ABSTRACT
Patients with borderline pulmonary hypertension (PH) often experience shortness of breath or exacerbation of PH during exercise, known as exercise‐induced PH. However, the pathogenesis of exercise‐induced post‐capillary PH (post‐EIPH) and its treatment strategies remain unclear. Recent guidelines and consensus documents have highlighted the benefits of sodium‐glucose cotransporter‐2 (SGLT2) inhibitors in heart failure and chronic kidney disease (CKD). This study aimed to investigate the effects of SGLT2 inhibitors in patients with post‐EIPH and CKD. This single‐center prospective cohort study enroled 10 patients with CKD (age, 68 years; female, 60%) who exhibited post‐EIPH between 1 July 2022 and 31 December 2023. Post‐EIPH was defined as a pulmonary capillary wedge pressure (PCWP)/cardiac output (CO) slope > 2 and peak PCWP during exercise ≥ 25 mmHg measured by catheterization. The patients received SGLT2 inhibitor treatment for 6 months. At rest, patients with post‐EIPH had borderline‐PH (21.5 ± 1.8 mmHg), with preserved left and right ventricular function. SGLT2 inhibitors treatment significantly reduced the PCWP/CO slope during exercise (3.9 ± 1.2 vs. 2.4 ± 1.2 mmHg/L/min, p = 0.013) and improved the 6‐min walking distance (489.9 ± 80.2 vs. 568.3 ± 91.9 m, p = 0.014). Magnetic resonance imaging revealed a lower left ventricular global longitudinal strain in patients with post‐EIPH, which was increased by SGLT2 inhibitor treatment (−13.8 ± 2.0 vs. −17.3 ± 2.0%, p = 0.003). SGLT2 treatment inhibitors mitigated post‐EIPH hemodynamic abnormalities and exercise intolerance, suggesting their potential as its therapeutic option.
Keywords: exercise induced pulmonary hypertension, MRI, right heart catheterization during exercise, SGLT2 inhibitor
Abbreviations
- CO
cardiac output
- CTEPH
chronic thromboembolic pulmonary hypertension
- EIPH
exercise induced pulmonary hypertension
- mPAP
mean pulmonary hypertension
- PCWP
pulmonary capillary wedge pressure
- PH
pulmonary hypertension
Pulmonary hypertension (PH) is a significant threat to patient health that requires effective treatment. While current approaches have advanced with the use of pulmonary vasodilators or balloon pulmonary angioplasty, many patients with normalised or borderline PH still experience symptoms such as shortness of breath or desaturation during exercise, highlighting the limitations of resting assessments [1]. The 2022 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines introduced algorithms and criteria for diagnosing exercise‐induced PH and acknowledged the need to evaluate PH dynamics during physical activity [2]. Exercise‐induced PH encompasses various pathophysiological mechanisms, including pre‐capillary PH originating from pulmonary arterial or right heart dysfunction and post‐capillary PH deriving from left heart dysfunction. These distinctions are characterised by specific haemodynamic criteria, such as the change rate of mean pulmonary artery pressure (mPAP)/cardiac output (CO) > 3 for pre‐capillary PH or pulmonary capillary wedge pressure (PCWP)/CO > 2 during exercise for post‐capillary PH [2]. Additionally, the 2021 Heart Failure Association (HFA) of ESC outlined recommendations for diagnosing heart failure with preserved ejection fraction (HFpEF) using exercise stress tests, such as observing peak PCWP ≥ 25 mmHg during exercise [3]. Consequently, this study defined exercise‐induced post‐capillary PH (post‐EIPH) as PCWP/CO > 2 and peak PCWP during exercise to investigate its underlying mechanisms and potential treatment avenues.
Despite the pressing need, there is a lack of clinical trials exploring treatment options for post‐EIPH. The 2022 ESC/ERS guideline advises against the use of certain medications, including angiotensin‐converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARBs), angiotensin receptor‐neprilysin inhibitors (ARNIs), sodium‐glucose cotransporter‐2 (SGLT2) inhibitors, beta‐blockers, or ivabradine in patients with pulmonary arterial hypertension (PAH), unless necessitated by comorbidities [2]. Among these medications, SGLT2 inhibitors have demonstrated efficacy beyond type 2 diabetes or chronic heart failure, with guidelines from the Kidney Disease Improving Global Outcomes (KDIGO), international societies, and the Japanese Society of Nephrology endorsing their use to attenuate the decline in the estimated glomerular filtration rate (eGFR) in chronic kidney disease (CKD), partly through diuretic and natriuretic effects or blood pressure reduction [4]. Additionally, robust evidence suggests that SGLT2 inhibitors offer prognostic benefits in patients with heart failure associated with CKD [5]. Therefore, this study aimed to explore the impact of SGLT2 inhibitors in patients with CKD and post‐EIPH and to assess their cardiac performance using cardiac magnetic resonance imaging (MRI).
1. Methods
1.1. Study Design and Participants
To investigate the association between exercise‐induced haemodynamic alterations and parameters measured by MRI, right heart catheterisation during exercise was conducted in 79 patients with borderline PH (mPAP 15–30 mmHg) and those experiencing symptoms during exertion at Tohoku University Hospital in Japan between 1 July 2022 and 31 December 2023. Patients were diagnosed or suspected with PH based on contrast‐enhanced lung computed tomography, perfusion lung scintigraphy, echocardiography, and right heart catheterisation, following the guidelines established by the ESC and ERS in 2015 and 2022 [2, 6]. Before right heart catheterisation or MRI, enroled patients received diuretics and appropriate targeted drugs for PH. The hemodynamic and MRI data obtained during follow‐up were compared before and after the initiation of treatment with empagliflozin 10 mg/day for CKD over a period of approximately 6 months (eGFR, 52.0 [44.2, 55.7] mL/min/1.73 m2). The EMPEROR‐Preserved Trial demonstrated that a 10 mg dose of empagliflozin improves clinical outcomes in patients with heart failure and preserved ejection fraction (HFpEF) [7]. Additionally, the 2023 ACC expert consensus recommends SGLT2 inhibitors as the primary treatment for HFpEF, with a target dose of 10 mg daily for empagliflozin [8]. Based on these findings and the preserved LV ejection fraction of all enroled patients, a 10 mg dose of empagliflozin was selected. Throughout this period, no changes were made to the medications or any other treatments, except for the addition of the SGLT2 inhibitor. In Japan, SGLT2 inhibitors were approved for reimbursement for the treatment of heart failure with reduced ejection fraction and CKD in August 2021.
All patients provided written informed consent for the use of their clinical data in this study, and the study protocol adhered to the principles of the Declaration of Helsinki and was approved by the Medical Ethics Review Committee of Tohoku University Graduate School of Medicine (approval no. 2021‐1‐684).
1.2. Right Heart Catheterisation During Exercise Using Bicycle Ergometer
The patients underwent right‐sided heart catheterisation and ventilatory gas analysis at rest and during exercise. Ventilatory gas analysis was performed using an expired gas analyser (AE‐100i; Minato Medical Science, Osaka, Japan) [7]. A Swan‐Ganz catheter (Edwards Life Sciences, Irvine, CA, USA) was inserted through the right internal jugular vein and placed in the right proximal pulmonary artery, while an arterial line was placed in the left radial artery. Exercise was performed using a bicycle ergometer in the supine position. Resting haemodynamics were evaluated with the feet on the bicycle pedals before exercise. The exercise protocol consisted of 1 min of unloaded cycling, followed by graded 20‐watt increments in workload at 1‐min intervals until self‐reported exhaustion, determining the peak workload and VO2 [9]. At peak workload, all measurements were taken within 1 min of the same workload. Before exercise and at peak workload, hemodynamic parameters were measured, including mPAP, PCWP, right atrial pressure (RAP), arterial blood pressure (BP), heart rate (HR), CO, mean aortic pressure (mAoP), and pulmonary vascular resistance (PVR). CO was calculated using the direct Fick method, utilising the results of expired gas and arterial/mixed venous blood gas analyses, and corrected for body surface area (cardiac index, CI). PCWP and RAP were measured at end expiration, and mPAP was calculated using the average systolic and diastolic PAP of consecutive 10 beats. According to the ESC and ERS guidelines, the mPAP/CO slope or PCWP/CO slope was calculated using the 2‐point method [(mPAP peak – mPAP foot on pedal)/(CO peak – CO foot on pedal)] or [(PCWP peak – PCWP foot on pedal)/(CO peak – CO foot on pedal)] [2].
1.3. MR Image Acquisition and Analysis
All MRI examinations without contrast media were performed using a 3.0‐T MR scanner (MAGNETOM Vida, Siemens Healthineers, Erlangen, Germany). Cardiac cine MR (CMR) images were acquired using a balanced steady‐state free precession sequence (echo time, 1.43 ms; repetition time, 3.26 ms; flip angle, 47°; slice thickness, 8 mm; and no. phases per 1 cardiac cycle, 25; matrix size, 155 × 224). A stack of short‐axis planes covering the entire left and right ventricles, as well as the four‐chamber and longitudinal two‐chamber planes, were imaged. CMR images were analysed using a dedicated workstation software (cvi42, Circle Cardiovascular Imaging, Montreal, Canada). Volumetric parameters of the left ventricle (LV) and right ventricle (RV), including the ejection fraction (EF) and end‐diastolic volume, index (EDVI), end‐systolic volume, index (ESVI), stroke volume, index (SI), and indices (CI), were measured using short‐axis cine images. Myocardial strain analysis included global radial strain (GRS) and circumferential strain (GCS) of the LV and RV derived from short‐axis images, as well as global longitudinal strain (GLS) derived from two‐ and four‐chamber images [10]. Additionally, the GLS of the RV, left atrium (LA), and right atrium (RA) were evaluated using four‐chamber images. Interobserver reproducibility analysis was performed by a cardiologist and radiologist (T.S. and S.H., with 14 and 11 years of experience, respectively) who independently measured the GLS of each chamber in 10 patients.
1.4. Case Definitions
According to the ESC/ERS guidelines 2022, a PCWP/CO slope > 2 indicates differentiated pre‐ and post‐capillary causes of exercise‐induced PH [2]. Additionally, the HFA of ESC 2021 specifies that a peak PCWP ≥ 25 mmHg during exercise is a criterion of HFpEF for high‐risk patients [3]. Combining these criteria, in this study, post‐EIPH is defined as a PCWP/CO slope > 2 and peak PCWP ≥ 25 mmHg during exercise.
1.5. Statistical Analyses
The results are expressed as mean ± standard deviation (SD). A two‐tailed Student's t‐test was applied to compare means between two groups with equal variances, and the Mann–Whitney U test was used for unequal variances [11]. Paired t‐tests were used for hemodynamic changes before and after treatment with SGLT2 inhibitor.
Statistical significance was set at a two‐sided p‐value < 0.05. Statistical analyses were conducted using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA) and R (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
2. Results
2.1. Characteristics of Patients With CKD and Post‐EIPH
Table 1 presents the baseline characteristics of patients with post‐EIPH and CKD. In patients with post‐EIPH and CKD, resting PCWP was 10.7 ± 1.9 mmHg and mean PAP was 21.5 ± 1.8 mmHg (Figure 1). During exercise, these patients showed increased PCWP/CO slope and peak PCWP (PCWP/CO slope: 3.9 ± 1.2; peak PCWP during exercise: 27.8 ± 2.1 mmHg) (Figure 1).
Table 1.
Baseline characteristics.
| Before administering SGLT2 inhibitor | After administering SGLT2 inhibitor | p‐value | |
|---|---|---|---|
| Number | 10 | 10 | |
| Age (years) | 67.8 ± 10.0 | ||
| Female (n, %) | 6 (60.0) | ||
| BMI (kg/m2) | 26.5 ± 4.8 | 25.6 ± 4.1 | 0.057 |
| eGFR (mL/min/1.73 m2) | 52.0 (44.2, 55.7) | 56.0 (44.7, 6152) | 0.101 |
| BNP (pg/mL) | 13.1 (7.9, 31.2) | 17.3 (11.6, 21.5) | 0.702 |
| 6MWD | 489.9 ± 80.2 | 568.3 ± 91.9 | 0.014 |
| Aetiology | |||
| Non‐PH | 1 (10) | ||
| Group 1 | 0 (0) | ||
| Group 2 | 0 (0) | ||
| Group 3 | 0 (0) | ||
| Group 4 | 8 (80) | ||
| Group 5 | 1 (10) | ||
| Medications (n, %) | |||
| Phosphodiesterase type 5 inhibitors | 0 (0) | ||
| sGC stimulator | 5 (50) | ||
| Endothelin receptor antagonists | 0 (0) | ||
| PGI2 receptor agonists | 1 (10) | ||
Note: Paired t‐tests were used for the changes in the patients before and after treatment with SGLT2 inhibitor.
Abbreviations: BMI, body mass index; BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; 6MWD, 6‐min walking distance; PG12, prostaglandin I2; PH, pulmonary hypertension, sGC, soluble guanylate cyclase.
Figure 1.
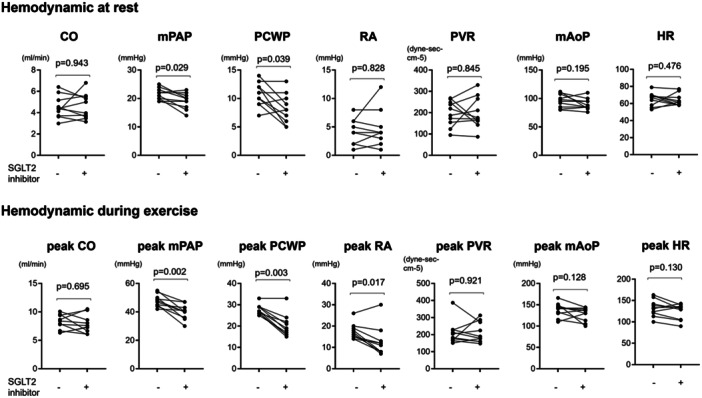
Haemodynamic at rest and during exercise before and after treatment with SGLT2 inhibitor. Paired t‐test were used for hemodynamic changes before and after treatment with SGLT2 inhibitor. CO, cardiac output; HR, heart rate; mAoP, mean aortic pressure; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrium; SGLT2, sodium‐glucose cotransporter‐2.
In the patients with post‐EIPH and CKD, the mean age was 67.8 ± 10.0 years old, and 60% of them were female. Their eGFR was reduced to a median of 52.0 [44.2, 55.7] mL/min/1.73 m2 (Table 1). These patients had a higher body mass index (BMI) (26.5 ± 4.8 kg/m2), considering that the Japanese standard BMI is < 25 kg/m2. The prevalence of Group 4 PH (80%) was highest in this cohort. Some patients were treated with pulmonary vasodilators as follows: sGC stimulators (5/10 patients, 50%) or PGI2 receptor agonists (1/10 patients, 0%) (Table 1).
For the assessment of cardiac function, echocardiography and cardiac MRI were performed to assess cardiac function. The echocardiography data showed normal left and right ventricular systolic/diastolic performance in the cohort (Table 2). However, MRI revealed a decrease in left ventricular GLS (LVGLS) in patients with post‐EIPH (−13.8 ± 2.0%) (Table 2).
Table 2.
Hemodynamic data before and after SGLT2 inhibitor treatment.
| Before administering SGLT2 inhibitor | After administering SGLT2 inhibitor | p‐value | |
|---|---|---|---|
| Number | 10 | 10 | |
| Echocardiography | |||
| LVEF (%) | 68.9 ± 3.2 | 69.0 ± 5.4 | 0.864 |
| LVDd (mm) | 44.1 ± 4.1 | 45.7 ± 3.8 | 0.163 |
| LA (mm) | 34.3 ± 7.4 | 37.6 ± 7.5 | 0.228 |
| E/e’ | 8.3 ± 2.9 | 10.5 ± 3.5 | 0.246 |
| RVFAC (mm) | 39.4 ± 2.7 | 38.4 ± 6.8 | 0.479 |
| TAPSE | 22.2 ± 3.1 | 21.2 ± 4.6 | 0.089 |
| TRPG (mmHg) | 36.9 ± 14.8 | 37.5 ± 13.4 | 0.132 |
| MRI | |||
| LVEDVi | 70.1 ± 15.3 | 75.7 ± 20.4 | 0.121 |
| LVESVi | 29.5 ± 7.5 | 31.0 ± 7.5 | 0.348 |
| LV: GLS | −13.8 ± 2.0 | −17.3 ± 2.0 | 0.003 |
| LV: GRS | 25.1 ± 2.6 | 29.3 ± 6.3 | 0.070 |
| LV: GCS | −18.8 ± 2.0 | −20.3 ± 3.1 | 0.095 |
| RVEDVi | 79.0 ± 16.9 | 80.9 ± 24.6 | 0.634 |
| RVESVi | 36.4 ± 16.1 | 36.7 ± 12.4 | 0.893 |
| RV: GLS | −13.8 ± 2.1 | −15.2 ± 3.3 | 0.284 |
| Peak LA strain | −14.1 ± 2.9 | −17.3 ± 5.0 | 0.297 |
| Peak RA strain | −15.8 ± 5.6 | −14.2 ± 5.4 | 0.105 |
Note: Paired t‐test were used for changes in parameters of echocardiography and MRI before and after treatment with SGLT2 inhibitor.
Abbreviations: GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; LA, left atrium; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; LVDd, left ventricular diameter at end‐diastole; LVEDVi, left ventricular end‐diastolic volume index; LVESi, left ventricular end‐systolic volume index; RA, right atrial; RVFAC, right ventricular fractional area change; TAPSE, tricuspid annular plane systolic excursion; TRPG, tricuspid regurgitation peak gradient.
2.2. Treatment of Post‐EIPH With SGLT2 Inhibitor and MRI Findings
At rest, treatment with SGLT2 inhibitors notably reduced the PCWP (before vs. after; 10.7 ± 1.9 vs. 8.2 ± 2.4 mmHg, p = 0.039) and mPAP (before vs. after; 21.5 ± 1.9 vs. 19.1 ± 2.6 mmHg, p = 0.029), indicating an additional improvement in borderline PH at rest (Figure 1).
During exercise, following SGLT2 inhibitor treatment, patients with CKD exhibited a significant reduction in exercise‐induced increase in peak PCWP (before vs. after; 27.8 ± 2.1 vs. 20.1 ± 5.1 mmHg, p < 0.001), peak mPAP (before vs. after; 47.0 ± 2.0 vs. 38.6 ± 5.7 mmHg, p = 0.002), and peak RA strain (before vs. after; 17.4 ± 3.4 vs. 12.7 ± 6.5 mmHg, p = 0.005) during exercise, while CO, PVR, and mAoP did not exhibit significant changes (Figure 1). Consistently, PCWP/CO slope was significantly decreased (before vs. after; 3.9 ± 1.2 vs. 2.4 ± 1.2 mmHg/L/min, p = 0.013), and mPAP/CO slope showed a decreasing trend (before vs. after; 6.5 ± 3.5 vs. 4.6 ± 1.2 mmHg/L/min, p = 0.105) (Figure 2). Similar patterns of decrease in PAWP/CO slope were seen when the population was divided to female and male groups, while all data was not statistically significant due to the small numbers (Supporting Information S1: Figure II). These data suggest that treatment with SGLT2 inhibitors primarily mitigated exercise‐induced pressure overload on the left side of the heart, leading to a reduction in the pressure transmitted to the pulmonary arteries and RV (Central illustration 1).
Figure 2.
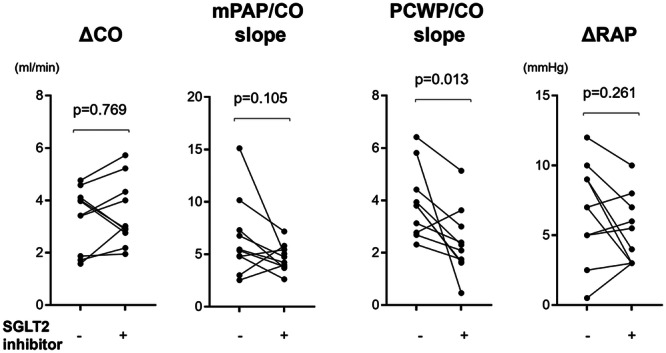
Changes in mPAP/CO and PCWP slopes before and after treatment with SGLT2 inhibitor. Paired t‐test were used for hemodynamic changes before and after treatment with SGLT2 inhibitor. CO, cardiac output; mPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; SGLT2, sodium‐glucose cotransporter‐2.
Central Illustration 1.
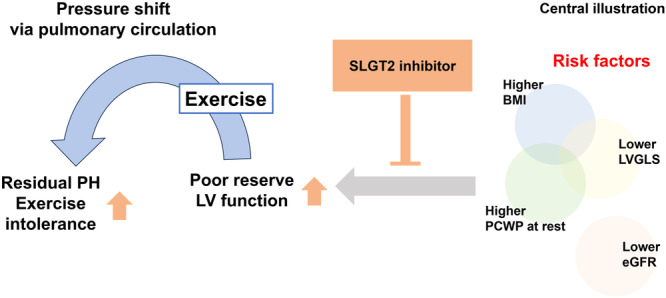
Effect of SGLT2 inhibition on exercise‐induced post‐capillary pulmonary hypertension. In this study, patients with exercise‐induced post‐capillary PH (post‐EIPH) exhibited higher BMI, increased PAWP at rest, and lower LVGLS. Those risk factors would lead to poor reserve of left ventricular function, which exacerbate pulmonary hypertension during exercise. This study demonstrated that SGLT2 inhibitor treatment alleviated haemodynamic abnormalities and exercise intolerance associated with post‐EIPH. BMI, body mass index; CO, cardiac output; eGFR, estimated glomerular filtration rate; LV, left ventricular; LVGLS, left ventricular global longitudinal strain; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; SGLT2, sodium‐glucose cotransporter‐2.
Furthermore, the 6‐min walking distance was extended following treatment with SGLT2 inhibitors (before vs. after; 489.9 ± 80.2 vs. 568.3 ± 91.9 m, p = 0.014), indicating an improvement in exercise tolerance (Figure 2). Although BMI exhibited a notable reduction after SGLT2 inhibitor treatment (before vs. after; 26.5 ± 4.87 vs. 25.6 ± 4.1 kg/m2, p = 0.057), this change was statistically not significant (Table 1).
Regarding cardiac function, MRI demonstrated a significant increase in LVGLS following SGLT2 inhibitor treatment (before vs. after; −13.8 ± 2.0 vs. −17.3 ± 2.0%, p = 0.003), with a tendency toward improvement in LVGRS (before vs. after; 25.8 ± 2.6 vs. 29.3 ± 6.3%, p = 0.074) and LVGCS (before vs. after; −18.8 ± 2.0 vs. −20.3 ± 3.1%, p = 0.097). The RVGLS remained unaltered after treatment with SGLT2 inhibitors (Table 2). These findings suggest that SGLT2 inhibitor treatment may primarily improve potential LV dysfunction rather than right cardiac function (Central illustration).
3. Discussion
The major finding of this study was that SGLT2 inhibitor treatment alleviated haemodynamic abnormalities and exercise intolerance associated with post‐EIPH and enhanced cardiac performance, as assessed through cardiac MRI in patients with concomitant CKD.
3.1. Enhancing Criteria for Post‐EIPH
While the ESC/ERS guidelines outline criteria for exercise‐induced PH, a significant number of patients may unexpectedly exhibit a PCWP/CO slope > 2, possibly due to inadequate exercise stress or inappropriate testing methods [7]. Additionally, the guideline primarily focuses on populations with a background of HFpEF, overlooking other conditions such as PAH, left ventricular systolic dysfunction, or congenital heart disease. For instance, studies have shown that patients with Fontan physiology or chronic thromboembolic pulmonary hypertension (CTEPH) may demonstrate a PCWP/CO slope > 2 or mPAP/CO > 3 mmHg/L/min, even in resting or borderline PH conditions [9, 12]. Furthermore, older patients with PAH often exhibit a PCWP/CO slope > 2 at relatively low exercise loads compared with younger patients [13]. Thus, the current criteria for exercise‐induced PH based solely on the mPAP/CO and PCWP/CO slopes may underestimate individual conditions. Therefore, this study proposes adding “peak PCWP during exercise ≥ 25 mmHg” to “PCWP/CO slope > 2” for defining post‐EIPH. This addition is supported by the recommendations of invasive haemodynamic assessment for high‐risk populations from the HFA of the ESC [3]. In this study, patients who met the new combined criteria exhibited lower LV function, as indicated by LVGLS, LVGRS, or LVGC. These findings suggest that this augmented criterion may better identify patients with potential LV dysfunction possibly associated with post‐EIPH.
3.2. LVGLS as a Marker of Ventricular Dysfunction in Post‐EIPH
The study revealed that patients with post‐EIPH showed lower LVGLS (−13.8%), implying that diminished LVGLS could be a crucial marker of compromised LV function, irrespective of the underlying conditions such as HFpEF or borderline PH. In exploring LV dysfunction and biventricular interactions in patients with PH, a previous study demonstrated that RV dysfunction could precipitate LV dysfunction [14]. Progressive RV dilation may contribute to mechanical dysfunction of the LV. This abnormal RV dilation predominantly affects the septal segments of the LV, resulting in impaired longitudinal and radial LV strain components despite a normal LV ejection fraction. These mechanisms likely lead to a reduced global filling capacity and an increase in pressure transmitted to the pulmonary circulation and RV during exercise (Central illustration). Further investigations with larger patient cohorts may provide deeper insight into the underlying pathophysiology of these observations.
3.3. Exploring SGLT2 Inhibitors as a Potential Treatment for Post‐EIPH
Current guidelines classify the treatment of left heart failure as Class III in patients with PH [2]. This classification indicates that such a treatment is not generally recommended for patients with PH unless specifically indicated because of comorbidities or other factors. Therefore, the present study emphasises CKD as an important comorbidity and incorporates diagnostic criteria involving exercise‐induced alterations, particularly peak PCWP ≥ 25 mmHg during exercise as potential LV dysfunction [3].
Exercise‐induced alteration or pressure extension gives various stress on the ventricles or vasculature via reactive oxygen species or inflammation [15, 16], which were exacerbated at conditions with metabolic dysfunction [17]. Previous basic research has reported that SGLT2 inhibition modifies soluble guanylate cyclase‐cGMP signalling by improving metabolic dysfunction, thereby alleviating exercise‐induced pulmonary artery dysfunction and PH in HFpEF rat models [17]. Furthermore, while exercise‐induced alterations increase ATP demands [17, 18], SGLT2 inhibitors can reprogramme cardiac substrate metabolism to meet these increased demands through mitochondrial function, or by rebalancing the utilisation of ketone bodies, free fatty acids, and branched‐chain amino acids [19, 20].
Moreover, one of the major factors of exercise‐induced vascular relaxation is shear stress, in which pulmonary endothelial cells have an important role. Especially, nitric oxide is an endothelium‐derived relaxing factors, which is promoted by exercise‐induced shear stress through oxidative stress [21]. In the pulmonary arteries of patients with PH, those pulmonary endothelial cells exhibited an abnormal response to increased fluid shear stress, plausibly due to the dysregulation of nitric oxide, resulting in pulmonary artery remodelling and dysfunction of relaxation [22]. Our findings [17], along with other reports [23], demonstrate that treatment with SGLT2 inhibitors modulates oxidative stress and nitric oxide production in both patients and animal models, which may contribute to the improvement of exercise‐induced post‐pulmonary hypertension observed in patients in this study.
In this study, one patient did not improve in mPAP/CO and PAWP/CO slopes post‐SGLT2 inhibitor treatment. With a BMI of 32.21 kg/m², her weight and BMI remained unchanged, unlike the trend of BMI reduction seen in the cohort. Her ongoing hyperlipidemia and hypertension may suggest limited hemodynamic benefits from SGLT2 inhibitors in patients with complex metabolic syndrome.
In total, although further research is needed to explore the mechanism of SGLT2 inhibitor on the cardiac/vascular function during exercise, these findings suggest that SGLT2 inhibitors can optimise the cardiac reserve in response to exercise‐induced alterations.
Recent clinical research suggests that SGLT2 inhibitors can improve LVGLS in patients with type 2 diabetes [24], possibly due to their diverse effects on metabolism, haemodynamic, inflammation, fibrosis and endothelial function. Our study observed a similar trend; treatment with SGLT2 inhibitors led to increased LVGLS in patients with post‐EIPH, coinciding with improvements in haemodynamic and exercise intolerance associated with post‐EIPH. Furthermore, in the present study, treatment with SGLT2 inhibitors resulted in a reduction in the BMI of this population, indicating an improvement in metabolic conditions, potentially contributing to the observed favourable effects.
In this study, the cohort was restricted to patients with CKD, which potentially limited the inclusion of patients with additional comorbidities or confounding factors. Given the multifaceted effects of SGLT2 inhibitors, treatment may modify their condition beyond cardiopulmonary function, possibly resulting in a more pronounced alleviation of post‐EIPH and broader benefits. Future research should aim to assess the impact of SGLT2 inhibitors in a larger population without CKD to elucidate their effects more comprehensively.
To explore the sex difference in effect of SGLT2 on exercise‐induced alteration, the groups were divided according to their sex (Supplemental Figure II). Due to the small numbers of each group (female n = 6, male n = 4), all data was not significant. However, both groups showed a tendency of decreased PCWP/CO slope after the treatment with SGLT2 inhibitor.
The present study had several limitations that warrant consideration. First, it was conducted at a single centre with a relatively small sample size, which limits the generalisability of the findings. The subgroup analyses were underpowered because of the limited number of participants, which may have affected our ability to draw more detailed conclusions. Additionally, most patients in this cohort had CTEPH, which may have influenced the observed outcomes. Furthermore, the use of pulmonary vasodilators among the participants could have altered the relationship between exercise‐induced alterations. Further investigations involving larger and more diverse patient populations are required to validate these findings.
In conclusion, administration of SGLT2 inhibitor alleviated post‐EIPH in patients with concomitant CKD.
Author Contributions
T. Satoh and S. Yasuda designed the study. N. Yaoita, S. Higuchi, S. Yamamoto, H. Sato, K. Nochioka, M. Nakada performed the catheterization and collected the data. S. Higuchi, R. Mori, and H. Ota performed and analysed cardiac MRI. All the authors participated in the development of the manuscript.
Ethics Statement
The study protocol was approved by the Medical Ethics Review Committee of Tohoku University Graduate School of Medicine (approval no. 2021‐1‐684).
Conflicts of Interest
Y.S. has received grant support from Takeda Pharmaceutical, Abbott, and Boston Scientific and lecture fees from Daiichi Sankyo and Bristol Myers Squibb. The remaining authors have nothing to disclose.
Supporting information
Supporting information.
Supporting information.
Acknowledgments
The authors wish to thank the staff and participants, Kaori Miyamura and Takeshi Kato of the Tohoku Graduate School of Medicine for their important contributions. The present study was supported by Japan Agency for Medical Research and Development under grant number 22ek0210149h0003.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Kovacs G., Avian A., Tscherner M., et al., “Characterization of Patients With Borderline Pulmonary Arterial Pressure,” Chest 146 (2014): 1486–1493. [DOI] [PubMed] [Google Scholar]
- 2. Humbert M., Kovacs G., Hoeper M. M., et al., “ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension,” European Heart Journal 2022, no. 43 (2022): 3618–3731. [DOI] [PubMed] [Google Scholar]
- 3. Pieske B., Tschöpe C., de Boer R. A., et al., “How to Diagnose Heart Failure With Preserved Ejection Fraction: The HFA‐PEFF Diagnostic Algorithm: A Consensus Recommendation From the Heart Failure Association (HFA) of the European Society of Cardiology (ESC),” European Journal of Heart Failure 22 (2020): 391–412. [DOI] [PubMed] [Google Scholar]
- 4. Kidney Disease: Improving Global Outcomes Diabetes Work G , “KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease,” Kidney International 102 (2022): S1–S127. [DOI] [PubMed] [Google Scholar]
- 5. Cho I. J. and Kang S. M., “Angiotensin Receptor‐Neprilysin Inhibitor in Patients With Heart Failure and Chronic Kidney Disease,” Kidney Research and Clinical Practice 40 (2021): 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galie N., Humbert M., Vachiery J. L., et al., “ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT),” European Heart Journal 2016, no. 37 (2015): 67–119. [DOI] [PubMed] [Google Scholar]
- 7. Anker S. D., Butler J., Filippatos G., et al., “Empagliflozin in Heart Failure With a Preserved Ejection Fraction,” New England Journal of Medicine 385 (2021): 1451–1461. [DOI] [PubMed] [Google Scholar]
- 8. Kittleson M. M., Panjrath G. S., Amancherla K., et al., “ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee,” Journal of the American College of Cardiology 2023, no. 81 (2023): 1835–1878. [DOI] [PubMed] [Google Scholar]
- 9. Aoki T., Sugimura K., Terui Y., et al., “Beneficial Effects of Riociguat on Hemodynamic Responses to Exercise in Cteph Patients After Balloon Pulmonary Angioplasty–A Randomized Controlled Study,” IJC Heart & Vasculature 29 (2020): 100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higuchi S., Ota H., Yaoita N., et al., “Update on the Roles of Imaging in the Management of Chronic Thromboembolic Pulmonary Hypertension,” Journal of Cardiology 81 (2023): 297–306. [DOI] [PubMed] [Google Scholar]
- 11. Brener M. I., Grayburn P., Lindenfeld J., et al., “Right Ventricular–Pulmonary Arterial Coupling in Patients With HF Secondary MR,” JACC: Cardiovascular Interventions 14 (2021): 2231–2242. [DOI] [PubMed] [Google Scholar]
- 12. Miranda W. R., Jain C. C., Borlaug B. A., et al. Exercise Catheterization in Adults Post‐fontan With Normal and Abnormal Hemodynamic Criteria: Insights Into Normal Fontan Physiology. European Journal of Heart Failure. 2023. [DOI] [PMC free article] [PubMed]
- 13. Muller J., Mayer L., Schneider S. R., et al., “Pulmonary Arterial Wedge Pressure Increase During Exercise in Patients Diagnosed With Pulmonary Arterial or Chronic Thromboembolic Pulmonary Hypertension,” ERJ Open Research 9, no. 5 (2023): 00379‐2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang H., Wang J., Shi R., et al., “Biventricular Dysfunction and Ventricular Interdependence in Patients With Pulmonary Hypertension: A 3.0‐T Cardiac MRI Feature Tracking Study,” Journal of Magnetic Resonance Imaging 60, no. 1 (2024): 350–362. [DOI] [PubMed] [Google Scholar]
- 15. Li H., Miao W., Ma J., et al., “Acute Exercise‐Induced Mitochondrial Stress Triggers an Inflammatory Response in the Myocardium via NLRP3 Inflammasome Activation With Mitophagy,” Oxidative Medicine and Cellular Longevity 2016 (2016): 1987149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bartolák‐Suki E., Imsirovic J., Nishibori Y., Krishnan R., and Suki B., “Regulation of Mitochondrial Structure and Dynamics by the Cytoskeleton and Mechanical Factors,” International Journal of Molecular Sciences 18 (2017): 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Satoh T., Wang L., Espinosa‐Diez C., et al., “Metabolic Syndrome Mediates ROS‐miR‐193b‐NFYA‐Dependent Downregulation of Soluble Guanylate Cyclase and Contributes to Exercise‐Induced Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction,” Circulation 144 (2021): 615–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wibom R., Hultman E., Johansson M., et al., “Adaptation of Mitochondrial ATP Production in Human Skeletal Muscle to Endurance Training and Detraining,” Journal of Applied Physiology 1992, no. 73 (1985): 2004–2010. [DOI] [PubMed] [Google Scholar]
- 19. Ostrominski J. W. and Vaduganathan M., “Chapter 2: Clinical and Mechanistic Potential of Sodium‐Glucose Co‐Transporter 2 (SGLT2) Inhibitors in Heart Failure With Preserved Ejection Fraction,” The American Journal of Medicine 137 (2024): S9–S24. [DOI] [PubMed] [Google Scholar]
- 20. Choi J., Matoba N., Setoyama D., et al., “The SGLT2 Inhibitor Empagliflozin Improves Cardiac Energy Status via Mitochondrial ATP Production in Diabetic Mice,” Communications biology 6 (2023): 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen H., Chen C., Spanos M., et al., “Exercise Training Maintains Cardiovascular Health: Signaling Pathways Involved and Potential Therapeutics,” Signal Transduction and Targeted Therapy 7 (2022): 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang A. and Valdez‐Jasso D., “Cellular Mechanosignaling in Pulmonary Arterial Hypertension,” Biophysical Reviews 13 (2021): 747–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yaribeygi H., Atkin S. L., Butler A. E., and Sahebkar A., “Sodium‐Glucose Cotransporter Inhibitors and Oxidative Stress: An Update,” Journal of Cellular Physiology 234 (2019): 3231–3237. [DOI] [PubMed] [Google Scholar]
- 24. Gamaza‐Chulián S., Díaz‐Retamino E., González‐Testón F., et al., “Effect of Sodium‐Glucose Cotransporter 2 (SGLT2) Inhibitors on Left Ventricular Remodelling and Longitudinal Strain: A Prospective Observational Study,” BMC Cardiovascular Disorders 21 (2021): 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


