Abstract
Patients with atrial fibrillation (AF) are linked to an increased risk of cognitive dysfunction, and serum uric acid levels play an important factor in cognitive dysfunction. However, the optimal serum uric acid level in patients with AF remains unclear. Therefore, we aimed to explore the relationship between serum uric acid and cognitive dysfunction. 583 patients were conducted in the Affiliated Hospital of Jining Medical University. Cognitive dysfunction was assessed by the Montreal Cognitive Assessment (MoCA). The relationship between serum uric acid levels and the risk of cognitive dysfunction in patients with AF was analyzed using the smoothing spline fitting model and threshold analysis. The average serum uric acid level was (383.26 ± 110.11) μmol/L, and the incidence of cognitive dysfunction was 79.76%. There was a non-linear relationship between serum uric acid levels and the risk of cognitive dysfunction in patients with AF, and the inflection point was 352 μmol/L. At the left of the inflection point, the relationship was significant (OR = 1.02, 95% CI = 1.00–1.04). At the right of the inflection point, there was no statistical difference (p=0.101). When serum uric acid levels are less than 352 μmol/L, the risk of cognitive dysfunction increases by 2% for each unit increase in serum uric acid levels in patients with AF. The study provides evidence for the treatment of serum uric acid levels in patients with AF.
Keywords: atrial fibrillation, cognitive dysfunction, nonlinear relationship, serum uric acid
1. Introduction
Atrial fibrillation (AF) is the most common type of arrhythmia in adults, with an estimated more than 8 million people in China suffering from AF at 45 years old [1]. One of the pathogenesis of AF is inflammation and oxidative stress [2]. Uric acid or enzymes in the producing pathway of uric acid are associated with oxidative stress, inflammation, and endothelial dysfunction, all of which may play a role in the development and progression of AF [3, 4]. High serum uric acid levels have been reported to increase the risk of stroke in patients with AF [5], which in turn increases the risk of cognitive impairment. However, there is growing evidence that the association between AF and cognitive impairment is independent of clinical stroke [6]. Studies have shown that patients with AF had a 1.4-fold increased risk of developing cognitive dysfunction compared to the general population [7, 8]. In addition, other studies have shown that elevated serum uric acid levels lead to decreased brain metabolism, which may be significantly related to the development of cognitive dysfunction [9–11]. It is worth emphasizing that the prevalence of hyperuricemia has risen dramatically, and the current incidence of hyperuricemia in China is 13.3% [12]. At present, hyperuricemia has become the second most common metabolic disease after diabetes. However, the effect of serum uric acid levels on cognitive dysfunction remains unclear in patients with AF. Further investigation is needed to determine the accurate levels of serum uric acid in improving the cognitive function in patients with AF. Therefore, this study aims to explore the relationship between serum uric acid levels and the occurrence of cognitive dysfunction in patients with AF, so as to help clinicians make the optimal decision in controlling serum uric acid.
2. Methods
This investigation was a descriptive cross-sectional design. From February 2020 to November 2022, 583 participants of AF who were hospitalized in the Affiliated Hospital of Jining Medical University were recruited into the investigation. The inclusion criteria were as follows: (1) according to the 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with AF [13], the diagnosis of AF was based on 12-lead electrocardiography or 24-h Holter monitoring; (2) all participants signed the informed consent forms. The exclusion criteria were as follows: (1) patients with severe liver and kidney function damage or malignant tumor; (2) patients with hearing or vision impairment and were unable to complete the questionnaire. Figure 1 is a flowchart of patient selection. This study was approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (NO: 2020C009).
Figure 1.
The flowchart of patients selection.
The following data were collected: (1) General information: including age, gender, life status, degree of education, marital status, smoking status, and alcohol consumption. (2) Clinical data: including valvular heart disease, hypertension, diabetes, heart failure, coronary heart disease, myocardial infarction, hyperlipidemia, cerebral infarction, AF, body mass index, red blood cell, hemoglobin, white blood cell, platelets, free triiodothyronine, free thyroxine, creatinine, left ventricular ejection fraction, left atrial diameter, and medicine use (aspirin, warfarin, clopidogrel, and amiodarone). All biochemistry analyses were performed in the biochemistry laboratory using standard automated procedures. Patients were asked to remove their coats and shoes during weight measurement. Echocardiography was performed by qualified sonographers, and researchers were responsible for collecting relevant indicators.
Cognitive function was measured using the MoCA-Beijing. The scale has some linguistic and cultural modifications to the original scale. The MoCA-Beijing is a 30 points scale with six cognitive subtests: (1) short-term memory; (2) visuospatial competence; (3) execution ability; (4) attention, numeracy, and working memory; (5) language; and (6) orientation. If the participant has less than 12 years of education, one point is added to the total score. Higher scores indicate better cognitive function. When the score is less than 26 points, the patient is recognized as having cognitive dysfunction. MoCA was performed face-to-face by the investigator in strict accordance with the guidelines, and it took about 10–15 min to complete. The MoCA had a high sensitivity of 0.85 [14].
K-S method was used to test the normality of continuous variables. Variables with normal distribution were represented by x ± s and one-way ANOVA analysis was used. Variables with non-normal distribution were represented by median (minimum, maximum), and the Kruskal–Wallis H test was used. N (%) and the Chi square test were used for categorical variables. First, univariate analysis was performed to compare baseline characteristics in patients with AF with different serum uric acid levels. Secondly, univariate analysis was used to analyze the occurrence of cognitive dysfunction. At last, three models were constructed in this study to explore the relationship between serum uric acid level and cognitive dysfunction in different models. Model 1, Model 2, and Model 3 represent rough models without adjusting any covariates, adjusted only for sociodemographic variables and adjusted the model for all covariates, respectively. The threshold effect test and smoothing spline fitting were used to manage the nonlinear relationship between the occurrence of serum uric acid and cognitive dysfunction. p < 0.05 was considered to be statistically significant. All analyses were conducted using SPSS 22.0 and EmpowerStats 4.1 software.
3. Results
In this study, 583 patients with AF were collected, with an average age of 66.96 ± 10.62 years. Of these, 333 were men, accounting for 57.12%. The average serum uric acid level was (383.26 ± 110.11) μmol/L, and the incidence of cognitive dysfunction was 79.76%. At present, the definition of hyperuricemia is not uniform. Considering that the lifelong risk of gout is significantly increased when the serum uric acid level exceeds 360 μmol/L (6 mg/dL), 360 μmol/L has been proposed as the threshold for defining hyperuricemia [15, 16]. In addition, when the serum uric acid concentration exceeds 420 μmol/L, monosodium urate crystals can directly adhere to and deposit in joints, surrounding soft tissues, kidney tissues, and blood vessels. This deposition can lead to damage in multiple organs, including the heart, brain, and kidneys [17, 18]. So some experts define hyperuricemia as a serum uric acid level over 420 μmol/L (7 mg/dL) [12]. Therefore, the patients were divided into three groups: T1 (≤ 360), T2 (> 360, ≤ 420), and T3 (> 420). There were significant differences in gender, live status, marital status, diabetes, heart failure, coronary heart disease, myocardial infarction, medicine use (aspirin, amiodarone), hemoglobin, platelets, free triiodothyronine, creatinine, and left ventricular ejection fraction among the three groups (p < 0.05). Table 1 presents the baseline characteristics.
Table 1.
Participants characteristics by serum uric acid (N = 583).
| Characteristic | ≤ 360 | > 360, ≤ 420 | > 420 | p |
|---|---|---|---|---|
| N | 277 (47.51) | 97 (16.64) | 209 (35.85) | |
| Age, year | 67.99 ± 10.41 | 66.63 ± 9.39 | 65.75 ± 11.31 | 0.267 |
| Gender, n (%) | < 0.001 | |||
| Male | 137 (49.46) | 67 (69.07) | 129 (61.72) | |
| Female | 140 (50.54) | 30 (30.93) | 80 (38.28) | |
| Degree of education, n (%) | 0.101 | |||
| Illiterate | 100 (36.10) | 29 (29.90) | 61 (29.19) | |
| Primary school | 125 (45.13) | 41 (42.27) | 111 (53.11) | |
| Junior school | 30 (10.83) | 20 (20.62) | 23 (11.00) | |
| High school and above | 22 (7.94) | 7 (7.22) | 14 (6.70) | |
| Marital status, n (%) | 0.015 | |||
| Unmarried | 2 (0.72) | 0 (0.00) | 0 (0.00) | |
| Married | 248 (89.53) | 82 (84.54) | 177 (84.69) | |
| Divorce | 0 (0.00) | 2 (2.06) | 0 (0.00) | |
| Widowed | 27 (9.75) | 13 (13.40) | 32 (15.31) | |
| Smoke, n (%) | 0.454 | |||
| Nonsmoker | 170 (61.37) | 53 (54.64) | 121 (57.89) | |
| Current smoker | 51 (18.41) | 26 (26.80) | 49 (23.44) | |
| Quit | 56 (20.22) | 18 (18.56) | 39 (18.66) | |
| Alcohol consumption, n (%) | 0.051 | |||
| Nondrinker | 211 (76.17) | 61 (62.89) | 144 (68.90) | |
| Current drinker | 46 (16.61) | 26 (26.80) | 39 (18.66) | |
| Quit | 20 (7.22) | 10 (10.31) | 26 (12.44) | |
| Valvular heart disease, n (%) | 0.126 | |||
| No | 192 (69.31) | 68 (70.10) | 128 (61.24) | |
| Yes | 85 (30.69) | 29 (29.90) | 81 (38.76) | |
| Hypertension, n (%) | 0.176 | |||
| No | 136 (49.10) | 37 (38.14) | 96 (45.93) | |
| Yes | 141 (50.90) | 60 (61.86) | 113 (54.07) | |
| Diabetes, n (%) | 0.008 | |||
| No | 218 (78.70) | 61 (62.89) | 158 (75.60) | |
| Yes | 59 (21.30) | 36 (37.11) | 51 (24.40) | |
| Heart failure, n (%) | < 0.001 | |||
| No | 144 (51.99) | 32 (32.99) | 65 (31.10) | |
| Yes | 133 (48.01) | 65 (67.01) | 144 (68.90) | |
| Coronary heart disease, n (%) | < 0.001 | |||
| No | 48 (17.33) | 15 (15.46) | 64 (30.62) | |
| Yes | 229 (82.67) | 82 (84.54) | 145 (69.38) | |
| Myocardial infarction, n (%) | 0.004 | |||
| No | 246 (88.81) | 91 (93.81) | 202 (96.65) | |
| Yes | 31 (11.19) | 6 (6.19) | 7 (3.35) | |
| Hyperlipidemia, n (%) | 0.567 | |||
| No | 267 (96.39) | 94 (96.91) | 198 (94.74) | |
| Yes | 10 (3.61) | 3 (3.09) | 11 (5.26) | |
| Cerebral infarction, n (%) | 0.537 | |||
| No | 237 (85.56) | 81 (83.51) | 171 (81.82) | |
| Yes | 40 (14.44) | 16 (16.49) | 38 (18.18) | |
| Aspirin, n (%) | 0.036 | |||
| No | 171 (61.73%) | 58 (59.79) | 150 (71.77) | |
| Yes | 106 (38.27%) | 39 (40.21) | 59 (28.23) | |
| Warfarin, n (%) | 0.166 | |||
| No | 239 (86.28) | 83 (85.57) | 191 (91.39) | |
| Yes | 38 (13.72) | 14 (14.43) | 18 (8.61) | |
| Clopidogrel, n (%) | 0.937 | |||
| No | 257 (92.78) | 89 (91.75) | 194 (92.82) | |
| Yes | 20 (7.22) | 8 (8.25) | 15 (7.18) | |
| Amiodarone, n (%) | 0.050 | |||
| No | 269 (97.11) | 95 (97.94) | 209 (100.00) | |
| Yes | 8 (2.89) | 2 (2.06) | 0 (0.00) | |
| Type of atrial fibrillation, n (%) | 0.066 | |||
| Paroxysmal | 87 (31.41) | 29 (29.90) | 48 (22.97) | |
| Persistent | 126 (45.49) | 53 (54.64) | 116 (55.50) | |
| Long-standing persistent | 32 (11.55) | 3 (3.09) | 18 (8.61) | |
| Permanent | 32 (11.55) | 12 (12.37) | 27 (12.92) | |
| Body mass index (kg/m2) | 24.87 ± 3.48 | 26.27 ± 4.22 | 25.66 ± 5.14 | 0.005 |
| Red blood cell (1012/L) | 4.42 ± 0.55 | 4.56 ± 0.73 | 4.32 ± 0.72 | 0.054 |
| Hemoglobin (g/L) | 135.65 ± 16.53 | 140.72 ± 20.15 | 132.07 ± 23.85 | 0.016 |
| White blood cell (109/L) | 7.11 ± 3.37 | 7.29 ± 2.68 | 7.03 ± 2.36 | 0.480 |
| Platelets (109/L) | 219.07 ± 67.74 | 191.80 ± 61.36 | 196.88 ± 66.39 | <0.001 |
| Free triiodothyronine (pmol/L) | 4.33 (2.13, 31.80) | 4.17 (1.95, 7.04) | 4.11 ± 0.99 | <0.001 |
| Free thyroxine (pmol/L) | 18.70 ± 9.12 | 18.20 (3.72, 100.00) | 18.22 ± 3.16 | 0.203 |
| Creatinine (umol/L) | 64.48 ± 18.22 | 74.56 ± 24.86 | 85.29 ± 23.95 | <0.001 |
| Left ventricular ejection fraction (%) | 53.19 ± 9.48 | 46.52 ± 13.85 | 47.18 ± 13.12 | <0.001 |
| Left atrial diameter (mm) | 45.48 ± 9.17 | 49.45 ± 7.92 | 49.29 ± 10.22 | <0.001 |
| MoCA | 19.06 ± 7.01 | 20.25 ± 6.14 | 19.37 ± 6.84 | 0.388 |
Univariate analysis showed that age (OR = 1.09, 95% CI: 1.07–1.12), gender (OR = 4.20, 95% CI: 2.55, 6.91), primary school (OR = 0.06, 95% CI: 0.02, 0.19), high school and above (OR = 0.01, 95% CI: 0.00, 0.03), hyperlipidemia (OR = 0.20, 95% CI: 0.09, 0.45), red blood cell (OR = 0.67, 95% CI: 0.49, 0.93), hemoglobin (OR = 0.98, 95% CI: 0.97, 0.99), free thyroxine (OR = 1.12, 95% CI: 1.06, 1.18) and body mass index (OR = 0.93, 95% CI: 0.89, 0.97) were significantly related to the occurrence of cognitive dysfunction(p < 0.05) (Table 2).
Table 2.
Univariate analysis for the risk of cognitive dysfunction.
| Covariate | Statistics | OR (95% CI) | p |
|---|---|---|---|
| Age, year | 66.96 ± 10.62 | 1.09 (1.07, 1.12) | < 0.001 |
| Gender, n (%) | < 0.001 | ||
| Male | 333 (57.12) | 1.0 | |
| Female | 250 (42.88) | 4.20 (2.55, 6.91) | |
| Marital status, n (%) | |||
| Unmarried | 2 (0.34) | 1.0 | |
| Married | 507 (86.96) | 0.00 (0.00, Inf) | 0.989 |
| Divorce | 2 (0.34) | 1.00 (0.00, Inf) | 1.000 |
| Widowed | 72 (12.35) | 0.00 (0.00, Inf) | 0.989 |
| Degree of education, n (%) | |||
| Illiterate | 190 (32.59) | 1.0 | |
| Primary school | 277 (47.51) | 0.06 (0.02, 0.19) | < 0.001 |
| Junior school | 73 (12.52) | 0.03 (0.01, 0.09) | < 0.001 |
| High school and above | 43 (7.38) | 0.01 (0.00, 0.03) | < 0.001 |
| Smoke, n (%) | |||
| Nonsmoker | 344 (59.01) | 1.0 | |
| Current smoker | 126 (21.61) | 0.42 (0.25, 0.69) 0.0006 | < 0.001 |
| Quit | 113 (19.38) | 0.32 (0.19, 0.53) < 0.0001 | < 0.001 |
| Alcohol consumption, n (%) | |||
| Nondrinker | 416 (71.36) | 1.0 | |
| Current drinker | 111 (19.04) | 0.37 (0.23, 0.59) | < 0.001 |
| Quit | 56 (9.61) | 0.64 (0.32, 1.24) | 0.186 |
| Valvular heart disease, n (%) | 0.158 | ||
| No | 388 (66.55) | 1.0 | |
| Yes | 195 (33.45) | 1.38 (0.88, 2.15) | |
| Hypertension, n (%) | 0.251 | ||
| No | 269 (46.14) | 1.0 | |
| Yes | 314 (53.86) | 1.27 (0.85, 1.90) | |
| Diabetes, n (%) | 0.412 | ||
| No | 437 (74.96) | 1.0 | |
| Yes | 146 (25.04) | 0.83 (0.52, 1.30) | |
| Heart failure, n (%) | 0.275 | ||
| No | 241 (41.34) | 1.0 | |
| Yes | 342 (58.66) | 1.25 (0.84, 1.88) | |
| Coronary heart disease, n (%) | 0.187 | ||
| No | 127 (21.78) | 1.0 | |
| Yes | 456 (78.22) | 1.37 (0.86, 2.19) | |
| Myocardial infarction, n (%) | 0.458 | ||
| No | 539 (92.45) | 1.0 | |
| Yes | 44 (7.55) | 1.37 (0.60, 3.16) | |
| Hyperlipidemia, n (%) | < 0.001 | ||
| No | 559 (95.88) | 1.0 | |
| Yes | 24 (4.12) | 0.20 (0.09, 0.45) | |
| Cerebral infarction, n (%) | 0.397 | ||
| No | 489 (83.88) | 1.0 | |
| Yes | 94 (16.12) | 1.28 (0.72, 2.30) | |
| Aspirin, n (%) | 0.021 | ||
| No | 379 (65.01) | 1.0 | |
| Yes | 204 (34.99) | 0.62 (0.41, 0.93) | |
| Warfarin, n (%) | 0.127 | ||
| No | 513 (87.99) | 1.0 | |
| Yes | 70 (12.01) | 0.64 (0.36, 1.14) | |
| Clopidogrel, n (%) | 0.073 | ||
| No | 540 (92.62) | 1.0 | |
| Yes | 43 (7.38) | 2.61 (0.91, 7.45) | |
| Amiodarone, n (%) | 0.131 | ||
| No | 573 (98.28) | 1.0 | |
| Yes | 10 (1.72) | 0.37 (0.10, 1.34) | |
| Type of atrial fibrillation, n (%) | |||
| Paroxysmal | 164 (28.13) | 1.0 | |
| Persistent | 295 (50.60) | 1.06 (0.66, 1.70) | 0.799 |
| Long-standing persistent | 53 (9.09) | 1.17 (0.53, 2.55) | 0.699 |
| Permanent | 71 (12.18) | 1.21 (0.60, 2.46) | 0.596 |
| Red blood cell (1012/L) | 4.41 ± 0.65 | 0.67 (0.49, 0.93) | 0.016 |
| Hemoglobin (g/L) | 135.22 ± 20.21 | 0.98 (0.97, 0.99) | < 0.001 |
| White blood cell (109/L) | 7.11 ± 2.93 | 1.09 (1.00, 1.18) | 0.055 |
| Platelets (109/L) | 206.54 ± 67.20 | 1.00 (1.00, 1.00) | 0.443 |
| Free triiodothyronine (pmol/L) | 4.86 ± 3.30 | 1.07 (0.98, 1.18) | 0.151 |
| Free thyroxine (pmol/L) | 18.78 ± 9.42 | 1.12 (1.06, 1.18) | < 0.001 |
| Creatinine (umol/L) | 73.56 ± 23.51 | 1.00 (0.99, 1.01) | 0.722 |
| Body mass index (kg/m2) | 25.39 ± 4.29 | 0.93 (0.89, 0.97) | 0.001 |
| Left ventricular ejection fraction (%) | 49.85 ± 12.10 | 1.00 (0.98, 1.01) | 0.700 |
| Left atrial diameter (mm) | 47.56 ± 9.56 | 0.97 (0.95, 1.00) | 0.018 |
In this study, three models were constructed to explore the effect of serum uric acid levels on cognitive dysfunction. In Model 3 which we adjusted for all variables, the effect size of serum uric acid was 1.01. For each unit increase in serum uric acid levels, the risk of cognitive dysfunction increased by 1% (95% CI: 1.00–1.02, p=0.117). The risk of cognitive dysfunction in the T2 group was 4.01 times that of the T1 group, and the risk of cognitive dysfunction in the T3 group was 2.32 times that of the T1 group. In the sensitivity analysis, when serum uric acid levels were transformed into a categorical variable, the p value of the trend of the risk of cognitive dysfunction with serum uric acid was consistent with that when serum uric acid was analyzed as a continuous variable (Table 3).
Table 3.
Effect of serum uric acid levels on the risk of cognitive dysfunction.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| UA | 1.00 (1.00, 1.00) | 0.753 | 1.00 (1.00, 1.00) | 0.053 | 1.01 (1.00, 1.02) | 0.002 |
| UA | ||||||
| T1 | Reference | Reference | Reference | |||
| T2 | 1.27 (0.71, 2.27) | 0.429 | 1.86 (0.97, 3.55) | 0.061 | 4.01 (0.71, 22.74) | < 0.001 |
| T3 | 1.30 (0.83, 2.04) | 0.257 | 2.20 (1.30, 3.73) | 0.003 | 2.32 (1.43, 4.11) | < 0.001 |
| p for trend | 1.14 (0.91, 1.43) | 0.244 | 1.50 (1.15, 1.95) | 0.002 | 3.15 (2.35, 5.44) | < 0.001 |
Note: Model 1: adjusted for none. Model 2: adjusted for age, gender. Model 3: adjusted for age, gender, degree of education, smoke, alcohol consumption, valvular heart disease, hypertension, diabetes, heart failure, coronary heart disease, myocardial infarction, hyperlipidemia, cerebral infarction, aspirin, warfarin, clopidogrel, amiodarone, type of atrial fibrillation, red blood cell, hemoglobin, free thyroxine, BMI, left atrial diameter.
Smooth spline fitting and threshold effect test results showed that serum uric acid levels were curve-related to the risk of cognitive dysfunction. Using multiple regression and recursion algorithms, the inflection point is calculated at 352 μmol/L. On the left side of the inflection point, the relationship was significant (OR = 1.02, 95% CI = 1.00–1.04). On the right side of the inflection point, there was no significant (p=0.101) (Table 4, Figure 2).
Table 4.
Threshold analysis of serum uric acid levels on the risk of cognitive dysfunction.
| Threshold (μmol/L) | Point of effect size | 95% CI | p |
|---|---|---|---|
| < 352 | 1.02 | (1.00, 1.04) | 0.030 |
| ≥ 352 | 0.98 | (0.95, 1.00) | 0.101 |
Figure 2.
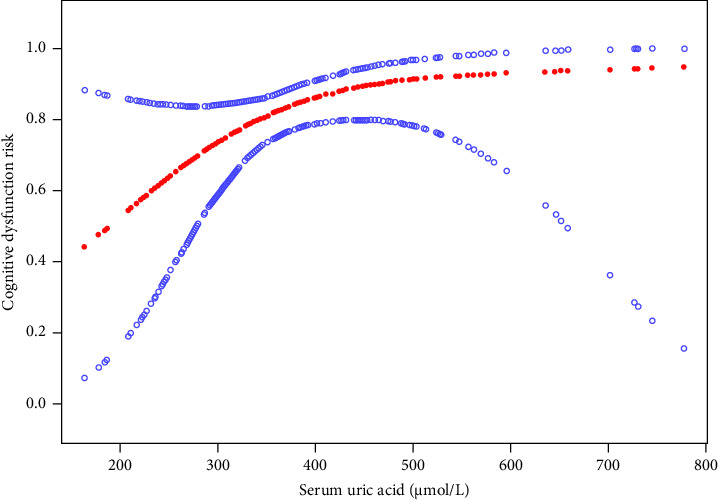
Association between serum uric acid and cognitive dysfunction risk of atrial fibrillation. A threshold, nonlinear relationship between serum uric acid and cognitive dysfunction risk was found in a generalized additive model. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit.
4. Discussion
The results of this study showed that there were differences in serum uric acid levels among people with different gender, living status, marital status, diabetes, coronary heart disease, myocardial infarction, heart failure, left ventricular ejection fraction, hemoglobin, creatinine, platelet, left atrial diameter, and medicine use (aspirin and amiodarone). Among the patients in the T3 group, the proportion of patients living alone was relatively high, which may be related to the patient's poor lifestyle and dietary patterns such as smoking, drinking, high-purine diet, and high-fat diet. In general, patients with a history of heart failure have a lower left ventricular ejection fraction and an increased left atrial diameter. In addition, myocardial infarction, heart failure, and elevated serum uric acid are associated with some common risk factors. For example, dyslipidemia, obesity, frequent consumption of fried foods, smoking, and alcohol consumption [19–24]. Serum uric acid also interacts with multiple serum markers (creatinine, hemoglobin, and platelets) as a dynamic oxidative stress indicator. Researches demonstrate that hyperuricemia is associated with type 2 diabetes. Serum uric acid induces insulin resistance and gluconeogenesis by inhibiting AMP-activated protein kinase, and insulin resistance also reduces urate excretion [25, 26]. Sex hormones also affect the excretion of uric acid, which is an important reason for the difference in serum uric acid in patients of different genders [27]. Studies have shown that patients with coronary heart disease have increased serum uric acid levels compared with patients without coronary heart disease, and the incidence of hyperuricemia in patients with coronary heart disease is greatly increased, which is considered to be an indicator of cardiovascular disease prognosis [28]. Aspirin can inhibit the aggregation of platelets, improve glomerular circulation, and excrete more uric acid [29]. As a result, patients taking aspirin had lower serum uric acid levels. Different marital status and amiodarone use may be affected by sample size. In general, individualized treatment is required when adjusting the patient's serum uric acid level.
Previous studies have shown that age, degree of education, heart failure, hypotension, diabetes, chronic kidney disease, and alcohol consumption are risk factors for cognitive dysfunction in patients with AF [30–32]. Results of this study showed that age, gender, degree of education, aspirin use for more than 1 month, body mass index, free thyroxine, and other indicators were significantly associated with the occurrence of cognitive dysfunction in patients with AF. However, the mechanism of cognitive dysfunction in patients with AF is not fully understood. The relevant possible mechanisms include the following [33]: (1) hypercoagulable state of blood; (2) the formation of inflammatory cytokine; (3) cerebral hypoperfusion; and (4) genes and other factors. Diener [30] also pointed out that further research and exploration of the mechanism of cognitive dysfunction in patients with AF is needed.
An independent association between elevated serum uric acid levels and diabetes is evident in some studies [34]. Diabetes is not only one of the risk factors for AF, but is also related to cognitive function [35, 36]. In addition, a positive correlation between education level and cognitive function has been consistently demonstrated [35]. Therefore, this study still showed a correlation between serum uric acid levels and cognitive impairment in patients with AF after controlling for relevant influencing factors. The inflection point of the relationship is 352 μmol/L, and there is no significant correlation on the right side of the inflection point. On the left side of the inflection point, the risk of cognitive dysfunction increases by 2% for each unit increase in serum uric acid levels in patients with AF. Previous studies have shown that high serum uric acid levels are an independent risk factor for the development of vascular dementia [37, 38], which is consistent with the results of our study. The mechanisms of cognitive dysfunction caused by elevated serum uric acid levels include the following four aspects: (1) uric acid has the effect of promoting inflammation and oxidative stress, which can lead to vascular endothelial dysfunction and directly promote the formation of atherosclerosis [39]; (2) elevated uric acid will increase the burden of cerebral ischemia, resulting in demyelinating changes in brain tissue and increasing brain protein hypersignal; (3) when serum uric acid levels increase, urate is deposited in the vessel wall. That leads to endothelial injury and interstitial fluid leakage, which promotes dilation of the perivascular space [10, 40]; and (4) high serum uric acid levels lead to an imbalance of intestinal flora, which may be closely related to the development of cognitive dysfunction [41, 42]. Although serum uric acid levels of less than 360 μmol/L are in the normal range, the relevant guidelines also recommend that uric acid levels be controlled at a lower level, especially in patients with comorbidities. The serum uric acid level should be controlled below 300 μmol/L as much as possible, and it is not recommended to be lower than 180 μmol/L [43–45]. For groups with uric acid levels less than 360 μmol/L, although it is not necessary to use drugs, uric acid levels can be controlled through lifestyle changes. In terms of diet, the consumption of vegetables, eggs, and milk is encouraged. Limit beef, lamb, pork, and purine-rich seafood. Avoid fructose-containing beverages, animal-offal, liquor, beer, and rice wine [46]. In addition to restricting a high-purine diet, smoking cessation, weight control (body mass index: 18.5–23.9 kg/m2), drinking more water (normal heart and kidney function to maintain daily urine output of 2000–3000 mL), moderate exercise (at least 150 min of moderate-intensity aerobic exercise per week) can also reduce serum uric acid levels [47–49]. Based on the results of our study, clinicians should take effective measures to control serum uric acid levels even if the serum uric acid level of patients is less than 352 μmol/L. We recommend patients improve their lifestyle and diet to reduce the risk of cognitive dysfunction.
This study explored the nonlinear correlation between serum uric acid levels and cognitive dysfunction in patients with AF. It is of great significance to establish a risk prediction model for cognitive dysfunction in patients with AF. However, this study also has some limitations. On the one hand, the number of patients with serum uric acid levels between 360 and 420 μmol/L was relatively small, possibly due to selection bias in this single-center study. This can be further verified by expanding the study's scope and increasing the sample size. On the other hand, the cross-sectional design has limitations in terms of causal inference. In addition, the use of statins and uric-lowering drugs can affect the level of serum uric acid [50]. However, information on the use of statins and uric-lowering drugs was not included in this study, which is the shortcoming of this study. We may do some in‐depth research about serum uric acid levels and cognitive function by improving research designs in the future.
5. Conclusions
Results of this study showed that when serum uric acid levels of patients with AF were lower than 352 μmol/L, the risk of cognitive dysfunction increased by 2% for each unit increase. However, when serum uric acid levels are more than 352 μmol/L, the relationship is not significant. This suggests that clinicians should take effective measures to control serum uric acid levels. We encourage patients to modify their lifestyle, dietary habits, and take antiacid medication in order to reduce the risk of cognitive dysfunction.
Acknowledgments
We are grateful to the entire staff for their work in collecting data and writing the article. Meanwhile, we also appreciate the funding from the Jining Key Research and Development Program (2023YXNS241).
Data Availability Statement
The datasets collected and analyzed in this study are available from the corresponding author upon reasonable request.
Disclosure
The paper has been published on the preprint server [51]. The DOI number is https://doi.org/10.21203/rs.3.rs-2838850/v1. In addition, permission has been granted to use MoCA in our research.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study was funded by the Jining Key Research and Development Program (2023YXNS241).
References
- 1.Du X., Guo L., Xia S., et al. Atrial Fibrillation Prevalence, Awareness and Management in a Nationwide Survey of Adults in China. Heart . 2021;107(7):535–541. doi: 10.1136/heartjnl-2020-317915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zakkar M., Ascione R., James A. F., Angelini G. D., Suleiman M. S. Inflammation, Oxidative Stress and Postoperative Atrial Fibrillation in Cardiac Surgery. Pharmacology & Therapeutics . 2015;154:13–20. doi: 10.1016/j.pharmthera.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Selçuk M., Çınar T., Şaylık F., et al. Predictive Value of Uric Acid/Albumin Ratio for the Prediction of New-Onset Atrial Fibrillation in Patients With ST-Elevation Myocardial Infarction. Revista De Investigacion Clinica; Organo Del Hospital De Enfermedades De La Nutricion . 2022;74(3):156–164. doi: 10.24875/RIC.22000072. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Hou Y., Wang X., et al. Relationship Between Serum Uric Acid Levels and Different Types of Atrial Fibrillation: An Updated Meta-Analysis. Nutrition, Metabolism, and Cardiovascular Diseases . 2021;31(10):2756–2765. doi: 10.1016/j.numecd.2021.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Bayar N., Küçükseymen S., Güven R., et al. Association Between Serum Uric Acid and Ischemic Stroke in Patients With Nonvalvular Paroxysmal Atrial Fibrillation. International Journal of the Cardiovascular Academy . 2017;3(3-4):118–121. doi: 10.1016/j.ijcac.2016.09.003. [DOI] [Google Scholar]
- 6.Rivard L., Friberg L., Conen D., et al. Atrial Fibrillation and Dementia: A Report From the AF-SCREEN International Collaboration. Circulation . 2022;145(5):392–409. doi: 10.1161/CIRCULATIONAHA.121.055018. [DOI] [PubMed] [Google Scholar]
- 7.Madhavan M., Graff-Radford J., Piccini J. P., Gersh B. J. Cognitive Dysfunction in Atrial Fibrillation. Nature Reviews Cardiology . 2018;15(12):744–756. doi: 10.1038/s41569-018-0075-z. [DOI] [PubMed] [Google Scholar]
- 8.Kokkinidis D. G., Zareifopoulos N., Theochari C. A., et al. Association Between Atrial Fibrillation and Cognitive Impairment in Individuals With Prior Stroke: A Meta-Analysis and Meta-Regression Analysis. Stroke . 2020;51(6):1662–1666. doi: 10.1161/STROKEAHA.119.027815. [DOI] [PubMed] [Google Scholar]
- 9.Alam A. B., Wu A., Power M. C., West N. A., Alonso A. Associations of Serum Uric Acid With Incident Dementia and Cognitive Decline in the ARIC-NCS Cohort. Journal of the Neurological Sciences . 2020;414:p. 116866. doi: 10.1016/j.jns.2020.116866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J. W., Byun M. S., Yi D., et al. Serum Uric Acid, Alzheimer-Related Brain Changes, and Cognitive Impairment. Frontiers in Aging Neuroscience . 2020;12:p. 160. doi: 10.3389/fnagi.2020.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niu W., Yang H., Lu C. The Relationship Between Serum Uric Acid and Cognitive Function in Patients With Chronic Heart Failure. BMC Cardiovascular Disorders . 2020;20(1):p. 381. doi: 10.1186/s12872-020-01666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinese Society of Endocrinology. Guideline for the Diagnosis and Management of Hyperuricemia and Gout in China (2019) Chinese Journal of Endocrinology and Metabolism . 2020;36:1–13. doi: 10.3760/cma.j.issn.1000-6699.2020.01.001. [DOI] [Google Scholar]
- 13.January C. T., Wann L. S., Calkins H., et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Journal of the American College of Cardiology . 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Lu J., Li D., Li F., et al. Montreal Cognitive Assessment in Detecting Cognitive Impairment in Chinese Elderly Individuals: A Population-Based Study. Journal of Geriatric Psychiatry and Neurology . 2011;24(4):184–190. doi: 10.1177/0891988711422528. [DOI] [PubMed] [Google Scholar]
- 15.Bardin T. Hyperuricemia Starts at 360 Micromoles (6 mg/dL) Joint Bone Spine . 2015;82(3):141–143. doi: 10.1016/j.jbspin.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Dalbeth N., Merriman T., Stamp L. Gout. The Lancet . 2016;388(10055):2039–2052. doi: 10.1016/s0140-6736(16)00346-9. [DOI] [PubMed] [Google Scholar]
- 17.Martillo M., Nazzal L., Crittenden D. The Crystallization of Monosodium Urate. Current Rheumatology Reports . 2014;16(2):p. 400. doi: 10.1007/s11926-013-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafsson D., Unwin R. The Pathophysiology of Hyperuricaemia and its Possible Relationship to Cardiovascular Disease, Morbidity and Mortalitymorbidity and Mortality. BMC Nephrology . 2013;14(1):p. 164. doi: 10.1186/1471-2369-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun D., Li W., Zhang H., Li Y., Zhang Q. Inverted U-Shaped Relationship Between Body Mass Index and Multivessel Lesions in Chinese Patients With Myocardial Infarction: A Cross-Sectional Study. Journal of International Medical Research . 2020;48(7):p. 300060520932820. doi: 10.1177/0300060520932820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye C., Huang X., Wang R., Halimulati M., Aihemaitijiang S., Zhang Z. Dietary Inflammatory Index and the Risk of Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients . 2021;13(12):p. 4504. doi: 10.3390/nu13124504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan R., Ning Y., Ma Y., et al. Incidence and Risk Factors of Hyperuricemia Among 2.5 Million Chinese Adults During the Years 2017–2018. International Journal of Environmental Research and Public Health . 2021;18(5):p. 2360. doi: 10.3390/ijerph18052360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ali N., Perveen R., Rahman S., et al. Prevalence of Hyperuricemia and the Relationship Between Serum Uric Acid and Obesity: A Study on Bangladeshi Adults. PLoS One . 2018;13(11):p. e0206850. doi: 10.1371/journal.pone.0206850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone S., Lavie C. J., Elagizi A., Arena R., Ventura H. O. The Impact of Obesity in Heart Failure. Cardiology Clinics . 2022;40(2):209–218. doi: 10.1016/j.ccl.2021.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Eckel N., Li Y., Kuxhaus O., Stefan N., Hu F. B., Schulze M. B. Transition From Metabolic Healthy to Unhealthy Phenotypes and Association With Cardiovascular Disease Risk Across BMI Categories in 90 257 Women (The Nurses’ Health Study): 30 Year Follow-Up From a Prospective Cohort Study. Lancet Diabetes & Endocrinology . 2018;6(9):714–724. doi: 10.1016/S2213-8587(18)30137-2. [DOI] [PubMed] [Google Scholar]
- 25.Sluijs I., Holmes M. V., van der Schouw Y. T., et al. A Mendelian Randomization Study of Circulating Uric Acid and Type 2 Diabetes. Diabetes . 2015;64(8):3028–3036. doi: 10.2337/db14-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicerchi C., Li N., Kratzer J., et al. Uric Acid‐Dependent Inhibition of AMP Kinase Induces Hepatic Glucose Production in Diabetes and Starvation: Evolutionary Implications of the Uricase Loss in Hominids. The FASEB Journal . 2014;28(8):3339–3350. doi: 10.1096/fj.13-243634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung J. H., Song G. G., Lee Y. H., Kim J. H., Hyun M. H., Choi S. J. Serum Uric Acid Levels and Hormone Therapy Type: A Retrospective Cohort Study of Postmenopausal Women. Menopause . 2018;25(1):77–81. doi: 10.1097/GME.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 28.Purnima S., El-Aal B. G. A. Serum Uric Acid as Prognostic Marker of Coronary Heart Disease (CHD) Clínica e Investigación en Arteriosclerosis . 2016;28(5):216–224. doi: 10.1016/j.arteri.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P., Wang H., Chen X. H., Liang W. Y., Liu W. W., Liu M. L. Effect of Low-Dose Aspirin on Serum Uric Acid Levels in Chinese Individuals Over 60: Subanalysis of a Multicentre Randomized Clinical Trial. European Review for Medical and Pharmacological Sciences . 2020;24(5):2719–2724. doi: 10.26355/eurrev_202003_20544. [DOI] [PubMed] [Google Scholar]
- 30.Diener H. Does Atrial Fibrillation Lead to Cognitive Decline and Dementia? European Heart Journal . 2017;38(34):2619–2620. doi: 10.1093/eurheartj/ehx320. [DOI] [PubMed] [Google Scholar]
- 31.Mailhot T., McManus D. D., Waring M. E., et al. Frailty, Cognitive Impairment, and Anticoagulation Among Older Adults With Nonvalvular Atrial Fibrillation. Journal of the American Geriatrics Society . 2020;68(12):2778–2786. doi: 10.1111/jgs.16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong N., Shen J., Wu B., et al. Factors Influencing Cognitive Function in Patients With Atrial Fibrillation: A Cross-Sectional Clinical Study. Journal of International Medical Research . 2019;47(12):6041–6052. doi: 10.1177/0300060519882556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh-Manoux A., Fayosse A., Sabia S., et al. Atrial Fibrillation as a Risk Factor for Cognitive Decline and Dementia. European Heart Journal . 2017;38(34):2612–2618. doi: 10.1093/eurheartj/ehx208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong Q., Liu J., Xu Y. Effects of Uric Acid on Diabetes Mellitus and Its Chronic Complications. International Journal of Endocrinology . 2019;2019:1–8. doi: 10.1155/2019/9691345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty A., Hegde S., Praharaj S. K., et al. Age Related Prevalence of Mild Cognitive Impairment in Type 2 Diabetes Mellitus Patients in the Indian Population and Association of Serum Lipids With Cognitive Dysfunction. Frontiers in Endocrinology . 2021;12:p. 798652. doi: 10.3389/fendo.2021.798652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang A., Green J. B., Halperin J. L., Piccini J. P. Atrial Fibrillation and Diabetes Mellitus: JACC Review Topic of the Week. Journal of the American College of Cardiology . 2019;74(8):1107–1115. doi: 10.1016/j.jacc.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Serdarevic N., Stanciu A. E., Begic L., Uncanin S. Serum Uric Acid Concentration in Patients With Cerebrovascular Disease (Ischemic Stroke and Vascular Dementia) Medical Archives . 2020;74(2):p. 95. doi: 10.5455/medarh.2020.74.95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latourte A., Soumaré A., Bardin T., Perez-Ruiz F., Debette S., Richette P. Uric Acid and Incident Dementia Over 12 Years of Follow-Up: A Population-Based Cohort Study. Annals of the Rheumatic Diseases . 2018;77(3):328–335. doi: 10.1136/annrheumdis-2016-210767. [DOI] [PubMed] [Google Scholar]
- 39.Gherghina M. E., Peride I., Tiglis M., Neagu T. P., Niculae A., Checherita I. A. Uric Acid and Oxidative Stress—Relationship With Cardiovascular, Metabolic, and Renal Impairment. International Journal of Molecular Sciences . 2022;23(6):p. 3188. doi: 10.3390/ijms23063188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tana C., Ticinesi A., Prati B., Nouvenne A., Meschi T. Uric Acid and Cognitive Function in Older Individuals. Nutrients . 2018;10(8):p. 975. doi: 10.3390/nu10080975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koszewicz M., Jaroch J., Brzecka A., et al. Dysbiosis Is One of the Risk Factor for Stroke and Cognitive Impairment and Potential Target for Treatment. Pharmacological Research . 2021;164:p. 105277. doi: 10.1016/j.phrs.2020.105277. [DOI] [PubMed] [Google Scholar]
- 42.Xu D., Lv Q., Wang X., et al. Hyperuricemia Is Associated With Impaired Intestinal Permeability in Mice. American Journal of Physiology—Gastrointestinal and Liver Physiology . 2019;317(4):G484–G492. doi: 10.1152/ajpgi.00151.2019. [DOI] [PubMed] [Google Scholar]
- 43.Yu K. H., Chen D. Y., Chen J. H., et al. Management of Gout and Hyperuricemia: Multidisciplinary Consensus in Taiwan. International Journal of Rheumatic Diseases . 2018;21(4):772–787. doi: 10.1111/1756-185X.13266. [DOI] [PubMed] [Google Scholar]
- 44.Richette P., Doherty M., Pascual E., et al. 2016 Updated EULAR Evidence-Based Recommendations for the Management of Gout. Annals of the Rheumatic Diseases . 2017;76(1):29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 45.Hisatome I., Li P., Miake J., et al. Uric Acid as a Risk Factor for Chronic Kidney Disease and Cardiovascular Disease-Japanese Guideline on the Management of Asymptomatic Hyperuricemia. Circulation Journal . 2021;85(2):130–138. doi: 10.1253/circj.CJ-20-0406. [DOI] [PubMed] [Google Scholar]
- 46.Kong B., Liu F., Zhang S., et al. Associations between Dietary Patterns and Serum Uric Acid Concentrations in Children and Adolescents: A Cross-Sectional Study. Food & Function . 2023;14(21):9803–9814. doi: 10.1039/d3fo03043a. [DOI] [PubMed] [Google Scholar]
- 47.Yang L., He Z., Gu X., Cheng H., Li L. Dose-Response Relationship Between BMI and Hyperuricemia. International Journal of General Medicine . 2021;14:8065–8071. doi: 10.2147/IJGM.S341622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W., Zhou X., Liu Y., Sang M., Sun Z., Qiu S. Association Between Regular Exercise and Urinary Uric Acid Excretion in Chinese Adults: A Cross-Sectional Study. Journal of Physiological Investigation . 2024;67(2):64–68. doi: 10.4103/ejpi.EJPI-D-24-00001. [DOI] [PubMed] [Google Scholar]
- 49.Jang Y. S., Nerobkova N., Yun I., Kim H., Park E. C. Association Between Smoking Behavior and Serum Uric Acid Among the Adults: Findings From a National Cross-Sectional Study. PLoS One . 2023;18(5):p. e0285080. doi: 10.1371/journal.pone.0285080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akbari A., Razmi M., Rafiee M., Watts G. F., Sahebkar A. The Effect of Statin Therapy on Serum Uric Acid Levels: A Systematic Review and Meta-Analysis. Current Medicinal Chemistry . 2024;31(13):1726–1739. doi: 10.2174/0929867330666230207124516. [DOI] [PubMed] [Google Scholar]
- 51.Shang M. M., Wang M. J., Cui Q., et al. The Association Between Serum Uric Acid Levels and the Risk of Cognitive Dysfunction in Patients With Atrial Fibrillation. Preprint . 2023 doi: 10.21203/rs.3.rs-2838850/v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets collected and analyzed in this study are available from the corresponding author upon reasonable request.


