Abstract
Background: Biosimilar pegylated L-asparaginase offers a promising alternative to the innovator molecule for treating acute lymphoblastic leukemia (ALL) in Indian children. It addresses challenges associated with drug availability and cost while providing similar therapeutic advantages. This biosimilar ensures wider access to essential treatment in resource-limited settings such as India.
Materials and methods: A retrospective study was conducted at the Pediatric Oncology unit of the Department of Medical Oncology, Sher-I-Kashmir Institute of Medical Sciences (SKIMS) Srinagar. The study evaluated the efficacy and safety of biosimilar polyethylene glycol-asparaginase (PEG-ASP) (Asviia) in newly diagnosed pediatric ALL patients treated between January 2021 and December 2023. Each patient received two induction doses of PEG-ASP.
Results: The study included 45 patients (29 boys, 16 girls) with a median age of 7.5 years (range: 1-16 years), with most patients diagnosed with Pre-B ALL. The median PEG-ASP dose administered intravenously was 1175 IU (range: 1125-3750 IU). Significant improvements in hemoglobin and platelet counts were observed following the first dose of PEG-ASP. The biosimilar PEG-ASP was well tolerated, with no life-threatening events reported. At the end of the induction phase, 40 patients (88.89%) achieved complete remission with minimal residual disease (MRD) negativity, while five patients had MRD positivity.
Conclusion: The study provides valuable insights into the efficacy and safety of biosimilar PEG-ASP for pediatric ALL in resource-limited settings, with strong data on remission rates and minimal adverse events.
Keywords: acute lymphoblastic leukemia (all), biosimilar peg-asparaginase, hematological parameters, minimal residual disease (mrd), pediatric oncology
Introduction
Acute lymphoblastic leukemia (ALL) constitutes nearly 77% of all cancers diagnosed in children under 15 years of age globally [1]. In India, leukemia accounts for approximately 40 to 50% of the total childhood cancer burden, with ALL being the most prevalent type with an incidence rate of up to 101.4 per million in boys and 62.3 per million in girls [2]. Chemotherapy remains the main treatment for pediatric ALL worldwide, with high-income countries achieving a 5-year overall survival rate of around 90% through effective protocols like Children’s Oncology Group (COG) and Berlin-Frankfurt-Münster (BFM), while India shows progress but still lags with survival rates ranging from 45% to 81% due to challenges in uniform treatment, supportive care, and treatment abandonment [3]. Currently, there are three asparaginase preparations available: L-asparaginase (native asparaginase), polyethylene glycol-asparaginase (PEG-ASP) (Asviia) derived from Escherichia coli, and Erwinia asparaginase sourced from Erwinia chrysanthemi [4]. PEG-ASP received FDA approval in February 1994 for intramuscular use in patients with ALL who have hypersensitivity to native forms of L-asparaginase. In November 2005, the FDA approved the intravenous administration of PEG-ASP, and in July 2006, expanded its approval for frontline treatment of ALL patients based on the COG study CCG-1962 [5]. PEG-ASP is the preferred formulation in ALL treatment regimens because of its longer half-life, which extends the circulation time of the enzyme and minimizes immunogenicity compared to native asparaginase [6].
PEG-ASP is a bioengineered compound synthesized through the covalent linkage between E. coli-L-asparaginase (EC-ASP) and PEG units. This innovative process, known as PEGylation, involves the modification of biological molecules by the non-toxic and non-immunogenic polymer, PEG [7]. The strategic application of PEGylation allows for the optimization of enzyme activity by facilitating unhindered access to the enzyme’s active sites, while concurrently impeding the uptake of EC-ASP by the reticuloendothelial system [7,8]. PEG-ASP has a longer half-life and lower immunogenicity than unmodified EC-ASP, enabling sustained asparagine depletion with fewer allergic reactions. This allows for less frequent dosing and fewer interruptions in ALL treatment. Additionally, PEG-ASP is more thermally stable and less temperature-dependent, facilitating easier transport and storage, which enhances accessibility in varied clinical settings. This dual mechanism not only enhances the therapeutic efficacy of ASP but also shields its antigenic determinants from immune recognition, thereby reducing the possibility of antibody development [9,10]. Leukemia cells require exogenous asparagine to survive, while normal cells can synthesize asparagine. Asparaginase depletes serum asparagine by converting it to aspartic acid and ammonia. Therefore, this anti-leukemic activity causes deprivation of the critical amino acid, leading to cell death [11].
In addition to its therapeutic benefits, the administration of Asviia in pediatric ALL treatment regimens requires a comprehensive understanding of its associated toxicities. Reported adverse effects include hepatotoxicity, pancreatitis, hypertriglyceridemia, and hypersensitivity reactions, all of which can significantly impact patient outcomes and treatment adherence [12,13]. Recognizing the importance of potential risks associated with PEG-ASP, our study evaluates the efficacy of PEG-ASP and its associated toxicities in pediatric ALL patients within the Indian population.
Materials and methods
Study objective
The objective of this study was to evaluate the efficacy and safety of the biosimilar PEG-ASP in newly diagnosed pediatric patients with ALL. Primary endpoints included the evaluation of efficacy through improvement in hematological parameters, such as hemoglobin, platelet count, total bilirubin, and serum albumin, following the administration of PEG-ASP. Secondary endpoints included the assessment of the incidence of adverse events, the overall safety profile, and the tolerance of the PEG-ASP among pediatric patients.
Study design and setting
This retrospective, single-center study was conducted at the Pediatric Oncology unit of the Department of Medical Oncology, Sher-I-Kashmir Institute of Medical Sciences (SKIMS) in Srinagar, India.
Participants included pediatric patients aged between 1 and 16 years who were newly diagnosed with ALL and enrolled in the SKIMS pediatric ALL treatment protocol, which incorporates PEG-ASP as part of the induction phase. Patients eligible for this study were those with a confirmed diagnosis of ALL and whose parents or guardians provided consent for participation.
Study participants and treatment protocol
The study included data from 45 pediatric patients diagnosed with ALL between 1st January 2021 and 31st December 2023. The treatment consisted of a standard induction chemotherapy regimen specifically designed for pediatric ALL patients, which included two doses of biosimilar PEG-ASP. Each dose was administered intravenously over a one-hour period at a dose of 2500 IU/m², in conjunction with other agents based on the treatment protocol.
Data collection and statistical analysis
Data for this retrospective analysis included patient demographics - age, gender, and diagnosis - alongside baseline and follow-up laboratory parameters, including hemoglobin, platelet count, total bilirubin, and serum albumin levels. These parameters were measured before and after the administration of PEG-ASP. Adverse events, including febrile neutropenia, allergic reactions, and liver function abnormalities, were recorded to assess the safety profile of PEG-ASP. Additionally, efficacy outcomes, specifically complete remission and minimal residual disease (MRD) status, were evaluated after the conclusion of the induction phase. Statistical analysis involved summarizing continuous variables like laboratory values and age as means or medians, while categorical variables, including gender and adverse events, were presented as frequencies and percentages. Efficacy was assessed through improvements in hematological parameters and MRD status at the end of induction therapy.
Ethical considerations
The local institutional review board (Institutional Ethics Committee- Sher-I-Kashmir Institute of Medical Sciences, Srinagar) approved the study (IEC/SKIMS Protocol # 227/2024), which complied with the Declaration of Helsinki regarding research involving humans.
Results
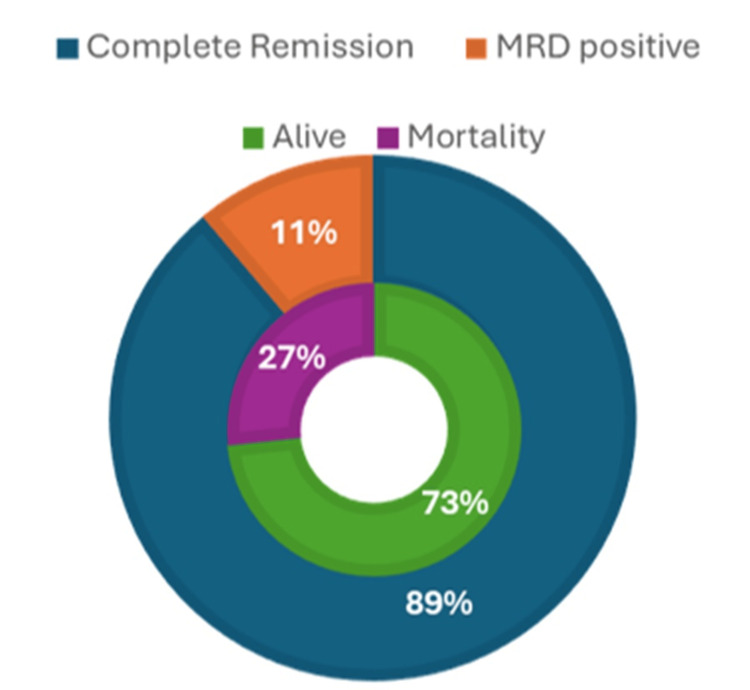
The study population data of 45 patients had a male predominance of 64.5%, with a median age of 7.5 years. The median dose of intravenously administered PEG-ASP was 2500 IU/m². Patient demographics and hematological parameters after PEG-ASP delivery are presented in Table 1. The efficacy data showed that 88.89% of patients achieved complete remission and 11.11% of patients were MRD positive with an overall mortality rate of 26.67% (Figure 1). The study reported 31 adverse events (AE), episodes with sepsis/febrile neutropenia (17.78%) being the most common AEs, followed by vomiting (13.33%), and allergy (13.33%) (Table 2).
Table 1. Patient’s demographic and clinical characteristics (n=45).
PEG-ASP: peg-asparaginase; IU: international unit
| Demography | ||
| Age in years (Median) | 7.7 | |
| Gender n (%) | Male | 29 (64.5%) |
| Female | 16 (35.5%) | |
| PEG-ASP dose (Median, IU) | 1175 | |
| Laboratory Parameters | ||
| Prior to first dose of PEG-ASP (mean + SD) | Prior to second dose of PEG-ASP (Mean + SD) | |
| Hemoglobin (g/dL) | 7.64 + 2.66 | 9.03 + 2.35 |
| Platelet /µl | 83673 + 94911 | 114075 + 149571 |
| Total bilirubin (mg/dL) | 0.54 + 0.32 | 1.68 + 5.97 |
| Serum albumin (g/dL) | 4.09 + 0.89 | 3.8 + 0.59 |
Table 2. Safety data of biosimilar Pegaspargase (Asviia).
| Adverse Events | Number (n=45) | (%) |
| Sepsis/febrile neutropenia | 8 | 17.8 |
| Allergy | 6 | 13.3 |
| Vomiting | 6 | 13.3 |
| Hyperglycemia | 5 | 11.1 |
| Transaminitis event | 2 | 4.44 |
| Pancreatitis | 2 | 4.44 |
| Bilirubin >3 mg/dL | 2 | 4.44 |
| Seizure | 0 | 0 |
| Encephalopathy syndrome | 0 | 0 |
| Albumin <2 g/dL | 0 | 0 |
| Life threat/intervention needed | 0 | 0 |
Figure 1. Efficacy data of biosimilar Pegaspargase (Asviia) (N=45).
MRD: minimal residual disease
Discussion
The efficacy of biosimilar PEG-ASP in treating pediatric ALL is evidenced by an 88.89% complete remission rate, accompanied by significant improvements in hematological parameters, including hemoglobin and platelet counts, showcasing its therapeutic effectiveness. The findings align well with established data on Oncaspar, highlighting the biosimilar’s comparable efficacy and safety profile [14,15]. The referenced pilot study by Venkatagiri et al. (2024) supports PEG-ASP’s therapeutic stability, demonstrating sustained serum asparaginase activity and an absence of significant hypersensitivity reactions or toxicities. This reinforces PEG-ASP’s potential as a cost-effective and reliable alternative in pediatric ALL treatment, with practical relevance for resource-limited settings [16].
The presence of MRD is a critical prognostic factor in ALL. This study highlights that early administration of PEG-ASP significantly accelerates MRD clearance. These findings are supported by the study of Popov et al. (2023), which demonstrated that early administration of PEG-ASP on day 3 of induction therapy improved MRD clearance and long-term outcomes in children with ALL [17]. However, patients who remain MRD-positive after treatment face a less favorable prognosis. Slow MRD responders (≥1% on day 15) generally have poorer outcomes compared to those with rapid MRD clearance. Despite this, PEG-ASP has been shown to improve overall outcomes even in slow responders, particularly in intermediate-risk (IR) patients, leading to better event-free survival (EFS) and overall survival (OS) rates [18].
The reported overall mortality rate of 26.67% in the present cohort shows the significant challenges in managing pediatric ALL patients. Sepsis and febrile neutropenia are common and severe complications in immunocompromised patients, often leading to high mortality rates if not promptly and effectively managed. Additionally, hyperglycemia, often exacerbated by steroid use in ALL treatment, can result in metabolic imbalances that further weaken a patient’s immune response, making them more susceptible to infections and other complications [19,20]. However, the mortality observed in our study could be attributed to the cumulative impact of multiple disease and treatment-related factors.
All patients in the study received PEG-ASP intravenously, a practice influenced by the FDA’s approval of IV PEG-ASP in 2005. The primary difference between administration routes lies in the duration of asparagine depletion; intravenous administration of PEG-ASP, with a half-life of 5.73 ± 3.24 days, sustains asparagine depletion significantly longer compared to the intramuscular administration of native EC-ASP, which has a shorter half-life of 1.28 ± 0.3 days, leading to quicker clearance and shorter depletion duration [21]. The study reported a higher incidence of allergic reactions, possibly due to the intravenous route, which results in prolonged asparagine depletion and an increased likelihood of AE. This observation is supported by studies from Abbott et al. (2015) and Hasan et al. (2017) [22-24].
The study noted an increase in total bilirubin levels and a decrease in serum albumin levels, indicating potential liver toxicity associated with PEG-ASP. Elevated liver enzymes and bilirubin levels, along with reduced albumin levels, suggest liver injury or inflammation, consistent with findings from other studies [17,25]. Therefore continuous monitoring of liver function tests is essential; Tölle et al. (2024) reported that hepatological parameters can be managed through the use of ursodeoxycholate and plasmapheresis [26].
The safety profile of biosimilar PEG-ASP in our study falls within acceptable benefit-risk parameters. Asviia enhances water solubility, improves mobility in solution, increases distribution, decreases immunogenicity, and reduces renal clearance [27,28]. These characteristics contribute to prolonged efficacy and a lower incidence of silent antibody formation. Asviia facilitates the rapid clearance of lymphoblasts-63% by day 7 and 96% by day 14 and maintains sustained asparaginase activity. The major advantages of Asviia include its 2-3-week duration of action and the flexibility of both intravenous and intramuscular administration, enhancing convenience for patients and healthcare providers [29]. Despite these promising findings, the study’s retrospective design and single-center setting may limit the generalizability of the results. The relatively small sample size restricts the ability to detect rare AE, and the lack of stringent inclusion criteria might introduce variability in patient characteristics. Further studies with larger, multi-center cohorts and prospective designs are necessary to validate these findings and provide more comprehensive insights into the long-term safety and efficacy of biosimilar PEG-ASP.
Conclusions
This study highlights that biosimilar PEG-ASP is an effective and safe option for treating pediatric ALL, achieving nearly 90% complete remission with MRD negativity. The manageable safety profile, with primarily mild and expected AE, highlights its suitability in clinical practice. Importantly, PEG-ASP provides a cost-effective alternative to the innovator drug, making essential leukemia treatment more accessible in resource-limited settings like India. The results show that biosimilar PEG-ASP offers comparable efficacy and addresses key challenges of affordability and availability, which are crucial for improving pediatric ALL treatment outcomes and expanding access to life-saving care for children in need. To improve PEG-ASP’s accessibility in resource-limited settings beyond India, initiatives such as local production to reduce cost, collaboration with WHO for essential drug listing, and government-backed financial support in healthcare programs can enhance affordability and distribution, potentially improving outcomes in underserved populations.
Acknowledgments
We express our sincere appreciation and gratitude to Dr. Jaymin Parikh and Dr. Devina Aswal, Medical Affairs at Digicare Health Solutions Private Limited (DHSPL), who contributed for the preparation of manuscript.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Institutional Ethics Committee - Sher-i-Kashmir Institute of Medical Sciences, Srinagar issued approval 227/2024.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Shumail Bashir, Faisal R Guru, Rukhsana Akhter, Syed Ahmed Nisar, Zafirah Zahir, Ulfat Ara Wani, Suyash Bharat, Mohmad Hussain Mir
Acquisition, analysis, or interpretation of data: Shumail Bashir, Faisal R Guru, Rukhsana Akhter, Syed Ahmed Nisar, Zafirah Zahir, Ulfat Ara Wani, Suyash Bharat, Richa Tripathi, Mohmad Hussain Mir
Drafting of the manuscript: Shumail Bashir, Faisal R Guru, Rukhsana Akhter, Syed Ahmed Nisar, Zafirah Zahir, Ulfat Ara Wani, Richa Tripathi, Mohmad Hussain Mir
Critical review of the manuscript for important intellectual content: Shumail Bashir, Faisal R Guru, Rukhsana Akhter, Syed Ahmed Nisar, Zafirah Zahir, Ulfat Ara Wani, Suyash Bharat, Richa Tripathi, Mohmad Hussain Mir
Supervision: Faisal R Guru, Suyash Bharat, Richa Tripathi
References
- 1.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries [Internet] Bethesda, MD: National Cancer Institute (US); 2024. Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®): Health Professional Version. [Google Scholar]
- 2.Childhood acute lymphoblastic leukemia: Progress through collaboration. Pui CH, Yang JJ, Hunger SP, et al. J Clin Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acute leukemia in children: A review of the current Indian data. Arora RS, Arora B. South Asian J Cancer. 2016;5:155–160. doi: 10.4103/2278-330X.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Tong WH, Pieters R, Kaspers GJ, et al. Blood. 2014;123:2026–2033. doi: 10.1182/blood-2013-10-534347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PEG-asparaginase. Fu CH, Sakamoto KM. Expert Opin Pharmacother. 2007;8:1977–1984. doi: 10.1517/14656566.8.12.1977. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Utility of Pegaspargase in children, adolescents and young adult patients with acute lymphoblastic leukemia: A review. Bender C, Maese L, Carter-Febres M, Verma A. Blood Lymphat Cancer. 2021;11:25–40. doi: 10.2147/BLCTT.S245210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pegylation: Engineering improved biopharmaceuticals for oncology. Molineux G. Pharmacotherapy. 2003;23:3–8. doi: 10.1592/phco.23.9.3s.32886. [DOI] [PubMed] [Google Scholar]
- 8.Treatment of children with advanced-stage lymphoblastic lymphoma with pegaspargase. Yu-tong Z, Li-hua F, Xiao-dan Z, Li-zhe W, Jian C. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4359608/ Iran J Pediatr. 2014;24:75–80. [PMC free article] [PubMed] [Google Scholar]
- 9.Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: The past, the present and recommendations for the future. Avramis VI, Panosyan EH. Clin Pharmacokinet. 2005;44:367–393. doi: 10.2165/00003088-200544040-00003. [DOI] [PubMed] [Google Scholar]
- 10.L-asparaginase and PEG asparaginase—Past, present, and future. Keating MJ, Holmes R, Lerner S, Ho DH. Leuk Lymphoma. 1993;10 Suppl:153–157. doi: 10.3109/10428199309149129. [DOI] [PubMed] [Google Scholar]
- 11.L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. Egler RA, Ahuja SP, Matloub Y. J Pharmacol Pharmacother. 2016;7:62–71. doi: 10.4103/0976-500X.184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammonia level as a proxy of asparaginase inactivation in children: A strategy for classification of infusion reactions. Santos AC, Land MG, Lima EC. J Oncol Pharm Pract. 2022;28:551–559. doi: 10.1177/1078155221998738. [DOI] [PubMed] [Google Scholar]
- 13.Pegaspargase hypersensitivity reactions: intravenous infusion versus intramuscular injection - a review. Beaupin LK, Bostrom B, Barth MJ, et al. Leuk Lymphoma. 2017;58:766–772. doi: 10.1080/10428194.2016.1218004. [DOI] [PubMed] [Google Scholar]
- 14.FDA drug approval summary: Pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL) Dinndorf PA, Gootenberg J, Cohen MH, Keegan P, Pazdur R. Oncologist. 2007;12:991–998. doi: 10.1634/theoncologist.12-8-991. [DOI] [PubMed] [Google Scholar]
- 15.Population pharmacokinetics to model the time-varying clearance of the PEGylated asparaginase Oncaspar(®) in children with acute lymphoblastic leukemia. Würthwein G, Lanvers-Kaminsky C, Hempel G, et al. Eur J Drug Metab Pharmacokinet. 2017;42:955–963. doi: 10.1007/s13318-017-0410-5. [DOI] [PubMed] [Google Scholar]
- 16.A pilot study conducted at a tertiary cancer care center, evaluating the serum asparaginase activity in children suffering from acute lymphoblastic leukemia after the administration of biosimilar Pegaspargase. Venkatagiri AM, Bhat VK, Asok A, Prabhu K. Indian J Med Paediatr Oncol. 2024;45:390–395. [Google Scholar]
- 17.A single dose of PEG-asparaginase at the beginning of induction not only accelerates MRD clearance but also improves long-term outcome in children with B-lineage ALL. Popov A, Henze G, Roumiantseva J, et al. Cancers. 2023;15:5547. doi: 10.3390/cancers15235547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Outstanding outcome for children with standard risk-low (SR-Low) acute lymphoblastic leukemia (ALL) and no benefit to intensified Peg-asparaginase (PEG-ASNase) therapy: Results of Children’s Oncology Group (COG) Study AALL0331. Mattano LA, Devidas M, Friedmann AM, et al. Blood. 2014;124:793. [Google Scholar]
- 19.Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Aldoss I, Douer D, Behrendt CE, Chaudhary P, Mohrbacher A, Vrona J, Pullarkat V. Eur J Haematol. 2016;96:375–380. doi: 10.1111/ejh.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PEG-asparaginase and native Escherichia coli L-asparaginase in acute lymphoblastic leukemia in children and adolescents: A systematic review. Medawar CV, Mosegui GB, Vianna CM, Costa TM. Hematol Transfus Cell Ther. 2020;42:54–61. doi: 10.1016/j.htct.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. van der Sluis IM, Vrooman LM, Pieters R, et al. Haematologica. 2016;101:279–285. doi: 10.3324/haematol.2015.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allergic reactions associated with intravenous versus intramuscular Pegaspargase: A retrospective chart review. Abbott LS, Zakova M, Shaikh F, Shewaramani N, Punnett A, Dupuis LL. Paediatr Drugs. 2015;17:315–321. doi: 10.1007/s40272-015-0129-1. [DOI] [PubMed] [Google Scholar]
- 23.Comparison of hypersensitivity rates to intravenous and intramuscular PEG-asparaginase in children with acute lymphoblastic leukemia: A meta-analysis and systematic review. Hasan H, Shaikh OM, Rassekh SR, Howard AF, Goddard K. Pediatr Blood Cancer. 2017;64:81–88. doi: 10.1002/pbc.26200. [DOI] [PubMed] [Google Scholar]
- 24.Peg-asparaginase associated toxicities in children with acute lymphoblastic leukemia: A single-center cross-sectional study. Awwad S, Abu Alnasr R, Almanjomi F, Al Sultan M, Howaidi J, Almotairi M, AlFayyad I. Pediatric Hematology Oncology Journal. 2024;9:54–62. [Google Scholar]
- 25.Pegaspargase: A review in acute lymphoblastic leukaemia. Heo YA, Syed YY, Keam SJ. Drugs. 2019;79:767–777. doi: 10.1007/s40265-019-01120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plasmapheresis effectively abrogates severe liver toxicity of pegaspargase in a patient with acute lymphoblastic leukemia. Tölle M, Gökbuget N, Habringer S, Keller U, Schwartz S. Ann Hematol. 2024;103:3269–3271. doi: 10.1007/s00277-024-05789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Henriksen LT, Harila-Saari A, Ruud E, et al. Pediatr Blood Cancer. 2015;62:427–433. doi: 10.1002/pbc.25319. [DOI] [PubMed] [Google Scholar]
- 28.Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Stock W, Douer D, DeAngelo DJ, et al. Leuk Lymphoma. 2011;52:2237–2253. doi: 10.3109/10428194.2011.596963. [DOI] [PubMed] [Google Scholar]
- 29.Levocarnitine for pegaspargase-induced hepatotoxicity in older children and young adults with acute lymphoblastic leukemia. Schulte R, Hinson A, Huynh V, et al. Cancer Med. 2021;10:7551–7560. doi: 10.1002/cam4.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]