouse heart was also found in human patients with HFpEF.50 Therefore, it is likely that the availability of that fuel source dictates the differential metabolism of ketones in HFpEF vs. HFrEF. That being said, fasting ketone levels are significantly higher in HFpEF mice,145 so another regulatory mechanism could potentially exist that also contributes to the lessening of cardiac ketone oxidation.
As discussed earlier in HFrEF section, increasing ketone supply to the heart may be beneficial as an extra fuel source for the failing heart. This approach has been tested in the setting of HFrEF with promising results. However, it remains unclear as whether this would be equivalently effective in the setting of HFpEF. Of relevance, SGLT2 inhibitors have recently been shown to lower hospitalization rates in patients with HFpEF.152 One of the main effects of SGLT2 inhibition is to induce a fasting mimetic state, therefore leading to endogenous ketogenesis and elevated plasma ketone levels,153 which might be one possible mechanism of SGLT2 inhibition, at least in part, in providing an extra source of fuel for the failing heart. However, direct flux measurements of ketone oxidation of the heart in the setting of HFpEF is still limited, and whether raising ketone availability to the heart, when the heart is already experiencing down-regulation of ketone metabolism, might be something beneficial or detrimental remains to be elucidated.
Interestingly, the severity of HFpEF in the ‘2-Hit’ model shows strong genetic and sex differences. Younger female mice are protected against the ‘2-Hit’ protocol, as evidenced by a smaller elevation in the E/E′ ratio and hypertrophy in female mice vs. male mice.154 However, it has not been determined if this is due to differences in cardiac energy metabolism. In contrast, Cao et al. suggested that female mice subjected to the HFpEF 2-Hit protocol develop a more severe diastolic dysfunction than male mice and linked this to differences in mitochondrial DNA (mtDNA) levels. Assessment of mitochondrial respiratory capacity showed a reduction in both basal and maximal respiration rates in mitochondrial from female compared to male hearts, as well as lower activity of Complex II and Complex IV of the ETC. In aging female mice subjected to the 2-Hit HFpEF protocol, we recently observed a marked increase in cardiac fatty acid oxidation and a decrease in insulin-stimulated glucose oxidation and ketone oxidation, similar to what we observed in younger male mice subjected to the 2-Hit protocol.121
Very little is known regarding what happens to cardiac amino acid metabolism in HFpEF. A clinical study from Hahn et al.143 found that BCAA levels are elevated in HFpEF myocardium, but metabolites of BCAA were lower, suggesting impaired BCAA oxidation. However, in this study, one of the major BCAA metabolites, BCKA was not measured. It is plausible that both BCKA and BCAA levels are elevated in HFpEF myocardium, as gene expression of enzymes that conve BCAA to BCKA, branched-chain amino acid transaminase 2 (BCAT2), is increased, in parallel with a decrease in downstream enzymes that catabolize BCKA. This is potentially important as BCKAs inhibit cardiac insulin-stimulated glucose oxidation,132 which is decreased in HFpEF.121
5. Cardiac energy metabolism in heart failure induced by obesity and T2D
Obesity and T2D are two prominent metabolic disorders that are closed related to heart failure progression.155,156 Obesity is a condition where the body accumulates excessive adipose tissue,157 whereas T2D is often characterized as a dysregulated glucose homeostasis.158 Both conditions are associated with insulin resistance.159 Insulin is an important regulator of energy metabolism and plays a key role in balancing glucose and fatty acid metabolism. In particular, obesity was proposed to be more strongly associated with risks of HFpEF than HFrEF.160 Similarly, another study showed that many patients with HFpEF also have T2D. Therefore, both obesity and T2D are highly prevalent in HFpEF,161 and are relevant for the progression of cardiac dysfunction.
In obesity, myocardial fatty acid oxidation rates are elevated.162–165 This is partially attributed to the higher circulating levels of free fatty acids in the bloodstream, so that and present themselves as readily available energy substrate to the heart (Figure 2). Many preclinical studies using isolated working heart perfusions have shown that myocardial fatty acid oxidation rates are elevated in obese mice.121,166–168 For example, in leptin-deficient ob/ob mice, obesity was progressively developed with concurrent development of insulin resistance and glucose intolerance.164 Systemic insulin resistance occurs in parallel with cardiac insulin resistance, where insulin stimulated glucose uptake and glucose oxidation is impaired at the level of the heart.164,169 Importantly, this suppression of glucose metabolism occurs simultanouesly with an increase in myocardial fatty acid oxidation.121,164,166–168 Overall, the heart in obesity switches from glucose oxidation towards fatty acid oxidation, becoming less metabolic flexible and less responsive to insulin.121,164,166–168 The paradoxical change of glucose and fatty acid metabolism170 is partly mediated through the inhibitory action of acetyl-CoA derived from fatty acid oxidation suppressing the activity of PDH and impairing glucose oxidation.16 Additionally, increased fatty acid oxidation flux will lead to more production of NADH and FADH2 from the TCA cycle, which would also negatively regulate PDH activity.171 Similar results were found by Buchanan et al.,165 showing that both ob/ob and db/db mice develop impaired glucose oxidation coupled with increased fatty acid oxidation as early as 4 weeks of age, which corresponds to the onset of systemic impaired glucose homeostasis and insulin resistance. This switch to fatty acid oxidation occurs with increased rates of myocardial oxygen consumption and lowered cardiac efficiency,165 as fatty acids are a less energy-efficient energy substrate compared to glucose.
Similar results were also found in the clinical setting.162 Peterson et al.162 reported that insulin resistance is closely related to high myocardial fatty acid uptake and oxidation in obese patients. Other studies have shown that patients with insulin resistance have increased lipolysis from adipose tissue,172 which would explain the higher circulating free fatty acids levels.
In diabetes, plasma glucose and fatty acids are also elevated, due to the combined effects of both stimulated hepatic glucose production and suppressed glucose uptake to peripheral tissue due to insulin resistance. Increased fatty acids levels in the bloodstream can be attributed to increased lipolysis of adipose tissues and elevated hepatic lipoprotein production. Yet, this increase in plasma substrate levels observed in diabetes is not directly correlated with substrate uptake rates in the heart.173 In particular, glucose uptake is controlled by GLUT1 and GLUT4. Insulin regulates the translocation of GLUT4 to sarcolemma membrane. In diabetes, GLUT4 translocation is impaired,174 which leads to a decrease of myocardial glucose uptake.175 Additionally, the expression of GLUT4 is also negatively regulated with increased intracellular levels of fatty acids. In fact, fatty acid uptake is increased in streptozotocin-induced diabetes.176,177 It is important to note that such increased uptake of fatty acids uptake might exceed mitochondrial oxidative capacity of metabolizing fatty acids, leading to incomplete ß-oxidation. This can cause an accumulation of unoxidized fatty acids, and possible conversion to harmful intracellular lipid intermediates, which suggest the danger of lipotoxicity.178–181
Since the heart switches from glucose oxidation to fatty acid oxidation in diabetes and obesity, it is reasonable to suggest that either inhibiting fatty acid oxidation or stimulating glucose oxidation may be a strategy to reconstruct the overall metabolic balance of the heart.182,183 Studies in a rat model of diabetic cardiomyopathy that is induced with streptozotocin injection showed that insulin resistance is improved with the PDK inhibitor, dichloroacetate (DCA), which leads to increased PDH flux, myocardial glucose oxidation, and overall improved cardiac function.182 On the other hand, treatment with a CPT1 inhibitor, oxfenicine, to suppress fatty acid oxidation also improves whole-body glucose tolerance and insulin sensitivity in mice fed with 12 weeks of HFD.183 Improved glucose homeostasis and clearance are also evidenced by increased expression of membrane GLUT4 and increased PDH activity in gastrocnemius skeletal muscle.183
6. Molecular mechanisms contributing to altered energy metabolism in heart failure
6.1. Alterations in mitochondrial function
Mitochondrial function is compromised in the failing heart due a number of reasons, including: (i) reduced transcriptional regulation of mitochondrial biogenesis, (ii) increased reactive oxygen species (ROS) production, (iii) impairments in mitofission, (iv) sustained mitophagy, and (v) increased autophagic cell death of cardiomyocytes (Figure 3).184–186 Mitochondrial biogenesis is also altered in the failing heart. For instance, peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α), a member of a family of transcription coactivators that plays a central role in regulating mitochondrial biogenesis, is decreased in HFrEF.93,187–189 In addition, the expression of p38 mitogen-activated protein kinase (MAPK), which modulates PGC-1α activity, is reduced in failing rat hearts caused by MI.190 Mitochondrial DNA content and mitochondrial DNA-encoded proteins are also decreased in patients with heart failure.191 An increased abundance of PGC-1α accompanies these alterations in mitochondrial DNA levels, but there is a decreased expression of oestrogen-related receptor α (ERRα) and mitochondrial transcription factor A.191 These findings highlight the role of PGC-1α in modulating mitochondrial biogenesis signalling in advanced heart failure.
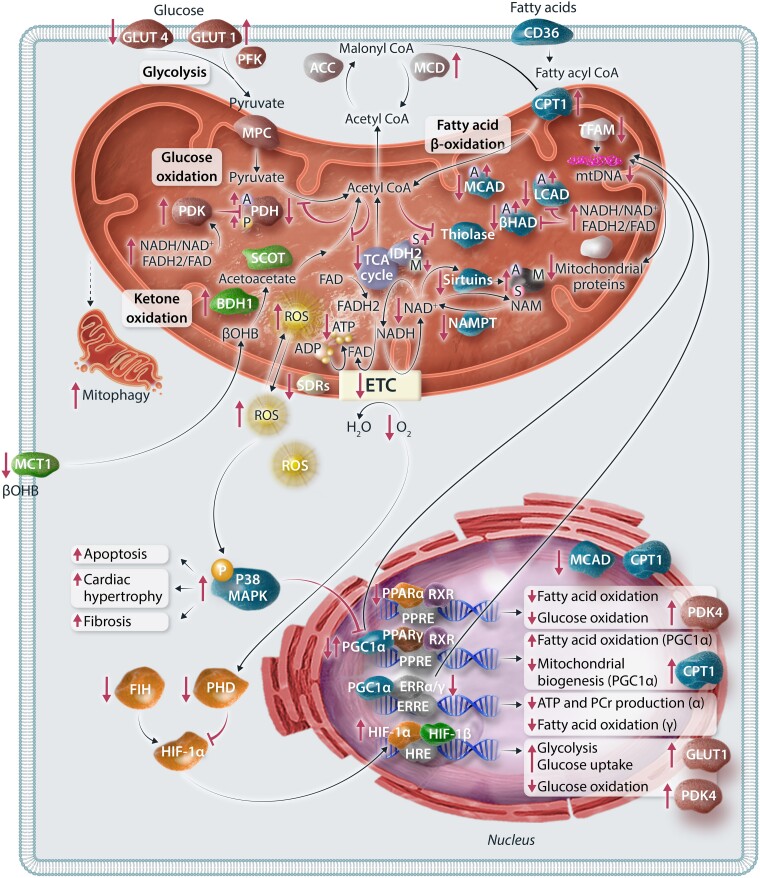
Figure 3.
Molecular mechanisms contributing to altered energy metabolism in heart failure. Changes and disruptions in energy metabolism in heart failure are governed by four distinct molecular mechanisms: mitochondrial function, substrate control, transcriptional control, and post-translational modifications. (i) Mitochondrial dysfunction in heart failure encompasses reduced transcriptional regulation of mitochondrial biogenesis, increased ROS production, impaired mitofission, sustained mitophagy, and enhanced autophagic cell death of cardiomyocytes. (ii) Regulation of carbon flux through metabolic pathways in the heart relies on tight control of CoA and its derivatives. In heart failure, the acetyl-CoA pool resulting from fatty acid oxidation can allosterically inhibit metabolic pathways through enzymes like PDH, thiolase, and ACAT. (iii) Transcriptional regulation in cardiac metabolism involves PPARs, ERRs, PGC-1α, and HIF-1α. (iv) PTMs play a crucial role in modulating the activities of mitochondrial enzymes and are implicated in heart failure progression. The small bubbles attached to specific proteins with letters P, A, S, M are representing PTMs. The representation of each letter are phosphorylation, P; acetylation, A; succinylation, S; malonylation, M. GLUT1/4, glucose transporter 1/4; PFK, phosphofructokinase; MPC, mitochondrial pyruvate carrier; PDH, pyruvate dehydrogenase; PDK, PDH kinase; CD36/FAT, fatty acid transporter; CPT1, carnitine palmitoyltransferase 1; LCAD, long-chain acyl-CoA dehydrogenase; βHAD, 3-OH-acyl-CoA dehydrogenase; MCAD, medium chain acyl-CoA dehydrogenase; βOHB, β-hydroxybutyrate; BDH1, βOHB dehydrogenase 1; SCOT, succinyl-CoA:3-ketoacid CoA transferase; MCT1, monocarboxylate transporter 1; ROS, reactive oxygen species; TCA, tricarboxylic acid; NADH, nicotinamide adenine dinucleotide; FADH2, flavin adenine dinucleotide; ETC, electron transport chain; ADP, adenosine diphosphate; ATP, adenosine triphosphate; SDR, short chain dehydrogenase; IDH2, isocitrate dehydrogenase; NAMPT, nicotinamide phosphoribosyltransferase; NAM, nicotinamide; TFAM, transcription factor A, mitochondrial; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α; MAPK, mitogen-activated protein kinase; PPAR, peroxisome proliferator-activated receptors; RXR, the retinoid X receptor; ERR, oestrogen-related receptor; HIF1, hypoxia-induced factor-1; HRE, hypoxia-response element.
Increased levels of cardiac ROS have been implicated in mitochondrial damage in heart failure.192,193 MAPK activation, cardiac hypertrophy, and fibrosis have also been linked to angiotensin II-induced mitochondrial ROS in HFrEF mice.194 In a mouse model of hypertensive cardiomyopathy induced by angiotensin II infusion,195 mitochondria-targeted antioxidant (N-acetyl cysteine) treatment, but not non-targeted ROS scavenging, mitigated cardiomyopathy. These findings emphasize the role of mitochondrial ROS in triggering structural remodelling and mitochondrial defects in heart failure. However, attempts to reduce ROS generation and/or levels in heart failure using antioxidant interventions in human studies have not been overwhelmingly encouraging.196,197 Therefore, the effectiveness of these antioxidant interventions in inhibiting cardiac ROS in patients with heart failure and whether ROS generation is a possible therapeutic target to limit mitochondrial damage and cardiac remodelling in heart failure are still outstanding questions that need to be answered in future studies.
Mitochondrial fission and mitophagy are closely linked, with mitochondrial fission facilitating the segregation of dysfunctional components of the mitochondria into an organelle that can undergo mitophagy (degradation). Cardiac-specific deletion of proteins involved in mitofission (preventing the assembly of these organelles) leads to the development of heart failure in vivo.185,186 Interestingly, disrupting the mitofission process also leads to the accumulation of defective mitochondria and an increased mitophagy of the entire mitochondria instead of mitophagy restricted to organelles containing the dysfunctional mitochondrial components.185,186 The heart responds to pressure overload stresses by increasing mitophagy, which removes damaged mitochondria to maintain ATP production. However, in cases of sustained mitophagy like heart failure, excessive mitochondrial clearance can occur, thereby reducing the number of mitochondria in the heart. On the other hand, when mitophagy is impaired, dysfunctional mitochondria cannot be properly degraded and continue to cause damage, leading to autophagy of the cardiomyocyte over time.186,198
6.2. Alterations in substrate control
Carbon flux through different energy metabolic pathways highly depends on the overall levels of coenzyme A (CoA) and its derivatives (Figure 3). These energy-baring molecules have major roles in controlling metabolic flux through these various pathways at three different levels: (i) as substrate and products of the metabolic pathways, (ii) as allosteric regulators of the metabolic pathways, and (iii) as a regulator of fatty acid oxidation via malonyl-CoA. Cytosolic CoA levels are very low in heart muscle (15–50 μM),199 but they are required to esterify fatty acids to long-chain acyl-CoA.200 In contrast, mitochondrial CoA levels are higher (2 mM), and they are prerequisites for acetyl-CoA production from pyruvate decarboxylation by PDH and catabolism of ketone bodies and amino acids.200,201 Acetyl-CoA production rates from these metabolic pathways are closely matched to its entry into the TCA cycle, allowing cardiac ATP production to match the heart's energy demand.202,203 At rest or during low cardiac workload, mitochondrial acetyl-CoA/CoA is increased, which feeds back and allosterically inhibits fatty acid oxidation at the level of 3-ketoacyl-CoA thiolase (KAT).202 High acetyl-CoA/CoA ratio also inhibits glucose oxidation and ketone oxidation at the levels of PDH and mThiolase, respectively.200,202 The increase in acetyl-CoA/CoA ratio controls carbohydrate oxidation by direct inhibition of PDH and/or stimulation of PDK, which phosphorylates and inhibits PDH due to the increase in acetyl-CoA/CoA ratios. However, increased energy demand and workload decrease the acetyl-CoA/CoA ratio due to increased entry of acetyl-CoA into the TCA cycle.199 As a result, the feedback inhibition on KAT, PDH, and mThiolase decreases, increasing the oxidation of fatty acids, carbohydrates, and ketones, respectively.199,201,202 Studies have shown a significant decrease in cardiac acetyl-CoA/CoA ratio in HFrEF and the infarcted heart.52,204 This is mainly due to the overall reduction in mitochondrial oxidative metabolism and in acetyl-CoA generation through the different metabolic pathways.52,90,204
Mitochondrial fatty acid β-oxidation is regulated by cytosolic malonyl-CoA levels and their effect on CPT1.36,205 Malonyl-CoA inhibits CPT1 and decreases fatty acid uptake into the mitochondria by inhibiting the conversion of long-chain acyl-CoA to long-chain acylcarnitine, thereby inhibiting fatty acid β-oxidation. Malonyl-CoA levels are rapidly turned over,206 with its production controlled by ACC and its degradation by MCD. Therefore, ACC activity negatively correlates to fatty acid β-oxidation rates, while MCD activity positively correlates with fatty acid β-oxidation rates.207–211 For example, genetic deletion or pharmacological inhibition of MCD is associated with an increase malonyl-CoA levels and a decrease in cardiac fatty acid oxidation.212,213 In obesity and diabetes, cardiac fatty acid ß-oxidation rates increase, leading to an increase in acetyl-CoA generation.163,204 Impaired mitochondrial TCA cycle activity, such as during ischaemia and severe heart failure, can also increase mitochondrial acetyl-CoA levels.52,204 The mitochondrial acetyl-CoA production in these conditions may exceed the oxidative capacity of the TCA cycle and, therefore, increase acetyl-CoA levels. Augmented levels of fatty acids seen in these conditions also increase cardiac MCD expression, contributing to a decrease in malonyl-CoA levels.214
Another layer of allosteric regulation of the flux through different metabolic pathways is the levels of reduced equivalents, namely NADH and FADH2. NADH and FADH2 are mainly generated from the metabolism of fatty acids, glucose, ketone bodies, amino acids, and TCA intermediates. Reduced equivalents can also be generated cytosolic glycolysis and shuttled into the mitochondria via the malate-aspartate shuttle or the glycerol phosphate shuttle.200 Similar to the acetyl-CoA/CoA ratio, the ratios of NADH/NAD+ and FADH2/FAD are important regulatory mechanisms of the dehydrogenases involved in fatty acid β-oxidation, glucose oxidation, ketone oxidation, amino acid oxidation, and TCA cycle activity. Under low energy demand, the ratios of NADH/NAD+ and FADH2/FAD increase, that feeds back and slows down carbon flux through the different metabolic pathways. High NADH/NAD+ and/or FADH2/FAD ratios also allosterically trigger PDK activity, phosphorylating, and inhibiting PDH activity, thereby decreasing glucose and lactate oxidation.215 NAD+ availability is a crucial determinant for Sirtuin activity (as discussed in the next section). In myocardial ischaemia, there is a decrease in the NAD+/NADH ratio as a result of reduced ETC activity, which contributes to the inactivation of SIRT3 and leads to the hyperacetylation of mitochondrial proteins.52,216 It is important to note that during I/R injury, nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme that converts nicotinamide (NAM) to nicotinamide mononucleotide in the NAD+ salvage synthesis pathway, is down-regulated in the heart. Consequently, the NAD+ levels in the heart decrease and this results in a reduction in sirtuin activity.217,218 Studies have shown the cardioprotective effect of enhancing NAD+ levels in I/R injury either by exogenous NAD+ supplementation or enzymatic manipulation.217,219,220
6.3. Transcriptional regulation
Expressed in cardiomyocytes, peroxisome proliferator-activated receptors (PPARs) constitute a pivotal pathway regulating cardiac fatty acid uptake and oxidation at the transcriptional level (Figure 3). Within the heart, three forms of PPARs are present, namely the more prevalent PPAR-α and PPAR-β/δ, alongside PPAR-γ. Extensive research has focused on elucidating the role of PPAR-α in governing fatty acid metabolism in cardiac tissue. Studies employing gain/loss-of-function approaches in mice have underscored the significance of PPAR-α in modulating the expression of genes involved in most steps of cardiac fatty acid utilization, such as the expression of CPT1 and medium chain acyl-CoA dehydrogenase 1 (MCAD1).221–223 Nevertheless, understanding the regulatory mechanisms of PPAR-α in heart failure remains complex. Notably, elevated levels of PPAR-α have been associated with diabetes, with chronic activation of PPAR-α resulting in a diabetic-like cardiac phenotype characterized by heightened fatty acid utilization alongside reduced glucose oxidation.165,180,224 Additionally, increased levels of PPAR-α have been demonstrated to exacerbate cardiac recovery impairment following ischaemia/reperfusion injury ex vivo.225 Similarly, PPAR-α agonism in hypertrophic rat hearts diminishes cardiac power and efficiency.226 Conversely, Kaimoto et al.227 showed that early-stage activation of PPAR-α in heart failure improves cardiac function and enhances the expression of fatty acid oxidation genes in mice. To reconcile these disparate findings, studies have suggested that PPAR-α can function as both an activator and repressor of transcription and the down-regulation of PPAR-α in heart failure may primarily affect a subset of fatty acid oxidative genes rather than entail global down-regulation.228,229
As a transcriptional coactivator of PPAR-γ, gene expression analysis has revealed that PGC-1α targets pathways including fatty acid oxidation, mitochondrial biogenesis, and OXPHOS.230–232 Although PGC-1α has been implicated as a crucial pathway in metabolic deterioration and cardiac remodelling in the failing heart, evidenced by accelerated development of heart failure, deficits in cardiac energy reserve and function, as well as accelerated cardiac remodelling in PGC-1α KO mice,189,233,234 the regulation of PGC-1α expression is intricate and may strongly depend on the cause and timing of disease progression. Animal studies have reported decreases in PGC-1α expression in heart failure.235–238 Similarly, evidence from patients at an advanced-stage of heart failure has shown decreased gene or protein expression.239,240 However, both murine and human studies have not consistently found changes in PGC-1α gene expression in heart failure191,241; some even demonstrated an increase in expression. For example, in a mouse model of angiotensin II-induced hypertrophied heart, an increase in mRNA expression of PGC-1α was associated with a concurrent increase in mitochondrial turnover.194 Moreover, patients with advanced-stage heart failure have also exhibited slight increases in gene or protein expression, suggesting heightened fatty acid oxidation in the failing heart.242
ERR also play a crucial role in the transcriptional regulation of cardiac metabolism. This family comprises three members (ERR-α, ERR-β, and ERR-γ), with ERR-α and ERR-γ being the predominant isoforms in cardiac tissue.243 Studies have shown that by stimulating ERR-α on PPAR-α, these receptors facilitate fatty acid uptake and β-oxidation.244 In pressure overload-induced heart failure, the deletion of ERR-α has been linked to reduced maximal ATP production and depletion of phosphocreatine, underscoring its significance in the bioenergetics and adaptive functions of a stressed heart.243 Recent findings have also highlighted the potential of novel pan-ERR agonists in mitigating heart failure in vivo.245 Primarily activating ERR-γ, agonism up-regulates genes associated with fatty acid metabolism and mitochondrial function. Consequently, this up-regulation significantly improved EF, improved fibrosis, and enhanced survival in pressure overload-induced heart failure.
As a subunit of the heterodimeric transcription factor hypoxia-inducible factor 1 (HIF-1), the primary function of HIF-1α is to regulate oxygen (O2) levels by balancing O2 supply and demand.246 Under hypoxic conditions, the activity of HIF-α increases due to decreased activities of its regulators prolyl hydroxylase domain (PHD) and factor inhibiting HIF, caused by the up-regulation of mitochondrial α-ketoglutarate dehydrogenases.247 In heart failure, HIF-1α responds to cardiac stress and hypoxic states by promoting glycolysis to cope with reduced oxygen levels, as evidenced by its direct activation of GLUT1 to facilitate myocardial glucose uptake.248,249 However, the increased glucose uptake and utilization is not accompanied by a rise in pyruvate oxidation. Significantly, HIF-1α suppresses mitochondrial function and O2 consumption by up-regulating and activating PDK, therefore inhibiting PDH activity and mitochondrial pyruvate/glucose oxidation.248
6.4. Post-translational modifications
Through the addition or deletion of distinct carbon and non-carbon groups, post-translational modifications (PTMs) [including phosphorylation, acetylation, succinylation, malonylation, glutarylation, and O-GlcNAcylation (O-GlcNAc)] are instrumental in modifying the activities of mitochondrial enzymes, and alterations in these PTMs are implicated in the progression of heart failure. Phosphorylation has been extensively studied in the heart given its substantial role in modulating glucose and fatty acid metabolism.52,84,204 Recent attention has shifted towards investigating other PTMs in the context of heart failure (Figure 3).
Lysine acetylation is a highly regulated process involved in various metabolic changes observed in heart failure, diabetes, and obesity.130 This modification occurs through enzymatic and non-enzymatic pathways, wherein an acetyl group is transferred from acetyl-CoA to a lysine residue. Moreover, acetylation can be reversed by histone deacetylases (KDACs), encompassing two main families: Zn2+-dependent DACs (KDAC1–11) and NAD+ -dependent KDACs (sirtuins 1–7). Notably, SIRT3, the major mitochondrial deacetylase, acts as a crucial link between NAD+ homeostasis and changes in myocardial energetics by modulating enzymes involved in, but not limited to, glucose, fatty acid, and ketone metabolism.218 In heart failure, depletion of SIRT3 expression results in an overall hyperacetylated state, disrupting cardiac metabolism, and exacerbating disease progression.145,250 Besides a decrease in phosphorylation of PDH in heart failure due to hyperacetylation of PDH phosphatase,251 studies have demonstrated the inhibition of PDH activity through direct acetylation in the failing myocardium of mice, leading to an overall decrease in glucose metabolism.252,253 Similarly, SIRT3 also regulates fatty acid oxidation enzymes such as long-chain acyl-CoA dehydrogenase (LCAD) and 3-hydroxy acyl-CoA dehydrogenase (β-HAD). Interestingly, there is conflicting evidence regarding the role of acetylation in fatty acid oxidation in the failing heart. Alrob et al.166 showed that fatty acid oxidation rates are increased in SIRT3 KO hearts, associated with increased acetylation and activity of LCAD and β-HAD. Conversely, other studies found that heart failure associated with decreased SIRT3 expression or KO was linked to increased LCAD acetylation and impaired fatty acid oxidation, with the activation of SIRT3 mediating deacetylation of LCAD and promoting fatty acid oxidation.254–256 Recently, SIRT3 activity has also been associated with ketone metabolism and its corresponding protective effects in heart failure. SIRT3 KO in neonatal rat cardiomyocytes leads to reductions in the ketone metabolism enzyme (SCOT and MCT1) expression while abolishing the beneficial effects of choline on Ang-II-induced hypertrophy.257 Additionally, Yan et al. also demonstrated that decreased SIRT3 expression in HFpEF in mice is associated with decreases in ketone metabolism proteins, including BDH1, SCOT, and acetyl-CoA acetyltransferase 1 (ACAT1). They further found that SIRT3 KO in HFpEF mice subsequently worsened HFpEF phenotypes such as cardiac hypertrophy and myocardial fibrosis.258
Regulated by SIRT5, succinylation, malonylation, and glutarylation are lesser investigated lysine acylation modifications involved in mitochondrial energetics. Although little is known about the specific mitochondrial enzymes succinylation catalyzes, increased succinylation in SIRT5KO cardiac mitochondria has been shown to associate with increased 3-hydroxyacyl dehydrogenase flux, suggesting an increase in fatty acid oxidation.259 Additionally increased succinylation of fatty acid oxidation enzymes in newborn hearts is associated with enhanced activity of these enzymes.260 However, the precise impact of succinylation on heart failure metabolism remains unresolved. TAC-induced heart failure in mice is associated with inhibited SIRT5 expression in the mitochondria, increased isocitrate dehydrogenase 2 (IDH2) succinylation, and decreased cardiac function attributable to decreased mitochondrial energy metabolism.261 Interestingly, supplementation with quercetin, a flavonoid, increased SIRT5 expression and promoted IDH2 desuccinylation, concomitantly inhibiting myocardial fibrosis and improving cardiac function.261 Additionally, Takada et al.262 observed that the overall myocardial mitochondrial protein succinylation levels were significantly lower in MI mice. However, some TCA cycle enzymes and membrane proteins, such as IDH2, had higher succinylation levels, which decreased subsequent to additions of succinyl-CoA.262 Similar to succinylation, the significance of malonylation in cardiac energy metabolism and other cellular processes in heart failure is not yet fully understood. In the normal heart, lysine malonylation involves proteins associated with protein translation, amino acid catabolism, the TCA cycle, and ATP synthesis. It has also been demonstrated that enhanced malonylation is associated with greater PDH activity in MCD KO heart mitochondria, suggesting malonylation's role in promoting glucose oxidation.259 Furthermore, increased malonylation through inhibition of MCD activity stimulates glucose oxidation, thereby protecting the ischaemic heart.213 Recently, LC-MS-based label-free lysine malonylation profiling of mice hearts after TAC surgery identified 679 malonylated sites in 330 proteins.263 Specifically, a decrease in IDH2 malonylation was detected both in vitro and in vivo, potentially linking malonylation to cardiac hypertrophy. Lastly, while hyperglutarylation of proteins involved in fatty acid metabolism, aerobic metabolism, the TCA cycle, and coenzyme metabolism has been demonstrated,264 the significance of this type of modification in heart failure has yet to be determined.
7. Targeting cardiac energy metabolism to treat heart failure
7.1. Targeting fatty acid oxidation
As discussed, there is considerable confusion about what alterations in fatty acid oxidation occur in heart failure, with decreases,88,99,108 no changes,81,82 or increases78 in fatty acid oxidation reported in the failing heart.
This has led to opposing studies aimed at either using pharmacological strategies to increase265,266 or decrease267,268 fatty acid oxidation in the failing heart. While no large clinical trials have yet been performed, currently avaliable clinical evidence does support the concept of inhibiting fatty acid oxidation to treat heart failure (Figure 4).269–274 Chronic treatment with trimetazidine, a partial inhibitor of fatty acid oxidation, improves functional class, quality of life, and left ventricular function in patients with HFrEF, regardless of its aetiology, and diabetic status.269 A meta-analysis also confirmed that trimetazidine has significant protective effects against all-cause mortality, cardiovascular events, and hospitalization in patients with heart failure.271 These beneficial effects of trimetazidine are thought to be due to increased cardiac glucose oxidation, secondary to fatty acid oxidation inhibition, resulting in an increase in cardiac efficiency.275 Clinical studies with the fatty acid oxidation inhibitors, perhexiline and etomoxir, have also shown decreased heart failure severity in HFrEF patients.270,272,273 Although cardiac fatty acid oxidation rates are increased in HFpEF,121 no clinical data are available on whether fatty acid oxidation inhibition benefits these patients.
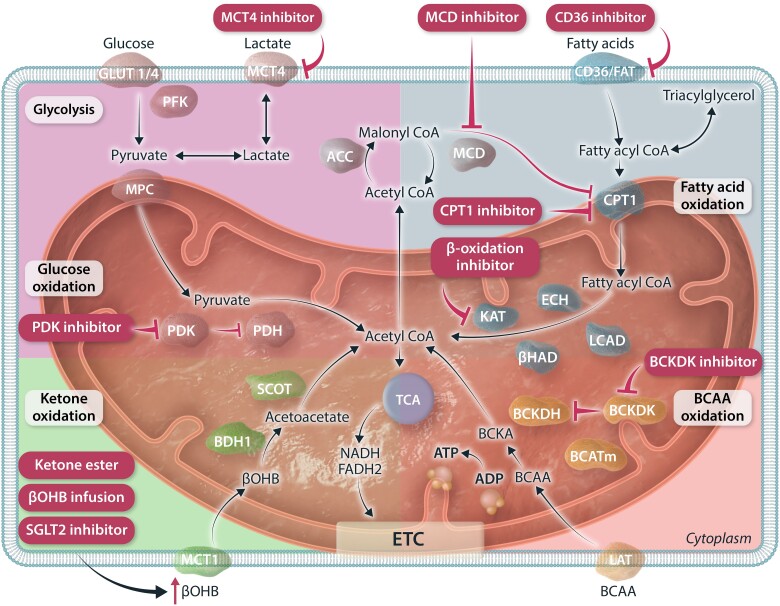
Figure 4.
Current available pharmacological therapies that target cardiac energy metabolism. Collection of drugs that target different metabolic pathways through modifying the action of key enzymes involved. PDK inhibitor alleviates the inhibitory action of PDK on PDH, thus can stimulate glucose oxidation. MCT4 inhibition decreases lactate efflux, which can redirect glycolytic carbon flux into mitochondrial pyruvate oxidation/glucose oxidation. CD36 inhibition decrease fatty acid uptake into myocardium. Both CPT1 inhibitors and MCD inhibitors decrease the action of CPT1 for uptake of fatty acids into mitochondria. β-oxidation inhibitors interferes with the action of KAT, one of the major β-oxidation enzymes. Ketone therapy includes, ketone ester, βOHB infusion, SGLT2 inhibitor all which increases circulating βOHB levels, thus elevated accessibility of βOHB for further uptake and oxidation by the heart. BCKDK inhibitors alleviate the inhibitory action of BCKDK, thus stimulating the action of BCKDH and inducing the flux of BCAA oxidation. GLUT1/4, glucose transporter 1/4; MCT4, monocarboxylate transporter 4; PFK, phosphofructokinase; MPC, mitochondrial pyruvate carrier; PDH, pyruvate dehydrogenase; PDK, PDH kinase; ACC, acetyl-CoA carboxylase; MCD, malonyl-CoA decarboxylase; CD36/FAT, fatty acid transporter; CPT1, carnitine palmitoyltransferase 1; LCAD, long-chain acyl-CoA dehydrogenase; ECH, enoyl CoA hydratase; βHAD, 3-OH-acyl-CoA dehydrogenase; KAT, 3-ketoacyl-CoA thiolase; BCAA, branched-chain amino acids; BCATm, mitochondrial BCAA amimotransferase; BCKDH, branched-chain α-ketoacid dehydrogenase; BCKDK, BCKDH kinase; LAT, L-type amino acid transporters; βOHB, β-hydroxybutyrate; BDH1, βOHB dehydrogenase 1; SCOT, succinyl-CoA:3-ketoacid CoA transferase; SGLT2, sodium-glucose co-transporter 2; TCA, tricarboxylic acid; NADH; nicotinamide adenine dinucleotide; FADH2, flavin adenine dinucleotide; ETC, electron transport chain; ADP, adenosine diphosphate; ATP, adenosine triphosphate.
Ranolazine is approved in the USA and Europe as a second-line agent in the management of chronic coronary syndromes. It not only blocks sodium channels (INa) but also inhibits cardiac fatty acid oxidation.276 A meta-analysis of eight clinical trials in HFpEF patients showed that ranolazine has good efficacy in improving diastolic performance, while not affecting blood pressure, heart rate, or rate of ventricular repolarisation (shortening of the QT interval).277 However, it remains unclear how much of this benefit originates from inhibition of cardiac fatty acid oxidation vs. INa inhibition.
At an experimental level, inhibiting cardiac fatty acid oxidation using a MCD inhibitor can improve heart function in rats subjected to heart failure due to permanent left anterior descending artery occlusion.97 In contrast, inhibition of ACC, which lowers malonyl-CoA and stimulates fatty acid oxidation, is cardioprotective in failing mice hearts due to pressure overload.265,266 While these beneficial effects of MCD and ACC inhibition may seem contradictory, both approaches result in an improved coupling of glycolysis to glucose oxidation, suggesting a decrease in proton production from uncoupled glucose metabolism. An alternate possible mechanism is inhibiting fatty acid oxidation in cardiomyocytes (due to CPT1ß deficiency), which has been proposed to improve resistance to hypoxia and to stimulate cardiomyocyte proliferation, allowing heart regeneration after ischaemia–reperfusion injury.268
Numerous mouse models have been produced in which myocardial fatty acid uptake or oxidation is either decreased or increased. This includes mice fed a high-fat diet, lipoprotein lipase KO mice, MCD KO mice, ACC KO mice, CPT1ß KO mice, CD36 KO mice, FATP1 overexpressing mice, acyl-CoA synthetase 1 overexpressing mice, diglyceride acyltransferase 1 (DGAT1) overexpressing mice, adipose triglyceride lipase (ATGL) overexpressing mice, ATGL KO mice, LCAD KO mice, PPARα KO mice, PPARα overexpressing mice, PGC1-α overexpressing mice, and PGC1α KO mice. In these mice there is no consensus on whether inhibiting or stimulating fatty acid oxidation is beneficial or harmful for either ischaemic or non-ischaemic heart failure (see Abudurrachim et al.278 for review). In general, if fatty acid oxidation is accompanied by increased glucose oxidation, then beneficial effects on cardiac function occur. However, contractile dysfunction occurs if fatty acid oxidation inhibition causes a cardiac energic deficiency or lipid accumulation.
A potential problem with mitochondrial fatty acid oxidation inhibition is that decreasing cardiac fatty acid oxidation with maintained fatty acid uptake has the potential to contribute to lipotoxicity.279 Inhibition of sarcolemmal fatty acid uptake by inhibiting FAT/CD36 is one potential approach to overcoming this issue. In support of this, deletion of cardiac FAT/CD36 reduces lipid accumulation and improves contractile function in mice prone to lipotoxic cardiomyopathy.280 The FAT/CD36 inhibitor sulfo-N-succinimidyl oleate also decreases fatty acid oxidation, decreases triacylglycerol accumulation, and improves cardiac function in diabetic rat hearts subjected to hypoxia-reoxygenation.280 Additionally, it is important to note that, even though pharmacological modulation of fatty acid metabolism has shown beneficial effects in improving cardiac function, but the results are not always consistent.270 As such, there should be a note of caution that the evidence has not been sufficient for the drugs to be adopted in international clinical practice guidelines.
7.2. Targeting glucose oxidation
Although glycolysis increases in heart failure, the subsequent oxidation of glycolytically derived pyruvate (glucose oxidation) is impaired.6 This results in an uncoupling of glycolysis from glucose oxidation, increasing proton production, and decreasing cardiac efficiency.6 Two potential therapeutic approaches to overcome this problem are to increase mitochondrial pyruvate uptake or to stimulate PDH (the rate-limiting enzyme for glucose oxidation) (Figure 4).
In heart failure patients, expression of the MPC is decreased, while the lactate exporter monocarboxylate transporter 4 (MCT4) is increased (which transports lactate-derived from glycolytically derived pyruvate that is not oxidized out of the cardiomyocyte). An inhibitor of MCT4 (VB124) has been developed that inhibits lactate efflux, which is thought to redirect glycolytic carbon flux into mitochondrial pyruvate oxidation.102 In support of this concept, treating cardiomyocytes with VB124 decreases their response to hypertrophic stress and increases mitochondrial pyruvate uptake.102 However, whether MCT4 inhibition can improve contractile function in either clinical or experimental heart failure has yet to be determined. Similarly, while a decrease in MPC function contributes to cardiac dysfunction,101,102 it is not yet known if stimulation of MPC can benefit the failing heart.
One approach to increasing glucose oxidation is to inhibit PDK, which normally phosphorylates and inhibits PDH. The most widely studied approach to do this is with DCA. DCA administration to patients with angina and coronary artery disease augments left ventricle stroke volume and enhances myocardial efficiency.281 DCA also improves left ventricle mechanical efficiency and enhances cardiac work in patients with New York Heart Association Classes III and IV congestive heart failure.282 Unfortunately, the potency and pharmacokinetics of DCA are not optimal for chronic treatment of heart failure patients. However, the concept of stimulating glucose oxidation with DCA to treat heart failure has been shown in a number of experimental studies. DCA improves function in hypertrophied rat hearts75 and post-ischaemic cardiac function and efficiency in rat hearts by stimulating glucose oxidation and improving the coupling between glycolysis and glucose oxidation.283 In hypertrophic rat hearts (induced by hyperthyroidism), DCA treatment significantly reduces the severity of hypertrophy and maintains cardiac output.284 Using hyperpolarized [13C] pyruvate and magnetic resonance imaging, DCA reverses the impaired glucose oxidation seen in the hyperthyroid heart.284 In a porcine model of volume overload heart failure, using magnetic resonance spectroscopy with hyperpolarized [13C] pyruvate it was found that DCA treatment can improve contractile reserve and decrease hypertrophy by augmenting carbohydrate metabolism.285 In HFpEF, myocardial glucose oxidation rates are also markedly depressed.121 We recently demonstrated that treatment of heart failure in mice with a PDK inhibitor (MMR-0013) increases cardiac glucose oxidation and improves survival and cardiac function.151 Combined, these data support the concept that increasing glucose oxidation, by inhibiting PDK is a potential promising approach to treating heart failure.
Glucose oxidation studies have shown an accumulation of pyruvate and other glycolytic intermediates in myocardial biopsies of patients with end-stage heart failure.92,102,104,105 The accumulation of these metabolites was accompanied by a reduction in the expression levels of the MPC and an increase in the phosphorylation of PDH.92,102,104,105 In line with these studies, direct measurement of glucose oxidation rates revealed that these rates are reduced in the failing heart.52,204 The expression of MPC is decreased in human and animal heart failure,286,287 which might contribute to the overall reduction in cardiac glucose oxidation in heart failure, a reproducible signature of the failing heart. The decrease in mitochondrial uptake and oxidation of pyruvate contribute to ‘energy starvation’ in heart failure.
Inhibition of mitochondrial pyruvate uptake by MPC subunits (MPC1 or MPC2) deletion or pharmacological inhibitors induces pathologic cardiac hypertrophy and heart failure.101,102,286,287 These functional and structural changes are associated with increased flux into non-oxidative pathways such as the HBP, glycogen synthesis, the PPP, and amino acid biosynthetic pathways. Consistent with this, overexpression of MPC in hearts subjected to pressure overload or treated isoproterenol and an inhibitor of the lactate transporter MCT4, which increased the generation of pyruvate and flux through the MPC, limits the flux through glucose non-oxidative metabolic pathways and decreases the severity of adverse remodelling.102,286 MPC deletion markedly reduces glucose oxidation and causes acceleration of both glycolysis and fatty acid oxidation to compensate for the reduction in glucose contribution to cardiac ATP production.101 Ketogenic diet (high-fat/low-carbohydrate diet) feeding mitigates adverse remodelling by further increasing fatty acid oxidation and blunting glycolysis, lactate and pyruvate accumulation and flux of glucose carbons into non-oxidative metabolic pathways such as the HBP and glycogen in MPC KO heart.101 However, a ketogenic diet feeding exacerbates cardiac insulin resistance and does not improve cardiac function in a mouse model of MI.288
7.3. Targeting ketone oxidation
Increasing cardiac ketone oxidation has recently attracted attention as a potential therapeutic strategy to treat heart failure (Figure 4).289 In HFrEF, cardiac ketone oxidation rates are elevated, which has been proposed as an adaptive mechanism to compensate for the impaired overall myocardial energy production.90,124 Although the benefits of increasing cardiac ketone oxidation have been suggested to ketones being a thrifty fuel,85,86,290 our studies do not support this,90 and rather suggest that increasing ketone supply to the heart provides the failing heart with an extra fuel source and helps to improve overall cardiac energetics. In HFpEF, cardiac ketone oxidation rates are not increased, but can be decreased.121,145 This may contribute to an energy deficit in the heart. Several strategies have been evaluated to increase ketone supply to the heart, including direct ketone infusions, ketone ester supplementation, administering a ketogenetic diet, and using SGLT2 inhibitor treatment.128 Most of these increasing ketone supply strategies were tested in pre-clinical and clinical studies only for HFrEF.
Acute infusions of the ketone βOHB into HFrEF patients improves haemodynamic function, as evidenced by an increase in %EF.70 But the decreased cardiac efficiency in HFrEF patients was not improved with βOHB infusions, supporting the concept that ketones are not a more efficient fuel for the heart. In canines subjected to HFrEF using a chronic cardiac pacing protocol, the direct infusion of the βOHB ameliorates pathological cardiac remodelling.126 This infusion of βOHB elicits changes in the myocardial metabolic profile, with a significant increase in ketone body uptake and a decrease in glucose uptake.126 This suggests that ketones might compete with other myocardial substrates such as glucose. On the contrary, another study found that increasing βOHB supply to the heart does not decrease glucose oxidation or glycolysis rates in mice subjected to TAC-induced heart failure mice.90 Regardless, further studies are needed to ascertain the benefits of ketones in heart failure and to investigate how ketone oxidation fits into the overall bigger picture of myocardial energy metabolism in heart failure.291
A ketogenic diet and ketone ester supplements can also be used to increase circulating ketone concentrations. Ketogenic diets are characterized as low-carbohydrate high-fat diets that induces the body to undergo a ‘pseudo-fasted’ state,292,293 which stimulates the liver to use free fatty acids to produce ketone bodies, known as the process of ketogenesis.294,295 The liver is the main organ for ketogenesis, although some recent studies also suggest that the heart is capable of ketogenesis.296,297 Although the ketogenic diet has been widely used as an effective weight loss strategy, its applicability in patients with heart disease is limited. In healthy individuals consuming the ketogenic diet, there is evidence of elevated PCr/ATP ratios.298 However, whether this would induce comparable results in patients with heart failure remains unclear. The results gathered to date are rather conflicting, with some studies showing improved cardiac function in pre-clinical mice models of heart failure receiving ketogenic diets,299,300 while others showing adverse effects with continuous ketogenic diet feeding.301 In support of this, a study found that increasing βOHB through ketogenic diet can lead to decreased mitochondrial biogenesis, increased cardiomyocyte apoptosis, and the development of cardiac fibrosis.302 Guo et al.301 proposed that an alternate-day ketogenic diet can improve the effects of the ketogenic diet, while maintaining its protective effects. Additionally, supplementing more medium chain triglyceride within the ketogenic diets was shown to recover an age-related decrease in succinate dehydrogenase activity and coverage of metabolically active mitochondria within the myocardium.303 We recently demonstrated that a ketogenic diet is not cardioprotective in a post-MI mouse model of heart failure.288 This appeared to be due to a marked increase in myocardial fatty acid oxidation and decrease in glucose oxidation seen in mice on the ketogenic diet. Considering that ketogenic diet has a high-fat content, more research is required to ascertain the safety and feasibility of the ketogenic diet in usage for patients with heart failure.
Administration of ketone esters is another approach to increase circulating ketone levels. Ketone ester is palatable by oral administration, as such, would be more convenient to ingest compared to direct infusion with ketone bodies. Ketone esters are precursors of ketones and will be converted to βOHB. Two examples of ketone esters are R-1,3-butanediol and ethyl (R)-3-hydroxybutyrate, which can increase blood ketone levels up to 6 mM in humans.304,305 Chronic supplementation with ketone ester has demonstrated cardioprotective effects against MI-induced heart failure in both mice and rats.306 Around the same time, another study published the results showing that acute ketone ester intake can improve cardiac function in healthy participants.307 This suggests the potential to use ketone ester in treating a wide range of cardiovascular diseases given its ease of administration, relatively safe profile, and strong cardioprotective echocardiographic and haemodynamic effects. That being said, to this day, there is no human study that shows cardioprotective effects with the use of ketone ester in patients with heart failure. Some of the on-going and/or completed yet not published clinical trials include: NCT04442555, which investigate the haemodynamic effect of exogenous ketones in acute heart failure and cardiogenic shock; NCT04633460, which assessed the effect of ketone ester, DeltaG, on exercise performance in people with heart failure; NCT05159570, which investigate the effect of 14 days ketone ester supplementation on whole body and skeletal metabolism; NCT06108076, which evaluates both acute and chronic effects of oral ketone monoester in subjects with HFrEF and diabetes; NCT06078683, which studies the effects of acute and chronic ketone ester consumption on exercise tolerance and cardiac function in subjects with the metabolic phenotype of HFpEF; NCT05348460, which assess effects of a single oral dose of ketone ester on exercise performance in patients with chronic heart failure; and NCT04698005, which investigates the effects of ketone monoester in acutely decompensated heart failure. The results from these clinical trials will offer valuable information for guiding the potential usage and application of ketone ester as a potential therapeutic strategy for heart failure.
Another approach to increase circulating ketones in heart failure patients is with the used of SGLT2 inhibitors. SGLT2 inhibitors were first developed as anti-diabetic drugs308–310 but have recently been shown to be effective in treating heart failure.152,309 In the large-scale clinical trial, EMPA-REG OUTCOME, the SGLT2 inhibitor empagliflozin decreased both the rate of hospitalization and death due to cardiovascular outcomes in patients with T2D.308 The SGLT2 inhibitors dapagliflozin and canagliflozin can also reduce heart failure severity and incidence rates of cardiovascular hospitalization in patients with HFrEF both with and without diabetes, suggesting that the effects of SGLT2 inhibitor lie beyond its glucose-lowering effects.309 SGLT2 inhibitors have also been shown to benefit patients with HFpEF.152 The mechanisms by which SGLT2 inhibitors exert their cardioprotective effects are still unclear.311 The primary action of SGLT2 inhibitor is to decrease glucose reabsorption from the proximal tubule of the kidney.312 This can elicit a ‘pseudo-fasting’ state and induce ketogenesis by the liver. This can increase the ketone supply to the heart, fuelling the energy-starved/failure in midst of plenty failing heart.313 However, this may not be the case in HFpEF, as myocardial ATP production is not compromised in HFpEF mice.121 As such, SGLT2 inhibitors may also act through other pathways to improve cardiac function in HFpEF. In addition to ketone acting as an extra source of fuel for the heart, ketones can also act as a signalling molecule, exert anti-inflammatory effects and ameliorate oxidative stress.127,314 In particular, activation of NLRP3 inflammasome has been shown to be suppressed with βOHB, as well as the lowering of NLRP3 inflammasome-mediated production of IL-1β and IL-18.314 In addition, cardiac-specific overexpression of BDH1 in mice showed reduced oxidative stress in response to TAC-induced heart failure.127 As such, ketones likely have several pleiotropic roles in the setting of heart failure to exert its beneficial effects.
Although most studies aimed at increasing circulating ketones have been promising, some studies have raised concerns that chronic ketone exposure may impair cardiac insulin sensitivity through perturbation in the PI3K/PKB signalling cascade.315,316 Therefore, more studies are required to delineate the proper dosage and duration of ketone therapy for heart failure treatment.
7.4. Targeting BCAA oxidation
Impaired cardiac BCAA oxidation in HFrEF increases cardiac levels of BCAAs and BCKAs, which have been linked to adverse effects on heart function.317–319 Since the contribution of BCAA oxidation to ATP production in the heart is minimal compared to their oxidative substrates (glucose, fatty acid, and ketone bodies), it seems unlikely the detrimental effects associated with increased levels of BCAAs and BCKAs are mediated by increased BCAA contribution to cardiac ATP production. Instead, it seems likely that cardiac BCAAs and BCKAs have more important roles as signalling molecules to mediate the observed detrimental effects.129 Studies have shown that reducing the accumulation of cardiac BCAAs and BCKAs is a cardioprotective approach in different models of heart failure (Figure 4).52,131,135,320,321 This increase in BCAAs and BCKAs is likely due to downstream inhibition of BCKDH in the failing heart.49,129,131,135,320,322 Pharmacological enhancement of BCAA oxidation by stimulating BCKDH activity with BT2 (3,6-dichlorobenzo[b]thiophene-2-carboxylic acid, an inhibitor of BCKDK) treatment mitigates contractile dysfunction in pressure overload-induced heart failure mouse models.131,135 BT2 treatment also accelerates cardiac BCAA oxidation49,135 and improves post-MI cardiac function in a mouse model of myocardial ischaemia.320 Similarly, accelerating BCAA oxidation reduces infarct size in a mouse model of ischaemia/reperfusion injury.321 Of interest is that a recent study suggested that systemic activation of BCAA oxidation contributes to the cardioprotective effects of BT2.134 Systemic activation of BCAA oxidation is associated with reduced blood pressure in a mouse model of MI.134 However, it is unknown how BCAA modulates blood pressure and whether the effect of BCAA on blood pressure is specific to this mouse model of MI.
Another approach to decreasing cardiac BCAA and BCKA levels is by reducing whole-body BCAA intake. Interestingly, restricting BCAA levels in the diet stimulates cardiac fatty acid contribution to ATP production and limits cardiac triacylglycerol levels in Zucker fatty rats.323 Accumulation of cardiac BCAA levels has also been reported in patients with HFpEF.143 Similar to HFrEF, cardiac insulin resistance is also present in HFpEF, and it negatively influences cardiac energy metabolism and function in a mouse model of HFpEF.121,151,324 However, it is unknown whether cardiac BCAA oxidation is altered in HFpEF or whether stimulating cardiac BCAA oxidation influences cardiac insulin resistance and function in HFpEF. Taken together, these studies demonstrate that perturbations in cardiac BCAA oxidation and accumulation of BCAAs and BCKAs are strongly linked to the occurrence of cardiac insulin resistance, cardiac malfunction, and adverse remodelling in the failing heart.
7.5. Targeting non-glucose oxidation fates of glucose
In addition to being used for glycolysis and glucose oxidation, glucose taken up by the heart can also be directed to a number of other metabolic pathways. These include the polyol pathway, the HBP, the PPP, or the one-carbon cycle pathway.101,105,286,287
Aldose reductase (AR) is an important enzyme in the polyol pathway that converts glucose to sorbitol and mediates a number of pathways that exacerbate contractile dysfunction and adverse remodelling of heart failure.325,326 Studies have shown that the expression of AR in the heart also increases following ischaemia/reperfusion (I/R) injury, and this enhanced activity of cardiac AR has been linked to worsening post-ischaemic cardiac recovery.326,327 Consistent with this, whole body and cardiac-specific overexpression of AR in mice exacerbates post-ischaemic cardiac recovery following I/R injury.326,327 We have recently demonstrated that pharmacological inhibition of AR activity lessens diastolic dysfunction in a mouse model of diabetic cardiomyopathy.328 Interestingly, we found that this cardioprotective effect of AR inhibition was not associated with modifying glycolysis or glucose oxidation. Instead, AR inhibition inhibits cardiac fatty acid oxidation rates and mitigates cardiac fibrosis in mice with diabetic cardiomyopathy.328
The regulation of the cellular redox environment is closely linked to substrate metabolism, mainly through the PPP, a significant source of NADPH. NADPH plays a crucial role in proliferation and survival signalling.329 Additionally, NADPH maintains the pool of reduced antioxidants such as glutathione, which is a crucial defence against oxidative stress.329 Thus, the PPP may perform a dual role in regulating redox homeostasis. While much research has been conducted to study glycolysis and glucose oxidation, little is known about regulating f the PPP in heart failure. In dogs with pacing-induced heart failure, increased superoxide levels were attributed to the heightened activity of G6P dehydrogenase, which is the key oxidative enzyme of the PPP.330 If superoxide's effects are damaging, this activation of the PPP in the failing heart could be considered detrimental. However, G6P dehydrogenase-deficient mice develop higher oxidative stress and worsened contractile function after MI or aortic constriction.331 In Dahl salt-sensitive rats, Kato et al. found that flux through the PPP progressively increased during the development of heart failure. Significantly, they demonstrated that DCA treatment further increased this flux, which was associated with improved cardiac function.75 Taken together, the PPP is activated in heart failure. Although superoxide production may increase, currently available data support the notion that higher flux through the PPP in HF may represent a compensatory mechanism whose further activation could be of therapeutic relevance.
O-GlcNAc is one of the post-translational modifications that add the sugar moiety, β-D-N-acetylglucosamine, to a serine or a threonine residue along the peptide chain. Such PTM is uncharged, but the added sugar moiety is five times larger than the size of a phosphate group. The substrate for O-GlcNAc, β-N-acetylglucosamine, is produced through the HBP, which consumes ∼5% of intracellular glucose. Following glucose uptake, it is converted to fructose-6-phosphate (F6P) by hexokinase and isomerase. F6P is then converted to uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc) in four enzymatic reactions. Among all the steps, glutamine:fructose-6-phosphate amidotransferase (GFAT) is the first and rate-limiting enzyme of the HBP. As for the enzyme that carries out the actual transfer of β-N-acetylglucosamine to the target protein, it would be the O-GlcNAc transferase. Wu et al.332 showed that salidroside administration is cardioprotective against I/R injury and improves cardiomyocyte glucose uptake by 1.7-fold, which was accompanied by a 1.6-fold increase in O-GlcNAc levels. Besides affecting glucose metabolism, fatty acid oxidation was also suggested to be under the impact of O-GlcNAc modification. Isolated rat hearts perfused with glucosamine (0–10 mM) showed an increase in O-GlcNAc levels and an increase in palmitate oxidation.333 This could be, at least in part, mediated by higher plasma membrane levels of the fatty acid transporter CD36.334 Interestingly, Li et al. demonstrated that chronic accumulation of BCAAs inhibits the HBP, thereby suppressing O-GlcNAc modification on cardiac proteins and sensitizing the heart to I/R injury.321 Excessive O-GlcNAc has also been linked to the development of cardiomyopathy, and attenuation of O-GlcNAc decreases the severity of pressure overload-induced contractile dysfunction and adverse cardiac remodelling.335 However, the data are not consistent regarding whether O-GlcNAc is beneficial or detrimental to the heart. For example, Kronlage et al.336 demonstrated that O-GlcNAc of HDAC4 is cardioprotective in diabetes mellitus and offsets pathological Ca2+/calmodulin-dependent protein kinase II signalling. Therefore, the effect of O-GlcNAc on cardiac function and structure needs further investigation.
8. Conclusions
In HFrEF, HFpEF, and heart failure associated with obesity and T2D, the energy metabolic profile of the heart undergoes dramatic changes, contributing to contractile dysfunction. The main metabolic change in all these forms of heart failure is a decrease in glucose oxidation. In HFpEF, diabetes and obesity, this decrease in glucose oxidation is accompanied by an increase in fatty acid oxidation, contributing to a decrease in cardiac efficiency (cardiac work/O2 consumed). Ketone oxidation is increased in HFrEF, which may be an adaptive process to provide the energy-starved failing heart with an extra fuel source. However, in HFpEF, diabetes, and obesity, ketone oxidation is decreased, contributing to a decrease in metabolic flexibility. These energy metabolic changes observed in the failing heart can be attributed to altered fuel availability, altered expression, altered allosteric control, and altered PTMs of key enzymes involved in various metabolic pathways. Targeting cardiac energy metabolism and/or optimizing the metabolic profile of the heart is a promising therapeutic strategy for treating heart failure. This includes stimulating glucose oxidation, stimulating ketone oxidation, or inhibiting fatty acid oxidation as an approach to increase metabolic flexibility and cardiac efficiency in the failing heart.
Contributor Information
Qiuyu Sun, Cardiovascular Research Center, University of Alberta, Edmonton, AB T6G 2S2, Canada; Department of Pediatrics, University of Alberta, Edmonton, AB T6G 2S2, Canada.
Qutuba G Karwi, Division of BioMedical Sciences, Faculty of Medicine, Memorial University of Newfoundland, Saint John’s, NL A1B 3V6, Canada.
Nathan Wong, Cardiovascular Research Center, University of Alberta, Edmonton, AB T6G 2S2, Canada; Department of Pediatrics, University of Alberta, Edmonton, AB T6G 2S2, Canada.
Gary D Lopaschuk, Cardiovascular Research Center, University of Alberta, Edmonton, AB T6G 2S2, Canada; Department of Pediatrics, University of Alberta, Edmonton, AB T6G 2S2, Canada.
Funding
G.D.L. was funded by a Canadian Institutes for Health Research Foundation Grant. Q.G.K. was funded by a Janeway Hospital Foundation Research Grant and a Medical Research Fund Cox Award. Q.S. was supported by the Alberta Diabetes Institute (ADI)—Helmholtz Research School for Diabetes (HRD) Graduate Studentship.
Data availability
No new data were generated or analyzed in this review article.
References
- 1. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020;22:1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lüscher TF. Heart failure subgroups: HFrEF, HFmrEF, and HFpEF with or without mitral regurgitation. Eur Heart J 2018;39:1–4. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors A, Anker S, Bueno H, Cleland J, Coats A, Falk V, González-Juanatey J, Harjola V, Jankowska E, Authors/Task Force Members . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 5. Neubauer S. The failing heart — an engine out of fuel. N Engl J Med 2007;356:1140–1151. [DOI] [PubMed] [Google Scholar]
- 6. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res 2021;128:1487–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol 2004;555:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res 2009;81:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tuomainen T, Tavi P. The role of cardiac energy metabolism in cardiac hypertrophy and failure. Exp Cell Res 2017;360:12–18. [DOI] [PubMed] [Google Scholar]
- 10. Azevedo PS, Minicucci MF, Santos PP, Paiva SA, Zornoff LA. Energy metabolism in cardiac remodeling and heart failure. Cardiol Rev 2013;21:135–140. [DOI] [PubMed] [Google Scholar]
- 11. Nagoshi T, Yoshimura M, Rosano GMC, Lopaschuk GD, Mochizuki S. Optimization of cardiac metabolism in heart failure. Curr Pharm Des 2011;17:3846–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res 1997;34:25–33. [DOI] [PubMed] [Google Scholar]
- 13. Heusch G, Libby P, Gersh B, Yellon D, Böhm M, Lopaschuk G, Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014;383:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions potential for pharmacological interventions. Cardiovasc Res 1997;33:243–257. [DOI] [PubMed] [Google Scholar]
- 15. Iemitsu M, Miyauchi T, Maeda S, Sakai S, Fujii N, Miyazaki H, Kakinuma Y, Matsuda M, Yamaguchi I. Cardiac hypertrophy by hypertension and exercise training exhibits different gene expression of enzymes in energy metabolism. Hypertens Res 2003;26:829–837. [DOI] [PubMed] [Google Scholar]
- 16. Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res 2007;101:335–347. [DOI] [PubMed] [Google Scholar]
- 17. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 2005;85:1093–1129. [DOI] [PubMed] [Google Scholar]
- 18. Balaban RS, Kantor HL, Katz LA, Briggs RW. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science 1986;232:1121–1123. [DOI] [PubMed] [Google Scholar]
- 19. Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am J Physiol Heart Circ Physiol 1994;267:H1862–H1871. [DOI] [PubMed] [Google Scholar]
- 20. Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem 1998;273:29530–29539. [DOI] [PubMed] [Google Scholar]
- 21. Van der Lee KA, Willemsen PH, Samec S, Seydoux J, Dulloo AG, Pelsers MM, Glatz JF, Van der Vusse GJ, Van Bilsen M. Fasting-induced changes in the expression of genes controlling substrate metabolism in the rat heart. J Lipid Res 2001;42:1752–1758. [PubMed] [Google Scholar]
- 22. Suzuki J, Shen W-J, Nelson BD, Selwood SP, Murphy GM Jr, Kanefara H, Takahashi S, Oida K, Miyamori I, Kraemer FB. Cardiac gene expression profile and lipid accumulation in response to starvation. Am J Physiol Endocrinol Metab 2002;283:E94–E102. [DOI] [PubMed] [Google Scholar]
- 23. Saddik M, Lopaschuk G. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 1991;266:8162–8170. [PubMed] [Google Scholar]
- 24. Wisneski JA, Stanley WC, Neese R, Gertz EW. Effects of acute hyperglycemia on myocardial glycolytic activity in humans. J Clin Invest 1990;85:1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van der Vusse GJ, van Bilsen M, Glatz JF. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc Res 2000;45:279–293. [DOI] [PubMed] [Google Scholar]
- 26. Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab 2003;284:E331–E339. [DOI] [PubMed] [Google Scholar]
- 27. Niu YG, Hauton D, Evans RD. Utilization of triacylglycerol-rich lipoproteins by the working rat heart: routes of uptake and metabolic fates. J Physiol 2004;558:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saddik M, Gamble J, Witters L, Lopaschuk G. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem 1993;268:25836–25845. [PubMed] [Google Scholar]
- 29. Glatz JF, Luiken JJ, Bonen A. Involvement of membrane-associated proteins in the acute regulation of cellular fatty acid uptake. J Mol Neurosci 2001;16:123–132. [DOI] [PubMed] [Google Scholar]
- 30. Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res 2008;79:249–258. [DOI] [PubMed] [Google Scholar]
- 31. Murthy M, Pande S. Mechanism of carnitine acylcarnitine translocase-catalyzed import of acylcarnitines into mitochondria. J Biol Chem 1984;259:9082–9089. [PubMed] [Google Scholar]
- 32. McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system — from concept to molecular analysis. Eur J Biochem 1997;244:1–14. [DOI] [PubMed] [Google Scholar]
- 33. Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 2013;113:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mcgarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest 1977;60:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGarry JD, Leatherman GF, Foster DW. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem 1978;253:4128–4136. [PubMed] [Google Scholar]
- 36. McGarry J, Foster D. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 1980;49:395–420. [DOI] [PubMed] [Google Scholar]
- 37. Savage DB, Choi CS, Samuel VT, Liu Z-X, Zhang D, Wang A, Zhang X-M, Cline GW, Yu XX, Geisler JG. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J Clin Invest 2006;116:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res 2009;50:S138–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dyck JR, Lopaschuk GD. Malonyl CoA control of fatty acid oxidation in the ischemic heart. J Mol Cell Cardiol 2002;34:1099–1109. [DOI] [PubMed] [Google Scholar]
- 40. Ussher JR, Lopaschuk GD. The malonyl CoA axis as a potential target for treating ischaemic heart disease. Cardiovasc Res 2008;79:259–268. [DOI] [PubMed] [Google Scholar]
- 41. Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation — a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 2011;18133:1333–1350. [DOI] [PubMed] [Google Scholar]
- 42. Paulson DJ, Ward KM, Shug AL. Malonyl CoA inhibition of carnitine palmityltransferase in rat heart mitochondria. FEBS Lett 1984;176:381–384. [DOI] [PubMed] [Google Scholar]
- 43. Abel ED. Glucose transport in the heart. Front Biosci 2004;9:201–215. [DOI] [PubMed] [Google Scholar]
- 44. Herzig S, Raemy E, Montessuit S, Veuthey J-L, Zamboni N, Westermann B, Kunji ER, Martinou J-C. Identification and functional expression of the mitochondrial pyruvate carrier. Science 2012;337:93–96. [DOI] [PubMed] [Google Scholar]
- 45. Holness M, Sugden M. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans 2003;31:1143–1151. [DOI] [PubMed] [Google Scholar]
- 46. Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol 2013;304:H1060–H1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karwi QG, Biswas D, Pulinilkunnil T, Lopaschuk GD. Myocardial ketones metabolism in heart failure. J Card Fail 2020;26:998–1005. [DOI] [PubMed] [Google Scholar]
- 48. Ho KL, Karwi QG, Wagg C, Zhang L, Vo K, Altamimi T, Uddin GM, Ussher JR, Lopaschuk GD. Ketones can become the major fuel source for the heart but do not increase cardiac efficiency. Cardiovasc Res 2021;117:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fillmore N, Wagg CS, Zhang L, Fukushima A, Lopaschuk GD. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am J Physiol Endocrinol Metab 2018;315:E1046–E1052. [DOI] [PubMed] [Google Scholar]
- 50. Murashige D, Jang C, Neinast M, Edwards JJ, Cowan A, Hyman MC, Rabinowitz JD, Frankel DS, Arany Z. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 2020;370:364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jackson RH, Singer TP. Inactivation of the 2-ketoglutarate and pyruvate dehydrogenase complexes of beef heart by branched chain keto acids. J Biol Chem 1983;258:1857–1865. [PubMed] [Google Scholar]
- 52. Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, Yan H, Ussher JR, Oudit GY, Lopaschuk GD. Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Cardiovasc Diabetol 2019;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neishabouri SH, Hutson S, Davoodi J. Chronic activation of mTOR complex 1 by branched chain amino acids and organ hypertrophy. Amino Acids 2015;47:1167–1182. [DOI] [PubMed] [Google Scholar]
- 54. Davoodi J, Hutson S. Constitutive activation of mTOR pathway by leucine causes heart hypertrophy which can be blocked by rapamycin. FASEB J 2012;26:1013.16–1013.16. [Google Scholar]
- 55. Shao D, Villet O, Zhang Z, Choi SW, Yan J, Ritterhoff J, Gu H, Djukovic D, Christodoulou D, Kolwicz SC Jr. Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat Commun 2018;9:2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM. Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab 2004;286:E64–E76. [DOI] [PubMed] [Google Scholar]
- 58. Herrmann G, Decherd GM Jr. The chemical nature of heart failure. Ann Intern Med 1939;12:1233–1244. [Google Scholar]
- 59. Wollenberger A. On the energy-rich phosphate supply of the failing heart. Am J Physiol 1947;150:733–745. [DOI] [PubMed] [Google Scholar]
- 60. Olson RE, Schwartz WB. Myocardial metabolism in congestive heart failure. Medicine 1951;30:21–42. [DOI] [PubMed] [Google Scholar]
- 61. Olson RE. Myocardial metabolism in congestive heart failure. J Chronic Dis 1959;9:442–464. [DOI] [PubMed] [Google Scholar]
- 62. Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 2004;95:135–145. [DOI] [PubMed] [Google Scholar]
- 63. Taegtmeyer H. Metabolism—the lost child of cardiology∗∗Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology. J Am Coll Cardiol 2000;36:1386–1388. [DOI] [PubMed] [Google Scholar]
- 64. Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Am Heart Assoc 2004;110:894–896. [DOI] [PubMed] [Google Scholar]
- 65. Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: General concepts. Circulation 2002;105:1727–1733. [DOI] [PubMed] [Google Scholar]
- 66. Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: Potential mechanisms. Circulation 2002;105:1861–1870. [DOI] [PubMed] [Google Scholar]
- 67. Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G, Weiss RG. Metabolic rates of ATP transfer through creatine kinase (CK flux) predict clinical heart failure events and death. Sci Transl Med 2013;5:215re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation 2019;139:1435–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997;96:2190–2196. [DOI] [PubMed] [Google Scholar]
- 70. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Masoud WG, Ussher JR, Wang W, Jaswal JS, Wagg CS, Dyck JR, Lygate CA, Neubauer S, Clanachan AS, Lopaschuk GD. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc Res 2014;101:30–38. [DOI] [PubMed] [Google Scholar]
- 72. Jüllig M, Hickey AJ, Chai CC, Skea GL, Middleditch MJ, Costa S, Choong SY, Philips AR, Cooper GJ. Is the failing heart out of fuel or a worn engine running rich? A study of mitochondria in old spontaneously hypertensive rats. Proteomics 2008;8:2556–2572. [DOI] [PubMed] [Google Scholar]
- 73. Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci 1999;318:36–42. [DOI] [PubMed] [Google Scholar]
- 74. Rosenblatt-Velin N, Montessuit C, Papageorgiou I, Terrand J, Lerch R. Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res 2001;52:407–416. [DOI] [PubMed] [Google Scholar]
- 75. Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 2010;3:420–430. [DOI] [PubMed] [Google Scholar]
- 76. Dávila-Román VG, Vedala G, Herrero P, De Las Fuentes L, Rogers JG, Kelly DP, Gropler RJ. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 2002;40:271–277. [DOI] [PubMed] [Google Scholar]
- 77. Neglia D, De Caterina A, Marraccini P, Natali A, Ciardetti M, Vecoli C, Gastaldelli A, Ciociaro D, Pellegrini P, Testa R. Impaired myocardial metabolic reserve and substrate selection flexibility during stress in patients with idiopathic dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2007;293:H3270–H3278. [DOI] [PubMed] [Google Scholar]
- 78. Taylor M, Wallhaus TR, DeGrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F] fluoro-6-thia-heptadecanoic acid and [18F] FDG in patients with congestive heart failure. J Nucl Med 2001;42:55–62. [PubMed] [Google Scholar]
- 79. Funada J, Betts TR, Hodson L, Humphreys SM, Timperley J, Frayn KN, Karpe F. Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLoS One 2009;4:e7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Voros G, Ector J, Garweg C, Droogne W, Van Cleemput J, Peersman N, Vermeersch P, Janssens S. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ Heart Fail 2018;11:e004953. [DOI] [PubMed] [Google Scholar]
- 81. Zhabyeyev P, Gandhi M, Mori J, Basu R, Kassiri Z, Clanachan A, Lopaschuk GD, Oudit GY. Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc Res 2013;97:676–685. [DOI] [PubMed] [Google Scholar]
- 82. Zhang L, Jaswal JS, Ussher JR, Sankaralingam S, Wagg C, Zaugg M, Lopaschuk GD. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ Heart Fail 2013;6:1039–1048. [DOI] [PubMed] [Google Scholar]
- 83. Degens H, de Brouwer KF, Gilde AJ, Lindhout M, Willemsen PH, Janssen BJ, van der Vusse GJ, van Bilsen M. Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res Cardiol 2006;101:17–26. [DOI] [PubMed] [Google Scholar]
- 84. Karwi QG, Uddin GM, Ho KL, Lopaschuk GD. Loss of metabolic flexibility in the failing heart. Front Cardiovasc Med 2018;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114. [DOI] [PubMed] [Google Scholar]
- 86. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016;39:1115–1122. [DOI] [PubMed] [Google Scholar]
- 87. Lopaschuk GD, Verma S. Empagliflozin’s fuel hypothesis: not so soon. Cell Metab 2016;24:200–202. [DOI] [PubMed] [Google Scholar]
- 88. Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 1996;94:2837–2842. [DOI] [PubMed] [Google Scholar]
- 89. Dodd MS, Ball DR, Schroeder MA, Le Page LM, Atherton HJ, Heather LC, Seymour A-M, Ashrafian H, Watkins H, Clarke K, Tyler DJ. In vivo alterations in cardiac metabolism and function in the spontaneously hypertensive rat heart. Cardiovasc Res 2012;95:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 2019;115:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Allard M, Schonekess B, Henning S, English D, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol Heart Circ Physiol 1994;267:H742–H750. [DOI] [PubMed] [Google Scholar]
- 92. Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: implications for cardiac reloading and conditioning. JACC Basic Transl Sci 2016;1:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Riehle C, Wende AR, Zaha VG, Pires KM, Wayment B, Olsen C, Bugger H, Buchanan J, Wang X, Moreira AB, Doenst T, Medina-Gomez G, Litwin SE, Lelliott CJ, Vidal-Puig A, Abel ED. PGC-1β deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ Res 2011;109:783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schroeder MA, Lau AZ, Chen AP, Gu Y, Nagendran J, Barry J, Hu X, Dyck JR, Tyler DJ, Clarke K. Hyperpolarized (13)C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur J Heart Fail 2013;15:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Seymour A-ML, Giles L, Ball V, Miller JJ, Clarke K, Carr CA, Tyler DJ. In vivo assessment of cardiac metabolism and function in the abdominal aortic banding model of compensated cardiac hypertrophy. Cardiovasc Res 2015;106:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dodd MS, Atherton HJ, Carr CA, Stuckey DJ, West JA, Griffin JL, Radda GK, Clarke K, Heather LC, Tyler DJ. Impaired in vivo mitochondrial Krebs cycle activity after myocardial infarction assessed using hyperpolarized magnetic resonance spectroscopy. Circ Cardiovasc Imaging 2014;7:895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang W, Zhang L, Battiprolu PK, Fukushima A, Nguyen K, Milner K, Gupta A, Altamimi T, Byrne N, Mori J. Malonyl CoA decarboxylase inhibition improves cardiac function post-myocardial infarction. JACC Basic Transl Sci 2019;4:385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Paolisso G, Gambardella A, Galzerano D, D’Amore A, Rubino P, Verza M, Teasuro P, Varricchio M, D’Onofrio F. Total-body and myocardial substrate oxidation in congestive heart failure. Metabolism 1994;43:174–179. [DOI] [PubMed] [Google Scholar]
- 99. Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, Hintze TH, Lopaschuk GD, Recchia FA. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-α in pacing-induced heart failure. Circulation 2002;106:606–612. [DOI] [PubMed] [Google Scholar]
- 100. Sidhu S, Gangasani A, Korotchkina LG, Suzuki G, Fallavollita JA, Canty JM Jr, Patel MS. Tissue-specific pyruvate dehydrogenase complex deficiency causes cardiac hypertrophy and sudden death of weaned male mice. Am J Physiol Heart Circ Physiol 2008;295:H946–H952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zhang Y, Taufalele PV, Cochran JD, Robillard-Frayne I, Marx JM, Soto J, Rauckhorst AJ, Tayyari F, Pewa AD, Gray LR, Teesch LM, Puchalska P, Funari TR, McGlauflin R, Zimmerman K, Kutschke WJ, Cassier T, Hitchcock S, Lin K, Kato KM, Stueve JL, Haff L, Weiss RM, Cox JE, Rutter J, Taylor EB, Crawford PA, Lewandowski ED, Des Rosiers C, Abel ED. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat Metab 2020;2:1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cluntun AA, Badolia R, Lettlova S, Parnell KM, Shankar TS, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, Cunningham CN, Van Ry T, Bott AJ, Krokidi AT, Fogarty S, Skedros S, Swiatek WI, Yu X, Luo B, Merx S, Navankasattusas S, Cox JE, Ducker GS, Holland WL, McKellar SH, Rutter J, Drakos SG. The pyruvate-lactate axis modulates cardiac hypertrophy and heart failure. Cell Metab 2021;33:629–648.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bing R, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart: II. Studies on fat, ketone and amino acid metabolism. Am J Med 1954;16:504–515. [DOI] [PubMed] [Google Scholar]
- 104. Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, Stevens RD, Wenner B, Ilkayeva O, Loebe M, Peterson LE, Lyon CJ, Wong STC, Newgard CB, Torre-Amione G, Taegtmeyer H, Hsueh WA. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet 2014;7:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Badolia R, Ramadurai DK, Abel ED, Ferrin P, Taleb I, Shankar TS, Krokidi AT, Navankasattusas S, McKellar SH, Yin M. The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure. Circulation 2020;142:259–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fiolet J, Baartscheer A. Cellular calcium homeostasis during ischemia; a thermodynamic approach. Cardiovasc Res 2000;45:100–106. [DOI] [PubMed] [Google Scholar]
- 107. Kemi O, Arbo I, Høydal M, Loennechen J, Wisløff U, Smith G, Ellingsen Ø. Reduced pH and contractility in failing rat cardiomyocytes. Acta Physiol 2006;188:185–193. [DOI] [PubMed] [Google Scholar]
- 108. Doenst T, Pytel G, Schrepper A, Amorim P, Färber G, Shingu Y, Mohr FW, Schwarzer M. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res 2010;86:461–470. [DOI] [PubMed] [Google Scholar]
- 109. Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 2005;115:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, Stepkowski S, Davies PJ, Taegtmeyer H. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med 1998;4:1269–1275. [DOI] [PubMed] [Google Scholar]
- 111. Lommi J, Kupari M, Yki-Järvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol 1998;81:45–50. [DOI] [PubMed] [Google Scholar]
- 112. Nørrelund H, Wiggers H, Halbirk M, Frystyk J, Flyvbjerg A, Bøtker H, Schmitz O, Jørgensen J, Christiansen J, Møller N. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic heart failure. J Intern Med 2006;260:11–21. [DOI] [PubMed] [Google Scholar]
- 113. Tuunanen H, Engblom E, Naum A, Scheinin M, Någren K, Airaksinen J, Nuutila P, Iozzo P, Ukkonen H, Knuuti J. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail 2006;12:644–652. [DOI] [PubMed] [Google Scholar]
- 114. Tuunanen H, Ukkonen H, Knuuti J. Myocardial fatty acid metabolism and cardiac performance in heart failure. Curr Cardiol Rep 2008;10:142–148. [DOI] [PubMed] [Google Scholar]
- 115. Sung MM, Byrne NJ, Robertson IM, Kim TT, Samokhvalov V, Levasseur J, Soltys C-L, Fung D, Tyreman N, Denou E. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Am J Physiol Heart Circ Physiol 2017;312:H842–H853. [DOI] [PubMed] [Google Scholar]
- 116. Byrne NJ, Levasseur J, Sung MM, Masson G, Boisvenue J, Young ME, Dyck JR. Normalization of cardiac substrate utilization and left ventricular hypertrophy precede functional recovery in heart failure regression. Cardiovasc Res 2016;110:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qanud K, Mamdani M, Pepe M, Khairallah RJ, Gravel J, Lei B, Gupte SA, Sharov VG, Sabbah HN, Stanley WC. Reverse changes in cardiac substrate oxidation in dogs recovering from heart failure. Am J Physiol Heart Circ Physiol 2008;295:H2098–H2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fillmore N, Mori J, Lopaschuk G. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 2014;171:2080–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001;414:799–806. [DOI] [PubMed] [Google Scholar]
- 120. Pherwani S, Connolly D, Sun Q, Karwi QG, Carr M, Ho KL, Wagg CS, Zhang L, Levasseur J, Silver H. Ketones provide an extra source of fuel for the failing heart without impairing glucose oxidation. Metabolism 2024;154:155818. [DOI] [PubMed] [Google Scholar]
- 121. Sun Q, Guven B, Wagg CS, de Oliveira AA, Silver H, Zhang L, Chen B, Wei K, Ketema E, Karwi QG, Persad KL, Vu J, Wang F, Dyck JRB, Oudit GY, Lopaschuk GD. Mitochondrial fatty acid oxidation is the major source of cardiac adenosine triphosphate production in heart failure with preserved ejection fraction. Cardiovasc Res 2024;120:360–371. [DOI] [PubMed] [Google Scholar]
- 122. Watson WD, Green PG, Lewis AJ, Arvidsson P, De Maria GL, Arheden H, Heiberg E, Clarke WT, Rodgers CT, Valkovič L. Retained metabolic flexibility of the failing human heart. Circulation 2023;148:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Du Z, Shen A, Huang Y, Su L, Lai W, Wang P, Xie Z, Xie Z, Zeng Q, Ren H, Xu D. 1H-NMR-based metabolic analysis of human serum reveals novel markers of myocardial energy expenditure in heart failure patients. PLoS One 2014;9:e88102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Krüger M, Hoppel CL. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, Ono K, Yamanaka R, Hato D, Fushimura Y. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Circ Heart Fail 2017;10:e004417. [DOI] [PubMed] [Google Scholar]
- 128. Lopaschuk GD, Karwi QG, Ho KL, Pherwani S, Ketema EB. Ketone metabolism in the failing heart. Biochim Biophys Acta Mol Cell Biol Lipids 2020;1865:158813. [DOI] [PubMed] [Google Scholar]
- 129. Karwi QG, Lopaschuk GD. Branched-chain amino acid metabolism in the failing heart. Cardiovasc Drugs Ther 2023;37:413–420. [DOI] [PubMed] [Google Scholar]
- 130. Lai L, Leone TC, Keller MP, Martin OJ, Broman AT, Nigro J, Kapoor K, Koves TR, Stevens R, Ilkayeva OR. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail 2014;7:1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui WJ, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 2016;133:2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Uddin GM, Karwi QG, Pherwani S, Gopal K, Wagg CS, Biswas D, Atnasious M, Wu Y, Wu G, Zhang L. Deletion of BCATm increases insulin-stimulated glucose oxidation in the heart. Metabolism 2021;124:154871. [DOI] [PubMed] [Google Scholar]
- 133. Sansbury BE, DeMartino AM, Xie Z, Brooks AC, Brainard RE, Watson LJ, DeFilippis AP, Cummins TD, Harbeson MA, Brittian KR. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 2014;7:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Murashige D, Jung JW, Neinast MD, Levin MG, Chu Q, Lambert JP, Garbincius JF, Kim B, Hoshino A, Marti-Pamies I, McDaid KS, Shewale SV, Flam E, Yang S, Roberts E, Li L, Morley MP, Bedi KC Jr, Hyman MC, Frankel DS, Margulies KB, Assoian RK, Elrod JW, Jang C, Rabinowitz JD, Arany Z. Extra-cardiac BCAA catabolism lowers blood pressure and protects from heart failure. Cell Metab 2022;34:1749–1764.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, Al Batran R, Pherwani S, Ho KL, Boisvenue J, Karwi QG, Altamimi T, Wishart DS, Dyck JRB, Ussher JR, Oudit GY, Lopaschuk GD. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol 2019;18:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, Chuang DT, Wang Y, Sun H. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J Am Heart Assoc 2019;8:e011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014;11:507–515. [DOI] [PubMed] [Google Scholar]
- 138. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. [DOI] [PubMed] [Google Scholar]
- 139. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 140. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Roh J, Houstis N, Rosenzweig A. Why don’t we have proven treatments for HFpEF? Circ Res 2017;120:1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang C-C, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015;131:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hahn VS, Petucci C, Kim MS, Bedi KC Jr, Wang H, Mishra S, Koleini N, Yoo EJ, Margulies KB, Arany Z, Kelly DP, Kass DA, Sharma K. Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation 2023;147:1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Deng Y, Xie M, Li Q, Xu X, Ou W, Zhang Y, Xiao H, Yu H, Zheng Y, Liang Y. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res 2021;128:232–245. [DOI] [PubMed] [Google Scholar]
- 146. Noll NA, Lal H, Merryman WD. Mouse models of heart failure with preserved or reduced ejection fraction. Am J Pathol 2020;190:1596–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Fisher SM, Murally AR, Rajabally Z, Almas T, Azhar M, Cheema FH, Malone A, Hasan B, Aslam N, Saidi J. Large animal models to study effectiveness of therapy devices in the treatment of heart failure with preserved ejection fraction (HFpEF). Heart Fail Rev 2023;29:257–276. [DOI] [PubMed] [Google Scholar]
- 148. Gao S, Liu X-p, Li T-t, Chen L, Feng Y-p, Wang Y-k, Yin Y-j, Little PJ, Wu X-q, Xu S-w, Jiang X-D. Animal models of heart failure with preserved ejection fraction (HFpEF): from metabolic pathobiology to drug discovery. Acta Pharmacol Sin 2024;45:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Valero-Muñoz M, Backman W, Sam F. Murine models of heart failure with preserved ejection fraction: a “fishing expedition”. JACC Basic Transl Sci 2017;2:770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tong D, Schiattarella GG, Jiang N, Altamirano F, Szweda PA, Elnwasany A, Lee DI, Yoo H, Kass DA, Szweda LI. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ Res 2021;128:1629–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Sun Q, Wagg CS, Güven B, Wei K, de Oliveira AA, Silver H, Zhang L, Vergara A, Chen B, Wong N, Wang F, Dyck JRB, Oudit GY, Lopaschuk GD. Stimulating cardiac glucose oxidation lessens the severity of heart failure in aged female mice. Basic Res Cardiol 2023;119:133–150. [DOI] [PubMed] [Google Scholar]
- 152. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner–La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. [DOI] [PubMed] [Google Scholar]
- 153. Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016;65:1190–1195. [DOI] [PubMed] [Google Scholar]
- 154. Tong D, Schiattarella GG, Jiang N, May HI, Lavandero S, Gillette TG, Hill JA. Female sex is protective in a preclinical model of heart failure with preserved ejection fraction. Circulation 2019;140:1769–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham Study. JAMA 1979;241:2035–2038. [DOI] [PubMed] [Google Scholar]
- 156. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 157. Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–643. [DOI] [PubMed] [Google Scholar]
- 158. DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI. Type 2 diabetes mellitus. Nat Rev Dis Primers 2015;1:1–22. [DOI] [PubMed] [Google Scholar]
- 159. Seidell JC. Obesity, insulin resistance and diabetes — a worldwide epidemic. Br J Nutr 2000;83:S5–S8. [DOI] [PubMed] [Google Scholar]
- 160. Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. Washington, DC: American College of Cardiology Foundation; 2016. p200–203. [DOI] [PubMed] [Google Scholar]
- 161. Triposkiadis F, Giamouzis G, Parissis J, Starling RC, Boudoulas H, Skoularigis J, Butler J, Filippatos G. Reframing the association and significance of co-morbidities in heart failure. Eur J Heart Fail 2016;18:744–758. [DOI] [PubMed] [Google Scholar]
- 162. Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T, Gropler RJ. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 2004;109:2191–2196. [DOI] [PubMed] [Google Scholar]
- 163. Lopaschuk GD, Tsang H. Metabolism of palmitate in isolated working hearts from spontaneously diabetic “BB” wistar rats. Circ Res 1987;61:853–858. [DOI] [PubMed] [Google Scholar]
- 164. Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004;53:2366–2374. [DOI] [PubMed] [Google Scholar]
- 165. Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology 2005;146:5341–5349. [DOI] [PubMed] [Google Scholar]
- 166. Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res 2014;103:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 2009;119:2818–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Gao S, McMillan RP, Zhu Q, Lopaschuk GD, Hulver MW, Butler AA. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol Metab 2015;4:310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Olefsky JM. Insulin's effect on glucose oxidation independent of glucose transport. Biochem Biophys Res Commun 1976;71:106–113. [DOI] [PubMed] [Google Scholar]
- 170. Randle P, Garland P, Hales C, Newsholme E. The glucose fatty-acid cycle its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;281:785–789. [DOI] [PubMed] [Google Scholar]
- 171. Fukushima A, Lopaschuk GD. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim Biophys Acta 2016;18611:1525–1534. [DOI] [PubMed] [Google Scholar]
- 172. Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie 2016;125:259–266. [DOI] [PubMed] [Google Scholar]
- 173. Taha M, Lopaschuk GD. Alterations in energy metabolism in cardiomyopathies. Ann Med 2007;39:594–607. [DOI] [PubMed] [Google Scholar]
- 174. Maria Z, Campolo AR, Lacombe VA. Diabetes alters the expression and translocation of the insulin-sensitive glucose transporters 4 and 8 in the atria. PLoS One 2015;10:e0146033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Huang X, Wu D, Cheng Y, Zhang X, Liu T, Liu Q, Xia P, Zhang G, Hu S, Liu S. Restoration of myocardial glucose uptake with facilitated myocardial glucose transporter 4 translocation contributes to alleviation of diabetic cardiomyopathy in rats after duodenal-jejunal bypass. J Diabetes Investig 2019;10:626–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Luiken JJ, Arumugam Y, Bell RC, Calles-Escandon J, Tandon NN, Glatz JF, Bonen A. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am J Physiol Endocrinol Metab 2002;283:E612–E621. [DOI] [PubMed] [Google Scholar]
- 177. Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest 2003;111:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Chiu H-C, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 2005;96:225–233. [DOI] [PubMed] [Google Scholar]
- 179. Chiu H-C, Kovacs A, Ford DA, Hsu F-F, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 2001;107:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J Clin Invest 2002;109:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Zhou Y-T, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A 2000;97:1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Diabetes 2015;64:2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Keung W, Ussher JR, Jaswal JS, Raubenheimer M, Lam VH, Wagg CS, Lopaschuk GD. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes 2013;62:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Dorn GW II, Vega RB, Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev 2015;29:1981–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Knowlton AA, Chen L, Malik ZA. Heart failure and mitochondrial dysfunction: the role of mitochondrial fission/fusion abnormalities and new therapeutic strategies. J Cardiovasc Pharmacol 2014;63:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Song M, Mihara K, Chen Y, Scorrano L, Dorn GW II. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab 2015;21:273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bugger H, Schwarzer M, Chen D, Schrepper A, Amorim PA, Schoepe M, Nguyen TD, Mohr FW, Khalimonchuk O, Weimer BC, Doenst T. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc Res 2010;85:376–384. [DOI] [PubMed] [Google Scholar]
- 188. Garnier A, Fortin D, Deloménie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 2003;551:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci U S A 2006;103:10086–10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Amorim PA, Nguyen TD, Shingu Y, Schwarzer M, Mohr FW, Schrepper A, Doenst T. Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. J Thorac Cardiovasc Surg 2010;140:1160–1167. [DOI] [PubMed] [Google Scholar]
- 191. Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res 2010;106:1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med 2019;51:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kornfeld OS, Hwang S, Disatnik MH, Chen CH, Qvit N, Mochly-Rosen D. Mitochondrial reactive oxygen species at the heart of the matter: new therapeutic approaches for cardiovascular diseases. Circ Res 2015;116:1783–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintrón M, Chen T, Marcinek DJ, Dorn GW II, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 2011;108:837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Dai DF, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 2011;58:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 2000;342:154–160. [DOI] [PubMed] [Google Scholar]
- 197. Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR, HOPE and HOPE-TOO Trial Investigators . Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 2005;293:1338–1347. [DOI] [PubMed] [Google Scholar]
- 198. Shires SE, Gustafsson AB. Mitophagy and heart failure. J Mol Med (Berl) 2015;93:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Idell-Wenger JA, Grotyohann LW, Neely JR. Coenzyme A and carnitine distribution in normal and ischemic hearts. J Biol Chem 1978;253:4310–4318. [PubMed] [Google Scholar]
- 200. Karwi QG, Jörg AR, Lopaschuk GD. Allosteric, transcriptional and post-translational control of mitochondrial energy metabolism. Biochem J 2019;476:1695–1712. [DOI] [PubMed] [Google Scholar]
- 201. Oram JF, Bennetch SL, Neely JR. Regulation of fatty acid utilization in isolated perfused rat hearts. J Biol Chem 1973;248:5299–5309. [PubMed] [Google Scholar]
- 202. Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol 1974;36:413–459. [DOI] [PubMed] [Google Scholar]
- 203. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;281:785–789. [DOI] [PubMed] [Google Scholar]
- 204. Karwi QG, Zhang L, Altamimi TR, Wagg CS, Patel V, Uddin GM, Joerg AR, Padwal RS, Johnstone DE, Sharma A, Oudit GY, Lopaschuk GD. Weight loss enhances cardiac energy metabolism and function in heart failure associated with obesity. Diabetes Obes Metab 2019;21:1944–1955. [DOI] [PubMed] [Google Scholar]
- 205. McGarry JD, Takabayashi Y, Foster DW. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem 1978;253:8294–8300. [PubMed] [Google Scholar]
- 206. Reszko AE, Kasumov T, Comte B, Pierce BA, David F, Bederman IR, Deutsch J, Des Rosiers C, Brunengraber H. Assay of the concentration and 13C-isotopic enrichment of malonyl-coenzyme A by gas chromatography-mass spectrometry. Anal Biochem 2001;298:69–75. [DOI] [PubMed] [Google Scholar]
- 207. Alam N, Saggerson ED. Malonyl-CoA and the regulation of fatty acid oxidation in soleus muscle. Biochem J 1998;334:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD. Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol 1998;275:H2122–H2129. [DOI] [PubMed] [Google Scholar]
- 209. Awan MM, Saggerson ED. Malonyl-CoA metabolism in cardiac myocytes and its relevance to the control of fatty acid oxidation. Biochem J 1993;295:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Goodwin GW, Taegtmeyer H. Regulation of fatty acid oxidation of the heart by MCD and ACC during contractile stimulation. Am J Physiol 1999;277:E772–E777. [DOI] [PubMed] [Google Scholar]
- 211. Lee GY, Kim NH, Zhao ZS, Cha BS, Kim YS. Peroxisomal-proliferator-activated receptor alpha activates transcription of the rat hepatic malonyl-CoA decarboxylase gene: a key regulation of malonyl-CoA level. Biochem J 2004;378:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, Kawase Y, Jishage K, Lopaschuk GD. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation 2006;114:1721–1728. [DOI] [PubMed] [Google Scholar]
- 213. Dyck JR, Cheng JF, Stanley WC, Barr R, Chandler MP, Brown S, Wallace D, Arrhenius T, Harmon C, Yang G, Nadzan AM, Lopaschuk GD. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res 2004;94:e78–e84. [DOI] [PubMed] [Google Scholar]
- 214. Young ME, Goodwin GW, Ying J, Guthrie P, Wilson CR, Laws FA, Taegtmeyer H. Regulation of cardiac and skeletal muscle malonyl-CoA decarboxylase by fatty acids. Am J Physiol Endocrinol Metab 2001;280:E471–E479. [DOI] [PubMed] [Google Scholar]
- 215. Berthiaume JM, Kurdys JG, Muntean DM, Rosca MG. Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxid Redox Signal 2019;30:375–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Lee CF, Chavez JD, Garcia-Menendez L, Choi Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE, Tian R. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 2016;134:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res 2009;105:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Ketema EB, Lopaschuk GD. Post-translational acetylation control of cardiac energy metabolism. Front Cardiovasc Med 2021;8:723996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS One 2014;9:e98972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Liu L, Wang P, Liu X, He D, Liang C, Yu Y. Exogenous NAD(+) supplementation protects H9c2 cardiac myoblasts against hypoxia/reoxygenation injury via Sirt1-p53 pathway. Fundam Clin Pharmacol 2014;28:180–189. [DOI] [PubMed] [Google Scholar]
- 221. Mistry NF, Cresci S. PPAR transcriptional activator complex polymorphisms and the promise of individualized therapy for heart failure. Heart Fail Rev 2010;15:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Smeets PJ, de Vogel-van den Bosch HM, Willemsen PH, Stassen AP, Ayoubi T, van der Vusse GJ, van Bilsen M. Transcriptomic analysis of PPARα-dependent alterations during cardiac hypertrophy. Physiol Genomics 2008;36:15–23. [DOI] [PubMed] [Google Scholar]
- 223. Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res 2004;95:568–578. [DOI] [PubMed] [Google Scholar]
- 224. Park S-Y, Cho Y-R, Finck BN, Kim H-J, Higashimori T, Hong E-G, Lee M-K, Danton C, Deshmukh S, Cline GW. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-α causes insulin resistance in heart and liver. Diabetes 2005;54:2514–2524. [DOI] [PubMed] [Google Scholar]
- 225. Sambandam N, Morabito D, Wagg C, Finck BN, Kelly DP, Lopaschuk GD. Chronic activation of PPARα is detrimental to cardiac recovery after ischemia. Am J Physiol Heart Circ Physiol 2006;290:H87–H95. [DOI] [PubMed] [Google Scholar]
- 226. Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor α is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem 2001;276:44390–44395. [DOI] [PubMed] [Google Scholar]
- 227. Kaimoto S, Hoshino A, Ariyoshi M, Okawa Y, Tateishi S, Ono K, Uchihashi M, Fukai K, Iwai-Kanai E, Matoba S. Activation of PPAR-α in the early stage of heart failure maintained myocardial function and energetics in pressure-overload heart failure. Am J Physiol Heart Circ Physiol 2017;312:H305–H313. [DOI] [PubMed] [Google Scholar]
- 228. Oka S-i, Zhai P, Yamamoto T, Ikeda Y, Byun J, Hsu C-P, Sadoshima J. Peroxisome proliferator activated receptor-α association with silent information regulator 1 suppresses cardiac fatty acid metabolism in the failing heart. Circ Heart Fail 2015;8:1123–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Oka S, Alcendor R, Zhai P, Park JY, Shao D, Cho J, Yamamoto T, Tian B, Sadoshima J. PPARα-Sirt1 complex mediates cardiac hypertrophy and failure through suppression of the ERR transcriptional pathway. Cell Metab 2011;14:598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 2000;106:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator-1α promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 2004;94:525–533. [DOI] [PubMed] [Google Scholar]
- 232. Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu P-H. Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab 2005;1:259–271. [DOI] [PubMed] [Google Scholar]
- 233. Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 2005;3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Chen H-J, Pan X-X, Ding L-L-Q, Ruan C-C, Gao P-J. Cardiac fibroblast-specific knockout of PGC-1α accelerates AngII-induced cardiac remodeling. Front Cardiovasc Med 2021;8:664626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 2010;13:1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Barth AS, Kumordzie A, Frangakis C, Margulies KB, Cappola TP, Tomaselli GF. Reciprocal transcriptional regulation of metabolic and signaling pathways correlates with disease severity in heart failure. Circ Cardiovasc Genet 2011;4:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Watanabe S, Horie T, Nagao K, Kuwabara Y, Baba O, Nishi H, Sowa N, Narazaki M, Matsuda T, Takemura G. Cardiac-specific inhibition of kinase activity in calcium/calmodulin-dependent protein kinase kinase-β leads to accelerated left ventricular remodeling and heart failure after transverse aortic constriction in mice. PLoS One 2014;9:e108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Piquereau J, Moulin M, Zurlo G, Mateo P, Gressette M, Paul J-L, Lemaire C, Ventura-Clapier R, Veksler V, Garnier A. Cobalamin and folate protect mitochondrial and contractile functions in a murine model of cardiac pressure overload. J Mol Cell Cardiol 2017;102:34–44. [DOI] [PubMed] [Google Scholar]
- 239. Garnier A, Zoll J, Fortin D, N’Guessan B, Lefebvre F, Geny B, Mettauer B, Veksler V, Ventura-Clapier R. Control by circulating factors of mitochondrial function and transcription cascade in heart failure: a role for endothelin-1 and angiotensin II. Circ Heart Fail 2009;2:342–350. [DOI] [PubMed] [Google Scholar]
- 240. Sebastiani M, Giordano C, Nediani C, Travaglini C, Borchi E, Zani M, Feccia M, Mancini M, Petrozza V, Cossarizza A. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol 2007;50:1362–1369. [DOI] [PubMed] [Google Scholar]
- 241. Hu X, Xu X, Lu Z, Zhang P, Fassett J, Zhang Y, Xin Y, Hall JL, Viollet B, Bache RJ. AMP activated protein kinase-α2 regulates expression of estrogen-related receptor-α, a metabolic transcription factor related to heart failure development. Hypertension 2011;58:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol 2009;46:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Aubert G, Vega RB, Kelly DP. Perturbations in the gene regulatory pathways controlling mitochondrial energy production in the failing heart. Biochim Biophys Acta 2013;18333:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Huss JM, Torra IP, Staels B, Giguère V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 2004;24:9079–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Xu W, Billon C, Li H, Wilderman A, Qi L, Graves A, Rideb JRDC, Zhao Y, Hayes M, Yu K. Novel pan-ERR agonists ameliorate heart failure through enhancing cardiac fatty acid metabolism and mitochondrial function. Circulation 2024;149:227–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 2014;76:39–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. da Luz Sousa Fialho M, Abd Jamil AH, Stannard GA, Heather LC. Hypoxia-inducible factor 1 signalling, metabolism and its therapeutic potential in cardiovascular disease. Biochim Biophys Acta Mol Basis Dis 2019;18655:831–843. [DOI] [PubMed] [Google Scholar]
- 248. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006;3:187–197. [DOI] [PubMed] [Google Scholar]
- 249. Cole MA, Abd Jamil AH, Heather LC, Murray AJ, Sutton ER, Slingo M, Sebag-Montefiore L, Tan SC, Aksentijević D, Gildea OS. On the pivotal role of PPARα in adaptation of the heart to hypoxia and why fat in the diet increases hypoxic injury. FASEB J 2016;30:2684–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Parodi-Rullán RM, Chapa-Dubocq XR, Javadov S. Acetylation of mitochondrial proteins in the heart: the role of SIRT3. Front Physiol 2018;9:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Fan J, Shan C, Kang H-B, Elf S, Xie J, Tucker M, Gu T-L, Aguiar M, Lonning S, Chen H. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell 2014;53:534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am J Physiol Heart Circ Physiol 2013;304:H1103–H1113. [DOI] [PubMed] [Google Scholar]
- 253. Zhang X, Ji R, Liao X, Castillero E, Kennel PJ, Brunjes DL, Franz M, Möbius-Winkler S, Drosatos K, George I. MicroRNA-195 regulates metabolism in failing myocardium via alterations in sirtuin 3 expression and mitochondrial protein acetylation. Circulation 2018;137:2052–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Chen T, Liu J, Li N, Wang S, Liu H, Li J, Zhang Y, Bu P. Mouse SIRT3 attenuates hypertrophy-related lipid accumulation in the heart through the deacetylation of LCAD. PLoS One 2015;10:e0118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Grillon JM, Johnson KR, Kotlo K, Danziger RS. Non-histone lysine acetylated proteins in heart failure. Biochim Biophys Acta 2012;18222:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Liu GZ, Xu W, Zang YX, Lou Q, Hang PZ, Gao Q, Shi H, Liu QY, Wang H, Sun X. Honokiol inhibits atrial metabolic remodeling in atrial fibrillation through sirt3 pathway. Front Pharmacol 2022;13:813272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Xu M, Xue R-Q, Lu Y, Yong S-Y, Wu Q, Cui Y-L, Zuo X-T, Yu X-J, Zhao M, Zang W-J. Choline ameliorates cardiac hypertrophy by regulating metabolic remodelling and UPRmt through SIRT3-AMPK pathway. Cardiovasc Res 2019;115:530–545. [DOI] [PubMed] [Google Scholar]
- 258. Zhou B, Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest 2018;128:3716–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Fisher-Wellman KH, Draper JA, Davidson MT, Williams AS, Narowski TM, Slentz DH, Ilkayeva OR, Stevens RD, Wagner GR, Najjar R. Respiratory phenomics across multiple models of protein hyperacylation in cardiac mitochondria reveals a marginal impact on bioenergetics. Cell Rep 2019;26:1557–1572.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Fukushima A, Alrob OA, Zhang L, Wagg CS, Altamimi T, Rawat S, Rebeyka IM, Kantor PF, Lopaschuk GD. Acetylation and succinylation contribute to maturational alterations in energy metabolism in the newborn heart. Am J Physiol Heart Circ Physiol 2016;311:H347–H363. [DOI] [PubMed] [Google Scholar]
- 261. Chang X, Zhang T, Wang J, Liu Y, Yan P, Meng Q, Yin Y, Wang S. SIRT5-related desuccinylation modification contributes to quercetin-induced protection against heart failure and high-glucose-prompted cardiomyocytes injured through regulation of mitochondrial quality surveillance. Oxid Med Cell Longev 2021;20211:5876841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Takada S, Maekawa S, Furihata T, Kakutani N, Setoyama D, Ueda K, Nambu H, Hagiwara H, Handa H, Fumoto Y. Succinyl-CoA-based energy metabolism dysfunction in chronic heart failure. Proc Natl Acad Sci U S A 2022;119:e2203628119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Wu L-F, Wang D-P, Shen J, Gao L-J, Zhou Y, Liu Q-H, Cao J-M. Global profiling of protein lysine malonylation in mouse cardiac hypertrophy. J Proteomics 2022;266:104667. [DOI] [PubMed] [Google Scholar]
- 264. Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab 2014;19:605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Shao D, Kolwicz SC Jr, Wang P, Roe ND, Villet O, Nishi K, Hsu Y-WA, Flint GV, Caudal A, Wang W. Increasing fatty acid oxidation prevents high-fat diet–induced cardiomyopathy through regulating Parkin-mediated mitophagy. Circulation 2020;142:983–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Kolwicz SC Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res 2012;111:728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Fragasso G, Salerno A, Lattuada G, Cuko A, Calori G, Scollo A, Ragogna F, Arioli F, Bassanelli G, Spoladore R. Effect of partial inhibition of fatty acid oxidation by trimetazidine on whole body energy metabolism in patients with chronic heart failure. Heart 2011;97:1495–1500. [DOI] [PubMed] [Google Scholar]
- 268. Li X, Wu F, Günther S, Looso M, Kuenne C, Zhang T, Wiesnet M, Klatt S, Zukunft S, Fleming I. Inhibition of fatty acid oxidation enables heart regeneration in adult mice. Nature 2023;622:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O, Margonato A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 2006;48:992–998. [DOI] [PubMed] [Google Scholar]
- 270. Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation 2005;112:3280–3288. [DOI] [PubMed] [Google Scholar]
- 271. Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart 2011;97:278–286. [DOI] [PubMed] [Google Scholar]
- 272. Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci 2000;99:27–35. [PubMed] [Google Scholar]
- 273. Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, Rhein S. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO (etomoxir for the recovery of glucose oxidation) study. Clin Sci 2007;113:205–212. [DOI] [PubMed] [Google Scholar]
- 274. Peng S, Zhao M, Wan J, Fang Q, Fang D, Li K. The efficacy of trimetazidine on stable angina pectoris: a meta-analysis of randomized clinical trials. Int J Cardiol 2014;177:780–785. [DOI] [PubMed] [Google Scholar]
- 275. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Reviol Rev 2010;90:207–258. [DOI] [PubMed] [Google Scholar]
- 276. McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation 1996;93:135–142. [DOI] [PubMed] [Google Scholar]
- 277. Putri DKSC, Andrianto A, Al-Farabi MJ, Saputra PBT, Nugraha RA. Efficacy of ranolazine to improve diastolic performance in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur Cardiol 2023;18:e02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Abdurrachim D, Luiken JJ, Nicolay K, Glatz JF, Prompers JJ, Nabben M. Good and bad consequences of altered fatty acid metabolism in heart failure: evidence from mouse models. Cardiovasc Res 2015;106:194–205. [DOI] [PubMed] [Google Scholar]
- 279. Yan R, Wei J, Gao D. Inhibition of fatty acid oxidation to treat heart failure in patients. In: Lopaschuk GD, Dhalla NS (eds.), Cardiac Energy Metabolism in Health and Disease. New York: Springer; 2014. p249–263. [Google Scholar]
- 280. Mansor LS, Sousa Fialho ML, Yea G, Coumans WA, West JA, Kerr M, Carr CA, Luiken JJ, Glatz JF, Evans RD. Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc Res 2017;113:737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Wargovich TJ, MacDonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am J Cardiol 1988;61:65–70. [DOI] [PubMed] [Google Scholar]
- 282. Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, Khorrami P, Henderson GN, de Marco T, Chatterjee K. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol 1994;23:1617–1624. [DOI] [PubMed] [Google Scholar]
- 283. Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol 2000;36:1378–1385. [DOI] [PubMed] [Google Scholar]
- 284. Atherton HJ, Dodd MS, Heather LC, Schroeder MA, Griffin JL, Radda GK, Clarke K, Tyler DJ. Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: a combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation 2011;123:2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Bøgh N, Hansen ES, Omann C, Lindhardt J, Nielsen PM, Stephenson RS, Laustsen C, Hjortdal VE, Agger P. Increasing carbohydrate oxidation improves contractile reserves and prevents hypertrophy in porcine right heart failure. Sci Rep 2020;10:8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Fernandez-Caggiano M, Kamynina A, Francois AA, Prysyazhna O, Eykyn TR, Krasemann S, Crespo-Leiro MG, Vieites MG, Bianchi K, Morales V, Domenech N, Eaton P. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat Metab 2020;2:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. McCommis KS, Kovacs A, Weinheimer CJ, Shew TM, Koves TR, Ilkayeva OR, Kamm DR, Pyles KD, King MT, Veech RL, DeBosch BJ, Muoio DM, Gross RW, Finck BN. Nutritional modulation of heart failure in mitochondrial pyruvate carrier-deficient mice. Nat Metab 2020;2:1232–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Ho KL, Karwi QG, Wang F, Wagg C, Zhang L, Panidarapu S, Chen B, Pherwani S, Greenwell AA, Oudit GY, Ussher JR, Lopaschuk GD. The ketogenic diet does not improve cardiac function and blunts glucose oxidation in ischaemic heart failure. Cardiovasc Res 2024;120:1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Lopaschuk GD, Dyck JR. Ketones and the cardiovascular system. Nat Cardiovasc 2023;2:425–437. [DOI] [PubMed] [Google Scholar]
- 290. Sato K, Kashiwaya Y, Keon C, Tsuchiya N, King M, Radda G, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 1995;9:651–658. [DOI] [PubMed] [Google Scholar]
- 291. Yurista SR, Nguyen CT, Rosenzweig A, de Boer RA, Westenbrink BD. Ketone bodies for the failing heart: fuels that can fix the engine? Trends Endocrinol Metab 2021;32:814–826. [DOI] [PubMed] [Google Scholar]
- 292. Paoli A, Rubini A, Volek J, Grimaldi K. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 2013;67:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Bueno NB, de Melo ISV, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr 2013;110:1178–1187. [DOI] [PubMed] [Google Scholar]
- 294. Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr 2006;26:1–22. [DOI] [PubMed] [Google Scholar]
- 295. Balasse EO, Féry F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev 1989;5:247–270. [DOI] [PubMed] [Google Scholar]
- 296. Song J-P, Chen L, Chen X, Ren J, Zhang N-N, Tirasawasdichai T, Hu Z-L, Hua W, Hu Y-R, Tang H-R. Elevated plasma β-hydroxybutyrate predicts adverse outcomes and disease progression in patients with arrhythmogenic cardiomyopathy. Sci Transl Med 2020;12:eaay8329. [DOI] [PubMed] [Google Scholar]
- 297. Mishra PK. Why the Diabetic Heart is Energy Inefficient: A Ketogenesis and Ketolysis Perspective. Rockville, MD: American Physiological Society; 2021. pH751–H755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Holloway CJ, Cochlin LE, Emmanuel Y, Murray A, Codreanu I, Edwards LM, Szmigielski C, Tyler DJ, Knight NS, Saxby BK. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am J Clin Nutr 2011;93:748–755. [DOI] [PubMed] [Google Scholar]
- 299. Nakamura M, Odanovic N, Nakada Y, Dohi S, Zhai P, Ivessa A, Yang Z, Abdellatif M, Sadoshima J. Dietary carbohydrates restriction inhibits the development of cardiac hypertrophy and heart failure. Cardiovasc Res 2021;117:2365–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. McCommis KS, Kovacs A, Weinheimer CJ, Shew TM, Koves TR, Ilkayeva OR, Kamm DR, Pyles KD, King MT, Veech RL. Nutritional modulation of heart failure in mitochondrial pyruvate carrier–deficient mice. Nat Metab 2020;2:1232–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Guo Y, Liu X, Li T, Zhao J, Yang Y, Yao Y, Wang L, Yang B, Ren G, Tan Y, Jiang S. Alternate-day ketogenic diet feeding protects against heart failure through preservation of ketogenesis in the liver. Oxid Med Cell Longev 2022;20222:4253651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Xu S, Tao H, Cao W, Cao L, Lin Y, Zhao S-M, Xu W, Cao J, Zhao J-Y. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct Target Ther 2021;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Balietti M, Fattoretti P, Giorgetti B, Casoli T, Di Stefano G, Solazzi M, Platano D, Aicardi G, Bertoni-Freddari C. A ketogenic diet increases succinic dehydrogenase activity in aging cardiomyocytes: potential protective role against apoptosis-induced heart failure. Ann N Y Acad Sci 2009;11711:377–384. [DOI] [PubMed] [Google Scholar]
- 304. Clarke K, Tchabanenko K, Pawlosky R, Carter E, Knight NS, Murray AJ, Cochlin LE, King MT, Wong AW, Roberts A. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul Toxicol Pharmacol 2012;63:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Clarke K, Tchabanenko K, Pawlosky R, Carter E, King MT, Musa-Veloso K, Ho M, Roberts A, Robertson J, VanItallie TB. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 2012;63:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Yurista SR, Matsuura TR, Silljé HH, Nijholt KT, McDaid KS, Shewale SV, Leone TC, Newman JC, Verdin E, van Veldhuisen DJ. Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ Heart Fail 2021;14:e007684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Selvaraj S, Hu R, Vidula MK, Dugyala S, Tierney A, Ky B, Margulies KB, Shah SH, Kelly DP, Bravo PE. Acute echocardiographic effects of exogenous ketone administration in healthy participants. J Am Soc Echocardiogr 2022;35:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 309. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 310. Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation 2018;138:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 2020;5:632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Hattersley AT, Thorens B. Type 2 diabetes, SGLT2 inhibitors, and glucose secretion. N Engl J Med 2015;373:974–976. [DOI] [PubMed] [Google Scholar]
- 313. Verma S, Rawat S, Ho KL, Wagg CS, Zhang L, Teoh H, Dyck JE, Uddin GM, Oudit GY, Mayoux E. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci 2018;3:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'agostino D, Planavsky N, Lupfer C, Kanneganti TD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015;21:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Pelletier A, Tardif A, Gingras M-H, Chiasson J-L, Coderre L. Chronic exposure to ketone bodies impairs glucose uptake in adult cardiomyocytes in response to insulin but not vanadate: the role of PI3-K. Mol Cell Biochem 2007;296:97–108. [DOI] [PubMed] [Google Scholar]
- 316. Tardif A, Julien N, Pelletier A, Thibault G, Srivastava AK, Chiasson J-L, Coderre L. Chronic exposure to β-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am J Physiol Endocrinol Metab 2001;281:E1205–E1212. [DOI] [PubMed] [Google Scholar]
- 317. Bhattacharya S, Granger CB, Craig D, Haynes C, Bain J, Stevens RD, Hauser ER, Newgard CB, Kraus WE, Newby LK, Shah SH. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 2014;232:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet 2010;3:207–214. [DOI] [PubMed] [Google Scholar]
- 319. Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, Haynes C, Hauser ER, Kraus WE, Granger CB, Newgard CB, Califf RM, Newby LK. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J 2012;163:844–850.e1. [DOI] [PubMed] [Google Scholar]
- 320. Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, Lee Y, Li C, Zhang L, Lian K, Gao E, Cheng H, Tao L. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 2016;311:H1160–H1169. [DOI] [PubMed] [Google Scholar]
- 321. Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M, Zhou B, Cao Y, Ritterhoff J, Gu H, Raftery D, Sun H, Tian R. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab 2017;25:374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Karwi Q, Uddin GM, Wagg CS, Lopaschuk GD. Abstract MP125: branched-chain keto acids, not branched-chain amino acids, impairs cardiac insulin sensitivity by disrupting insulin signaling in the mitochondria. Circ Res 2020;127:AMP125. [Google Scholar]
- 323. McGarrah RW, Zhang GF, Christopher BA, Deleye Y, Walejko JM, Page S, Ilkayeva O, White PJ, Newgard CB. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am J Physiol Endocrinol Metab 2020;318:E216–E223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Guven B, Sun Q, Wagg CS, de Oliveira AA, Silver H, Persad KL, Onay-Besikci A, Vu J, Oudit GY, Lopaschuk GD. Obesity is a major determinant of impaired cardiac energy metabolism in heart failure with preserved ejection fraction. J Pharmacol Exp Ther 2024;388:145–155. [DOI] [PubMed] [Google Scholar]
- 325. Jannapureddy S, Sharma M, Yepuri G, Schmidt AM, Ramasamy R. Aldose reductase: an emerging target for development of interventions for diabetic cardiovascular complications. Front Endocrinol (Lausanne) 2021;12:636267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes 1997;46:292–300. [DOI] [PubMed] [Google Scholar]
- 327. Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, Skopicki H, Homma S, Schmidt AM, Oates PJ, Szabolcs M, Ramasamy R. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J 2004;18:1192–1199. [DOI] [PubMed] [Google Scholar]
- 328. Gopal K, Karwi QG, Tabatabaei Dakhili SA, Wagg CS, Zhang L, Sun Q, Saed CT, Panidarapu S, Perfetti R, Ramasamy R, Ussher JR, Lopaschuk GD. Aldose reductase inhibition alleviates diabetic cardiomyopathy and is associated with a decrease in myocardial fatty acid oxidation. Cardiovasc Diabetol 2023;22:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 2012;111:1091–1106. [DOI] [PubMed] [Google Scholar]
- 330. Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol 2006;41:340–349. [DOI] [PubMed] [Google Scholar]
- 331. Hecker PA, Lionetti V, Ribeiro RF Jr, Rastogi S, Brown BH, O'Connell KA, Cox JW, Shekar KC, Gamble DM, Sabbah HN, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circ Heart Fail 2013;6:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Wu T, Zhou H, Jin Z, Bi S, Yang X, Yi D, Liu W. Cardioprotection of salidroside from ischemia/reperfusion injury by increasing N-acetylglucosamine linkage to cellular proteins. Eur J Pharmacol 2009;613:93–99. [DOI] [PubMed] [Google Scholar]
- 333. Laczy B, Fülöp N, Onay-Besikci A, Des Rosiers C, Chatham JC. Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation. PLoS One 2011;6:e18417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Lauzier B, Vaillant F, Merlen C, Gélinas R, Bouchard B, Rivard ME, Labarthe F, Dolinsky VW, Dyck JR, Allen BG, Chatham JC, Des Rosiers C. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol 2013;55:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Umapathi P, Mesubi OO, Banerjee PS, Abrol N, Wang Q, Luczak ED, Wu Y, Granger JM, Wei AC, Reyes Gaido OE, Florea L, Talbot CC Jr, Hart GW, Zachara NE, Anderson ME. Excessive O-GlcNAcylation causes heart failure and sudden death. Circulation 2021;143:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Kronlage M, Dewenter M, Grosso J, Fleming T, Oehl U, Lehmann LH, Falcao-Pires I, Leite-Moreira AF, Volk N, Grone HJ, Muller OJ, Sickmann A, Katus HA, Backs J. O-GlcNAcylation of histone deacetylase 4 protects the diabetic heart from failure. Circulation 2019;140:580–594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in this review article.