Abstract
Allicin is a sulfide extracted from garlic bulbs responsible for various physiological and pathophysiological effects, including antioxidant, antibacterial, and anti-parasite activities. However, its efficacy and mechanism of protecting UVB-induced photodamage have not been studied. The research explores Allicin’s protective roles and underlying mechanisms in UVB-induced photodamage of keratinocytes. UVB was employed to generate photodamage in the HaCaT cell line. DCFH-DA fluorescent and Biochemical analyses were carried out to evaluate reactive oxygen species (ROS) and oxidative stress on UVB-induced photodamage to HaCaT cells. RT-qPCR and western blot were performed to measure mRNA and protein expression. Allicin pretreatment (10 and 25 µM) improved cell proliferation and reduced apoptotic rates in UVB-induced HaCaT cells. Allicin (10 and 25 µM) inhibited tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) expressions (all, P < 0.001). Allicin reduced the intracellular ROS level and attenuated oxidative stress, with reduced malondialdehyde (MDA) level while increasing the levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSh-Px) (all, P < 0.001) in UVB-induced HaCaT cells. Allicin pretreatment inhibited autophagy and reduced the protein expression of Beclin-1 while increasing the p62 protein expression (all, P < 0.001). We also observed that Allicin pretreatment reduced the NLRP3-related protein, such as Caspase-1 (P < 0.001) and increased the protein expressions of the PI3K/Akt pathway molecules, such as PI3K and Akt (all, P < 0.001). Our research data demonstrated that Allicin might inhibit UVB-induced photodamage of keratinocytes via inhibiting NLRP3 inflammasomes and activating the PI3K/Akt pathway.
Keywords: Allicin, ROS, Oxidative stress, Autophagy, NLRP3 inflammasomes
Introduction
The skin, comprising the epidermis, dermis, and subcutaneous tissue, is the largest organ in the human body. The skin aging process, a natural phenomenon, is influenced by intrinsic and extrinsic factors, leading to a gradual decline in structural integrity and physiological function [1]. Intrinsic skin aging, characterized by inevitable changes in the skin's condition, is primarily dictated by immutable genetic factors. Conversely, extrinsic factors, such as exposure to ultraviolet (UV) rays, pollution, nicotine, repetitive facial expressions, dietary habits, sleep posture, and overall well-being, can be managed to some extent [2].
Environmental factors and UV exposure have been reported as the primary agents of age-related skin damage, such as oxidative stress, inflammation, ECM degradation and skin aging. For example, skin is most exposed to UV radiation, which includes UVA (315–400 nm), UVB (280–315 nm) and UVC (100–280 nm) [3]. UVB and UVA can penetrate deep inside the skin, but UVC is absorbed and filtered by the ozone. UVB is especially significant, to say the least, because it has the longest wavelength out of all UV rays. UV light can damage the cells in the body through DNA damage, among other causes [4, 5].
Furthermore, UVB increases the generation of ROS, the leading cause of skin disorders, including wrinkling, skin cancer, and inflammation [6]. Oxidative stress, the production of ROS, can cause programmed cell death using apoptosis and autophagy or unprogrammed cell death using necrosis [7]. Therefore, the generation of ROS by UVB is responsible for skin damage, and substances with antioxidant properties are expected to protect against UVB-induced skin damage.
Allicin, a sulfide derived from garlic bulbs, is recognized for its involvement in most of garlic's functions. The molecular configuration is depicted in Fig. 1A. Its physiological and pathophysiological impacts encompass anti-oxidative, antibacterial, and anti-parasitic properties [8]. Recent investigations have unveiled the protective properties of Allicin against cardiovascular disorders. Notably, it has exhibited mitigating effects on cardiomyocyte apoptosis and enhancement of cardiac performance in myocardial infarction-afflicted rats [9]. Furthermore, it has demonstrated efficacy in averting myocardial apoptosis in diabetic rats and cardiac hypertrophy induced by pathological overload [10, 11]. However, the influence of Allicin on UVB-triggered photodamage of keratinocytes remains ambiguous.
Fig. 1.
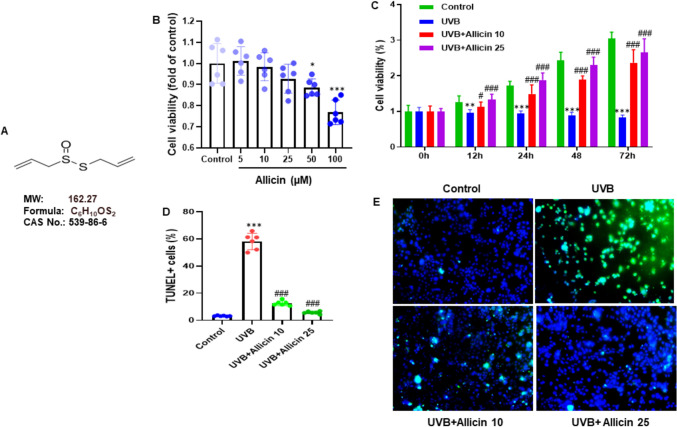
Allicin attenuated keratinocyte proliferation and apoptosis induced by UVB. A Structural formula, molecular weight, chemical formula and CAS number of Allicin. B HaCaT cells were incubated with different concentrations of Allicin (0, 5, 10, 25, 50, and 100 μM) for 24 h, and the cell viability was assessed using an MTT assay. C HaCaT cells were pretreated with Allicin (10 and 25 µM) for 2 h, followed by UVB irradiation (50 mJ/cm2) and incubated for further 6, 12, 24, 48, and 72 h, and the effect of Allicin on the viability of UVB-exposed HaCaT cells was assessed using an MTT assay. D HaCaT cells were stained with TUNEL and DAPI, and cell apoptotic rate was assessed by calculating the percentage of TUNEL-positive cells to all DAPI-positive cells. E Representative images of microphotographs of HaCaT cells with TUNEL and DAPI staining. Data are presented as mean ± SD in triplicates and analyzed using one-way ANOVA, and LSD was used for the post-hoc test. Scale 50 µm. *P < 0.05, ***P < 0.001 vs. control group; ###P < 0.001 vs. UVB group
The NLRP3 inflammasome, which consists of NLRP3, an apoptosis-associated speck-like protein with a CARD (ASC) and procaspase-1, mediates the innate immune system. Upon activation, this inflammasome triggers caspase-1 activation, resulting in the generation of interleukin 18 (IL-18) and interleukin 1 beta (IL-1β), subsequently initiating inflammation [12]. Furthermore, the phosphatidylinositol 3-kinase (PI3K) pathway regulates the autophagic response of cells to changes in ROS levels. The PI3K α catalytic subunit inhibits the autophagy process, while the β catalytic subunit promotes autophagy in response to ROS level alterations [13]. However, the precise regulatory mechanisms of NLRP3 and the PI3K/Akt/autophagy in UVB-induced photodamage of keratinocytes remain unknown. Therefore, in this research, we evaluated the potential effects of Allicin on UVB-induced photodamage of keratinocytes and the underlying mechanisms.
Materials and methods
Cell culture
The human immortal epidermal keratinocyte (HaCaT) cells were purchased from Shanghai Jikai Gene Medical Technology Co., Ltd and cultured in high-glucose DMEM supplied with 10% FBS, 100 µg/mL of streptomycin, 100 U/mL of penicillin, and 10% FBS at 37 °C in a humidified incubator at 5% CO2. Cells from passages 2–3 were used in experiments. HaCaT cells received UVB irradiation to establish an in vitro skin photodamage model.
UVB irradiation procedure
HaCaT cells were seeded in 96-well plates at 1 × 105/well density. After growing to 70–80% confluence, the monolayer of cells was washed with PBS solution. Then, the cells were covered with a thin layer of PBS and exposed to a single dose of UVB radiation (range: 280–315 nm), with the total UVB irradiation dose as 50 mJ/cm2 for 30 min using a UVB lamp (VL-6. L M, Vilber Lourmat). After irradiation, cells were washed with PBS and incubated with DMEM supplemented with 10% FBS and Allicin at 10 and 25 μM concentrations. Cells were cultured for a further 24 h for the experiments.
Cell grouping
HaCaT cells were randomly divided into four groups. Control group: Cells were not only exposed to UVB irradiation and culture with DMEM. UVB group: Cells were exposed to UVB irradiation and culture with DMEM. UVB + Allicin 10 group: Cells were exposed to UVB irradiation and culture with DMEM containing 10 μM Allicin. UVB + Allicin 25 group: Cells were exposed to UVB irradiation and culture with DMEM containing 25 μM Allicin.
3-(4, 5-dimethylthiazol-2-yl)−2, 5-diphenyltetrazolium bromide (MTT) assay
To measure the cytotoxicity of Allicin, HaCaT cells were plated at a concentration of 1 × 105 cells/well in 24-well plates and treated with various concentrations of Allicin for 24 h. Then, to identify the cytoprotective effect of Allicin on photodamaged HaCaT cells, the cells were treated with Allicin (10 and 25 µM) for 2 h and incubated with UVB (50 mJ/cm2) for 24 h. After treatment, the cells were subjected to MTT (0.2 mg/mL) for 30 min to ascertain the presence of viable cells. Subsequently, any formazan crystals produced within the wells were dissolved by adding 300 µL of dimethyl sulfoxide. The optical absorbance of the resulting solution was then measured at 550 nm using a microplate reader (Spectra MAX; Molecular Devices, Sunnyvale, CA, USA).
Measurement of inflammatory cytokines
HaCaT cells were placed into the six-well plate, and the supernatants were obtained to identify inflammatory factors. To assess the levels of TNF-α (MTA00B, R&D Systems), IL-1β (MLB00C, R&D Systems), and IL-6 (M6000B, R&D Systems), the culture supernatants were collected and subjected to further analysis using an enzyme-linked immunosorbent assay (ELISA) kit obtained from R&D Systems, as per the instructions provided by the manufacturer.
Intracellular reactive oxygen species (ROS) level evaluation
The DCFH-DA fluorescent probe was used to evaluate intracellular ROS generation in cells treated with Allicin (10 and 25 µM) for 1 h and exposed to UVB (50 mJ/cm2). After treatment, cells were stained with 10 µM DCFH-DA (S0033S, Beyotime, Shanghai, China) for 30 min at 37◦C in the dark, rinsed with PBS, and then collected. The formation of intracellular ROS was monitored using a fluorescent microplate reader (Gemini; Molecular Devices) with excitation/emission wavelengths of 485 nm/530 nm, respectively.
Measurement of oxidative stress indicators
HaCaT cells were homogenized, and protein concentrations were determined for measuring oxidative stress indicators, MDA (S0131S, Beyotime), SOD (S0109, Beyotime), and CAT (S0051, Beyotime) according to the instructions of the manufacture guidelines.
Cellular immunofluorescence
HaCaT cells were formalized using a 4% paraformaldehyde solution and permeabilized with 1% Triton X-100. Subsequently, the cells underwent incubation with 50 μL of TUNEL (C1086; from Beyotime Institute of Biotechnology, Jiangsu, China) at 37 °C for 1 h. After two washes with PBS, HaCaT cells were incubated with the LC3-II (ab192890; rabbit monoclonal, Abcam) reaction mixture for 1 h at 37 °C to achieve nuclear staining. Ultimately, cells were examined using a fluorescence microscope (wavelength range: 515–565 nm; Olympus, BX71, Japan). TUNEL-positive cells were quantified using five images (magnification × 200) for each sample.
Real-time quantitative PCR (RT-qPCR)
Total RNA was isolated from HaCaT cells utilizing TRIzol (Invitrogen) by the manufacturer's guidelines and was employed as templates (1 μg) for cDNA synthesis using a PrimeScript RT Reagent kit (TaKaRa Bio Inc, Dalian, China). RT-qPCR analysis employed a SYBR Green RT‑qPCR kit (Takara) on an Applied Biosystems 7500 real-time PCR system (Applied Biosystems, CA, USA) with GAPDH as an internal reference. The primer sequences for PCR are outlined in Table 1. The RT-qPCR procedure involved denaturation at 95˚C for 7 min, followed by 40 cycles at 95˚C for 15 s and 60˚C for 1 min. Each measurement was duplicated, and the relative mRNA expression level was determined using the 2−ΔΔCt method [14].
Table 1.
List of primer sequences used in the study
| Genes | Forward primer (5′−3′) | Reverse primer (5′−3′) |
|---|---|---|
| Human TNF-α | CTGGGCAGGTCTACTTTGGG | CTGGAGGCCCCAGTTTGAAT |
| Human IL-1β | GCTCGCCAGTGAAATGATGG | TCGTGCACATAAGCCTCGTT |
| Human IL-6 | TCCACAAGCGCCTTCGGTC | GGTCAGGGGTGGTTATTGCAT |
| Human GAPDH | CATGTTGCAACCGGGAAGGA | CGCCCAATACGACCAAATCAG |
Western blotting
Protein extraction involved the utilization of RIPA lysis buffer for total protein and Extraction Reagents from Pierce Biotechnology, Inc., for nuclear protein isolation. Subsequent protein concentration was determined using a BCA protein assay kit obtained from Beyotime Biotechnology in China. Following this, protein samples amounting to 50 μg were subjected to separation on 10% SDS-PAGE and subsequent transfer onto PVDF membranes. A blocking step using 5% low-fat milk was followed by incubation with primary antibodies including Beclin-1 (1:500, sc-48341, mouse monoclonal, Santa Cruz), P62 (1:500, sc-28359, mouse monoclonal, Santa Cruz), p-PI3K (p85, Tyr458) (1:400, ab278545, rabbit monoclonal, Abcam), t-PI3K (1:500, ab302958, rabbit monoclonal, Abcam), p-Akt (Ser473) (1:400, ab81283, rabbit monoclonal, Abcam), t-Akt (1:400, ab8805, rabbit polyclonal, Abcam), NLRP3 (1:500, Cat# ab214185, Abcam, UK), Caspase-1 p10 (1: 200, Cat# sc-392736, Santa Cruz, USA). Subsequently, HRP-linked secondary antibodies were applied to the membranes, with GAPDH (1:2000, ab8245, mouse monoclonal, Abcam) serving as the internal control. The bands were visualized through ECL (Thermo, Waltham, MA, USA), followed by ImageJ software analysis.
Statistical analysis
The data are displayed as mean ± standard deviation in triplicates. These data sets underwent analysis through one-way analysis of variance (ANOVA), with the post-hoc test utilizing the least significant difference (LSD) method. The statistical evaluations were executed using Prism 8 software developed by GraphPad. A P value less than 0.05 (P < 0.05) was deemed statistically significant.
Results
Allicin reduced keratinocyte proliferation and apoptosis induced by UVB
To examine the potential effect of Allicin on UVB-induced keratinocyte proliferation and apoptosis, HaCaT cells were treated with different concentrations of Allicin (0, 5, 10, 25, 50, and 100 μM) for 24 h. The results showed no adverse effects of Allicin observed on HaCaT cells at concentrations of 5, 10 and 25 µM (Fig. 1B). HaCaT cells were pretreated with Allicin (10 and 25 µM) for 2 h, followed by UVB irradiation (50 mJ/cm2) and incubated for further 6, 12, 24, 48, and 72 h. Then, the effect of Allicin on the cell viability of UVB-exposed HaCaT cells was assessed by MTT assay, and no adverse effects were observed (Fig. 1C). In addition, HaCaT cells were stained with TUNEL and DAPI, and cell apoptotic rate was assessed by calculating the percentage of TUNEL-positive cells to all DAPI-positive cells. The results showed that apoptotic rates were reduced in the UVB + Allicin 10 group and UVB + Allicin 25 group (Fig. 1D and 1E).
Allicin attenuated UVB-induced inflammation of keratinocytes
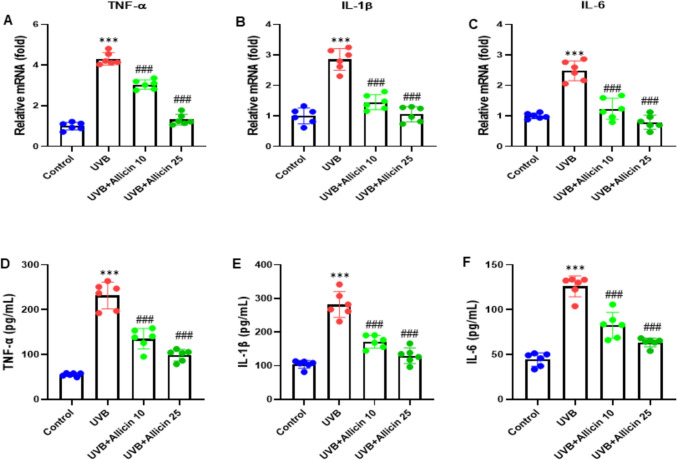
In this experiment, we aimed to evaluate the possible impact of Allicin on UVB-induced inflammation of keratinocytes, HaCaT cells were treated with Allicin 10 µM and 25 µM. Cell supernatant was collected, and RT-qPCR was performed to determine the mRNA levels of pro-inflammatory cytokines in HaCaT cells, including TNF-α, IL-1β, and IL-6. The results showed that Allicin treatment decreased the mRNA levels of TNF-α, IL-1β, and IL-6 (all, P < 0.001) while they were elevated by UVB induction (Fig. 2A-C). In addition, we carried out an ELISA assay to measure the release of TNF-α, IL-1β, and IL-6 in the culture media of HaCaT cells. We observed that the release of TNF-α, IL-1β, and IL-6 was inhibited (all, P < 0.001) while stimulated by UVB induction (Fig. 2D-F). Thus, Allicin suppressed UVB-induced inflammation of keratinocytes.
Fig. 2.
Allicin suppresses UVB-induced inflammation of keratinocytes. A, B, C RT-qPCR was performed to determine the mRNA levels of pro-inflammatory cytokines in HaCaT cells, including TNF-α, IL-1β, and IL-6. D-F. D, E, F ELISA was used to determine the release of TNF-α, IL-1β, and IL-6 in the culture media of HaCaT cells. Data are presented as mean ± SD in triplicates and analyzed using one-way ANOVA, and LSD was used for the post-hoc test. ***P < 0.001 vs. control group; ###P < 0.001 vs. UVB group
Allicin inhibited intracellular ROS generation and oxidative stress in UVB-induced keratinocytes
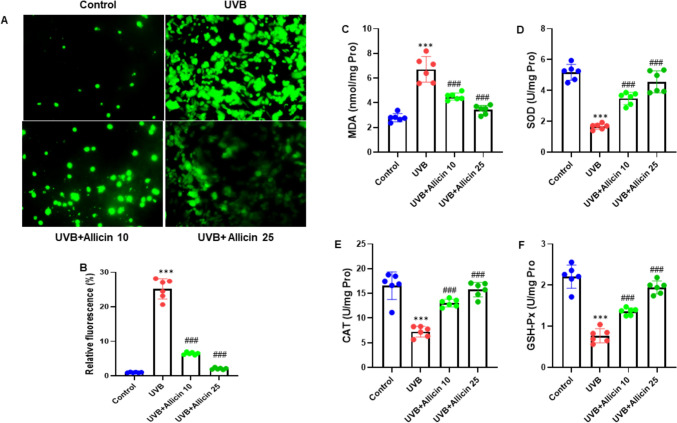
In this experiment, we examined the potential effect on the intracellular ROS generation and oxidative stress in UVB-induced keratinocytes. HaCaT cells were stained with DCFH-DA, and the representative images were taken at 200x. The results indicated that Allicin attenuated the intracellular ROS production in UVB-induced HaCaT cells (Fig. 3A). After quantification analysis of the relative DCFH-DA fluorescence intensity of HaCaT cells, we observed the same consistent findings (Fig. 3B). Additionally, the cell lysate of HaCaT cells was used to measure the oxidative stress markers such as MDA, SOD, CAT and GSH-Px. Our study outcomes showed that Allicin reduced the MDA level while increasing the levels of SOD, CAT and GSH-Px (all, P < 0.001) in the HaCaT cell lysate (Fig. 3C-F). Thus, the experimental findings demonstrated that Allicin suppressed the intracellular ROS generation and oxidative stress in UVB-induced keratinocytes.
Fig. 3.
Allicin inhibits intracellular ROS generation and oxidative stress in UVB-induced keratinocytes. A HaCaT cells were stained with DCFH-DA, and the representative images were shown (200 ×). B Quantitative analysis of the relative DCFH-DA fluorescence intensity of HaCaT cells. The cell lysate of HaCaT cells was used to measure the oxidative stress markers for C MDA, D SOD, E CAT and F GSH-Px. Data are presented as mean ± SD in triplicates and analyzed using one-way ANOVA, and LSD was used for the post-hoc test. Scale 50 µm. ***P < 0.001 vs. control group; ###P < 0.001 vs. UVB group
Allicin inhibits autophagy in UVB-induced HaCaT cells
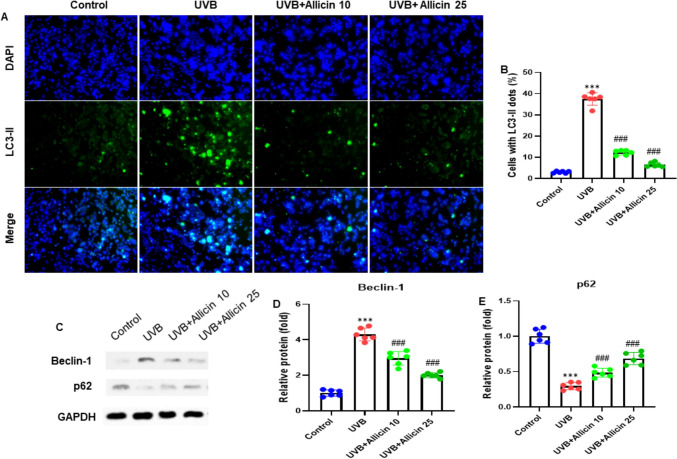
This experiment was carried out to investigate the potential effect of Allicin on autophagy in UVB-induced HaCaT cells. HaCaT cells were stained with the LC3-II antibody (green), and the nucleus was stained with DAPI (blue). The images were taken at a magnification of 200x. The study findings showed that Allicin treatment reduced the autophagy in UVB-induced HaCaT cells (Fig. 4A). After quantifying the relative LC3-II fluorescence intensity, as presented by cells with LC3-II dots to DAPI + cells, we observed the same consistent findings (Fig. 4B). Next, we carried out the western blot analysis to evaluate the expression patterns of autophagy-related proteins such as Beclin-1 and p62. The results demonstrated that Allicin treatment reduced the protein expression of Beclin-1 while increasing the p62 protein expression (all, P < 0.001) (Fig. 4C). After quantification of the blots, we observed consistent expression patterns for Beclin-1 and p62 (Fig. 4D, E). Therefore, the study results indicated that Allicin inhibited autophagy in UVB-induced HaCaT cells.
Fig. 4.
Allicin inhibits autophagy in UVB-induced HaCaT cells. A HaCaT cells were stained with LC3-II antibody. The nucleus was stained with DAPI (blue). B Quantifying relative LC3-II fluorescence intensity, as presented by cells with LC3-II dots to DAPI + cells. C Representative blots of autophagy-related proteins by western blot analysis. Allicin significantly increases the protein expression of D Beclin-1 and decreases the protein expression of (F) p62. Data are presented as mean ± SD in triplicates and analyzed using one-way ANOVA, and LSD was used for the post-hoc test. Scale 50 µm. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group; ###P < 0.001 vs UVB group
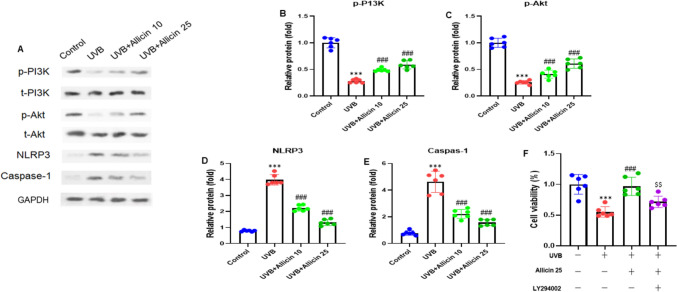
Allicin activated the PI3K/Akt pathway whereas inhibiting the NLRP3 inflammasome in UVB-induced keratinocytes
We performed the Western blot analysis to examine the potential effect of Allicin on the PI3K/Akt pathway and the NLRP3 inflammasome in UVB-induced keratinocytes. The western blot results showed that Allicin increased the protein expressions of the PI3K/Akt pathway molecules, such as PI3K and Akt (all, P < 0.001). On the other hand, Allicin treatment decreased the NLRP3-related protein, such as Caspase-1 (P < 0.001) (Fig. 5A). After quantifying the gel blots, we observed the consistent expression patterns in UVB-induced keratinocytes (Fig. 5B-E). The phosphorylated PI3K was normalized to total PI3K; phosphorylated Akt was normalized to total Akt, and NLRP3 and Caspase-1 were normalized to GAPDH. The protective effect of Allicin on UVB-induced damage of keratinocytes was reversed by co-incubation with a PI3K/Akt pathway inhibitor LY294002 (10 μM) (Fig. 6F). Thus, the experiment results demonstrated that Allicin activated the PI3K/Akt pathway and inhibited the NLRP3 inflammasome in UVB-induced keratinocytes.
Fig. 5.
Allicin activates the PI3K/Akt pathway and inhibits NLRP3 inflammasome in UVB-induced keratinocytes. A Representative gel blots of phosphorylated PI3K and Akt, and NLRP3-related protein by Western blotting. Quantification analysis of (B) phosphorylated PI3K (normalized to total PI3K), C phosphorylated Akt (normalized to total Akt), D NLRP3 (normalized to GAPDH), and E Caspase-1 (normalized to GAPDH) protein bands. F HaCaT cells were pretreated with Allicin (25 μM) or PI3K/Akt pathway inhibitor LY294002 (10 μM), followed by irradiated with UVB for further 24 h. Cell viability was evaluated with MTT assay. Data are presented as mean ± SD in triplicates and analyzed using one-way ANOVA, and LSD was used for the post-hoc test. ***P < 0.001 vs. control group; ###P < 0.001 vs. UVB group; $$P < 0.001 vs. UVB + Allicin 25 group
Fig. 6 .
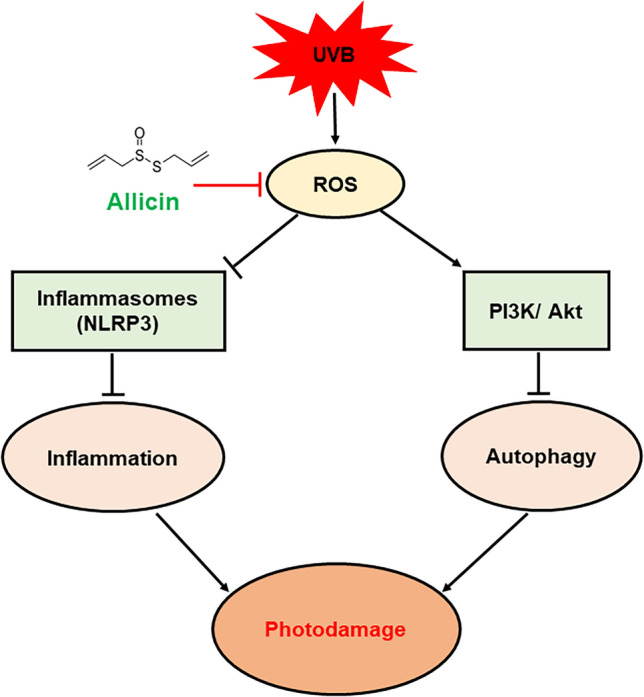
Schematic illustration of the protective effects of Allicin against UVB-induced photodamage of keratinocytes by inhibiting NLRP3 inflammasomes and activating the PI3K/Akt/autophagy pathway
Discussion
Our present research examined the effects of Allicin on UVB-induced photodamage of keratinocytes. The study revealed that Allicin reduced Ang II-induced HaCaT cell proliferation and apoptosis, inhibited UVB-induced pro-inflammatory cytokines production, attenuated intracellular ROS production and oxidative stress, and inhibited autophagy in UVB-induced HaCaT cells. Additionally, Allicin treatment effectively inhibited the NLRP3 inflammasome while activating the PI3K/Akt pathway. Therefore, the current research findings demonstrated that Allicin could have a protective therapeutic effect against UVB-induced photodamage of keratinocytes.
Ultraviolet (UV) radiation poses a significant environmental hazard impacting all living organisms. The largest human organ, the skin, is promptly exposed to UV radiation, rendering it more susceptible to adverse effects. Reactive oxygen species (ROS) are identified as the leading cause of skin aging and inflammation from UVB radiation exposure. Consequently, individuals with fair skin tones face an elevated risk of developing skin cancer [15]. Allicin, an essential compound found in crushed garlic used in traditional medicine, exhibits diverse pharmacological properties that aid in anti-cancer activities. Notably, Allicin impedes cell proliferation in colon cancer cells by reducing intracellular glutathione levels [16].
Furthermore, Allicin prompts apoptosis in gastric cancer cells by activating the p38 mitogen-activated protein kinase/caspase-3 signaling pathway [17]. Additionally, Allicin enhances cell death in human renal clear cell carcinoma cells by obstructing the hypoxia-inducible factor-1α pathway [18]. Previous research has demonstrated that Allicin effectively eliminates cancer cells and diminishes tumor growth in various carcinomas. However, our current investigation reveals that treatment with Allicin suppresses cell growth and the apoptosis rate in Ang II-induced HaCaT cells.
UVB exposure enhances skin damage and inflammation by stimulating various cytokines and immunological regulators released by cells. To prevent the initiation of skin inflammation, UVB-damaged keratinocytes need to undergo apoptosis. UVB can penetrate the epidermis and extend into the upper dermis, exhibiting higher activity than UVA in initiating skin cancer and modulation of cell-cycle-control pathways [19]. The impact of UVB radiation is predominantly observed on keratinocytes, known for their secretion of cytokines like IL-1, IL-6, IL-8, IL-10, GM-CSF, and TNF-α, ultimately leading to inflammatory and immunomodulatory responses [20]. Recent investigations have suggested that Allicin can mitigate the inflammation of keratinocytes triggered by UVB exposure.
UVB exposure imbalances the redox system in the epidermis. Oxidative stress can increase intracellular ROS and damage mitochondria, resulting in a drop in mitochondrial membrane potential (MMP) and an increase in intracellular Ca2+ [21, 22]. The formation of ROS will lead to lipid oxidation and the generation of MDA [23]. Vitagene expression is related to the preservation of cellular homeostasis under stress and has a role in the development of several degenerative disorders. SOD is one of the vitagene components, and glutathione peroxidase is part of the vitagene network. They contribute to the cellular oxidative stress response [24, 25]. Our results indicated that following UVB exposure, ROS levels in cells increased dramatically, antioxidants GSH-Px and SOD reduced, and lipid oxidation products, MDA, increased, which is consistent with earlier research. Mitochondria are the principal generator of ROS, and excessive ROS can cause mitochondrial damage [26]. In the current work, we observed that UVB radiation reduced the mitochondrial membrane potential of HaCaT cells and that Allicin treatment restored this effect.
Autophagy contributes to maintaining cellular homeostasis by recycling damaged organelles [3]. Autophagy is characterized by the accumulation of LC3-II and the decrease of p62. Beclin-1 is a crucial regulator of autophagy and endocytosis and involves the maintenance of the epidermal barrier [27]. In autophagy, the expression of the p62 protein was downregulated, and an autophagy inhibitor, 3-MA, increased the p62 protein in HaCaT cells [28]. However, our study showed that Allicin treatment decreased the protein expression of Beclin-1 while increasing the p62 protein expression in UVB-induced HaCaT cells.
The NLRP3 inflammasome, comprising NLRP3, an apoptosis-associated speck-like protein with a CARD (ASC) and procaspase-1, plays a pivotal role in the innate immune response. Activation of this inflammasome leads to the activation of caspase-1, resulting in the production of interleukin 18 (IL-18) and interleukin 1 beta (IL-1β), thus initiating the inflammatory process [12]. Previous studies have revealed that excessive UVB exposure increases the synthesis of inflammasome components, such as NLRP3, ASC, Caspase-1, and IL-1β, and down-regulates antioxidant genes in the HaCaT cell line [29]. However, our research showed that Allicin treatment reduced the NLRP3-related protein, such as Caspase-1. In addition, Allicin elevated the protein expression levels of molecules within the PI3K/Akt signaling pathway, specifically PI3K and Akt.
Limitations
Allicin's cytoprotective properties and action method in additional keratinocytes, such as primary cells, human N/TERT keratinocytes, and skin fibroblasts, remain to be investigated. Furthermore, in vivo investigations are needed to establish Allicin as a supplementary option for UVB-induced photodamage. Although these limitations could be addressed to consolidate and expand on current findings, the study indicated that Allicin might be a potential contender against UVB-induced photodamage.
Conclusions
In the present research, we investigated the possible protective impact of Allicin on UVB-induced photodamage of keratinocytes in vitro. Our results demonstrated that Allicin treatment protected against UVB-induced photodamage of keratinocytes by inhibiting NLRP3 inflammasomes and activating the PI3K/Akt/autophagy pathway (Fig. 6). In future studies, these findings will need to be validated in preclinical and clinical settings.
Author contributions
Y.Y. designed the project, supervised the project, and revised the manuscript. J.K. performed experiments, collected data, analyzed the data, performed statistical analysis, and wrote the first draft of the manuscript.
Funding
This investigation is supported by the Shanghai Pudong Hospital Talent Introduction and Research Launch Project (YJRCJJ201802).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests. The authors report no conflict of interest.
Ethical approval
Ethical approval is not required for this study in accordance with local or national guidelines.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Friedman O (2005) Changes associated with the aging face. Facial Plast Surg Clin N Am 13:371–380 [DOI] [PubMed] [Google Scholar]
- 2.Keaney TC (2016) Aging in the male face: intrinsic and extrinsic factors. Dermatol Surg 42(7):797–803 [DOI] [PubMed] [Google Scholar]
- 3.Sample A, He YY (2017) Autophagy in UV damage response. Photochem Photobiol 93:943–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinha RP, Häder DP (2002) UV-induced DNA damage and repair: A review. Photochem Photobiol Sci 1:225–236 [DOI] [PubMed] [Google Scholar]
- 5.Hegedűs C, Juhász T, Fidrus E, Janka EA, Juhász G, Boros G, Paragh G, Uray K, Emri G, Remenyik É, Bai P (2021) Cyclobutane pyrimidine dimers from UVB exposure induce a hypermetabolic state in keratinocytes via mitochondrial oxidative stress. Redox Biol 38:101808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T (2003) UV-induced skin damage. Toxicology 189:21–39 [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Han J, Jiang C, Zhang Y (2020) Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev 59:101036 [DOI] [PubMed] [Google Scholar]
- 8.Hayat S, Cheng Z, Ahmad H, Ali M, Chen X, Wang M (2016) Garlic, from remedy to stimulant: evaluation of antifungal potential reveals diversity in phytoalexin allicin content among garlic cultivars; allicin containing aqueous garlic extracts trigger antioxidants in cucumber. Front Plant Sci 7:1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma LN, Li LD, Li SC, Hao XM, Zhang JY, He P, Li YK (2017) Allicin improves cardiac function by protecting against apoptosis in a rat model of myocardial infarction. Chin J Integr Med 23(8):589–597 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Qi H, Wang Y, Wu M, Cao Y, Huang W, Li L, Ji Z, Sun H (2012) Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine 19(8–9):693–698 [DOI] [PubMed] [Google Scholar]
- 11.Ba L, Gao J, Chen Y, Qi H, Dong C, Pan H, Zhang Q, Shi P, Song C, Guan X, Cao Y, Sun H (2019) Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine 58:152765 [DOI] [PubMed] [Google Scholar]
- 12.Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225 [DOI] [PubMed] [Google Scholar]
- 13.Kma L, Baruah TJ (2022) The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol Appl Biochem 69(1):248–264 [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 15.Swalwell H, Latimer J, Haywood RM, Birch-Machin MA (2020) Investigating the role of melanin in UVA/UVB-and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radical Biol Med 52:626–634 [DOI] [PubMed] [Google Scholar]
- 16.Hirsch K, Danilenko M, Giat J, Miron T, Rabinkov A, Wilchek M, Mirelman D, Levy J, Sharoni Y (2000) Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer 38(2):245–254 [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Zhu Y, Duan W, Feng C, He X (2015) Allicin induces apoptosis of the MGC-803 human gastric carcinoma cell line through the p38 mitogen-activated protein kinase/caspase-3 signaling pathway. Mol Med Rep 11(4):2755–2760 [DOI] [PubMed] [Google Scholar]
- 18.Song B, Shu Y, Cui T, Fu P (2015) Allicin inhibits human renal clear cell carcinoma progression via suppressing HIF pathway. Int J Clin Exp Med 8(11):20573–20580 [PMC free article] [PubMed] [Google Scholar]
- 19.Girolamo ND, Wakefield D, Coroneo MT (2006) UVB-mediated induction of cytokines and growth factors in pterygium epithelial cells involves cell surface receptors and intracellular signaling. Invest Ophthalmol Vis Sci 47(6):2430–2437 [DOI] [PubMed] [Google Scholar]
- 20.Ansary TM, Hossain MR, Kamiya K, Komine M, Ohtsuki M (2021) Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int J Mol Sci 22(8):3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs D, Raffa S, Flori E, Aspite N, Briganti S, Cardinali G, Torrisi MR, Picardo M (2009) Keratinocyte growth factor down-regulates intracellular ROS production induced by UVB. J Dermatol Sci 54:106–113 [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Das B, Sen R, Kundu P, Manna A, Sarkar A, Chowdhury C, Chatterjee M, Das P (2015) Andrographolide Analogue Induces Apoptosis and Autophagy Mediated Cell Death in U937 Cells by Inhibition of PI3K/Akt/mTOR Pathway. PLoS ONE 10:e0139657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mali VR, Palaniyandi SS (2014) Regulation and therapeutic strategies of 4-hydroxy-2-nonenal metabolism in heart disease. Free Radic Res 48:251–263 [DOI] [PubMed] [Google Scholar]
- 24.Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ (2009) Vitagenes, cellular stress response, and acetylcarnitine: Relevance to hormesis. BioFactors 35(2):146–160 [DOI] [PubMed] [Google Scholar]
- 25.Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13(11):1763–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox CS, McKay SE, Holmbeck MA, Christian BE, Scortea AC, Tsay AJ, Newman LE, Shadel GS (2018) Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling. Cell Metab 28:776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noguchi S, Honda S, Saitoh T, Matsumura H, Nishimura E, Akira S, Shimizu S (2019) Beclin 1 regulates recycling endosome and is required for skin development in mice. Commun Biol 2:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Zeng B, Pan Y, Huang P, Wang C (2018) Autophagy participates in isoliquiritigenin-induced melanin degradation in human epidermal keratinocytes through PI3K/AKT/mTOR signaling. Biomed Pharmacother 97:248–254 [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Luna A, Ávila-Román J, Oliveira H, Motilva V, Talero E (2019) Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 Inflammasome in UVB-Exposed HaCaT Keratinocytes. Mar Drugs 17(8):451 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. No datasets were generated or analysed during the current study.