Abstract
Portulaca oleracea L. (POL) has a long history of medicinal use worldwide, and numerous clinical and experimental studies demonstrated the therapeutic effects of POL and its active ingredients in the treatment of Ulcerative colitis (UC). In this review, we summarized the underlying mechanisms and roles of POL in UC treatment based on experimental and clinical studies. The research articles cited in this study were obtained by employing specific keywords, such as “purslane”, “IBD”, “UC”, “inflammation”, “gut microbiota”, and “intestinal barrier”, in PubMed, Web of Science, Google Scholar, and China National Knowledge Infrastructure databases. Clinical studies found that both POL monotherapy and POL traditional Chinese medicine compound are effective in treating UC. Meanwhile, experimental studies found that POL intervenes in UC by regulating intestinal flora, repairing mucosal barrier, and regulating immune response. Increasing evidence suggests the therapeutic potential of POL in UC treatment.
Keywords: ulcerative colitis, Portulaca oleracea L., gut microbiota, intestinal barrier, immunoregulation
Introduction
Ulcerative colitis (UC) is a lifelong chronic non-specific inflammatory bowel disease (IBD), characterized by bloody mucous stools, abdominal pain, diarrhea, and tenesmus. UC lesions primarily occur in the colon and rectum and are limited to the mucosa and submucosa.1,2 The incidence of UC is increasing worldwide, with an estimated global prevalence of 5 million cases in 2023.1,3 Epidemiological studies found that the incidence and prevalence of UC in some Asian countries had increased by 1.5 to nearly 20-fold in the past four decades.4 Current therapeutic agents for UC include 5-aminosalicylic acid, glucocorticoids, immunosuppressants, biologics, and small-molecule agents. However, these drugs are insufficient at targeting the complex pathophysiology of UC, which is highly variable and susceptible to comorbidities, such as infections, thrombosis, and risk of carcinoma.5
Traditional Chinese medicine (TCM) preparations have multi-component, multi-target, multi-pathway characteristics, which allow for a comprehensive treatment of several diseases with fewer adverse effects. Therefore, in recent years, TCM has become a research hotspot for the identification of novel drugs for UC treatment.6 In TCM, UC is categorized under “Jiu Li” and “Chang Pi”, which are characterized by dampness and heat in the intestines and an imbalance of qi and blood, during the active stage. According to TCM, dampness–heat syndrome of the large intestine is the most common type of UC, which can be treated by clearing heat and transforming dampness and regulating qi and harmonizing blood.7,8
Portulaca oleracea L. (POL) is a representative anti-dysentery TCM herb that is known to clear heat, transform dampness, remove toxins, cool the blood, and stop bleeding. It is cooling and sour and is consumed as a food and medicinal herb. It is also known as a “natural antibiotic”. Additionally, POL has been recorded to treat heat toxic blood dysentery in the TCM books, “Tai Ping Sheng Hui Fang” and “Jing Xiao Chan Bao”.9 Modern pharmacological studies found that POL contained flavonoids, alkaloids, fatty acids, terpenoids, polysaccharides, vitamins, sterols, proteins, minerals, and other components, which have anti-inflammatory, immunomodulatory, antibacterial, antiviral, antioxidant, anticarcinogenic, nephroprotective, hepatoprotective, gastrointestinal (GI) protective, metabolic, muscle relaxant, antiasthmatic, and antioxidant properties.10
In this review, we summarized the mechanisms of action and clinical research progress of POL in the treatment of UC and indicated directions for subsequent research on the role of POL in UC treatment.
Overview of POL
History of POL in the Treatment of Dysentery
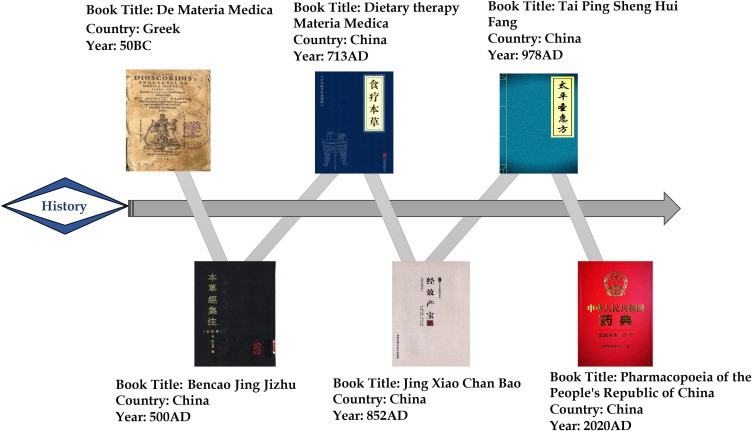
POL, belonging to the Amaranthaceae family, is an annual herb with reddish stems and alternate leaves. It is widely distributed, especially in the tropical and subtropical regions.11 It is used in many countries as a food source as well as a traditional medicine for relieving a wide range of ailments, including GI disorders, respiratory disorders, and liver inflammation.12,13 Moreover, Pedanius Dioscorides (40–90 AD), known as the father of pharmacology, described POL (under the name “andrachne”) as an astringent in De Materia Medica and recorded it as a treatment for dysentery.14 In China, POL was first described in the “Collected Notes on the Materia Medica (Ben Cao Jing Ji Zhu)” as having curative effects of clearing heat, removing toxins, dissipating blood, and resolving swelling. Additionally, POL has been recorded to treat infantile malnutrition and dysentery in the “Food Therapy Materia Medica (Shi Liao Ben Cao)” and heat toxic blood dysentery in “Tai Ping Sheng Hui Fang” and “Jing Xiao Chan Bao”. In the Chinese Pharmacopoeia, POL has been recorded to treat heat toxic blood dysentery, swollen welling-abscess and clove sores (Figure 1).
Figure 1.
Historical texts documenting the use of Portulaca oleracea L. (POL) in the treatment of dysentery.
Active Ingredients of POL
POL primarily contains flavonoids, alkaloids, organic acids, and terpenoids. Kaempferol, myricetin, rutin, luteolin, quercetin, apigenin (API), genistin, and genistein are some of the flavonoids isolated from stems, leaves, and roots of POL.15 Norepinephrine, dopamine, dopa, uracil, thymine, adenosine, allantoin, N, N-dicyclohexylurea, and N-trans-feruloyltyl tyrosine (dominated by isoquinoline-like structures and indole-like structures of the parent nucleus) as well as cyclic dipeptide alkaloids and amides are a few alkaloid compounds found in POL.16 α-Linolenic acid, palmitic acid, p-coumaric acid, linoleic acid, stearic acid, ferulic acid, oleic acid, caffeic acid, and oxalic acid are some organic acids found in POL.17 Purslane monoterpene, lupeol, eleutheroside A, taraxerol, oleanolic acid, and cycloartenol are some terpene constituents reported in POL.11 Other pharmacological compounds found in POL include trans-p-coumaric acid, 7-hydroxycoumarin, bergapten, isopimpinellin, scopoletin, lutein, β-carotene, melatonin, and vitamin C (Table 1).18,19
Table 1.
Active Compounds Isolated from POL and Their Structural Formulas
| Classification | Chemical component | Part of plant | Molecular Formula | Structural Formula | Reference |
|---|---|---|---|---|---|
| Flavonoids | Kaempferol | Whole plant | C15H10O6 | 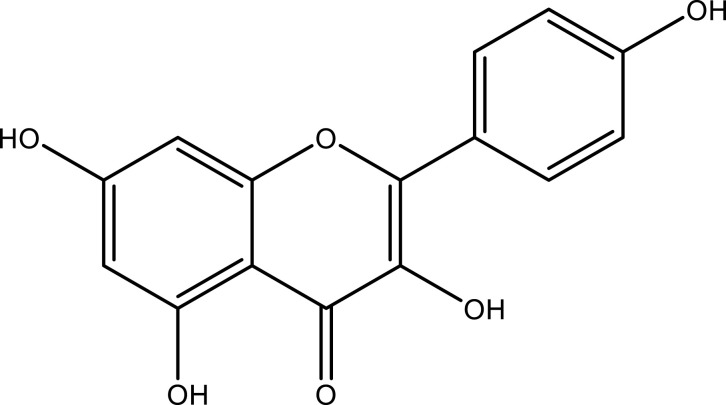 |
[20] |
| Apigenin | Leaf and stem | C15H10O5 | 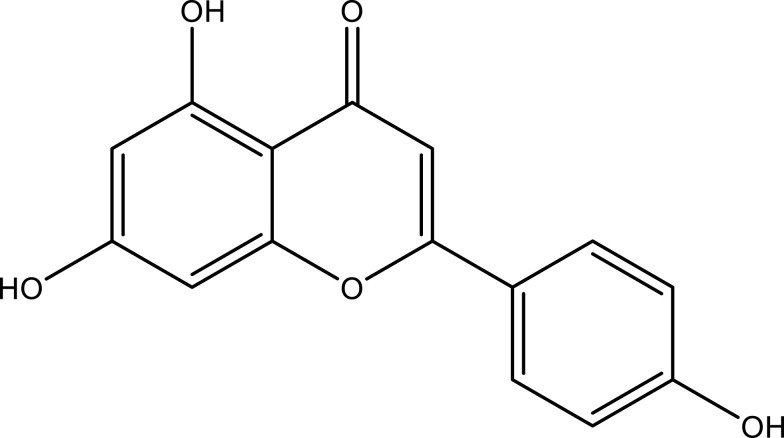 |
[20] | |
| Myricetin | Whole plant | C15H10O8 | 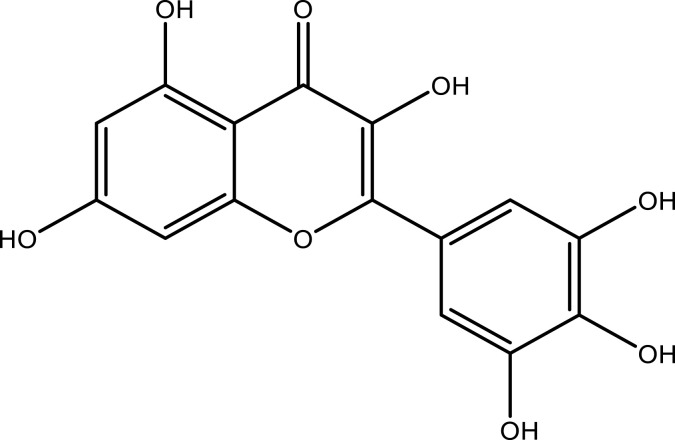 |
[20] | |
| Rutin | C27H30O16 | 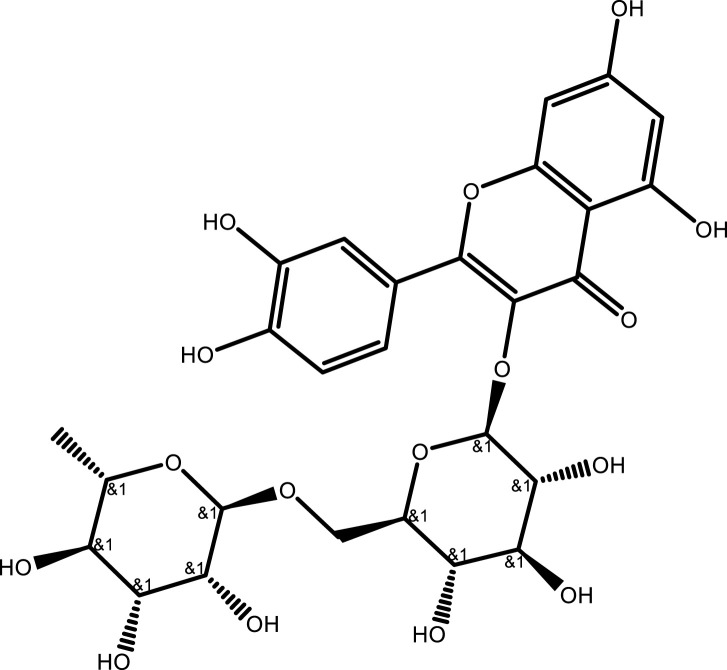 |
[21] | ||
| Luteolin | Whole plant | C15H10O6 | 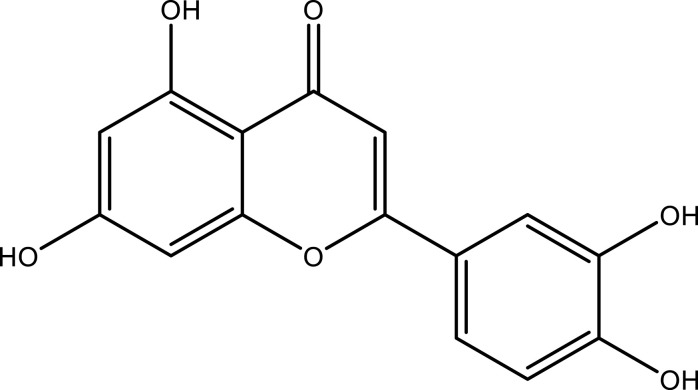 |
[20] | |
| Quercetin | Whole plant | C15H10O7 | 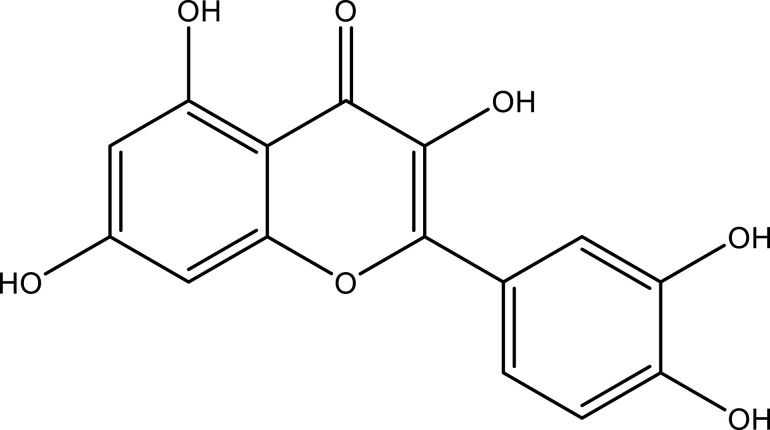 |
[20] | |
| Genistin | Whole plant | C21H20O10 | 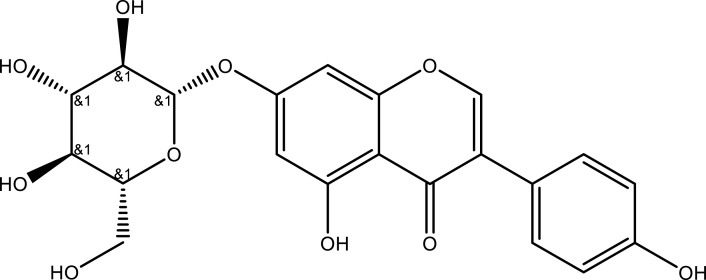 |
[22] | |
| Genistein | Whole plant | C15H10O5 | 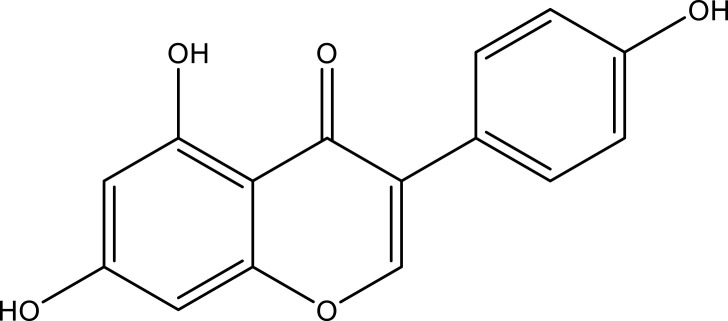 |
[22] | |
| Alkaloids | Noradrenalin | Stem, leaf and seed | C8H11NO3 | 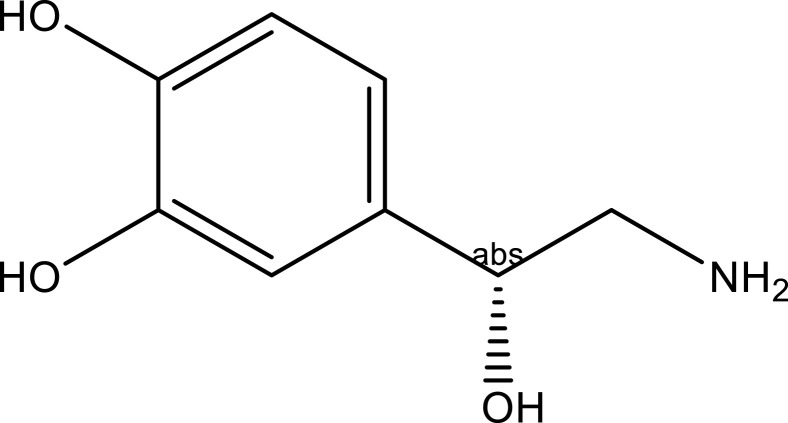 |
[23] |
| Dopamine | Stem, leaf and seed | C8H11NO2 | 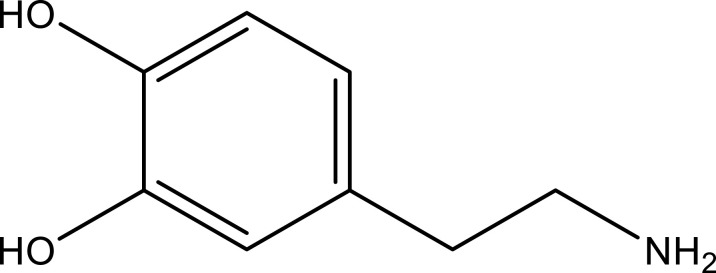 |
[24] | |
| Dopa | C39H74NaO8P | 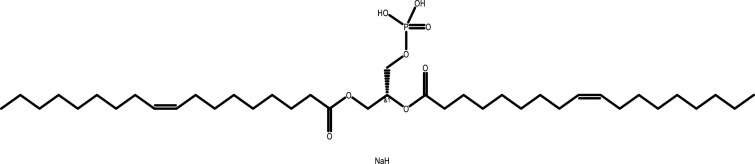 |
[25] | ||
| Uracil | Aerial parts | C4H4N2O2 | 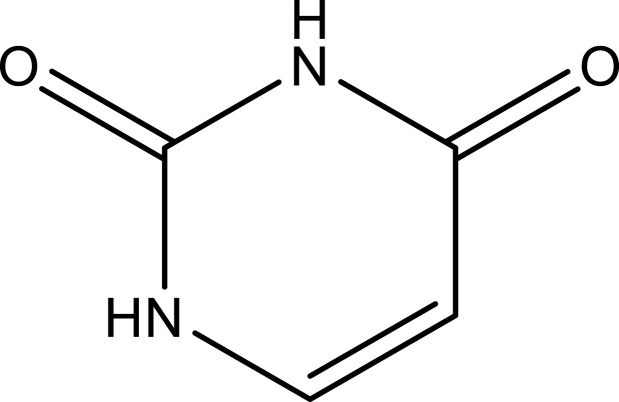 |
[26] | |
| Thymine | Aerial parts | C5H6N2O2 | 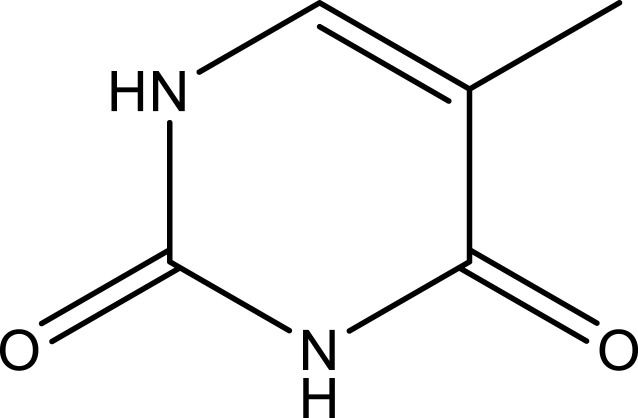 |
[26] | |
| Adenosine | Whole plant | C10H13N5O4 | 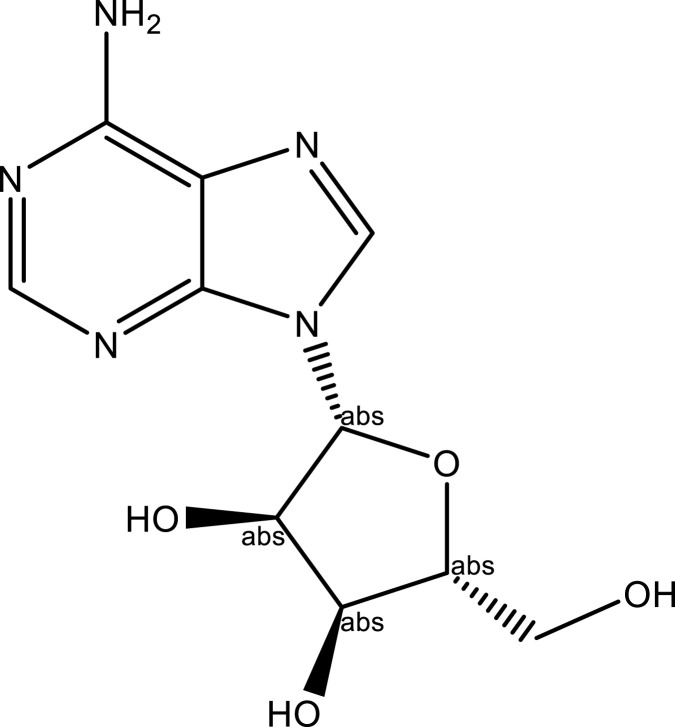 |
[25] | |
| Allantoin | C4H6N4O3 | 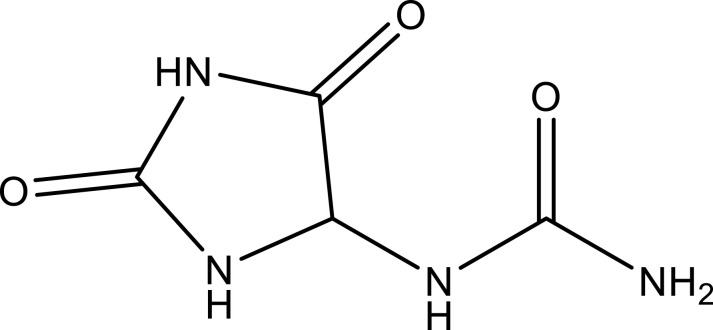 |
[27] | ||
| N, N-dicyclohexylurea | C13H24N2O | 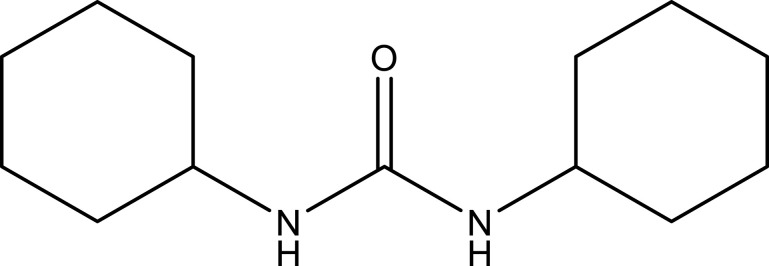 |
[27] | ||
| N-trans-feruloyltyramine | Aerial part | C18H19NO4 | 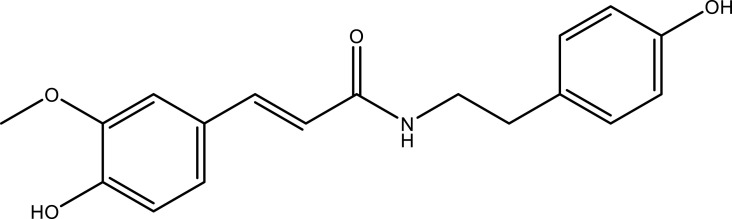 |
[28] | |
| Organic acids | a-Linolenic acid | Leaf | C18H30O2 | 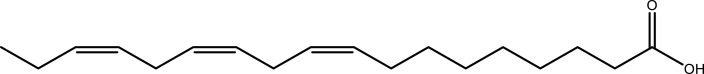 |
[29] |
| Palmitic acid | Leaf | C16H32O2 | 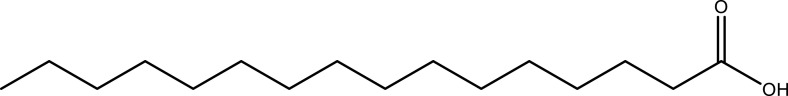 |
[30] | |
| p-Coumaric acid | Whole plant | C9H8O3 | 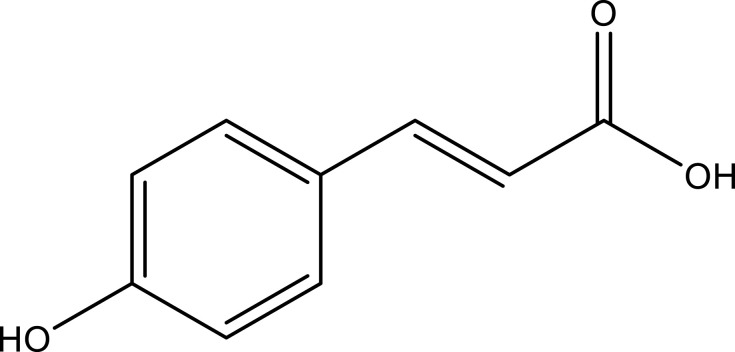 |
[25] | |
| Linoleic acid | Leaf | C18H32O2 | 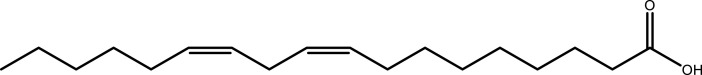 |
[31] | |
| Stearic acid | C18H36O2 | 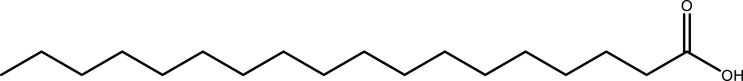 |
[30] | ||
| Ferulic acid | Whole plant | C10H10O4 | 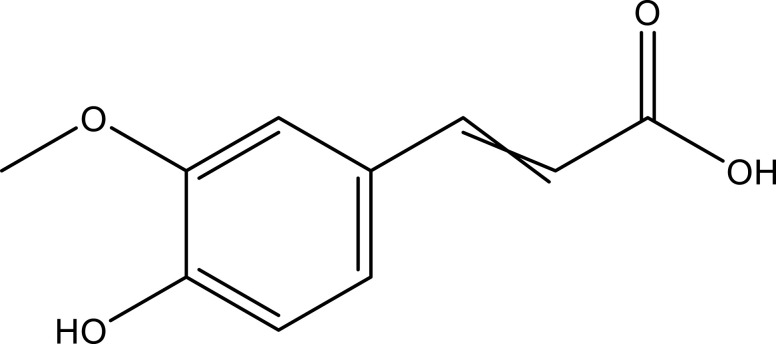 |
[25] | |
| Oleic acid | Leaf | C18H34O2 | 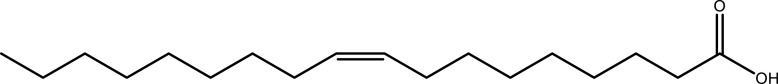 |
[30] | |
| Caffeic acid | Aerial part | C9H8O4 | 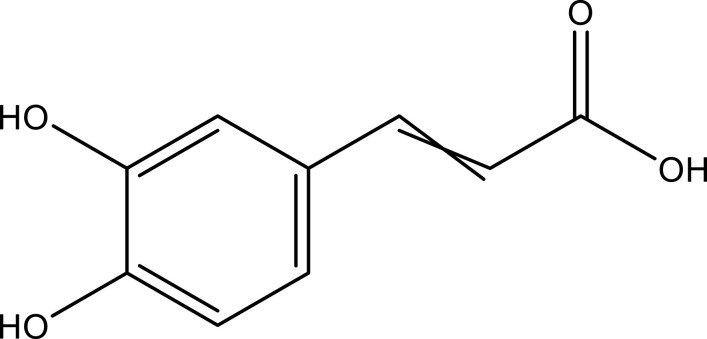 |
[32] | |
| Oxalic acid | Leaf | C2H2O4 | 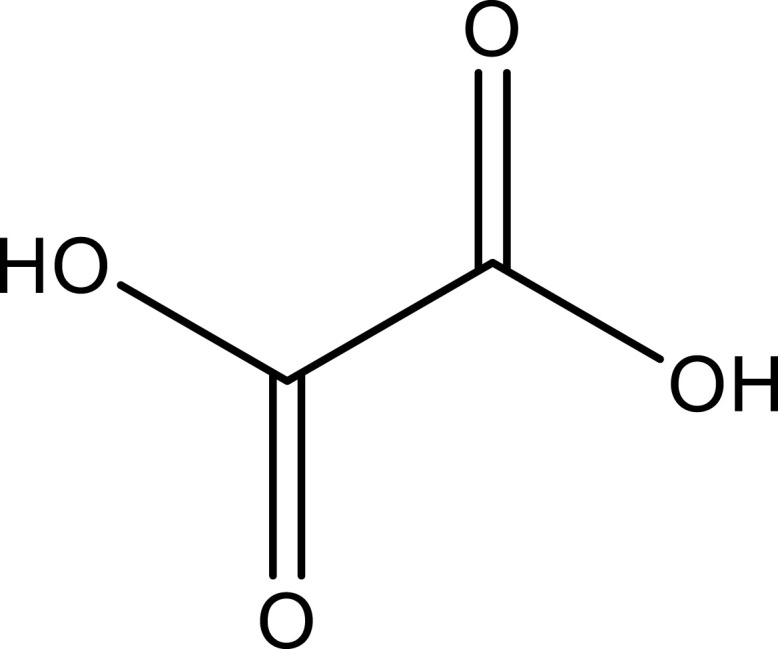 |
[33] | |
| Terpenoids | Lupeol | Aerial part | C30H50O | 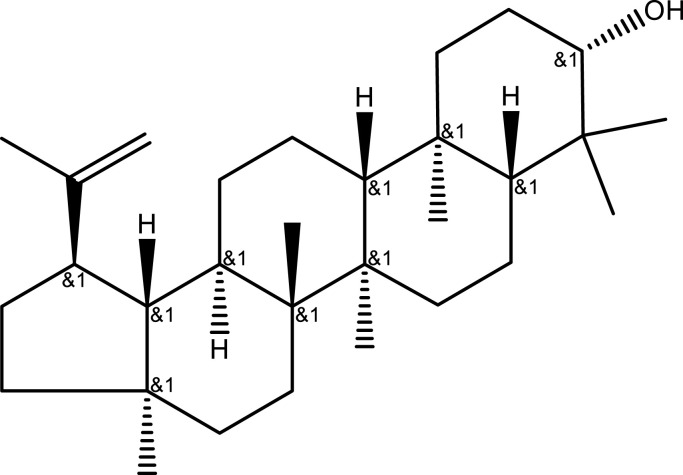 |
[34] |
| Eleutheroside A | C35H60O6 | 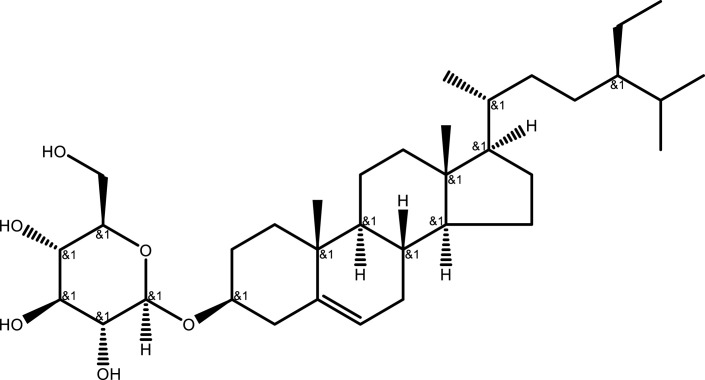 |
[15] | ||
| Taraxerol | C30H50O | 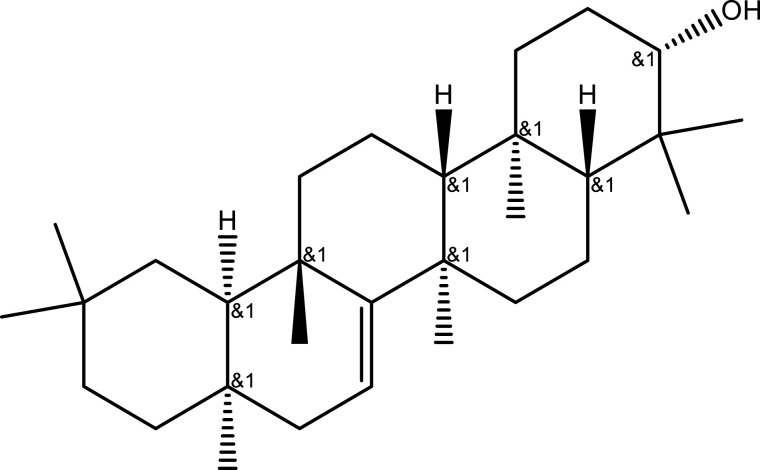 |
[15] | ||
| Oleanolic acid | C30H48O3 | 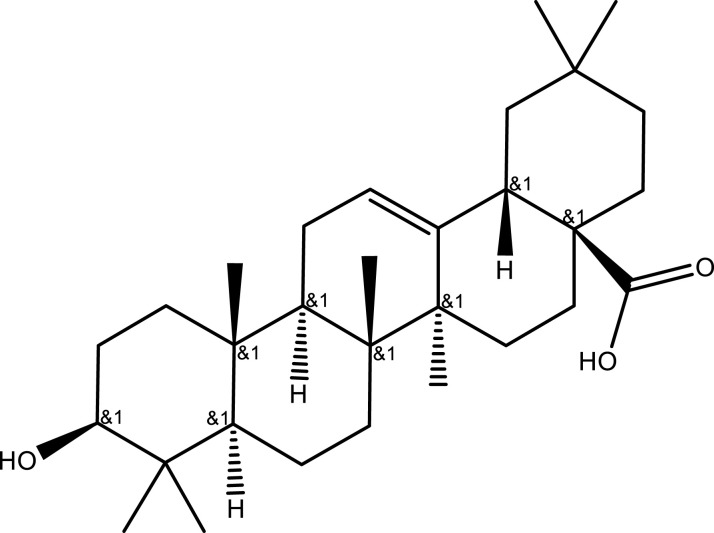 |
[15] | ||
| Cycloartenol | C30H50O | 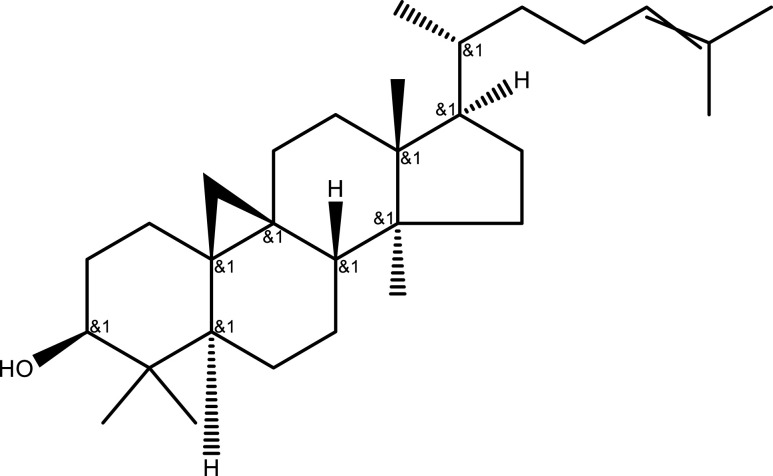 |
[15] | ||
| Other compounds | 7-Hydroxycoumarin | C9H6O3 | 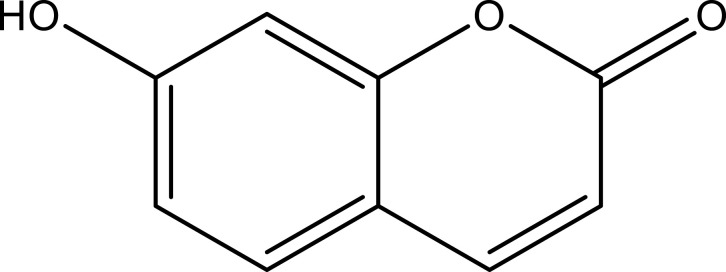 |
[18] | |
| Bergapten | C12H8O4 | 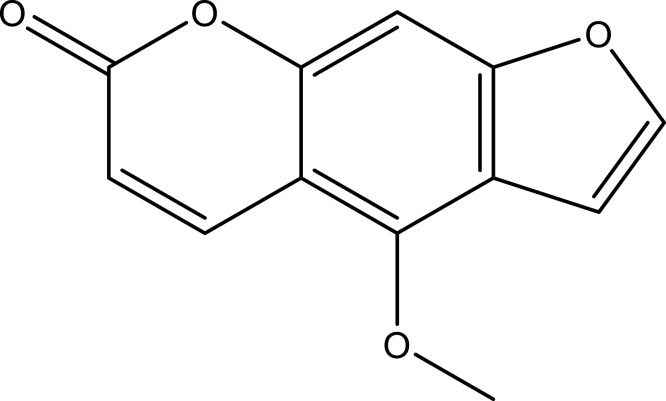 |
[18] | ||
| Isopimpinellin | C13H10O5 | 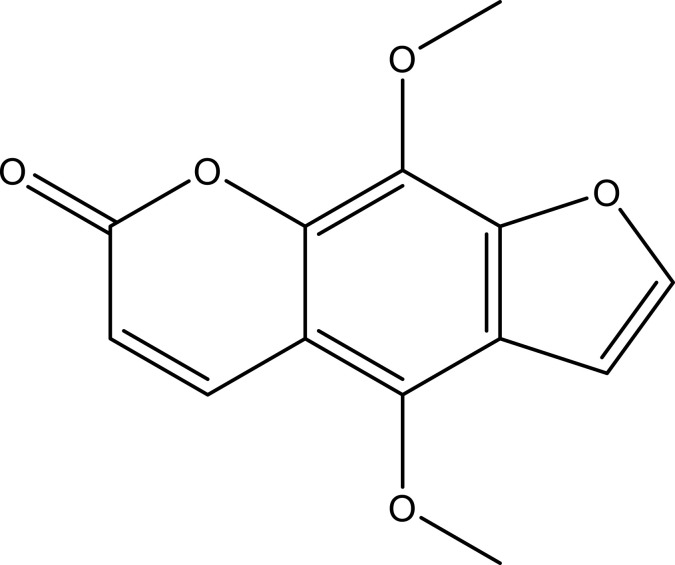 |
[35] | ||
| Scopoletin | Leaf | C10H8O4 | 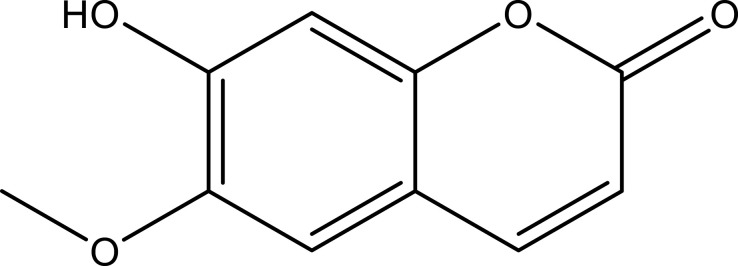 |
[18] | |
| β-Carotene | C40H56 | 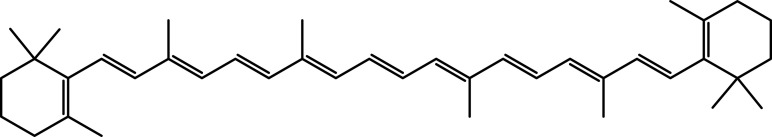 |
[30] | ||
| Melatonin | Leaf | C13H16N2O2 | 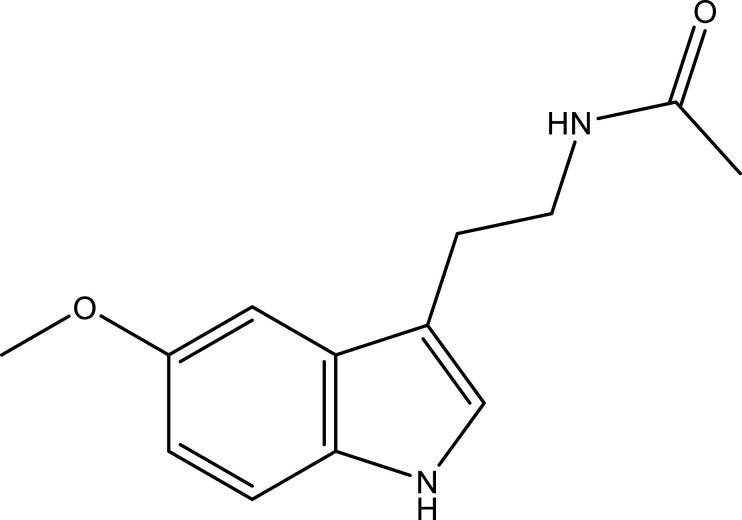 |
[29] | |
| Vitamin C | Leaf | C6H8O6 | 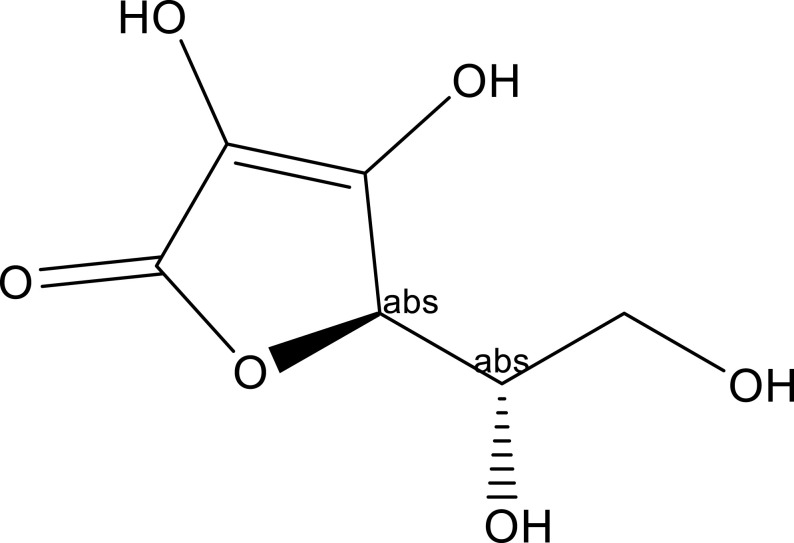 |
[31] |
Mechanisms of Action of POL in UC Treatment
UC has a complex pathogenesis, which is influenced by multiple risk factors, including genetic susceptibility, mucosal barrier defects, dysregulated immune response, intestinal flora dysbiosis, persistent inflammatory interactions, and environmental stress.1,36 Several studies have shown that POL might intervene in UC by modulating the above pathways.
POL Mediates UC Treatment by Regulating the Intestinal Flora
Several studies have found that intestinal flora dysbiosis played an important role in the pathogenesis of UC. The intestinal microbiota is a complex and rich ecosystem. Microorganisms settle in the nutrient-rich environment of the colon and provide energy and nutrients to the host, protect the host, and regulate the host’s immune system.37–40 Feng et al41 and Zhou et al42 found that treatment with 0.3 mL of 0.5 g/mL P. oleracea polysaccharide (POP) significantly increased the abundance of intestinal Bifidobacteria and Lactobacilli and significantly decreased the abundance of Enterobacteriaceae and Enterococcus in dextran sulfate sodium (DSS)-induced UC mice. Additionally, they found that POP treatment increased the level of anti-inflammatory cytokine interleukin (IL)-10 in the intestinal mucosa and reduced endotoxin content in the peripheral blood and the levels of pro-inflammatory cytokines, tumor necrosis factor (TNF)-α, and IL-6 in UC mice. In addition, they found that POP treatment attenuated the DSS-induced colonic histopathological changes in UC mice. Ren et al43 found that POL-Cinnamomum cassia Presl treatment significantly regulated the 5% DSS-induced Bifidobacteriacea, Moryella, Lachnospiraceae-ND-3007-group, Bacteroidales-BS11-gut-group, Coriobacteriales, Negativibacillus, Jeotgalicoccus, and Rikenellaceae-RC9-gut-group. Moreover, intestinal flora analysis revealed that POL-Cinnamomum cassia Presl treatment partially restored intestinal flora structure and reduced intestinal inflammation.
Myricetin is a natural dietary flavonoid with a wide range of pharmacological activities, including antioxidant, anti-inflammatory, anticarcinogenic, and antiproliferative activities.44 Moreover, it maintains the composition of Firmicutes and Actinomycetes in the intestinal flora. Myricetin-3-O-β-d-lactose sodium salt (M10) has been demonstrated to be highly effective in preventing DSS-induced chronic inflammation of colonic tissues and intestinal flora dysbiosis in mice.45 Quercetin supplementation was found to inhibit Citrobacter rodentium-induced inflammation in mice by inhibiting pro-inflammatory cytokines, such as IL-17, TNF-α, and IL-6; increasing the abundance of Bacteroides, Bifidobacterium, Lactobacillus, and Clostridium; and significantly decreasing the abundance of Fusobacterium and Enterococcus.46 Notably, melatonin was found to exert anti-colitis effects by recognizing bacteria through toll-like receptor (TLR)-4, regulating bacteria through adenosine monophosphate, and altering goblet cells.47 Vitamin C supplementation was found to attenuate acute inflammation and subchronic inflammation during early recovery from colon cancer by modulating inflammatory mediators and cytokines and regulating the abundance of inflammation-associated gut bacteria.48 However, vitamin C deficiency increased inflammatory cell infiltration and oxidative stress.49
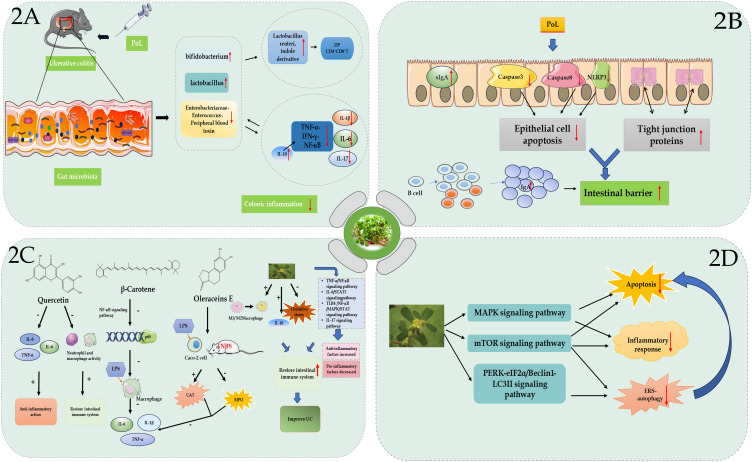
Elkhayat et al reported that the ethanolic extract of POL exhibited antimicrobial activity against both Gram-positive and Gram-negative bacteria.34 Moreover, previous studies have shown that the ethanolic extract of POL modulated bacterial proliferation and growth, thereby down-regulating the levels of IL-1, −6, and −17; TNF-α; interferon (IFN)-γ; and nuclear factor kappa B (NFκB).50,51 Ning et al obtained extracts of P. oleracea macromolecules (POEM) (>10 kDa) and small molecules (POES) (<1 kDa) by membrane separation and found that POEM contained higher protein content, while POES contained higher organic acid and alkaloid content.52 Additionally, they found that POEM alleviated DSS-induced UC in mice by regulating the antioxidant capacity and intestinal flora. Zhu et al found that P. oleracea L-derived exosome-like nanoparticles (PELNs) showed excellent stability and safety in the GI tract and exhibited affinity for the inflammation site in mice colon. Moreover, they found that oral administration of PELNs maintained intestinal flora diversity and homeostasis, enhanced the growth of Lactobacillus reuteri, elevated the levels of indole derivatives, activated aryl hydrocarbon receptor (AhR) in conventional CD4+ T cells, down-regulated Zbtb7b gene expression, and reprogrammed the conversion of conventional CD4+ T cells into double-positive CD4+CD8+ T cells (Figure 2A).53
Figure 2.
(A) P. oleracea L. alleviates intestinal inflammation by regulating gut microbiota in mice. (B) P. oleracea L. alleviates ulcerative colitis (UC) by repairing the mucosal barrier. (C) The active components of P. oleracea L., including quercetin, β-carotene, and Oleracein E, alleviate UC by regulating the inflammatory response and immune system through related signaling pathways. (D) P. oleracea L. alleviates UC by regulating apoptosis, autophagy, endoplasmic reticulum stress, and other pathways.
POL Mediates UC Treatment by Repairing the Mucosal Barrier
The intestinal mucosal barrier is composed of intestinal mucosal epithelium, intestinal mucus, intestinal flora, secretory immunoglobulins (sIg), and intestine-associated lymphoid tissues, including biological, chemical, mechanical, and immune barriers.54 The four barriers maintain the stability of intestinal mucosal barrier function (MBF) and the integrity of intestinal mucosa by effectively identifying and eliminating harmful substances both inside and outside the intestines. Impaired intestinal MBF is considered to play a central role in the development and progression of UC.55 Apigenin (API), a POL flavonoid, ameliorates DSS-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways.56 Additionally, a study found that API-Mn(II) loaded sodium hyaluronate nanoparticles (prepared by incorporating API in Mn2+ framework and subsequently coated with hyaluronic acid) significantly improved the damaged colonic tissues and repaired the intestinal barrier by mediating inflammatory factors, attenuating the damage to tight junctions, increasing cellular density, and narrowing cellular gaps.57 M10, prevents DSS-induced UC by inhibiting colonic mucosal cell necrosis and remodeling the intestinal barrier.58 Dai et al found that POP increased the sIg-A content of intestinal mucosa, improved colonic histopathological changes, and exerted therapeutic effects against DSS-induced UC in mice.59 Additionally, POP exerts therapeutic effects against UC by reducing the expression of caspase-3 and caspase-8 in colonic epithelial cells, regulating epithelial cell apoptosis, and promoting mucosal repair.60 Ning et al found that POP maintained intestinal retinol and short-chain fatty acid levels, promoted colonic B-cell proliferation and differentiation, and increased intestinal IgA expression, thereby improving the intestinal barrier and maintaining intestinal homeostasis.61 Finally, purslane juice was found to improve DSS-induced UC by decreasing the expression of NOD-like receptor protein 3 inflammasomes to inhibit cellular pyroptosis and by up-regulating the expression of tight junction proteins to repair intestinal barrier dysfunction (Figure 2B).62
POL Mediates UC Treatment by Regulating the Immune Response
Studies on the immune response and inflammatory pathways in UC have shown that the imbalance between pro-inflammatory and anti-inflammatory cytokines mediated UC development. The induction of C-reactive proteins, IFN-γ, lipopolysaccharide (LPS), and TNF-α can induce M1 polarization of macrophages during the active phase of a disease.63 The M1 macrophages release pro-inflammatory cytokines and chemokines, such as IL-1, IL-6, IL-8, IL-18, IL-12, IL-23, IFN-γ, inducible nitric oxide synthase, and reactive oxygen intermediates, which participate in acute inflammatory responses and disrupt the intestinal mucosal barrier, as well as mediate cellular immunity and intervene in disease prognosis.64,65 During intestinal injury, large amounts of neutrophils are rapidly recruited to the site of injury, where they trigger the recruitment of other immune cells, consequently generating an over-immune response, which is associated with the aggravation of intestinal inflammation and mucosal damage in the early stages of UC.66,67 In contrast, macrophage polarization leads to the accumulation and release of various pro-inflammatory factors or regulatory anti-inflammatory cytokines in the local intestinal segments of IBD lesions to exert biological effects.68 Studies have shown that M2 macrophages primarily secreted anti-inflammatory factors, such as IL-10, transforming growth factor-β, and arginase 1, which induced Th2-type immune response, suppressed inflammatory response, and promoted tissue repair and tumor progression.65
Several studies have shown that the active ingredients of POL could play a preventive and therapeutic role against the onset, development, drug resistance, and other related processes of UC by modulating the inflammatory response and the immune system (Figure 2C). Studies have found that quercetin, which was abundant in POL, could inhibit LPS-induced production of IL-6, TNF-α, and IL-8, thus exerting an anti-inflammatory effect.69 Additionally, a study found that quercetin treatment partially restored DSS-induced loss of epithelial integrity in C57BL/6 mice by inducing tight junction proteins via AhR-mediated anti-inflammatory mechanisms. Moreover, quercetin restores innate and adaptive intestinal immune system homeostasis by down-regulating neutrophil and macrophage activity and by shifting the Th17/ T-cell (Treg) ratio in favour of the Treg subpopulation, respectively.70 Meanwhile, β-carotene exhibits anti-inflammatory functions by inhibiting LPS-induced secretion of macrophage inflammatory factors, such as IL-1β, IL-6, and TNF-α, via down-regulating the expression of NF-κB p65 protein in the NF-κB pathway.71 Myricetin exerts anti-inflammatory effects in UC mice by increasing Treg proliferation, decreasing Th1 and Th17 cell proliferation, and promoting immune cell homeostasis.72 Huang et al demonstrated that Oleracein E (OE) significantly increased catalase activity, decreased myeloperoxidase (MPO) activity, and decreased IL-1β, IL-6, and TNF-α levels in LPS-induced Caco-2 cells and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced UC rats.73 In addition, OE may reduce the level of oxidative stress by activating the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 pathway, thereby improving intestinal mucosal barrier damage. Previous studies have found that genistein and lupeol mediated the anti-inflammatory effects against IBD by promoting M1 to M2 polarization of macrophages. Intriguingly, lupeol promotes M1 to M2 polarization of infiltrating macrophages by inhibiting IRF5 and possibly other transcription factors critical for M1-type polarization via a specific receptor and down-stream signaling pathways, such as p38 MAPK.74,75 In addition, genistein flavonoids reduce serum cytokine expression levels, reduce MPO activity, and significantly down-regulate COX-2 protein expression levels in TNBS-induced UC rats.76 Zhou et al found that POL might mediate its therapeutic effects against UC by restoring Th1 and Th2 homeostasis, which further led to a significant decrease in IFN-γ expression and a significant increase in IL-10 expression in the colon of TNBS-induced UC rats.77 Vanden Braber et al found that microencapsulated genistein significantly attenuated intestinal inflammation and tissue damage in mice and restored the expression of IL-10, which was a key factor in mucosal homeostasis.78
Among the immunomodulatory factors, oxidative stress is considered to be one of the main mechanisms associated with the pathophysiology of IBD.79 Studies have found that chronic intestinal inflammation was associated with the overproduction of reactive oxygen and nitrogen species.80 Induced oxidative stress injury disrupts intracellular homeostasis, which may lead to intestinal flora disorders, dysregulated immune response, altered MBF, abnormal autophagy, and intestinal fibrosis, thus further exacerbating intestinal inflammation.81,82 Quercetin, a constituent of POL, protects against indomethacin-induced oxidative stress and GI inflammation through its antioxidant and anti-inflammatory properties.83 Luteolin significantly attenuates disease activity index colon shortening, and histological damage in DSS-induced UC mice by activating Nrf2-mediated antioxidant activity.84
A few studies found that POPs could regulate TNF-α/NF-κB and IL-6/signal transducer and activator of transcription 3 (STAT3) signaling pathways and reduce soluble IL-6 receptor-alpha (sIL-6Rα) and glycoprotein 130 levels, as well as colonic MPO and NF-κB levels in rats. Additionally, POPs can attenuate sIL-6Rα–IL-6-induced inflammatory responses; inhibit p-STAT3, COX-2, and ikB-alpha expression; and inhibit CXC chemokine ligand 1 and its receptor CXC chemokine receptor 2, thereby decreasing neutrophil recruitment to the inflammatory sites and reducing UC symptoms.85–88 Ai et al found that API inhibited inflammation and inflammation-induced carcinogenesis under normal conditions by regulating the STAT3/NF-κB signaling pathway.89
Bian Yifei found that the aqueous POL extract played an anti-inflammatory role by inhibiting the expression of inflammatory factors, such as TNF-α, IL-1β, and IL-6, and TLR4/NFκB pathway proteins. In addition, quercetin and cannabinol, the active ingredients of POL, inhibit the expression of TLR4/NFκB/MAPK/STAT pathway proteins in LPS-induced rat microvascular endothelial cells.90
A study reported that IL-17 levels in peripheral blood monocytes were associated with the severity of IBD.91 By using cyberpharmacological analysis, Chen et al found that combination treatment with pannotoginseng and purslane regulated the IL-17, TNF, and proteoglycan signaling pathways and exerted a preventive effect against the onset, progression, and resistance of UC.92
Other Mechanisms of Action of POL in UC Treatment
Studies have found that the active components in POL exhibited anti-inflammatory effects by reducing the release of inflammatory transmitters, such as TNF-α, by inhibiting MAPK.93,94 Zhang et al used network pharmacology to screen 10 active ingredients of POL, including arachidonic acid, β-sitosterol, kaempferol, luteolin, isobetaine, and quercetin, and found 62 core targets, which were closely associated with cancer, viral infection, IBD, and immune regulation. Additionally, the study found that 17 of core targets played a multi-targeted, multi-pathway role in alleviating UC through anti-inflammatory, antioxidant, and cell proliferation and apoptosis regulation, including multidrug resistance-associated protein 4, serum albumin, IFN gamma, inhibitor of NFκB kinase subunit alpha, MPO, nitric oxide synthase, inducible, nuclear receptor subfamily 1 group I member 2, peroxisome proliferator-activated receptor gamma, TNF, xanthine dehydrogenase/oxidase.95 Yang et al found that POP mediated UC treatment by regulating the TLR4/MyD88/NF-κB signaling pathway in DSS-induced UC mice.94
In experiments, some active ingredients of POL were found to be associated with the MAPK signaling pathway and important inflammatory transmitters, such as TNF, IL-1β, IL-4, and IL-6. Additionally, the anti-inflammatory effects of POL components, such as 1-carbomethoxy-β-carboline, luteolin, kaempferol, quercitrin, and cis-N- feruloyl-3’-methoxytyramine, were also confirmed in these studies.96–99 Ma et al suggested that POL might mediate its therapeutic effects in UC by regulating the mTOR signaling pathway based on network pharmacology analysis.9 The mTOR is a serine/threonine protein kinase consisting of mTORC1 complexes and mTORC2 complexes. The mTORC1 and mTORC2 complexes form a signaling pathway with other proteins, respectively, to regulate cell growth, proliferation, and survival, as well as organismal growth and internal homeostasis based on external signals.100 Additionally, the mTOR signaling pathway participates in autophagy, which is essential for UC pathogenesis.
Numerous studies have indicated that endoplasmic reticulum stress (ERS)-induced autophagy synergistically promoted UC pathogenesis by targeting intestinal epithelial cells (IECs).101 A study found that POL extracts attenuated ERS-induced autophagy of IECs through the protein kinase R-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/Beclin1/microtubule-associated protein light chain 3II pathway, thereby mediating its therapeutic effects in UC.102 M10 significantly increases the number of CD8+ and CD4+ T lymphocytes and reduces macrophage phagocytosis, thereby attenuating ERS-induced autophagy and apoptosis and preventing chronic inflammation and colorectal tumorigenesis.103 Melatonin slows the progression of UC-associated colorectal cancer by modulating autophagy and Nrf2 signaling pathways.104 Another study found that POL extract alleviated UC in mice by modulating inflammatory response, apoptosis, and PPAR-γ levels (Figure 2D). 105,106
Clinical Studies of POL in UC Treatment
POL Monotherapy
Liu et al found that treatment with POL capsules (n = 6, 0.35 g/capsule, tid po) and traditional Chinese herbal colitis enema solution rapidly alleviated clinical UC symptoms, repaired colonic mucosa, and reduced inflammatory response, without any adverse reactions.107–109 Clinical observations revealed that this combination treatment was safer and more effective than Sulfasalazine Tablets (91.42% vs 76.47).107
POL Compounds
Clinical studies have found that the combination of compound POL decoction (containing POL 150 g, Granatum 100 g, Elaeagnus angustifolia Linn. 300 g, Cichorium intybus L. 20 g, etc.) and compound Rhizoma coptidis decoction could obtain ideal therapeutic effects in the treatment of colitis, especially when consumed in the earlier stages of UC.110 Wang et al found that a self-made POL decoction (containing POL 30 g, Sargentodoxa cuneata (Oliv). Rehd. and E. H. Wilson in C. S. Sargent 20 g, Taraxacum mongolicum Hand.-Mazz. 10 g, Fraxini Cortex10 g, etc.) showed a cure rate of 80.5% and a total effective rate of 95.1% when used as the primary treatment for 41 UC cases.111 These results suggest that this formula is simple and easy to implement and is reliable for the treatment of mild and moderate chronic UC, with a low rate of recurrence. Pan et al found that a POL decoction, POL as a sovereign medicine (containing POL 30 g, Hedyotis diffusa 20 g, Codonopsis Radix 12 g, Poria 10 g, Atractylodes macrocephala 10 g, etc.) internal and POL enema showed a total effective rate of 93.75% when used to treat 32 UC cases.112 A clinical study found that treatment with POL enema alone showed a total effective rate of 84.21% in 38 UC cases.113 Another study found that Jingfang and Purslane decoction (containing POL 30 g, Gentianae Macrophyllae Radix 30 g, Nepeta cataria L. 15 g, and Saposhnikoviae Radix 15 g) showed a total effective rate of 95.7% in 68 UC cases, suggesting that this prescription improves intestinal function and has immunoregulatory and antibacterial effects.114
However, the existing clinical studies on POL monotherapy and its combination therapy in the treatment of UC are small-scale, single-center studies. Therefore, more standardized, scientific, and rigorous clinical trials are needed to provide evidence-based reports on the role of POL in UC treatment. In addition, previous studies have indicated that POL had a certain degree of nephrotoxicity, making it necessary to standardize its optimal dosage in clinical applications.115
Conclusion
POL has a long history of use as food and medicine, and it shows significant efficacy in the treatment of UC. In addition to inhibiting IEC apoptosis, POL inhibits the release of inflammatory factors and promotes colonic mucosal repair in UC rats. TCM POL effectively improves the pathological state of intestinal mucosa in UC patients, thereby relieving their clinical symptoms and promoting mucosal healing. This review summarizes the mechanisms of action and clinical research progress of P. oleracea L. in the treatment of UC, indicating the novel targets and more effective treatment modalities for UC. Despite these promising findings, further research is essential to optimize POL’s clinical application. As a herbal medicine, POL is a chemical entity formed by the polymerization of multiple components, but the active ingredients or active molecular groups of various herbs have not been clearly defined, the interactions between various components in herbal medicines are not clear, and its toxic effects are of concern. In addition, clinical data describing the mode of action of the naturally active compounds of POL are still lacking. Therefore, long-term clinical studies assessing the safety and efficacy of POL as a potential treatment against UC remain necessary.
Funding Statement
This study was funded by the grants from the National Natural Science Foundation of China, No. 81873253; the Shanghai Natural Science Foundation, No. 22ZR1458800; the scientific research Project Plan of Shanghai Municipal Health Commission, No. 202240385; the XingLin Scholar Program of Shanghai University of Traditional Chinese Medicine, No. [2020]23; and the Science and Technology Innovation Cultivation Program of LongHua, No.YD202220.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402(10401):571–584. doi: 10.1016/s0140-6736(23)00966-2 [DOI] [PubMed] [Google Scholar]
- 2.Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94(7):1357–1373. doi: 10.1016/j.mayocp.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 3.Du L, Ha C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol Clin North Am. 2020;49(4):643–654. doi: 10.1016/j.gtc.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 4.Wei SC, Sollano J, Hui YT, et al. Epidemiology, burden of disease, and unmet needs in the treatment of ulcerative colitis in Asia. Expert Rev Gastroenterol Hepatol. 2021;15(3):275–289. doi: 10.1080/17474124.2021.1840976 [DOI] [PubMed] [Google Scholar]
- 5.Gros B, Kaplan GG. Ulcerative colitis in adults: a review. JAMA. 2023;330(10):951–965. doi: 10.1001/jama.2023.15389 [DOI] [PubMed] [Google Scholar]
- 6.Wu K, Chen M, Qian J, Inflammatory Bowel Disease Group CSoG, Chinese Medical Association, China IBDQCCo. Chinese clinical practice guideline on the management of ulcerative colitis (2023, Xi′an). Chin J Inflamm Bowel Dis. 2024;01:33–58. doi: 10.3760/cma.j.cn101480-20240123-00017. [DOI] [Google Scholar]
- 7.Medicine CAo I. Experts consensus on diagnosis and treatment of ulcerative colitis by integrative medicine. Chinese Journal of Integrated Traditional and Western Medicine. 2023;43(01):5–11. [Google Scholar]
- 8.Zhang S, Zhao L, Shen H, et al. International clinical practice guideline on the use of traditional Chinese medicine for ulcerative colitis by Board of Specialty Committee of Digestive System Disease of World Federation of Chinese Medicine Societies (2023). Phytother Res. 2024;38(2):970–999. doi: 10.1002/ptr.8087 [DOI] [PubMed] [Google Scholar]
- 9.Ma X, Du X, Wang X, et al. Mechanism of machixian (portulacae herba) on ulcerative colitis based on network pharmacology. Shandong J Tradition Chin Med. 2020;39(11):1240–1247. doi: 10.16295/j.cnki.0257-358x.2020.11.020 [DOI] [Google Scholar]
- 10.Li K, Xia T, Jiang Y, et al. A review on ethnopharmacology, phytochemistry, pharmacology and potential uses of Portulaca oleracea L. J Ethnopharmacol. 2024;319(Pt 2):117211. doi: 10.1016/j.jep.2023.117211 [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Sreedharan S, Kashyap AK, Singh P, Ramchiary N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon. 2022;8(1):e08669. doi: 10.1016/j.heliyon.2021.e08669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahimi VB, Ajam F, Rakhshandeh H, Askari VR. A pharmacological review on portulaca oleracea l.: focusing on anti-inflammatory, anti- oxidant, immuno-modulatory and antitumor activities. J Pharmacopuncture. 2019;22(1):7–15. doi: 10.3831/kpi.2019.22.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalali J, Ghasemzadeh Rahbardar M. Ameliorative effects of Portulaca oleracea L. (purslane) on the metabolic syndrome: a review. J Ethnopharmacol. 2022;299:115672. doi: 10.1016/j.jep.2022.115672 [DOI] [PubMed] [Google Scholar]
- 14.Iranshahy M, Javadi B, Iranshahi M, et al. A review of traditional uses, phytochemistry and pharmacology of Portulaca oleracea L. J Ethnopharmacol. 2017;205:158–172. doi: 10.1016/j.jep.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Gao S. Research progress on active components and pharmacological effects of purslane. J Anhui Agric Sci. 2023;51(10):21–24. [Google Scholar]
- 16.Li K. Research progress on flavonoids and alkaloids in portulaca oleracea L. Pharm Inform. 2023;12(04):317–337. doi: 10.12677/pi.2023.124040 [DOI] [Google Scholar]
- 17.Ghorani V, Saadat S, Khazdair MR, Gholamnezhad Z, El-Seedi H, Boskabady MH. Phytochemical characteristics and anti-inflammatory, immunoregulatory, and antioxidant effects of portulaca oleracea l.: a comprehensive review. Evid Based Complement Alternat Med. 2023;2023(1):2075444. doi: 10.1155/2023/2075444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Liu Y, Xiao F, et al. Chemical composition and pharmacological activity of portulaca olerace. Chin J Exp Traditional Med Formulae. 2018;24(06):224–234. doi: 10.13422/j.cnki.syfjx.20180671 [DOI] [Google Scholar]
- 19.Dias MG, Camões MFGFC, Oliveira L. Carotenoids in traditional Portuguese fruits and vegetables. Food Chem. 2009;113(3):808–815. doi: 10.1016/j.foodchem.2008.08.002 [DOI] [Google Scholar]
- 20.Xu X, Yu L, Chen G. Determination of flavonoids in Portulaca oleracea L. by capillary electrophoresis with electrochemical detection. J Pharmaceutical & Biomed Analysis. 2006;41(2):493–499. doi: 10.1016/j.jpba.2006.01.013 [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Qiu S, Huang H, et al. Transcriptome and metabolome analyses of flavonoids metabolism in leaf, stem and root of Portulaca oleracea L. Acta Botanica Boreali-Occidentalia Sinica. 2021;41(02):254–261. [Google Scholar]
- 22.Zhu H, Wang Y, Liu Y, Xia Y, Tang T. Analysis of flavonoids in portulaca oleracea L. by UV–Vis spectrophotometry with comparative study on different extraction technologies. Food Anal Methods. 2010;3(2):90–97. doi: 10.1007/s12161-009-9091-2 [DOI] [Google Scholar]
- 23.Chen J, Shi Y-P, Liu J-Y. Determination of noradrenaline and dopamine in Chinese herbal extracts from Portulaca oleracea L. by high-performance liquid chromatography. J Chromatogr A. 2003;1003(1–2):127–132. doi: 10.1016/s0021-9673(03)00786-6 [DOI] [PubMed] [Google Scholar]
- 24.Yue ME, Jiang TF, Shi YP. Simultaneous determination of noradrenaline and dopamine in Portulaca oleracea L. by capillary zone electrophoresis. J Separation Sci. 2005;28(4):360–364. doi: 10.1002/jssc.200400045 [DOI] [PubMed] [Google Scholar]
- 25.Xiang L, Xing D, Wang W, Wang R, Ding Y, Du L. Alkaloids from Portulaca oleracea L. Phytochemistry. 2005;66(21):2595–2601. doi: 10.1016/j.phytochem.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 26.Yan J, Sun L-R, Zhou Z-Y, et al. Homoisoflavonoids from the medicinal plant Portulaca oleracea. Phytochemistry. 2012;80:37–41. doi: 10.1016/j.phytochem.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Wang Z, Zhang X, et al. Recent advances in research on chemical components and antibacterial activities of Portulaca oleracea L. Food Sci. 2023;359–371. [Google Scholar]
- 28.Tian JL, Liang X, Gao PY, et al. Two new alkaloids from Portulaca oleracea and their cytotoxic activities. J Asian Nat Prod Res. 2014;16(3):259–264. doi: 10.1080/10286020.2013.866948 [DOI] [PubMed] [Google Scholar]
- 29.Simopoulos AP, Tan D-X, Manchester LC, Reiter RJ. Purslane: a plant source of omega-3 fatty acids and melatonin. J Pineal Res. 2005;39(3):331–332. doi: 10.1111/j.1600-079X.2005.00269.x [DOI] [PubMed] [Google Scholar]
- 30.Palaniswamy UR, McAvoy RJ, Bible BB. Stage of Harvest and Polyunsaturated Essential Fatty Acid Concentrations in Purslane (Portulaca oleraceae) Leaves. Journal of Agricultural and Food Chemistry. 2001;49(7):3490–3493. doi: 10.1021/jf0102113 [DOI] [PubMed] [Google Scholar]
- 31.Uddin MK, Juraimi AS, Hossain MS, Nahar MAU, Ali ME, Rahman MM. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci World J. 2014;2014:951019. doi: 10.1155/2014/951019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Liu C, Xiang L, Zheng Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytother Res. 2009;23(7):1032–1035. doi: 10.1002/ptr.2742 [DOI] [PubMed] [Google Scholar]
- 33.Palaniswamy U, Bible B, McAvoy R. Effect of nitrate: ammonium nitrogen ratio on oxalate levels of purslane. Trends in New Crops and New Uses. 2002;11:453–455. [Google Scholar]
- 34.Elkhayat ES, Ibrahim SRM, Aziz MA. Portulene, a new diterpene fromPortulaca oleraceaL. J Asian Nat Prod Res. 2008;10(11):1039–1043. doi: 10.1080/10286020802320590 [DOI] [PubMed] [Google Scholar]
- 35.Aljeboori KH, AL-Rubai OHK, Yassen Y. Study of pathological, effects of crude extract of Portulaca oleracea L. in the albino mice organs. Pharmacol Res Modern Chin Med. 2014;4:100101. [Google Scholar]
- 36.Cao Z, Zhou X. Ulcerative colitis and intestinal microecology. 2018;1. [Google Scholar]
- 37.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553–570. doi: 10.1161/circresaha.120.316242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Michalak M, Agellon LB. Importance of nutrients and nutrient metabolism on human health. Yale J Biol Med. 2018;91(2):95–103. [PMC free article] [PubMed] [Google Scholar]
- 39.Leslie JL, Vendrov KC, Jenior ML, Young VB. The gut microbiota is associated with clearance of clostridium difficile infection independent of adaptive immunity. mSphere. 2019;4(1). doi: 10.1128/mSphereDirect.00698-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sefik E, Geva-Zatorsky N, Oh S, et al. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORγ⁺ regulatory T cells. Science. 2015;349(6251):993–997. doi: 10.1126/science.aaa9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng L, Li S, Dai L, et al. Effects of polysaccharides from Portulaca L. on intestinal mucosal cytokines and intestinal flora in mice with ulcerative colitis. Chin J Microecol. 2015;27(02):139–142. doi: 10.13381/j.cnki.cjm.201502004 [DOI] [Google Scholar]
- 42.Zhou Z, Ma T, Feng L, et al. Effects of polysaccharides from Portulaca Oleracea L. on intestinal flora and blood endotoxin in mice with ulcerative colitis. Chin J Microecol. 2014;26(06):646–648. doi: 10.13381/j.cnki.cjm.201406006 [DOI] [Google Scholar]
- 43.Ren L, Tang Z, Liang Y, et al. Preparation of Portulacae herba-cinnamomi cortex granules and its effect on intestinal flora of ulcerative colitis mice. Chin J Microecol. 2019;31(02):130–137. doi: 10.13381/j.cnki.cjm.201902002 [DOI] [Google Scholar]
- 44.Zhu S, Yang C, Zhang L, et al. Development of M10, myricetin-3-O-β-d-lactose sodium salt, a derivative of myricetin as a potent agent of anti-chronic colonic inflammation. Eur J Med Chem. 2019;174:9–15. doi: 10.1016/j.ejmech.2019.04.031 [DOI] [PubMed] [Google Scholar]
- 45.Miao RR, Zhan S, Hu XT, et al. Myricetin and M10, a myricetin-3-O-β-d-lactose sodium salt, modify composition of gut microbiota in mice with ulcerative colitis. Toxicol Lett. 2021;346:7–15. doi: 10.1016/j.toxlet.2021.03.009 [DOI] [PubMed] [Google Scholar]
- 46.Lin R, Piao M, Song Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in citrobacter rodentium-infected mice. Front Microbiol. 2019;10:1092. doi: 10.3389/fmicb.2019.01092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SW, Kim S, Son M, Cheon JH, Park YS. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci Rep. 2020;10(1):2232. doi: 10.1038/s41598-020-59314-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeon HJ, Yeom Y, Kim YS, et al. Effect of vitamin C on azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colitis-associated early colon cancer in mice. Nutr Res Pract. 2018;12(2):101–109. doi: 10.4162/nrp.2018.12.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jo H, Lee D, Go C, et al. Preventive effect of vitamin C on Dextran Sulfate Sodium (DSS)-induced colitis via the regulation of IL-22 and IL-6 Production in Gulo(-/-) Mice. Int J Mol Sci. 2022;23(18):10612. doi: 10.3390/ijms231810612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alfwuaires MA, Algefare AI, Afkar E, Salam SA, El-Moaty HIA, Badr GM. Immunomodulatory assessment of Portulaca oleracea L. extract in a mouse model of colitis. Biomed Pharmacother. 2021;143:112148. doi: 10.1016/j.biopha.2021.112148 [DOI] [PubMed] [Google Scholar]
- 51.Peng C, Ouyang Y, Lu N, Li N. The NF-κB signaling pathway, the microbiota, and gastrointestinal tumorigenesis: recent advances. Front Immunol. 2020;11:1387. doi: 10.3389/fimmu.2020.01387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ning K, Duan Y, Tong W, et al. Protective effects of different molecular weights of purslane (Portulaca oleracea L.) aqueous extract on DSS-induced ulcerative colitis in mice. Antioxidants. 2023;12(7). doi: 10.3390/antiox12071400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu MZ, Xu HM, Liang YJ, et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4(+)CD8(+)T cells expansion. J Nanobiotechnology. 2023;21(1):309. doi: 10.1186/s12951-023-02065-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang K, Wu LY, Dou CZ, Guan X, Wu HG, Liu HR. Research advance in intestinal mucosal barrier and pathogenesis of Crohn’s disease. Gastroenterol Res Pract. 2016;2016:9686238. doi: 10.1155/2016/9686238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang WC, Liang J, Nagahashi M, et al. Sphingosine-1-phosphate phosphatase 2 promotes disruption of mucosal integrity, and contributes to ulcerative colitis in mice and humans. FASEB j. 2016;30(8):2945–2958. doi: 10.1096/fj.201600394R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Márquez-Flores YK, Villegas I, Cárdeno A, Rosillo M, Alarcón-de-la-Lastra C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J Nutr Biochem. 2016;30:143–152. doi: 10.1016/j.jnutbio.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 57.Lv F, Zhang Y, Peng Q, et al. Apigenin-Mn(II) loaded hyaluronic acid nanoparticles for ulcerative colitis therapy in mice. Front Chem. 2022;10:969962. doi: 10.3389/fchem.2022.969962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou XL, Yang J, Qu XJ, Meng J, Miao RR, Cui SX. M10, a myricetin-3-O-b-D-lactose sodium salt, prevents ulcerative colitis through inhibiting necroptosis in mice. Front Pharmacol. 2020;11:557312. doi: 10.3389/fphar.2020.557312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dai Y, Han Z, Yang C, et al. Effects of polysaccharides from Portulaca oleracea L. on intestinal mucosal slgA and pathological manifestations in mice ulcerative colitis. Chin J Microecol. 2016;28(08):903–905+915. doi: 10.13381/j.cnki.cjm.201608008 [DOI] [Google Scholar]
- 60.Pan F, Zhang T, Chen J. The POP interfered with the expression of caspase-3 and caspase-8 in a rat experimental model of TNBS-induced colitis. Chin Archiv Tradition Chin. 2010;28(08):1639–1641. doi: 10.13193/j.archtcm.2010.08.73.panf.074 [DOI] [Google Scholar]
- 61.Ning K, Shi C, Chi YY, et al. Portulaca oleracea L. polysaccharide alleviates dextran sulfate sodium-induced ulcerative colitis by regulating intestinal homeostasis. Int J Biol Macromol. 2024;256(Pt 2):128375. doi: 10.1016/j.ijbiomac.2023.128375 [DOI] [PubMed] [Google Scholar]
- 62.Fan YM, Wei YY, Wang HR, Yu G, Zhang YN, Hao Z. Inhibitory effect of Portulaca oleracea L. aqueous extract and juice on NLRP3 inflammasome activation in an ulcerative colitis mouse model. Environ Sci Pollut Res Int. 2023;30(36):86380–86394. doi: 10.1007/s11356-023-28365-4 [DOI] [PubMed] [Google Scholar]
- 63.Arnold CE, Whyte CS, Gordon P, Barker RN, Rees AJ, Wilson HM. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology. 2014;141(1):96–110. doi: 10.1111/imm.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J, Zhang F, Yu X, Wang X, Li Y. Pathogenesis of ulcerative colitis and research progress of traditional Chinese and Western medicine. J Liaoning Univ Traditional Chin Med. 2021;23(01):70–74. doi: 10.13194/j.issn.1673-842x.2021.01.017 [DOI] [Google Scholar]
- 65.Wang D, Wang H, Zhou Y, Fan H, Wang Z. Research progress of the role of macrophage polarization in inflammatory diseases. Chin Med. 2017;12(9):4.28077963 [Google Scholar]
- 66.Wu X, Liu Z. Recent advances in the pathogenesis of ulcerative colitis. Chin J Integrated Tradition Western Med Digestion. 2023;31(04):237–242. [Google Scholar]
- 67.Chen H, Wu X, Xu C, Lin J, Liu Z. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis Clin Med. 2021;4(4):246–257. doi: 10.1093/pcmedi/pbab025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lissner D, Schumann M, Batra A, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21(6):1297–1305. doi: 10.1097/mib.0000000000000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geraets L, Moonen HJ, Brauers K, Wouters EF, Bast A, Hageman GJ. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J Nutr. 2007;137(10):2190–2195. doi: 10.1093/jn/137.10.2190 [DOI] [PubMed] [Google Scholar]
- 70.Riemschneider S, Hoffmann M, Slanina U, Weber K, Hauschildt S, Lehmann J. Indol-3-carbinol and quercetin ameliorate chronic DSS-induced colitis in C57BL/6 mice by AhR-mediated anti-inflammatory mechanisms. Int J Environ Res Public Health. 2021;18(5):2262. doi: 10.3390/ijerph18052262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Zhang S, Wu M, Ji Y, Huang Y, Zheng X. Effects and mechanism of β-carotene on inflammatory factors LPS-induced RAW264.7 cells. Chin J Immunol. 2017;33(06):838–843. [Google Scholar]
- 72.Qu X, Li Q, Song Y, et al. Potential of myricetin to restore the immune balance in dextran sulfate sodium-induced acute murine ulcerative colitis. J Pharm Pharmacol. 2020;72(1):92–100. doi: 10.1111/jphp.13197 [DOI] [PubMed] [Google Scholar]
- 73.Huang Y, Su Y, Qin R, et al. Mechanism by which oleracein E alleviates TNBS-induced ulcerative colitis. Eur J Gastroenterol Hepatol. 2023;35(8):854–864. doi: 10.1097/meg.0000000000002597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abron JD, Singh NP, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS One. 2018;13(7):e0199631. doi: 10.1371/journal.pone.0199631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Y, Li X, Chen J, et al. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int Immunopharmacol. 2016;30:74–84. doi: 10.1016/j.intimp.2015.11.031 [DOI] [PubMed] [Google Scholar]
- 76.Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Diel P. Oral treatment with genistein reduces the expression of molecular and biochemical markers of inflammation in a rat model of chronic TNBS-induced colitis. Eur J Nutr. 2009;48(4):213–220. doi: 10.1007/s00394-009-0004-3 [DOI] [PubMed] [Google Scholar]
- 77.Zhou Y, Luo J, Zhang G, Zhang G, Zhang C. Effects of Portulaca oleracea L on expression of IFN-γ and IL-10 cytokine in rats with experimental colitis. Chin Pharmacol Bulletin. 2008;08:1113–1114. [Google Scholar]
- 78.Vanden Braber NL, Novotny Nuñez I, Bohl L, et al. Soy genistein administered in soluble chitosan microcapsules maintains antioxidant activity and limits intestinal inflammation. J Nutr Biochem. 2018;62:50–58. doi: 10.1016/j.jnutbio.2018.08.009 [DOI] [PubMed] [Google Scholar]
- 79.Borody T, Campbell J, Torres M, Nowak A, Leis S. Reversal of Idiopathic Thrombocytopenic Purpura [ITP] with Fecal Microbiota Transplantation [FMT]: 941. Official J Am Col Gastroenterol | ACG. 2011;106:S352. [Google Scholar]
- 80.Wendland BE, Aghdassi E, Tam C, et al. Lipid peroxidation and plasma antioxidant micronutrients in Crohn disease. Am J Clin Nutr. 2001;74(2):259–264. doi: 10.1093/ajcn/74.2.259 [DOI] [PubMed] [Google Scholar]
- 81.Guan G, Lan S. Implications of antioxidant systems in inflammatory bowel disease. Biomed Res Int. 2018;2018:1290179. doi: 10.1155/2018/1290179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gao Q. Oxidative stress and autophagy. Adv Exp Med Biol. 2019;1206:179–198. doi: 10.1007/978-981-15-0602-4_9 [DOI] [PubMed] [Google Scholar]
- 83.Carrasco-Pozo C, Castillo RL, Beltrán C, Miranda A, Fuentes J, Gotteland M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: role of NF-κB and Nrf2. J Nutr Biochem. 2016;27:289–298. doi: 10.1016/j.jnutbio.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Shen L, Luo H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int Immunopharmacol. 2016;40:24–31. doi: 10.1016/j.intimp.2016.08.020 [DOI] [PubMed] [Google Scholar]
- 85.Wang L. Extraction and Isolation of Polysaccharide from Portulaca Oleracea and Evaluation of Its Therapeutic Effect On Ulcerative Colitis and Its Mechanisms. Master. Shaanxi University of Chinese Medicine; 2017. [Google Scholar]
- 86.Zhang T, Shi B, Chen J, Pan F. Effects of polysaccharide from Portulaca oleracea on the expression of CINC-1 and its receptor CXCR2 in rats ulcerative colitis. Chinese Traditional Patent Med. 2009;31(12):1929–1932. [Google Scholar]
- 87.Fan W, Wang P, Wang Q. Effects of purslane polysaccharide on the intestinal tissue IL-6/STAT3 and NF-κB of ulcerative colitis. Chin J Appl Physiol. 2018;34(03):263–267. [Google Scholar]
- 88.Wang Z, Liang Y, Zhang D, et al. Protective effects of polysaccharide extracted from Portulacae oleracea l. on colitis induced by dextran sulfate sodium. J Med Food. 2020;23(2):125–131. doi: 10.1089/jmf.2019.4414 [DOI] [PubMed] [Google Scholar]
- 89.Ai XY, Qin Y, Liu HJ, et al. Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-κB signaling. Oncotarget. 2017;8(59):100216–100226. doi: 10.18632/oncotarget.22145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bian Y. Protective Effect and Mechanisms of Portulaca Oleracea L. on Experimental Enteritis in Rats Doctor. China Agricultural University; 2018. [Google Scholar]
- 91.Abdelmegid AM, Abdo FK, Ahmed FE, Kattaia AAA. Therapeutic effect of gold nanoparticles on DSS-induced ulcerative colitis in mice with reference to interleukin-17 expression. Sci Rep. 2019;9(1):10176. doi: 10.1038/s41598-019-46671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S, Tang A, Zhang T, Huang L, Liu C. Analysis of the effective mechanism of panax notoginseng and portulaca oleracea in treating ulcerative colitis by network pharmacology. Mod J Integr Traditional Chinese and Western Med. 2021;30(25):2782–2787+2807. [Google Scholar]
- 93.Liu Y. Study on the Analgesia, Healing and Anti-Inflammation Mechanisms of Simiao Junyi Ointment Based on the Neuroendocrine-Immunoregulatory Network. Doctor. Hunan University of Traditional Chinese Medicine; 2016. [Google Scholar]
- 94.Yang Y, Zhou X, Jia G, et al. Network pharmacology based research into the effect and potential mechanism of Portulaca oleracea L. polysaccharide against ulcerative colitis. Comput Biol Med. 2023;161:106999. doi: 10.1016/j.compbiomed.2023.106999 [DOI] [PubMed] [Google Scholar]
- 95.Zhang Z, Yin B, Lian R, et al. Anti-colitis Mechanism of Herba Portulacae Based on Network Pharmacology. World Chin Med. 2020;15(24):3748–3760. [Google Scholar]
- 96.Kim KH, Park EJ, Jang HJ, et al. 1-Carbomethoxy-β-Carboline, derived from Portulaca oleracea L. ameliorates LPS-mediated inflammatory response associated with MAPK signaling and nuclear translocation of NF-κB. Molecules. 2019;24(22):4042. doi: 10.3390/molecules24224042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miao L, Tao H, Peng Y, et al. The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem. 2019;290:239–245. doi: 10.1016/j.foodchem.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 98.Kim Y, Lim HJ, Jang HJ, et al. Portulaca oleracea extracts and their active compounds ameliorate inflammatory bowel diseases in vitro and in vivo by modulating TNF-α, IL-6 and IL-1β signalling. Food Res Int. 2018;106:335–343. doi: 10.1016/j.foodres.2017.12.058 [DOI] [PubMed] [Google Scholar]
- 99.Yang X, Yan Y, Li J, et al. Protective effects of ethanol extract from Portulaca oleracea L on dextran sulphate sodium-induced mice ulcerative colitis involving anti-inflammatory and antioxidant. Am J Transl Res. 2016;8(5):2138–2148. [PMC free article] [PubMed] [Google Scholar]
- 100.Yao R, Wei H, Lei J, Wang Z. Progress on the role of mTOR signaling pathway in the pathogenesis and regulatory mechanisms. Chinese Bulletin of Life Sic. 2019;31(02):135–142. doi: 10.13376/j.cbls/2019020 [DOI] [Google Scholar]
- 101.Qiao D, Zhang Z, Zhang Y, et al. Regulation of endoplasmic reticulum stress-autophagy: a potential therapeutic target for ulcerative colitis. Front Pharmacol. 2021;12:697360. doi: 10.3389/fphar.2021.697360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Z, Qiao D, Zhang Y, et al. Portulaca oleracea L. extract ameliorates intestinal inflammation by regulating endoplasmic reticulum stress and autophagy. Mol Nutr Food Res. 2022;66(5):e2100791. doi: 10.1002/mnfr.202100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang F, Song ZY, Qu XJ, et al. M10, a novel derivative of Myricetin, prevents ulcerative colitis and colorectal tumor through attenuating robust endoplasmic reticulum stress. Carcinogenesis. 2018;39(7):889–899. doi: 10.1093/carcin/bgy057 [DOI] [PubMed] [Google Scholar]
- 104.Trivedi PP, Jena GB, Tikoo KB, Kumar V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Mol, Carcinog. 2016;55(3):255–267. doi: 10.1002/mc.22274 [DOI] [PubMed] [Google Scholar]
- 105.Kong R, Luo H, Wang N, et al. Portulaca extract attenuates development of dextran sulfate sodium induced colitis in mice through activation of PPARγ. PPAR Res. 2018;2018:6079101. doi: 10.1155/2018/6079101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kong R. The mechanism of portulaca attenuates development of dextran sulfate sodium induced colitis in mice master. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu L, Fan W. Clinical efficacy analysis of purslane capsules combining TCM enema in the treatment of ulcerative colitis. Modern Chin Med. 2007;05:6–8. [Google Scholar]
- 108.Liu L, Fan W, Zhang J, Zhang R. Treatment of 37 cases of ulcerative colitis with Purslane capsule and Chinese medicine enema. Shaanxi J Tradition Chin Med. 2007;10:1323–1325. [Google Scholar]
- 109.Ma X, Du X, Fan Z, Liu L. Experience of Professor Liu Li in diagnosis and treatment of ulcerative colitis. Shandong J Tradition Chin Med. 2017;36(07):585–587. doi: 10.16295/j.cnki.0257-358x.2017.07.015 [DOI] [Google Scholar]
- 110.Maimeti A, Maimeti A. Treatment of 32 cases of colitis with compound Purslane and coptis decoction. J Med Pharm Chin Minorities. 2009;15(11):29. doi: 10.16041/j.cnki.cn15-1175.2009.11.021 [DOI] [Google Scholar]
- 111.Wang X, Li Y. Treatment of 41 cases of ulcerative colitis with Purslane decoction. Guangxi J Tradition Chin Med. 2006;03:38. [Google Scholar]
- 112.Pan A, Yu H, Ren D, Ren J. Observation of therapeutic effect of Portulaca decoction on ulcerative colitis. Lishizhen Medicine and Materia Medica Res. 2002;06:359. [Google Scholar]
- 113.Peng X, Wang X, Wang X. Clinical Observation on 38 cases of ulcerative colitis treated by enema decoction. J Pract TRADITIONAL CHINESE INTERNAL MED. 2016;30(06):33–34+37. doi: 10.13729/j.issn.1671-7813.2016.06.14 [DOI] [Google Scholar]
- 114.Wang M, Lin B, Wei A. Treatment of 68 cases of ulcerative colitis with Jingfang Portulaca decoction. Guangming J Chin Med. 2010;25(07):1207. [Google Scholar]
- 115.Zheng T, Chen T, Cai L, Huang Z. Acute renal failure after eating purslane vegetable: report of 2 cases. Chinese J Integr Traditional and Western Nephrol. 2021;22(10):930–931+944. [Google Scholar]