Abstract
Background
Several conditions such as infertility, repeated implantation failure, and recurrent pregnancy loss can pose challenges in early pregnancy. These issues can be caused by the abnormal inflammatory response with various factors, including exogenous and endogenous agents, and pathogenic and nonpathogenic agents. In addition, they can be exacerbated by maternal immune response to the abovementioned factors.
Methods
This review aimed to assess the detrimental inflammatory effects of chronic endometritis, endometrial microbiota disturbance, and maternal immune system abnormalities on early pregnancy. Further, essential details such as ovulation, implantation, trophoblast invasion, and placental formation, were examined, thereby highlighting the beneficial roles of inflammation.
Main Findings
Excessive inflammation was associated with various early pregnancy disorders. Meanwhile, a lack of appropriate inflammation could also contribute to the development of different early pregnancy complications.
Conclusion
Excessive inflammation and insufficient inflammation can possibly lead to abnormal conditions in early pregnancy, and appropriate inflammation is required for a successful pregnancy.
Keywords: chronic endometritis, endometrial microbiota, inflammation, recurrent pregnancy loss, repeated implantation failure
Not only excess inflammation but also lack of inflammation can contribute to the development of complications in early pregnancy. Appropriate inflammation is required for successful offspring.
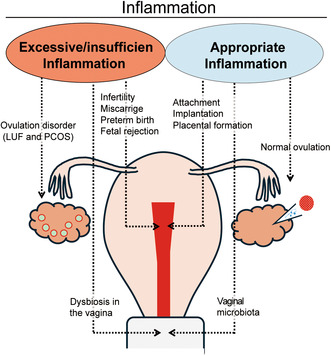
1. INTRODUCTION
Inflammation plays an important role in the defense mechanisms of hosts against microbial infections. It includes the phagocytic activity of macrophages and neutrophils during bacterial infections, and the recognition, activation, and cytotoxic functions of T cells and B cells in response to viral infections. These immune cells are essential for eliminating microbes. Further, inflammation that is not involved in pathogen infection is important. Independent of pathogen infection, abnormal, excessive, or suppressed inflammation is commonly observed in various pathologies, including cancer, 1 cardiovascular diseases, 2 , 3 , 4 diabetic kidney disease, 5 and pulmonary disorders. 6 Inflammation without evident pathogenic infection, referred to as sterile inflammation, involves the collaboration of the innate and acquired immune systems to regulate inflammatory reactions. These inflammatory abnormalities are often triggered by exogenous nonpathogenic agents, such as silica, asbestos, and particulate matter 2.5 (PM 2.5). 7 Thus, inflammation, whether due to infection or not, serves as a biological response that maintains homeostasis in the body in response to various stimuli.
Inflammatory responses are generally triggered by alarmins, which include infection‐related molecules and microparticles. Pathogen‐associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), peptidoglycan, flagellin, and viral particles, can function as alarmins. 8 , 9 These molecules are recognized by the immune system, which then triggers an inflammatory response that alerts the body to the presence of pathogens. As alarmins, PAMPs activate immune cells and initiate a cascade of immune responses, helping in the defense against infections. When cells are damaged, endogenous molecules, such as high‐mobility group box 1 (HMGB1), interleukin (IL)‐1α, IL‐33, heat shock proteins, and S100 proteins, are released into the extracellular space. These molecules, known as damage‐associated molecular patterns (DAMPs), play a critical role in triggering and inducing inflammation. They are also referred to as inflammatory alarmins. 9 Various factors can trigger the release of DAMPs during pregnancy, including hypoxia, ischemia, vascular dysfunction, and oxidative stress associated with placental insufficiency. 10 Additional factors include maternal alcohol consumption, smoking, poor nutrition, and trauma. The abovementioned nonpathogenic exogenous nanoparticles, such as asbestos, silica, PM 2.5, microplastics, also act as alarmins. Recently, the concept of resolution‐associated molecular patterns (RAMPs) has gained attention. Proteins such as heart shock protein (HSP)10, HSP27, HSPA5, HSPB5, and phosphatidylserine have been shown to be a type of RAMPs. 11 While HMGB1 is considered a DAMP, oxidized HMGB1, in which the cysteine of HMGB1 has been oxidized, has anti‐inflammatory properties as a RAMP. 12 RAMP stimulation can resolve excessive inflammation induced by DAPMs and PAMPs. 13
Alarmins are detected by pattern recognition receptors (PRRs) such as Toll‐like receptors (TLRs), C‐type lectin receptors, NOD‐like receptors, and receptors for advanced glycation end‐products mainly in antigen‐presenting cells, macrophages, and dendritic cells (DCs). 14 , 15 , 16 , 17 If these cells detect antigens via their PRRs, they can influence T cells and other effector cells either via direct interaction between the T‐cell receptor and the major histocompatibility complex or via indirect pathways involving cytokine networks. Various inflammatory responses in vivo are induced, starting with the response of alarmins and the PRRs that receive them.
At the fetal–maternal interface, different immune cells are present. Further, each type of cells is skillfully orchestrated, and they work together to maintain pregnancy. Therefore, if this immune imbalance occurs, various pregnancy complications are triggered. The onset of preterm labor/birth is significantly strongly associated with both pathogenic and nonpathogenic excess inflammation. 18 , 19 , 20 , 21 Preeclampsia, which is characterized by hypertension, proteinuria, and edema, involves an excessive maternal inflammatory response without microbial infection. 22 , 23 , 24 , 25 Excessive inflammation can also cause infertility, repeated implantation failure (RIF), recurrent pregnancy loss (RPL), and miscarriage in the early pregnancy period. 26 , 27 , 28 Notably, excessive inflammation induces these pregnancy complications. However, an appropriate level of inflammation is also required for a successful offspring production. Labor onset is caused by uterine inflammation. 29 Hence, an extremely powerful inflammation is required in late pregnancy and parturition. In early pregnancy, an appropriate level of inflammatory response is required for the attachment and implantation of an embryo, infiltration of trophoblast cells into the maternal tissue, and placentation (Figure 1 and Section 2.2). 30 , 31 , 32 Hence, the retention of a semiallogenic fetus in the maternal body requires the establishment of an anti‐inflammatory environment (i.e., maternal immune tolerance). However, an insufficient inflammation also occasionally contributes to the development of early pregnancy complications (Figure 1 and Section 2.2.3). Further, the mother must have a good immune system to eliminate infectious pathogens to protect the fetus. Thus, during the limited gestation period, the maternal immune system requires prompt alteration in the inflammatory/anti‐inflammatory environment in response to pathological conditions. 33
FIGURE 1.
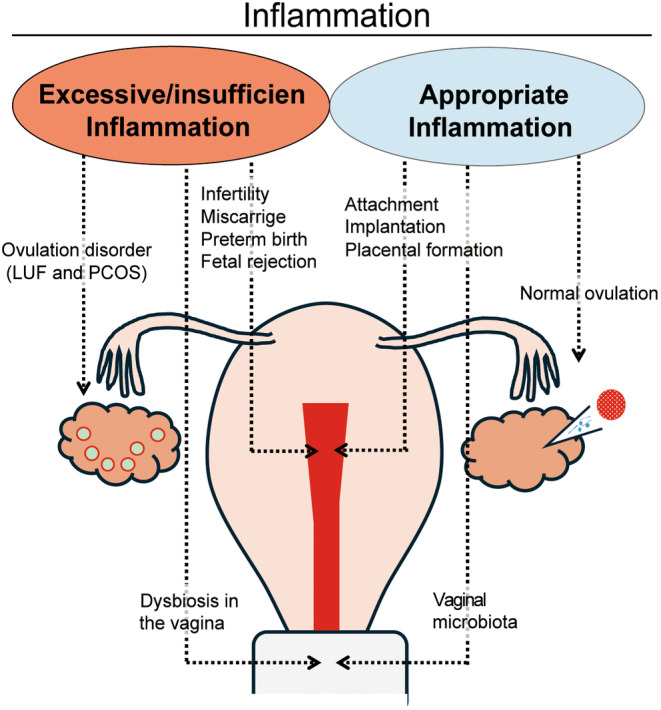
Dual nature of inflammation and its associated pathologies/phenomena during pregnancy. Excessive/insufficient inflammation leads to pregnancy complications. However, an appropriate degree of inflammation is required for a normal pregnancy process.
This review aimed to assess the beneficial and harmful effects of inflammation in the pathogenesis of various diseases in early pregnancy.
2. BENEFICIAL EFFECTS OF INFLAMMATION
2.1. Role of inflammation in ovulation
2.1.1. Appropriate inflammation in ovulation
The notion that ovulation involves an inflammatory response was first proposed by Espey 34 and has since become widely accepted. 35 Ovulation is triggered by a surge in luteinizing hormone (LH). This surge leads to the invasion of blood vessels into the granulosa cell area and disrupts the basal lamina of these cells, thereby allowing the infiltration of theca cells and leukocytes. After the dilation and increased permeability of these vessels, the cumulus–oocyte complex detaches from the surrounding granulosa cells and expands (cumulus expansion). The follicle eventually deteriorates and ruptures, releasing the cumulus‐enclosed oocyte. Then, the damaged tissue is repaired.
In essence, ovulation represents a complex interaction involving the oocyte, granulosa cells, theca cells, endothelial cells, and both resident and infiltrated immune cells. This process is accompanied by the secretion of various inflammatory mediators, including prostaglandins, reproductive hormones, matrix metalloproteinases, cytokines, and chemokines. The preovulatory follicle contains several inflammatory cytokines such as IL‐1, IL‐2, IL‐6, TNF‐α, granulocyte–macrophage colony‐stimulating factor, and macrophage colony‐stimulating factor. 36 , 37 , 38 , 39 , 40 , 41 The LH surge stimulates the production of chemokines that attract immune cells including neutrophils, monocytes, macrophages, natural killer (NK) cells, B cells, and T cells. 42 , 43 , 44 , 45 , 46 , 47 Macrophages release cytokines and chemokines that help in the migration of other immune cells. Thus, they are essential for follicular growth and rupture. 48 , 49 , 50 Ovarian DCs are important for cumulus expansion, ovulation, and management of ovulation‐related inflammatory response. 51 Neutrophils, which are recruited by bone morphogenetic protein 6, secrete cytotoxic peptides and proteases that degrade the follicular wall. 52 NK cells and their chemokine receptors play roles in ovulation and angiogenesis. 42 , 53
2.1.2. Inappropriate inflammation in ovulation
Luteinized unruptured follicle (LUF) is a common condition in individuals with infertility. It is characterized by the lack of follicular rupture that results in ovulatory dysfunction. 54 , 55 , 56 LUF may be associated with disruptions in the inflammatory processes involved in ovulation. In humans, the granulocyte‐colony stimulating factor (G‐CSF) levels in the peripheral blood increase during the late follicular phase of a normal ovulatory cycle. 57 Further, LUF can be alleviated by administering G‐CSF during a clomiphene citrate cycle. 58 , 59 In animal models, the systemic depletion of neutrophils leads to a reduced ovulation rate. 60 These findings indicate that an inappropriate inflammatory response by granulocytes can contribute to LUF development (Figure 1).
Nevertheless, excessive inflammation of course has a harmful effect on ovulation. Polycystic ovary syndrome (PCOS), a major cause of infertility, is associated with endocrine abnormalities, polycystic ovarian morphology, and ovulatory dysfunction. 61 Further, it is characterized by systemic low‐grade inflammation. 62 , 63 Patients with PCOS have elevated levels of C‐reactive protein and inflammatory cytokines. 64 , 65 , 66 The high levels of HMGB1 in adolescents with PCOS can be reduced by anti‐inflammatory treatments such as myo‐inositol and alpha‐lipoic acid. 67 Excessive inflammatory responses may contribute to the ovulatory dysfunction observed in PCOS (Figure 1). The inflammation involved in ovulation is typically sterile. However, infections caused by pathogens can impair follicular growth. Granulosa cells, which express LPS receptors such as TLR4, CD14, and MD‐2, exhibit disrupted estradiol production and follicular growth failure when exposed to LPS in animal models. 68 , 69
2.2. Appropriate inflammation in early pregnancy
2.2.1. Appropriate inflammatory cytokine
As mentioned in the previous text, a maternal immune tolerant and anti‐inflammatory environment is essential for retaining a fetus with semi‐allogeneic antigens. However, recent findings have revealed that, in addition to maternal tolerance to fetal allogeneic antigens, an adequate level of inflammation is also required for successful embryo attachment, implantation, and placentation during the early pregnancy period. Previously, inflammation represented by the secretion of IL‐1, IL‐6, IL‐17, interferon gamma (IFN‐γ), and tumor necrosis factor alpha (TNF‐α) is believed to have a harmful effect on the maintenance of pregnancy. However, attachment, implantation, and placentation require adequate levels of these inflammatory cytokines. 30 , 31 In humans, proinflammatory responses, such as the secretion of IL‐6, IL‐8, and TNF‐α, are essential for achieving uterine receptivity. 70 , 71 , 72 In addition, increased levels of IL‐12, IL‐1β, TNF‐α, IL‐6, and nitric oxide facilitate embryo attachment to the decidua. 32 Elevated levels of IFN‐γ, a key Th1 cytokine, are associated with early pregnancy complications. 73 , 74 , 75 Excessive IFN‐γ production caused by increased inflammation can result in fetal rejection. Nevertheless, IFN‐γ also plays a role in modifying uterine vascularization. 76 , 77 Further, patients with RPL present with a reduced proportion of IFN‐γ‐ and TNF‐α‐positive cells in the endometrium. 77 Therefore, IFN‐γ can also have beneficial effects in sustaining a successful pregnancy. Human chorionic gonadotropin, a glycoprotein hormone produced by the placenta, significantly increases during early pregnancy. It stimulates IL‐8 secretion from monocytes and helps in endometrial differentiation and implantation by modulating immune cell activity. 78 , 79
2.2.2. Inflammatory role of macrophages and DCs
Macrophages exhibit plasticity, and they are classified into M1 and M2 subtypes, each with different inflammatory effects. M1 macrophages have proinflammatory effects, and M2 macrophages have anti‐inflammatory effects. The presence of M1 macrophages is detrimental to the maintenance of pregnancy. However, emerging evidence shows that M1 macrophages may play an important role in initiating an appropriate inflammatory response during parturition and early pregnancy. 32 During the implantation period, macrophages are predominantly polarized toward the M1 phenotype, which is associated with the production of proinflammatory cytokines. 71 After implantation, to help prevent maternal rejection of the fetus as pregnancy progresses, macrophages transition to a mixed M1/M2 phenotype and then to a predominantly M2 phenotype. 32 , 80 Toward the end of pregnancy, the re‐emergence of M1 macrophages, which secrete proinflammatory cytokines, becomes essential for parturition. 32 , 81
In clinical cases, mechanical injury induced by endometrial biopsy leads to an increased production of macrophage inflammatory protein 3 beta, TNF‐α, CXCL1, osteopontin, and IL‐15, accompanied by an abundance of macrophages and DCs. 82 The induction of DC and macrophage accumulation via the upregulation of proinflammatory cytokines enhances implantation rates, in vitro fertilization (IVF) outcomes, and clinical pregnancy rates in patients experiencing unexplained infertility. 82 , 83 , 84 , 85 , 86 DCs are recruited to the uterus before implantation, and they play an important role in modulating the cytokine profile at the fetal–maternal interface. 87 , 88 , 89 , 90 Adequate inflammation driven by DCs is essential for successful implantation and for reducing the risk of miscarriage during the first trimester. 91
Our recent study found reduced levels of DC1s (CD141+ DCs), which are responsible for promoting Th1 polarization, in the uterine septum of patients with a septate uterus with low chemokine levels. 92 Considering that a septate uterus is generally a significant risk factor of RPL, we assumed that a diminished accumulation of DCs with an inflammatory phenotype can result in failed appropriate inflammatory response that creates an immune environment unfavorable for implantation in the uterine septum in early pregnancy.
2.2.3. Approaches according to the endometrial immune profile
Systemic and utero‐local reactions are extremely different; therefore, it is important to investigate the immune profile of the endometrium. The application of various biomarkers is recommended for assessing the immune profile of the human endometrium in patients with RIF. 93 , 94 The IL‐18/tumor necrosis factor‐like weak inducer of apoptosis (TWEAK) mRNA ratio is a useful endometrial biomarker of angiogenesis and Th1/Th2 balance during implantation. 93 , 95 The IL‐15/fibroblast growth factor‐inducible molecule 14 (Fn‐14) mRNA ratio is a biomarker of uterine NK cell activation and maturation. 93 Interestingly, neither over‐immune activation (extremely high) nor low‐immune activation (extremely low) that was evaluated using the IL‐18/TWEAK and IL‐15/Fn‐14 ratio is favorable for pregnancy outcomes. Hence, individualized treatment based on the patient's endometrial condition is required.
3. HARMFUL EFFECTS OF INFLAMMATION
3.1. Chronic endometritis (CE)
The concept of CE is now commonly recognized in clinical practice. CE is defined as inflammation of the endometrium. Clinically, patients with CE are usually asymptomatic or present only subtle symptoms. 96 However, previous studies have revealed that CE is associated with implantation failure and pregnancy loss. 97 , 98 , 99 , 100 The incidence rates of CE in patients with infertility and RPL are approximately 2.8%–39% and 60%, respectively. 101 CE induces endometrial dysfunction and reduces the endometrial receptivity of embryos. Hysteroscopic examinations typically detect stromal edema, thickening of the uterine lining, micropolyps, and focal or diffuse hyperemia in affected patients. 98 , 102 , 103 The pathological feature of CE is the infiltration of CD138 (syndecan‐1)‐positive plasma cells into the endometrial tissue. 98 , 104 , 105 , 106 , 107 Classically, CE is diagnosed by identifying the presence of CD138‐positive plasma cells in the endometrial stroma through immunohistochemical staining for CD138 108 ; however, this finding is not specific, and its effectiveness can vary depending on the menstrual cycle. Given these limitations, complementary methods such as hysteroscopy and microbial culture are frequently used. 101 Bacterial infections, such as Escherichia coli, Streptococcus spp., Staphylococcus spp., Enterococcus faecalis, Corynebacterium, Mycoplasma, and Ureaplasma, are major causes of CE. 98 , 103 Oral antibiotic therapy is the first‐line treatment for CE in patients with pregnancy complications. Notably, antibiotic therapy has been effective in improving implantation, clinical pregnancy, and live birth rates. 102 , 109 This finding supports the role of microbial infection in the pathogenesis of CE. However, the most frequently detected infectious agents at the endometrial level are common bacteria, such as E. coli, Streptococcus spp., and Staphylococcus spp. 103 A previous study evaluated the presence of various pathogens in patients with infertility, with or without CE, using reverse‐transcription polymerase chain reaction. No significant differences were found in the percentage of these pathogens between patients with and without CE. 101 These results indicate that no specific pathogen is responsible for CE development. In general, the dominance of Lactobacillus in the uterus indicates a healthy status (Section 3.2). However, patients with CE had a higher detection rate of Lactobacillus than healthy women. 110 According to these findings, the pathogenesis of CE may involve not only a direct attack by pathogens on the endometrium and embryo but also an abnormal interaction between these pathogens and the immune system in the endometrium. Several theories regarding the immune response related to CE have been proposed. Patients with CE and RIF presented with an abnormal distribution of a NK cell subsets, 111 and patients with CE exhibited elevated levels of IL‐6 and TNF‐α in menstrual effluents. 112 Other studies have reported that autophagy dysregulation promotes the production of proinflammatory cytokines and Th17‐dominant milieu in patients with CE 113 and the accumulation of Th1 cells surrounding CD138‐positive cells in patients with CE. 114 Abnormalities in maternal immune responses toward pathogens may contribute to reproductive failure.
Patients often do not respond to various antibiotic regimens, indicating the involvement of additional factors. Reports indicate that infections caused by viruses, such as herpes simplex virus, 115 , 116 Epstein–Barr virus, 117 and human immunodeficiency virus, 118 , 119 are correlated with CE. Furthermore, other nonpathogenic exogenous agents and endogenous alarmins related to immune disorders may also contribute to CE development.
Collectively, pathogens and the abnormal immune response of the endometrium induced by these pathogens can be responsible for CE development (Figure 2). Further detailed analysis may be required to achieve a more precise understanding of the pathogenesis of CE.
FIGURE 2.
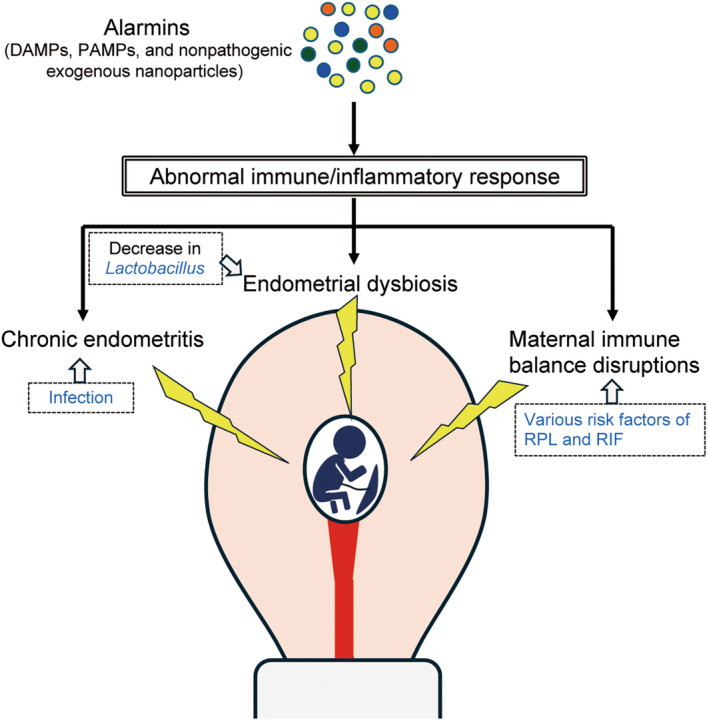
Schematic drawing of complications in early pregnancy via abnormal immune/inflammatory responses. Abnormal immune/inflammatory responses are involved in early pregnancy complications such as chronic endometritis, endometrial dysbiosis, and maternal immune balance disruptions. Alarmins may be deeply involved in triggering these immune/inflammatory abnormalities.
3.2. Endometrial microbiota disturbance
Previously, the uterine cavity is considered a sterile organ under normal conditions. Next‐generation sequencing of the 16S rRNA gene (16S analysis) is widely used to analyze the microbiota in the reproductive tract. Thus, there is now a consensus that the uterus is not a sterile environment. 120 , 121 , 122 The endometrial microbiota, in numerous bacterial species are present, significantly affect pregnancy outcomes. The Lactobacillus dominant microbiota is associated with favorable pregnancy outcomes, pregnancy rate, implantation rate, and live birth rate. Meanwhile, the low abundance of endometrial Lactobacillus is associated with poor reproductive outcomes. 123 , 124 , 125 Thus, investigating the endometrial microbiota has become a more frequent component of infertility evaluations. However, the notion that Lactobacillus dominance is beneficial for pregnancy outcomes remains controversial. The pregnancy outcomes, pregnancy, and implantation or miscarriage rates did not significantly differ in patients undergoing IVF between the Lactobacillus dominant (>80% Lactobacillus) and Lactobacillus nondominant (<80% Lactobacillus) groups. 126 Moreover, pregnancies are maintained even if the percentage of Lactobacillus is extremely low in some cases. 126 However, improving intrauterine dysbiosis, which is an environment with low abundance of Lactobacillus, with antibiotics and prebiotic and probiotic preparations did not improve pregnancy outcomes. 123 As mentioned in Section 3.1, patients with CE who are likely to present with poor pregnancy outcomes have higher Lactobacillus detection rates. 110 Given these conflicting reports, further investigations are needed to understand the effect of Lactobacillus on pregnancy outcomes.
Lactobacillus genus contains some species, L. crispatus, L. gasseris, L. iners, and L. jensenii. A recent in vitro study revealed that L. crispatus promotes trophoblast invasion into the maternal myometrium. 127 L. iners is the most common microbe detected in the endometrium during early pregnancy, and its presence is associated with defense mechanisms and essential functions. 125 However, a study reported that individuals with L. iners as the dominant microbe had the lowest implantation rate after IVF. 128 Lactobacillus produce different isomers of lactic acid. L. crispatus and L. gasseri produce both d‐lactic acid and l‐lactic acid; however, L. iners produces only l‐lactic acid, whereas L. jensenii produces only d‐lactic acid. 129 Importantly, these lactic acids have different acidity and resistance to bacterial infections. L. crispatus induces a lower pH in the vaginal environment and has a strong antibacterial effect on Gardneralla, which causes bacterial vaginosis. These structural differences in lactic acid may induce different immune responses and contribute to changes in the vaginal and uterine environment. To prove the effectiveness of Lactobacillus, the characteristics of pregnancy outcomes must be studied for each of these species.
The uterine cavity contains various innate and acquired immune cells that recognize these bacteria and trigger diverse immune responses. Thus, even with the same bacterial species, differences in individual immune responses are expected (Figure 1). Therefore, pregnancy outcomes may differ even with the same endometrial microbiota. Further investigations should be performed explore the correlation between endometrial dysbiosis and abnormal immune responses, CE, and decidualization and invasion disruptions.
3.3. Immunological disorder
A successful pregnancy requires a delicate balance in the maternal immune system (i.e., the mother's immune tolerance) to the paternal semi‐alloantigens of the fetus. 130 , 131 Disruption of this maternal tolerance can lead to infertility, implantation failure, miscarriage, and premature birth. Thus, maintaining an appropriate balance between Th1 and Th2 cells is crucial during pregnancy. 132 , 133 , 134 , 135 , 136 Recently, the balance between Th17 and regulatory T (Treg) cells, the Th1/Th2/Th17/Treg paradigm, the cytotoxic potential of NK cells, and the involvement of various cytokines have also been identified as important factors. 137 , 138 , 139 , 140 , 141 , 142 , 143 Patients experiencing complications, such as infertility, implantation failure, and early miscarriage often exhibit excessive inflammation characterized by Th1 dominance, Th17 dominance, and elevated NK cell cytotoxicity. Therefore, accurate diagnosis and treatment for these conditions during early pregnancy are essential. To obtain a diagnosis, the Th1/Th2 ratios, Th17/Treg ratios, NK cell activation, immune profiles of the endometrium, and levels of various autoantibodies in peripheral blood must be evaluated (Section 2.2.3). 94
In recent years, various immunotherapeutic approaches aimed at inhibiting excessive inflammation in early pregnancy complications have been proposed. In clinical cases, heparin is used in patients with RPL associated with antiphospholipid antibody syndrome. Some studies have indicated that heparin improves live birth rates in cases of RIF. 144 , 145 Steroid hormones possess powerful anti‐inflammatory and immunosuppressive effects and can induce an increase in Treg expression, 144 decrease in uNK cell expression, 146 and increase in HLA‐G expression in the trophoblasts. 147 , 148 Intravenous immunoglobulin G (IVIg) has powerful immunomodulating functions. IVIg can decrease the frequency and activity of NK cells, antibody production of B cells, and expression of activator receptors in monocytes. In contrast, IVIg can increase the expansion and suppressive function of Tregs. 149 Thus, IVIg has a powerful anti‐inflammatory effect. Recent studies have revealed that high‐dose IVIg administration in early pregnancy improves pregnancy outcomes in women with four or more RPL of unexplained etiology. 150 Intralipid, a fat emulsion containing soyabean oil, glycerol, and egg phospholipids, inhibits NK cell activity and has been found to be effective in patients with reproductive failure. 151 , 152 Additionally, tacrolimus, a major immunosuppressive agent used to prevent organ transplant rejection, has been shown to improve pregnancy outcomes in patients with RIF who present with high Th1/Th2 ratios. 153 , 154 , 155
As outlined, inflammation during pregnancy can have beneficial and harmful effects, and excessive inflammation and insufficient inflammation are risk factors for pregnancy complications. Table 1 shows some representative studies on inflammation and immune function related to early pregnancy, focusing on beneficial/harmful inflammation and immune reaction.
TABLE 1.
Representative studies on inflammation and immune function related to early pregnancy.
| Disease/pathology | Author/years | Description | Roles of inflammation/findings |
|---|---|---|---|
| Ovulation | Duffy (review)/2019 35 | LH a surge and inflammatory response | Beneficial inflammation |
| Buyalos/1992 37 | IL‐6 level in the follicular fluid | Beneficial inflammation | |
| Wang/1992 38 | TNFα in the follicular fluid | Beneficial inflammation | |
| Nishimura/1998 41 | M‐CSF in the follicular fluid | Beneficial inflammation | |
| Al‐Alem/2015 42 | Leukocytes and chemokines | Beneficial inflammation | |
| Kryczek/2005 46 | Chemokine and T cells | Beneficial inflammation | |
| Nio‐Kobayashi/2015 47 | Chemokine and macrophages | Beneficial inflammation | |
| Cohen‐Fredarow/2014 51 | Dendritic cells | Both pro‐ and anti‐inflammatory effects | |
| LUF b | Shibata/2016 59 | Administration of G‐CSF | Beneficial effect of inflammation for preventing LUF |
| Brannstrom/1995 60 | Neutrophils | Beneficial effect of inflammation for preventing LUF | |
| PCOS c | Escobar‐Morreale (review)/2011 63 | C‐reactive protein | Harmful inflammation |
| Cirillo/2020 67 | HMGB1 d | Harmful inflammation induced by HMGB1 | |
| Placenta formation/fetal growth | Griffith/2017 31 | Inflammation at attachment | Beneficial inflammation at attachment of embryo |
| Granot (review)/2012 70 | Inflammatory cytokines and Th1 inflammatory response in implantation | Beneficial inflammation in implantation | |
| Ashkar/2000 76 | IFN‐γ in placental formation | Beneficial role of IFN‐γ | |
| Valero‐Pacheco/2022 166 | IL‐33 in placental formation | Beneficial role of IL‐33 in placenta formation and fetal growth | |
| CE e | Matteo/2009 111 | Abnormal distribution of NK cell subsets | Harmful inflammation |
| Tortorella/2014 112 | Elevation of IL‐6 and TNF‐α levels | Harmful inflammation | |
| Wang/2019 113 | Autophagy dysregulation and Th17 dominancy | Harmful effects of Th17 dominanct in CE | |
| Kitazawa/2021 114 | Accumulation of Th1 cells | Harmful inflammation | |
| Endometrial microbiota disturbance | Moreno/2016 124 | Dominancy of Lactobacillus | Beneficial effects of Lactobacillus |
| Hashimoto/2019 126 | Eubiosis and dysbiosis related to Lactobacillus | No differences between eubiosis and dysbiosis for implantation | |
| Yoshida/2021 127 | L. crispatus | Beneficial role of L. crispatus in trophoblast invasion | |
| Kadogami/2023 128 | L. iners | Low implantation rate in L. iners dominant state | |
| Immunological disorder | Saito (review)/2010 137 | Th1/Th2/Th17/Treg f balance | Pregnancy complications and disruption of immune balance |
| Fukui/2008 143 | NK cell cytotoxicity | Harmful inflammation in RIF g | |
| Ledee (review)/2016 93 | IL‐18/TWEAK h and IL‐15/Fn‐14 i balance | Importance of appropriate balance |
LH, luteinizing hormone.
LUF, luteinized unruptured follicle.
PCOS, polycystic ovary syndrome.
HMGB1, high‐mobility group box 1.
CE, chronic endometritis.
Treg, regulatory T.
RIF, repeated implantation failure.
TWEAK, tumor necrosis factor‐like weak inducer of apoptosis.
Fn, fibroblast growth factor‐inducible molecule 14.
The occurrence of disruptions in maternal immune balance remains unclear. The disruptions of maternal immune balance may cause sterile inflammation induced by DAPMs, endometriosis, environmental factors, metabolic disorders, and pathogen‐associated inflammation induced by CE and PAMPs (Figure 2 and Section 3.4). Maternal immune abnormalities caused by these alarmins may be associated with early pregnancy complications, necessitating further elucidation of the efficacy of the abovementioned therapies and their therapeutic targets.
3.4. Alarmins and complications in early pregnancy
Some abnormal immune responses are expectedly involved in CE development, endometrial microbiota disturbances, and immunological disorders. However, the initial triggering substances for these abnormal immune responses remain unknown. Stimulations by alarmins, PAMPs and DAPMs, are thought to be significant candidates involved in initiating these abnormal immune responses. TLRs, which recognize PAMPs derived from pathogens during bacterial infections such as LPS, are expressed not only in maternal immune cells but also in trophoblasts and play an important role in miscarriage and early placental formation. 156 , 157 Given the involvement of bacterial kinetics in CE onset and endometrial microbiota disturbances, PAMPs derived from bacteria are likely responsible for these complications. Viral infections may also be involved in pregnancy complications. Trophoblasts express TLR3, which recognizes dsRNA, a PAMP of viral origin, and may contribute to pregnancy complications. 158 DAMPs, which are nonpathogenic, can also cause pregnancy complications. 10 HMGB1, one of the DAMPs, is released from damaged cells to induce inflammation. Several reports have suggested that an increase in HMGB1 negatively affects the early stages of pregnancy and is associated with miscarriages and implantation failure. 159 , 160 Additionally, the S100A8 protein, which is a calcium‐binding protein with a molecular weight of 10–13 kDa having two EF‐hand motifs, is present at high levels in the serum of patients with recurrent miscarriage. 161 These DAMPs may contribute to maternal immunological disorders that are unrelated to pathogen infections. DAMPs can also have beneficial effects on pregnancy outcomes. For instance, IL‐33, an IL‐1 family cytokine, is a representative DAMP associated with positive pregnancy outcomes. 162 , 163 , 164 , 165 A recent study revealed that appropriate maternal IL‐33 secretion contributes to placental formation in early pregnancy, leading to healthy fetal growth. 166 This suggests that certain DAMPs induce appropriate inflammation during pregnancy.
CE, endometrial microbiota disturbance, and maternal immune abnormalities induce excessive inflammation, which can lead to various pregnancy complications. Along with the direct stimulation of alarmins, infections, and the various risk factors of RPL and RIF, abnormal immune responses between the mother and fetus can be involved in this phenomenon (Figure 2).
4. CONCLUSIONS
Anti‐inflammatory effects represented by maternal immune tolerance and an appropriate level of proinflammatory response are essential for maintaining pregnancy and ensuring successful offspring. Therefore, rather than using a binary model of inflammation versus anti‐inflammation, the specific functions of various cytokines and immune cells should be elucidated in detail.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest for this article.
ACKNOWLEDGMENTS
We want to extend our gratitude to all the staff in the Department of Microbiology and Immunology and the Department of Obstetrics and Gynecology. Also, we want to thank Enago (www.enago.jp) for the English language review.
Negishi Y, Morita R. Inflammatory responses in early pregnancy: Physiological and pathological perspectives. Reprod Med Biol. 2024;23:e12619. 10.1002/rmb2.12619
REFERENCES
- 1. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, et al. Fatty acid‐induced mitochondrial uncoupling elicits inflammasome‐independent IL‐1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14(10):1045–1053. 10.1038/ni.2704 [DOI] [PubMed] [Google Scholar]
- 3. Ross R. Atherosclerosis‐‐an inflammatory disease. N Engl J Med. 1999;340(2):115–126. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- 4. Zhu X, Huang H, Zhao L. PAMPs and DAMPs as the bridge between periodontitis and atherosclerosis: the potential therapeutic targets. Front Cell Dev Biol. 2022;10:856118. 10.3389/fcell.2022.856118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang SC, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–222. 10.1038/s41581-019-0234-4 [DOI] [PubMed] [Google Scholar]
- 6. Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1666–1680. 10.1164/ajrccm.157.5.9707141 [DOI] [PubMed] [Google Scholar]
- 7. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826–837. 10.1038/nri2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. 10.1126/science.1068883 [DOI] [PubMed] [Google Scholar]
- 9. Rubartelli A, Lotze MT. Inside, outside, upside down: damage‐associated molecular‐pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–436. 10.1016/j.it.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 10. Nadeau‐Vallée M, Obari D, Palacios J, Brien MÈ, Duval C, Chemtob S, et al. Sterile inflammation and pregnancy complications: a review. Reproduction. 2016;152(6):R277–R292. 10.1530/REP-16-0453 [DOI] [PubMed] [Google Scholar]
- 11. Koncz G, Jenei V, Tóth M, Váradi E, Kardos B, Bácsi A, et al. Damage‐mediated macrophage polarization in sterile inflammation. Front Immunol. 2023;14:1169560. 10.3389/fimmu.2023.1169560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang D, Billiar TR, Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med (Camb Mass). 2012;18(1):1360–1362. 10.2119/molmed.2012.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shields AM, Panayi GS, Corrigall VM. Resolution‐associated molecular patterns (RAMP): RAMParts defending immunological homeostasis? Clin Exp Immunol. 2011;165(3):292–300. 10.1111/j.1365-2249.2011.04433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo B, Chen JH, Zhang JH, Fang Y, Liu XJ, Zhang J, et al. Pattern‐recognition receptors in endometriosis: a narrative review. Front Immunol. 2023;14:1161606. 10.3389/fimmu.2023.1161606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar V, Stewart JH. Pattern‐recognition receptors and Immunometabolic reprogramming: what we know and what to explore. J Innate Immun. 2024;16(1):295–323. 10.1159/000539278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lappas M. NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor‐kappa B. Biol Reprod. 2013;89(1):14. 10.1095/biolreprod.113.110056 [DOI] [PubMed] [Google Scholar]
- 17. Leso V, Fontana L, Iavicoli I. Nanomaterial exposure and sterile inflammatory reactions. Toxicol Appl Pharmacol. 2018;355:80–92. 10.1016/j.taap.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 18. Negishi Y, Shima Y, Kato M, Ichikawa T, Ino H, Horii Y, et al. Inflammation in preterm birth: novel mechanism of preterm birth associated with innate and acquired immunity. J Reprod Immunol. 2022;154:103748. 10.1016/j.jri.2022.103748 [DOI] [PubMed] [Google Scholar]
- 19. Romero R, Gómez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15(2):41–56. 10.1046/j.1365-3016.2001.00007.x [DOI] [PubMed] [Google Scholar]
- 20. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra‐amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–474. 10.1111/aji.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57. 10.1016/j.jri.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 22. Kim YM, Romero R, Oh SY, Kim CJ, Kilburn BA, Armant DR, et al. Toll‐like receptor 4: a potential link between danger signals, the innate immune system, and preeclampsia? Am J Obstet Gynecol. 2005;193(3 Pt 2):921–927. 10.1016/j.ajog.2005.07.076 [DOI] [PubMed] [Google Scholar]
- 23. Pradervand PA, Clerc S, Frantz J, Rotaru C, Bardy D, Waeber B, et al. High mobility group box 1 protein (HMGB‐1): a pathogenic role in preeclampsia? Placenta. 2014;35(9):784–786. 10.1016/j.placenta.2014.06.370 [DOI] [PubMed] [Google Scholar]
- 24. Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506. 10.1016/s0002-9378(99)70239-5 [DOI] [PubMed] [Google Scholar]
- 25. Zhu L, Zhang Z, Zhang L, Shi Y, Qi J, Chang A, et al. HMGB1‐RAGE signaling pathway in severe preeclampsia. Placenta. 2015;36(10):1148–1152. 10.1016/j.placenta.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 26. Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. Pathogenic mechanisms in endometriosis‐associated infertility. Fertil Steril. 2008;90(2):247–257. 10.1016/j.fertnstert.2008.02.093 [DOI] [PubMed] [Google Scholar]
- 27. Laird SM, Tuckerman EM, Cork BA, Linjawi S, Blakemore AI, Li TC. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update. 2003;9(2):163–174. 10.1093/humupd/dmg013 [DOI] [PubMed] [Google Scholar]
- 28. von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6(7):627–634. 10.1093/molehr/6.7.627 [DOI] [PubMed] [Google Scholar]
- 29. Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14(1):229–236. 10.1093/humrep/14.1.229 [DOI] [PubMed] [Google Scholar]
- 30. Förger F, Villiger PM. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat Rev Rheumatol. 2020;16(2):113–122. 10.1038/s41584-019-0351-2 [DOI] [PubMed] [Google Scholar]
- 31. Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci USA. 2017;114(32):E6566–E6575. 10.1073/pnas.1701129114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang YH, He M, Wang Y, Liao AH. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol. 2017;8:120. 10.3389/fimmu.2017.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Negishi Y, Shima Y, Takeshita T, Morita R. Harmful and beneficial effects of inflammatory response on reproduction: sterile and pathogen‐associated inflammation. Immunol Med. 2021;44(2):98–115. 10.1080/25785826.2020.1809951 [DOI] [PubMed] [Google Scholar]
- 34. Espey LL. Ovulation as an inflammatory reaction‐‐a hypothesis. Biol Reprod. 1980;22(1):73–106. 10.1095/biolreprod22.1.73 [DOI] [PubMed] [Google Scholar]
- 35. Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: parallels with inflammatory processes. Endocr Rev. 2019;40(2):369–416. 10.1210/er.2018-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang LJ, Norman RJ. Concentrations of immunoreactive interleukin‐1 and interleukin‐2 in human preovulatory follicular fluid. Hum Reprod. 1992;7(2):147–150. 10.1093/oxfordjournals.humrep.a137607 [DOI] [PubMed] [Google Scholar]
- 37. Buyalos RP, Watson JM, Martinez‐Maza O. Detection of interleukin‐6 in human follicular fluid. Fertil Steril. 1992;57(6):1230–1234. 10.1016/S0015-0282(16)55079-1 [DOI] [PubMed] [Google Scholar]
- 38. Wang LJ, Brännström M, Robertson SA, Norman RJ. Tumor necrosis factor alpha in the human ovary: presence in follicular fluid and effects on cell proliferation and prostaglandin production. Fertil Steril. 1992;58(5):934–940. 10.1016/s0015-0282(16)55438-7 [DOI] [PubMed] [Google Scholar]
- 39. Jasper MJ, Brännström M, Olofsson JI, Petrucco OM, Mason H, Robertson SA, et al. Granulocyte‐macrophage colony‐stimulating factor: presence in human follicular fluid, protein secretion and mRNA expression by ovarian cells. Mol Hum Reprod. 1996;2(8):555–562. 10.1093/molehr/2.8.555 [DOI] [PubMed] [Google Scholar]
- 40. Witt BR, Pollard JW. Colony stimulating factor‐1 in human follicular fluid. Fertil Steril. 1997;68(2):259–264. 10.1016/s0015-0282(97)81512-9 [DOI] [PubMed] [Google Scholar]
- 41. Nishimura K, Tanaka N, Kawano T, Matsuura K, Okamura H. Changes in macrophage colony‐stimulating factor concentration in serum and follicular fluid in in‐vitro fertilization and embryo transfer cycles. Fertil Steril. 1998;69(1):53–57. 10.1016/s0015-0282(97)00433-0 [DOI] [PubMed] [Google Scholar]
- 42. Al‐Alem L, Puttabyatappa M, Rosewell K, Brännström M, Akin J, Boldt J, et al. Chemokine ligand 20: a signal for leukocyte recruitment during human ovulation? Endocrinology. 2015;156(9):3358–3369. 10.1210/en.2014-1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arici A, Oral E, Bukulmez O, Buradagunta S, Bahtiyar O, Jones EE. Monocyte chemotactic protein‐1 expression in human preovulatory follicles and ovarian cells. J Reprod Immunol. 1997;32(3):201–219. 10.1016/s0165-0378(97)82476-x [DOI] [PubMed] [Google Scholar]
- 44. Castilla JA, Sampalo A, Molina R, Samaniego F, Mozas J, Vergara F, et al. Mononuclear cell subpopulations in human follicular fluid from stimulated cycles. Am J Reprod Immunol. 1990;22(3–4):127–129. 10.1111/j.1600-0897.1990.tb00655.x [DOI] [PubMed] [Google Scholar]
- 45. Fainaru O, Amsalem H, Bentov Y, Esfandiari N, Casper RF. CD56brightCD16‐ natural killer cells accumulate in the ovarian follicular fluid of patients undergoing in vitro fertilization. Fertil Steril. 2010;94(5):1918–1921. 10.1016/j.fertnstert.2009.12.067 [DOI] [PubMed] [Google Scholar]
- 46. Kryczek I, Frydman N, Gaudin F, Krzysiek R, Fanchin R, Emilie D, et al. The chemokine SDF‐1/CXCL12 contributes to T lymphocyte recruitment in human pre‐ovulatory follicles and coordinates with lymphocytes to increase granulosa cell survival and embryo quality. Am J Reprod Immunol. 2005;54(5):270–283. 10.1111/j.1600-0897.2005.00307.x [DOI] [PubMed] [Google Scholar]
- 47. Nio‐Kobayashi J, Kudo M, Sakuragi N, Kimura S, Iwanaga T, Duncan WC. Regulated C‐C motif ligand 2 (CCL2) in luteal cells contributes to macrophage infiltration into the human corpus luteum during luteolysis. Mol Hum Reprod. 2015;21(8):645–654. 10.1093/molehr/gav028 [DOI] [PubMed] [Google Scholar]
- 48. Dahm‐Kähler P, Ghahremani M, Lind AK, Sundfeldt K, Brännström M. Monocyte chemotactic protein‐1 (MCP‐1), its receptor, and macrophages in the perifollicular stroma during the human ovulatory process. Fertil Steril. 2009;91(1):231–239. 10.1016/j.fertnstert.2007.07.1330 [DOI] [PubMed] [Google Scholar]
- 49. Van der Hoek KH, Maddocks S, Woodhouse CM, van Rooijen N, Robertson SA, Norman RJ. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol Reprod. 2000;62(4):1059–1066. 10.1095/biolreprod62.4.1059 [DOI] [PubMed] [Google Scholar]
- 50. Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10(2):119–133. 10.1093/humupd/dmh011 [DOI] [PubMed] [Google Scholar]
- 51. Cohen‐Fredarow A, Tadmor A, Raz T, Meterani N, Addadi Y, Nevo N, et al. Ovarian dendritic cells act as a double‐edged pro‐ovulatory and anti‐inflammatory sword. Mol Endocrinol. 2014;28(7):1039–1054. 10.1210/me.2013-1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Akiyama I, Yoshino O, Osuga Y, Shi J, Takamura M, Harada M, et al. The role of bone morphogenetic protein 6 in accumulation and regulation of neutrophils in the human ovary. Reprod Sci. 2014;21(6):772–777. 10.1177/1933719113518988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yang Z, Kong B, Mosser DM, Zhang X. TLRs, macrophages, and NK cells: our understandings of their functions in uterus and ovary. Int Immunopharmacol. 2011;11(10):1442–1450. 10.1016/j.intimp.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 54. Dal J, Vural B, Caliskan E, Ozkan S, Yucesoy I. Power doppler ultrasound studies of ovarian, uterine, and endometrial blood flow in regularly menstruating women with respect to luteal phase defects. Fertil Steril. 2005;84(1):224–227. 10.1016/j.fertnstert.2004.12.059 [DOI] [PubMed] [Google Scholar]
- 55. Qublan H, Amarin Z, Nawasreh M, Diab F, Malkawi S, Al‐Ahmad N, et al. Luteinized unruptured follicle syndrome: incidence and recurrence rate in infertile women with unexplained infertility undergoing intrauterine insemination. Hum Reprod. 2006;21(8):2110–2113. 10.1093/humrep/del113 [DOI] [PubMed] [Google Scholar]
- 56. Wang L, Qiao J, Liu P, Lian Y. Effect of luteinized unruptured follicle cycles on clinical outcomes of frozen thawed embryo transfer in Chinese women. J Assist Reprod Genet. 2008;25(6):229–233. 10.1007/s10815-008-9225-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hock DL, Huhn RD, Kemmann E. Leukocytosis in response to exogenous gonadotrophin stimulation. Hum Reprod. 1997;12(10):2143–2146. 10.1093/humrep/12.10.2143 [DOI] [PubMed] [Google Scholar]
- 58. Makinoda S, Hirosaki N, Waseda T, Tomizawa H, Fujii R. Granulocyte colony‐stimulating factor (G‐CSF) in the mechanism of human ovulation and its clinical usefulness. Curr Med Chem. 2008;15(6):604–613. 10.2174/092986708783769740 [DOI] [PubMed] [Google Scholar]
- 59. Shibata T, Makinoda S, Waseda T, Tomizawa H, Fujii R, Utsunomiya T. Granulocyte colony‐stimulating factor as a potential inducer of ovulation in infertile women with luteinized unruptured follicle syndrome. Transl Res. 2016;171:63–70. 10.1016/j.trsl.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 60. Brännström M, Bonello N, Norman RJ, Robertson SA. Reduction of ovulation rate in the rat by administration of a neutrophil‐depleting monoclonal antibody. J Reprod Immunol. 1995;29(3):265–270. 10.1016/0165-0378(95)00941-d [DOI] [PubMed] [Google Scholar]
- 61. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova‐Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. 10.1016/j.fertnstert.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 62. Diamanti‐Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine. 2006;30(1):19–26. 10.1385/ENDO:30:1:19 [DOI] [PubMed] [Google Scholar]
- 63. Escobar‐Morreale HF, Luque‐Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048–1058. 10.1016/j.fertnstert.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, et al. Increased C‐reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89(5):2160–2165. 10.1210/jc.2003-031096 [DOI] [PubMed] [Google Scholar]
- 65. Peng Z, Sun Y, Lv X, Zhang H, Liu C, Dai S. Interleukin‐6 levels in women with polycystic ovary syndrome: a systematic review and meta‐analysis. PLoS One. 2016;11(2):e0148531. 10.1371/journal.pone.0148531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Seyam E, Hasan M, Khalifa EM, Ramadan A, Hefzy E. Evaluation of tumor necrosis factor alpha serum level in obese and lean women with clomiphene citrate‐resistant polycystic ovary disease. Gynecol Endocrinol. 2017;33(11):892–898. 10.1080/09513590.2017.1320383 [DOI] [PubMed] [Google Scholar]
- 67. Cirillo F, Catellani C, Lazzeroni P, Sartori C, Tridenti G, Vezzani C, et al. HMGB1 is increased in adolescents with polycystic ovary syndrome (PCOS) and decreases after treatment with MYO‐inositol (MYO) in combination with alpha‐lipoic acid (ALA). Gynecol Endocrinol. 2020;36(7):588–593. 10.1080/09513590.2020.1725967 [DOI] [PubMed] [Google Scholar]
- 68. Williams EJ, Sibley K, Miller AN, Lane EA, Fishwick J, Nash DM, et al. The effect of Escherichia coli lipopolysaccharide and tumour necrosis factor alpha on ovarian function. Am J Reprod Immunol. 2008;60(5):462–473. 10.1111/j.1600-0897.2008.00645.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, et al. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007;134(5):683–693. 10.1530/REP-07-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Granot I, Gnainsky Y, Dekel N. Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction. 2012;144(6):661–668. 10.1530/REP-12-0217 [DOI] [PubMed] [Google Scholar]
- 71. Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221(1):80–87. 10.1111/j.1749-6632.2010.05938.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Van Sinderen M, Menkhorst E, Winship A, Cuman C, Dimitriadis E. Preimplantation human blastocyst‐endometrial interactions: the role of inflammatory mediators. Am J Reprod Immunol. 2013;69(5):427–440. 10.1111/aji.12038 [DOI] [PubMed] [Google Scholar]
- 73. Hayakawa S, Karasaki‐Suzuki M, Itoh T, Ishii M, Kanaeda T, Nagai N, et al. Effects of paternal lymphocyte immunization on peripheral Th1/Th2 balance and TCR V beta and V gamma repertoire usage of patients with recurrent spontaneous abortions. Am J Reprod Immunol. 2000;43(2):107–115. 10.1111/j.8755-8920.2000.430207.x [DOI] [PubMed] [Google Scholar]
- 74. Hill JA, Polgar K, Anderson DJ. T‐helper 1‐type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273(24):1933–1936. 10.1001/jama.1995.03520480053039 [DOI] [PubMed] [Google Scholar]
- 75. Visvanathan S, McNeil HP. Cellular immunity to beta 2‐glycoprotein‐1 in patients with the antiphospholipid syndrome. J Immunol. 1999;162(11):6919–6925. 10.4049/jimmunol.162.11.6919 [DOI] [PubMed] [Google Scholar]
- 76. Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192(2):259–270. 10.1084/jem.192.2.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shimada S, Kato EH, Morikawa M, Iwabuchi K, Nishida R, Kishi R, et al. No difference in natural killer or natural killer T‐cell population, but aberrant T‐helper cell population in the endometrium of women with repeated miscarriage. Hum Reprod. 2004;19(4):1018–1024. 10.1093/humrep/deh159 [DOI] [PubMed] [Google Scholar]
- 78. Fujiwara H. Do circulating blood cells contribute to maternal tissue remodeling and embryo‐maternal cross‐talk around the implantation period? Mol Hum Reprod. 2009;15(6):335–343. 10.1093/molehr/gap027 [DOI] [PubMed] [Google Scholar]
- 79. Yu N, Yang J, Guo Y, Fang J, Yin T, Luo J, et al. Intrauterine administration of peripheral blood mononuclear cells (PBMCs) improves endometrial receptivity in mice with embryonic implantation dysfunction. Am J Reprod Immunol. 2014;71(1):24–33. 10.1111/aji.12150 [DOI] [PubMed] [Google Scholar]
- 80. Jaiswal MK, Mallers TM, Larsen B, Kwak‐Kim J, Chaouat G, Gilman‐Sachs A, et al. V‐ATPase upregulation during early pregnancy: a possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction. 2012;143(5):713–725. 10.1530/REP-12-0036 [DOI] [PubMed] [Google Scholar]
- 81. Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86(2):39. 10.1095/biolreprod.111.095505 [DOI] [PubMed] [Google Scholar]
- 82. Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, et al. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril. 2010;94(6):2030–2036. 10.1016/j.fertnstert.2010.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79(6):1317–1322. 10.1016/s0015-0282(03)00345-5 [DOI] [PubMed] [Google Scholar]
- 84. El‐Toukhy T, Sunkara S, Khalaf Y. Local endometrial injury and IVF outcome: a systematic review and meta‐analysis. Reprod Biomed Online. 2012;25(4):345–354. 10.1016/j.rbmo.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 85. Gibreel A, Badawy A, El‐Refai W, El‐Adawi N. Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. J Obstet Gynaecol Res. 2013;39(3):680–684. 10.1111/j.1447-0756.2012.02016.x [DOI] [PubMed] [Google Scholar]
- 86. Potdar N, Gelbaya T, Nardo LG. Endometrial injury to overcome recurrent embryo implantation failure: a systematic review and meta‐analysis. Reprod Biomed Online. 2012;25(6):561–571. 10.1016/j.rbmo.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 87. Kämmerer U. Antigen‐presenting cells in the decidua. Chem Immunol Allergy. 2005;89:96–104. 10.1159/000087951 [DOI] [PubMed] [Google Scholar]
- 88. Laskarin G, Kämmerer U, Rukavina D, Thomson AW, Fernandez N, Blois SM. Antigen‐presenting cells and materno‐fetal tolerance: an emerging role for dendritic cells. Am J Reprod Immunol. 2007;58(3):255–267. 10.1111/j.1600-0897.2007.00511.x [DOI] [PubMed] [Google Scholar]
- 89. Miyazaki S, Tsuda H, Sakai M, Hori S, Sasaki Y, Futatani T, et al. Predominance of Th2‐promoting dendritic cells in early human pregnancy decidua. J Leukoc Biol. 2003;74(4):514–522. 10.1189/jlb.1102566 [DOI] [PubMed] [Google Scholar]
- 90. Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118(12):3954–3965. 10.1172/JCI36682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am J Reprod Immunol. 2014;72(2):141–147. 10.1111/aji.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Negishi Y, Kato M, Ono S, Kuwabara Y, Morita R, Takahashi H, et al. Distribution of dendritic cells in the septate uterus: an immunological perspective. Am J Reprod Immunol. 2020;83(6):e13241. 10.1111/aji.13241 [DOI] [PubMed] [Google Scholar]
- 93. Lédée N, Petitbarat M, Chevrier L, Vitoux D, Vezmar K, Rahmati M, et al. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol. 2016;75(3):388–401. 10.1111/aji.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kwak‐Kim J, AlSubki L, Luu T, Ganieva U, Thees A, Dambaeva S, et al. The role of immunologic tests for subfertility in the clinical environment. Fertil Steril. 2022;117(6):1132–1143. 10.1016/j.fertnstert.2022.04.009 [DOI] [PubMed] [Google Scholar]
- 95. Petitbarat M, Serazin V, Dubanchet S, Wayner R, de Mazancourt P, Chaouat G, et al. Tumor necrosis factor‐like weak inducer of apoptosis (TWEAK)/fibroblast growth factor inducible‐14 might regulate the effects of interleukin 18 and 15 in the human endometrium. Fertil Steril. 2010;94(3):1141–1143. 10.1016/j.fertnstert.2009.10.049 [DOI] [PubMed] [Google Scholar]
- 96. Greenwood SM, Moran JJ. Chronic endometritis: morphologic and clinical observations. Obstet Gynecol. 1981;58(2):176–184. [PubMed] [Google Scholar]
- 97. Cicinelli E, Resta L, Nicoletti R, Tartagni M, Marinaccio M, Bulletti C, et al. Detection of chronic endometritis at fluid hysteroscopy. J Minim Invasive Gynecol. 2005;12(6):514–518. 10.1016/j.jmig.2005.07.394 [DOI] [PubMed] [Google Scholar]
- 98. Kitaya K, Matsubayashi H, Yamaguchi K, Nishiyama R, Takaya Y, Ishikawa T, et al. Chronic endometritis: potential cause of infertility and obstetric and neonatal complications. Am J Reprod Immunol. 2016;75(1):13–22. 10.1111/aji.12438 [DOI] [PubMed] [Google Scholar]
- 99. Polisseni F, Bambirra EA, Camargos AF. Detection of chronic endometritis by diagnostic hysteroscopy in asymptomatic infertile patients. Gynecol Obstet Investig. 2003;55(4):205–210. 10.1159/000072075 [DOI] [PubMed] [Google Scholar]
- 100. Park HJ, Kim YS, Yoon TK, Lee WS. Chronic endometritis and infertility. Clin Exp Reprod Med. 2016;43(4):185–192. 10.5653/cerm.2016.43.4.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Moreno I, Cicinelli E, Garcia‐Grau I, Gonzalez‐Monfort M, Bau D, Vilella F, et al. The diagnosis of chronic endometritis in infertile asymptomatic women: a comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am J Obstet Gynecol. 2018;218(6):602.e1–602.e16. 10.1016/j.ajog.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 102. Cicinelli E, Matteo M, Tinelli R, Lepera A, Alfonso R, Indraccolo U, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30(2):323–330. 10.1093/humrep/deu292 [DOI] [PubMed] [Google Scholar]
- 103. Cicinelli E, De Ziegler D, Nicoletti R, Colafiglio G, Saliani N, Resta L, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89(3):677–684. 10.1016/j.fertnstert.2007.03.074 [DOI] [PubMed] [Google Scholar]
- 104. Johnston‐MacAnanny EB, Hartnett J, Engmann LL, Nulsen JC, Sanders MM, Benadiva CA. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93(2):437–441. 10.1016/j.fertnstert.2008.12.131 [DOI] [PubMed] [Google Scholar]
- 105. Bayer‐Garner IB, Korourian S. Plasma cells in chronic endometritis are easily identified when stained with syndecan‐1. Mod Pathol. 2001;14(9):877–879. 10.1038/modpathol.3880405 [DOI] [PubMed] [Google Scholar]
- 106. Smith M, Hagerty KA, Skipper B, Bocklage T. Chronic endometritis: a combined histopathologic and clinical review of cases from 2002 to 2007. Int J Gynecol Pathol. 2010;29(1):44–50. 10.1097/PGP.0b013e3181ae81bb [DOI] [PubMed] [Google Scholar]
- 107. Gilmore H, Fleischhacker D, Hecht JL. Diagnosis of chronic endometritis in biopsies with stromal breakdown. Hum Pathol. 2007;38(4):581–584. 10.1016/j.humpath.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 108. McQueen DB, Perfetto CO, Hazard FK, Lathi RB. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil Steril. 2015;104(4):927–931. 10.1016/j.fertnstert.2015.06.044 [DOI] [PubMed] [Google Scholar]
- 109. Vitagliano A, Saccardi C, Noventa M, Di Spiezio SA, Saccone G, Cicinelli E, et al. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: a systematic review and meta‐analysis. Fertil Steril. 2018;110(1):103–112.e1. 10.1016/j.fertnstert.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 110. Fang RL, Chen LX, Shu WS, Yao SZ, Wang SW, Chen YQ. Barcoded sequencing reveals diverse intrauterine microbiomes in patients suffering with endometrial polyps. Am J Transl Res. 2016;8:1581–1592. [PMC free article] [PubMed] [Google Scholar]
- 111. Matteo M, Cicinelli E, Greco P, Massenzio F, Baldini D, Falagario T, et al. Abnormal pattern of lymphocyte subpopulations in the endometrium of infertile women with chronic endometritis. Am J Reprod Immunol. 2009;61(5):322–329. 10.1111/j.1600-0897.2009.00698.x [DOI] [PubMed] [Google Scholar]
- 112. Tortorella C, Piazzolla G, Matteo M, Pinto V, Tinelli R, Sabbà C, et al. Interleukin‐6, interleukin‐1beta, and tumor necrosis factor alpha in menstrual effluents as biomarkers of chronic endometritis. Fertil Steril. 2014;101(1):242–247. 10.1016/j.fertnstert.2013.09.041 [DOI] [PubMed] [Google Scholar]
- 113. Wang WJ, Zhang H, Chen ZQ, Zhang W, Liu XM, Fang JY, et al. Endometrial TGF‐β, IL‐10, IL‐17 and autophagy are dysregulated in women with recurrent implantation failure with chronic endometritis. Reprod Biol Endocrinol. 2019;17(1):2. 10.1186/s12958-018-0444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kitazawa J, Kimura F, Nakamura A, Morimune A, Hanada T, Amano T, et al. Alteration in endometrial helper T‐cell subgroups in chronic endometritis. Am J Reprod Immunol. 2021;85(3):e13372. 10.1111/aji.13372 [DOI] [PubMed] [Google Scholar]
- 115. Hollier LM, Scott LL, Murphree SS, Wendel GD Jr. Postpartum endometritis caused by herpes simplex virus. Obstet Gynecol. 1997;89(5 Pt 2):836–838. 10.1016/s0029-7844(97)00106-3 [DOI] [PubMed] [Google Scholar]
- 116. McGill AL, Bavaro MF, You WB. Postpartum herpes simplex virus endometritis and disseminated infection in both mother and neonate. Obstet Gynecol. 2012;120(2 Pt 2):471–473. 10.1097/AOG.0b013e318257245b [DOI] [PubMed] [Google Scholar]
- 117. Haeri S, Baker AM, Boggess KA. Prevalence of Epstein‐Barr virus reactivation in pregnancy. Am J Perinatol. 2010;27(9):715–719. 10.1055/s-0030-1253098 [DOI] [PubMed] [Google Scholar]
- 118. Okong P, Biryahwaho B, Bergström S. Post‐abortion endometritis‐myometritis and HIV infection. Int J STD AIDS. 2002;13(11):729–732. 10.1258/095646202320753664 [DOI] [PubMed] [Google Scholar]
- 119. Peuchmaur M, Emilie D, Vazeux R, Pons JC, Delfraissy JF, Lemaigre G, et al. HIV‐associated endometritis. AIDS. 1989;3(4):239–241. 10.1097/00002030-198904000-00008 [DOI] [PubMed] [Google Scholar]
- 120. Mitchell CM, Haick A, Nkwopara E, Garcia R, Rendi M, Agnew K, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol. 2015;212(5):611.e1–611.e9. 10.1016/j.ajog.2014.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine‐related diseases. Nat Commun. 2017;8(1):875. 10.1038/s41467-017-00901-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bardos J, Fiorentino D, Longman RE, Paidas M. Immunological role of the maternal uterine microbiome in pregnancy: pregnancies pathologies and alterated microbiota. Front Immunol. 2019;10:2823. 10.3389/fimmu.2019.02823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kyono K, Hashimoto T, Kikuchi S, Nagai Y, Sakuraba Y. A pilot study and case reports on endometrial microbiota and pregnancy outcome: an analysis using 16S rRNA gene sequencing among IVF patients, and trial therapeutic intervention for dysbiotic endometrium. Reprod Med Biol. 2019;18(1):72–82. 10.1002/rmb2.12250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Moreno I, Codoñer FM, Vilella F, Valbuena D, Martinez‐Blanch JF, Jimenez‐Almazán J, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol. 2016;215(6):684–703. 10.1016/j.ajog.2016.09.075 [DOI] [PubMed] [Google Scholar]
- 125. Moreno I, Garcia‐Grau I, Bau D, Perez‐Villaroya D, Gonzalez‐Monfort M, Vilella F, et al. The first glimpse of the endometrial microbiota in early pregnancy. Am J Obstet Gynecol. 2020;222(4):296–305. 10.1016/j.ajog.2020.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hashimoto T, Kyono K. Does dysbiotic endometrium affect blastocyst implantation in IVF patients? J Assist Reprod Genet. 2019;36(12):2471–2479. 10.1007/s10815-019-01630-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yoshida T, Takada K, Komine‐Aizawa S, Kamei Y, Ishihara O, Hayakawa S. Lactobacillus crispatus promotes invasion of the HTR‐8/SVneo trophoblast cell line. Placenta. 2021;111:76–81. 10.1016/j.placenta.2021.06.006 [DOI] [PubMed] [Google Scholar]
- 128. Kadogami D, Kimura F, Hanada T, Tsuji S, Nakaoka Y, Murakami T, et al. Impact of lactobacillus in the uterine microbiota on in vitro fertilization outcomes. J Reprod Immunol. 2023;160:104138. 10.1016/j.jri.2023.104138 [DOI] [PubMed] [Google Scholar]
- 129. Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic lactobacillus species in vaginal health. Res Microbiol. 2017;168(9–10):782–792. 10.1016/j.resmic.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 130. Finn R, St Hill CA, Davis JC, Hipkin LJ, Harvey M. Feto‐maternal bidirectional mixed lymphocyte reaction and survival of fetal allograft. Lancet. 1977;2(8050):1200–1202. 10.1016/s0140-6736(77)90439-1 [DOI] [PubMed] [Google Scholar]
- 131. Tafuri A, Alferink J, Möller P, Hämmerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270(5236):630–633. 10.1126/science.270.5236.630 [DOI] [PubMed] [Google Scholar]
- 132. Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2‐type cytokines at the maternal‐fetal interface. J Immunol. 1993;151(9):4562–4573. 10.4049/jimmunol.151.9.4562 [DOI] [PubMed] [Google Scholar]
- 133. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal‐fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. 10.1016/0167-5699(93)90235-D [DOI] [PubMed] [Google Scholar]
- 134. Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo‐Russo R, et al. A pivotal role for galectin‐1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. 10.1038/nm1680 [DOI] [PubMed] [Google Scholar]
- 135. Blois SM, Joachim R, Kandil J, Margni R, Tometten M, Klapp BF, et al. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol. 2004;172(10):5893–5899. 10.4049/jimmunol.172.10.5893 [DOI] [PubMed] [Google Scholar]
- 136. Shimada S, Nishida R, Takeda M, Iwabuchi K, Kishi R, Onoé K, et al. Natural killer, natural killer T, helper and cytotoxic T cells in the decidua from sporadic miscarriage. Am J Reprod Immunol. 2006;56(3):193–200. 10.1111/j.1600-0897.2006.00417.x [DOI] [PubMed] [Google Scholar]
- 137. Saito S, Nakashima A, Shima T, Ito MT. Th1/Th2/Th17 and regulatory T‐cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–610. 10.1111/j.1600-0897.2010.00852.x [DOI] [PubMed] [Google Scholar]
- 138. Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148(1):13–21. 10.1111/imm.12595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hosseini S, Shokri F, Ansari Pour S, Jeddi‐Tehrani M, Nikoo S, Yousefi M, et al. A shift in the balance of T17 and Treg cells in menstrual blood of women with unexplained recurrent spontaneous abortion. J Reprod Immunol. 2016;116:13–22. 10.1016/j.jri.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 140. Nakashima A, Ito M, Shima T, Bac ND, Hidaka T, Saito S. Accumulation of IL‐17‐positive cells in decidua of inevitable abortion cases. Am J Reprod Immunol. 2010;64(1):4–11. 10.1111/j.1600-0897.2010.00812.x [DOI] [PubMed] [Google Scholar]
- 141. Saito S, Nakashima A, Ito M, Shima T. Clinical implication of recent advances in our understanding of IL‐17 and reproductive immunology. Expert Rev Clin Immunol. 2011;7(5):649–657. 10.1586/eci.11.49 [DOI] [PubMed] [Google Scholar]
- 142. Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26(1):44–62. 10.1210/er.2003-0021 [DOI] [PubMed] [Google Scholar]
- 143. Fukui A, Kwak‐Kim J, Ntrivalas E, Gilman‐Sachs A, Lee SK, Beaman K. Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures. Fertil Steril. 2008;89(1):157–165. 10.1016/j.fertnstert.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 144. Mekinian A, Cohen J, Alijotas‐Reig J, Carbillon L, Nicaise‐Roland P, Kayem G, et al. Unexplained recurrent miscarriage and recurrent implantation failure: is there a place for immunomodulation? Am J Reprod Immunol. 2016;76(1):8–28. 10.1111/aji.12493 [DOI] [PubMed] [Google Scholar]
- 145. Potdar N, Gelbaya TA, Konje JC, Nardo LG. Adjunct low‐molecular‐weight heparin to improve live birth rate after recurrent implantation failure: a systematic review and meta‐analysis. Hum Reprod Update. 2013;19(6):674–684. 10.1093/humupd/dmt032 [DOI] [PubMed] [Google Scholar]
- 146. Quenby S, Kalumbi C, Bates M, Farquharson R, Vince G. Prednisolone reduces preconceptual endometrial natural killer cells in women with recurrent miscarriage. Fertil Steril. 2005;84(4):980–984. 10.1016/j.fertnstert.2005.05.012 [DOI] [PubMed] [Google Scholar]
- 147. Akhter A, Faridi RM, Das V, Pandey A, Naik S, Agrawal S. In vitro up‐regulation of HLA‐G using dexamethasone and hydrocortisone in first‐trimester trophoblast cells of women experiencing recurrent miscarriage. Tissue Antigens. 2012;80(2):126–135. 10.1111/j.1399-0039.2012.01884.x [DOI] [PubMed] [Google Scholar]
- 148. Alijotas‐Reig J. Treatment of refractory obstetric antiphospholipid syndrome: the state of the art and new trends in the therapeutic management. Lupus. 2013;22(1):6–17. 10.1177/0961203312465782 [DOI] [PubMed] [Google Scholar]
- 149. Abdolmohammadi‐Vahid S, Danaii S, Hamdi K, Jadidi‐Niaragh F, Ahmadi M, Yousefi M. Novel immunotherapeutic approaches for treatment of infertility. Biomed Pharmacother. 2016;84:1449–1459. 10.1016/j.biopha.2016.10.062 [DOI] [PubMed] [Google Scholar]
- 150. Yamada H, Deguchi M, Saito S, Takeshita T, Mitsui M, Saito T, et al. Intravenous immunoglobulin treatment in women with four or more recurrent pregnancy losses: a double‐blind, randomised, placebo‐controlled trial. EClinicalmedicine. 2022;50:101527. 10.1016/j.eclinm.2022.101527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Roussev RG, Acacio B, Ng SC, Coulam CB. Duration of intralipid's suppressive effect on NK cell's functional activity. Am J Reprod Immunol. 2008;60(3):258–263. 10.1111/j.1600-0897.2008.00621.x [DOI] [PubMed] [Google Scholar]
- 152. Coulam CB, Acacio B. Does immunotherapy for treatment of reproductive failure enhance live births? Am J Reprod Immunol. 2012;67(4):296–304. 10.1111/j.1600-0897.2012.01111.x [DOI] [PubMed] [Google Scholar]
- 153. Hisano M, Nakagawa K, Kwak‐Kim J, Sugiyama R, Sago H, Yamaguchi K. Changes in the T‐helper 1 and 2 cell populations during pregnancy in tacrolimus‐treated women with repeated implantation failure and recurrent pregnancy loss. Hum Fertil (Camb). 2022;25(5):975–982. 10.1080/14647273.2021.1955415 [DOI] [PubMed] [Google Scholar]
- 154. Nakagawa K, Kwak‐Kim J, Hisano M, Kasahara Y, Kuroda K, Sugiyama R, et al. Obstetric and perinatal outcome of the women with repeated implantation failures or recurrent pregnancy losses who received pre‐ and post‐conception tacrolimus treatment. Am J Reprod Immunol. 2019;82(2):e13142. 10.1111/aji.13142 [DOI] [PubMed] [Google Scholar]
- 155. Nakagawa K, Kwak‐Kim J, Ota K, Kuroda K, Hisano M, Sugiyama R, et al. Immunosuppression with tacrolimus improved reproductive outcome of women with repeated implantation failure and elevated peripheral blood TH1/TH2 cell ratios. Am J Reprod Immunol. 2015;73(4):353–361. 10.1111/aji.12338 [DOI] [PubMed] [Google Scholar]
- 156. Abrahams VM, Mor G. Toll‐like receptors and their role in the trophoblast. Placenta. 2005;26(7):540–547. 10.1016/j.placenta.2004.08.010 [DOI] [PubMed] [Google Scholar]
- 157. Koga K, Mor G. Toll‐like receptors at the maternal‐fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600. 10.1111/j.1600-0897.2010.00848.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, et al. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61(3):196–212. 10.1111/j.1600-0897.2008.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bhutada S, Basak T, Savardekar L, Katkam RR, Jadhav G, Metkari SM, et al. High mobility group box 1 (HMGB1) protein in human uterine fluid and its relevance in implantation. Hum Reprod. 2014;29(4):763–780. 10.1093/humrep/det461 [DOI] [PubMed] [Google Scholar]
- 160. Jin H, Wu J, Yang Q, Cai Y, He W, Liu C. High mobility group box 1 protein polymorphism affects susceptibility to recurrent pregnancy loss by up‐regulating gene expression in chorionic villi. J Assist Reprod Genet. 2015;32(7):1123–1128. 10.1007/s10815-015-0493-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Nair RR, Khanna A, Singh K. Association of increased S100A8 serum protein with early pregnancy loss. Am J Reprod Immunol. 2015;73(2):91–94. 10.1111/aji.12318 [DOI] [PubMed] [Google Scholar]
- 162. Kaitu'u‐Lino TJ, Tuohey L, Tong S. Maternal serum interleukin‐33 and soluble ST2 across early pregnancy, and their association with miscarriage. J Reprod Immunol. 2012;95(1–2):46–49. 10.1016/j.jri.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 163. Salker MS, Nautiyal J, Steel JH, Webster Z, Sućurović S, Nicou M, et al. Disordered IL‐33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS One. 2012;7(12):e52252. 10.1371/journal.pone.0052252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Yue J, Tong Y, Xie L, Ma T, Yang J. Genetic variant in IL‐33 is associated with idiopathic recurrent miscarriage in Chinese Han population. Sci Rep. 2016;6:23806. 10.1038/srep23806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zidan HE, Abdul‐Maksoud RS, Mowafy HE, Elsayed WSH. The association of IL‐33 and Foxp3 gene polymorphisms with recurrent pregnancy loss in Egyptian women. Cytokine. 2018;108:115–119. [DOI] [PubMed] [Google Scholar]
- 166. Valero‐Pacheco N, Tang EK, Massri N, Loia R, Chemerinski A, Wu T, et al. Maternal IL‐33 critically regulates tissue remodeling and type 2 immune responses in the uterus during early pregnancy in mice. Proc Natl Acad Sci USA. 2022;119(35):e2123267119. 10.1073/pnas.2123267119 [DOI] [PMC free article] [PubMed] [Google Scholar]


