Abstract
Background
Streptococcus intermedius is a commensal microflora commonly found in various mucosal sites in the respiratory, gastrointestinal, and genitourinary tracts. It causes invasive suppurative infections including liver and brain abscesses along with thoracic empyema. However, it rarely causes meningitis without abscess formation.
Case Presentation
A 56-year-old immunocompetent man who presented with fever and headache. Bacterial meningitis was confirmed using cerebrospinal fluid analysis. Magnetic resonance imaging of the brain revealed leptomeningitis and ventriculitis. However, conventional methods, such as microbiological culture failed to identify the causative pathogens. Metagenomic next-generation sequencing of cerebrospinal fluid revealed the presence of S. intermedius. It allowed us the optimal treatment for him. The patient underwent antibiotic treatment with 6-week duration of ceftriaxone administration accompanied by surgical intervention, resulting in a favorable prognosis.
Conclusion
Herein, we report a rare case of meningitis and ventriculitis caused by S. intermedius using metagenomic next-generation sequencing. The patient recovered well after antibiotic treatment and surgery. We present this rare case and summarize previous studies to remind clinicians that timely identification of the pathogen and optimal treatment are crucial for management of S. intermedius-induced infections.
Keywords: diagnosis, meningitis, metagenomic next-generation sequencing, Streptococcus intermedius, ventriculitis
Introduction
Central nervous system (CNS) infection is a life-threatening infectious disease that usually results in high morbidity and mortality.1,2 Accurate and timely identification of the causative pathogen is important for effective management of CNS infections.2 Cerebrospinal fluid (CSF) culture is the gold standard for the diagnosis of CNS infection and to guide appropriate antimicrobial therapy. However, the causative pathogen for CNS infection is not identified in approximately half of patients,3,4 accounting for poor patient outcomes and higher economic burdens to the health care system.3
Streptococcus intermedius is a human commensal microflora belonging to the Streptococcus anginosus group (SAG).5 It is associated with dental plaque and usually causes complicated invasive suppurative infections at various body sites, resulting in brain abscesses, dentoalveolar infections, infective endocarditis, and liver abscesses.5,6 This pathogen is mostly isolated from the pus of infection foci instead of from non-pyogenic lesions.6 However, identification of S. intermedius is difficult because of a lack of reliable phenotypic characteristics among SAG members. Therefore, a PCR amplification with multi-locus sequence analysis would be a more reliable tool to pathogen identification. Metagenomic next-generation sequencing (mNGS) has been used as a rapid and reliable tool for identifying microorganisms that exhibits promising detection ability in patients with infectious diseases, including meningitis.3,4,7–9 It can identify a broad spectrum of pathogens, including both commonly and rarely reported causative agents of CNS infections.7 In this report, we describe an immunocompetent man with meningitis and ventriculitis caused by S. intermedius, which was detected by the mNGS technique using a culture-negative CSF sample. Additionally, we have summarized previously reported cases of S. intermedius meningitis. The study was approved by the Institutional Review Board of Tri-Service General Hospital, which also consented to the publication of the case details.(TSGHIRB No.:C202415030).
Case Presentation
A 56-year-old Taiwanese male with a history of hypertension and dyslipidemia was taking regular medications. He presented to a hospital emergency department in December 2023 with headache, chills, nuchal rigidity, and nausea over 3-d. His vital signs showed body temperature of 38.7 °C, rapid heart rate of 112 beats/min, normal respiratory rate of 18/min, and normal blood pressure of 114/63 mmHg. Neurological examination revealed as the follows: Glasgow Coma Scale (GCS) E4V5M6, cognitively intact and orientation, fluent speech, steady gait, free and conjugated extraocular movement, isochoric pupils with a light reflex, positive Kernig’s sign, absent Babinski sign, and intact motor or sensory function. Laboratory results disclosed remarkable leukocytosis (white blood cell [WBC] count: 32,460/μL; normal reference range: 4500–11,000/μL) and elevated C-reactive protein level (18.58 mg/dL; normal reference range: <0.8 mg/dL). Influenza virus antigen (Ag) and SARS-CoV-2 rapid Ag test results were negative. Computed tomography of the brain revealed no remarkable findings. Lumbar puncture was performed at an opening pressure of 17 cmH2O to obtain the CSF sample, which appeared turbid and yellowish. CSF analysis revealed the following: WBC 1687/μL, glucose <10 mg/dL (normal reference range: 40–70 mg/dL), and total protein 772 mg/dL (normal reference range: 15–45 mg/dL). Empirical antibiotics, including ceftriaxone 2g and vancomycin 1g twice per day, were administered to treat probable bacterial meningitis. The CSF was negative for Group B Streptococcus Ag, Neisseria meningitidis Ag, S. pneumoniae Ag, Hemophilus influenza Ag, India Ink stain, Cryptococcus Ag test, and PCR for Mycobacterium tuberculosis complex. The results of a BIOFIRE® FILMARRAY® Meningitis/Encephalitis Panel also demonstrated negative results. The CSF fluid and blood cultures yielded no pathogen. Furthermore, CSF, obtained through a repeat lumbar puncture on the 7th d of hospitalization, was sent for mNGS. The results discovered the presence of the S. intermedius gene sequences (Table 1).
Table 1.
Metagenomic Next-Generation Sequencing Analysis of Cerebrospinal Fluid Sample in This Patient
| Type | Pathogen | Reads | Genus Relative Abundance (%)* | Pathogen | Reads | Coverage (%) | Species Relative Abundance (%)** |
|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Streptococcus | 4698 | 91.71 | Streptococcus intermedius | 1919 | 7.68 | 60.71 |
| Gram-negative bacteria | Fusobacterium | 108 | 2.12 | Fusobacterium nucleatum | 53 | 0.12 | 1.74 |
Notes: *Relative abundance at the genus level refers to the proportion of detected microorganisms within the genus in the entire sample. **Relative abundance at the species level refers to the proportion of the detected microorganism within the species in the overall sample.
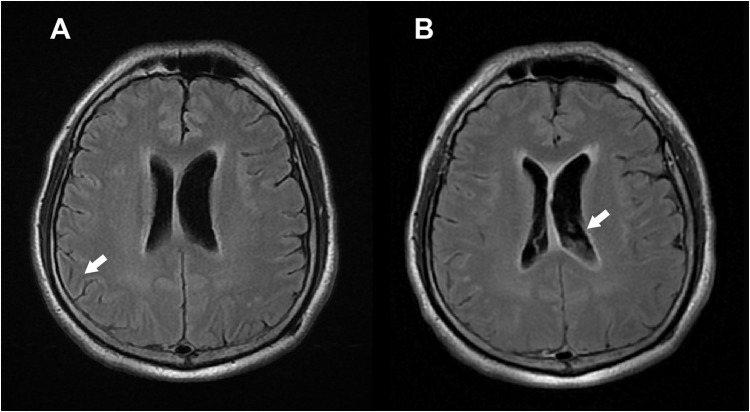
Contrast-enhanced magnetic resonance imaging of the brain revealed leptomeningeal enhancement in the bilateral cerebral sulci and brainstem surface, suggestive of meningitis and subependymal enhancement with sedimentation in the bilateral lateral ventricles (Figure 1). The patient was diagnosed with S. intermedius meningitis and ventriculitis, and treated with ceftriaxone administration and undergoing external ventricular drainage (EVD). Follow-up CSF analysis revealed the following: WBC 7/μL, glucose 61 mg/dL, and total protein 67 mg/dL. EVD was transitioned to a ventriculoperitoneal shunt for continuous drainage. The patient was discharged 6 weeks post-treatment without neurological sequelae.
Figure 1.
Magnetic resonance imaging of the brain (A) Leptomeningeal enhancement (white arrow) in bilateral cerebral sulci and brainstem surface. (T2-weighted Fluid-attenuated inversion recovery [T2-FLAIR]) (B) Subependymal enhancement with sedimentations (white arrow) in bilateral lateral ventricles. (Contrast-enhanced Fluid- attenuated inversion recovery [CE-FLAIR]).
Discussion
In this study, we reported the case of an immunocompetent man with S. intermedius infection presenting with meningitis and ventriculitis. The pathogen was identified in CSF samples using mNGS. The patient recovered well after antibiotic treatment and surgery.
Meningitis is a life-threatening infectious disease and accounts for an estimated 2.51 million cases annually and up to 236,000 deaths globally.10 The overall mortality rate of bacterial meningitis was 16.4% in adults, and increased to 22.7% in the elderly (≥ 65 years) in the United States.1 CSF culture is the gold standard for pathogen identification in CNS infections,2,11,12 however, the positive rate of CSF cultures was estimated to be only 30–40% in CNS infections.7 Patients who received antibiotic treatment > 6 h after disease presentation had higher in-hospital mortality and worse outcomes at discharge, compared to those who received treatment within 2 h of admission.13 Although timely antibiotic treatment for CNS infections management is important, the usage of antibiotics before CSF sampling would decrease pathogen detection rates.7,12,14 While S. intermedius is vulnerable to β-lactam antibiotics, antibiotic treatment before obtaining CSF samples can lower detection efficacy, which clarifies reason for the negative results of CSF pathogen detection tests in this case.
SAGs represent normal flora and can mostly be isolated from dental plaques and mucosa of respiratory, gastrointestinal, and urogenital tracts in human beings.6,15 The incidence of SAG bacteremia has increased gradually in recent years.16 As a member of SAG, S. intermedius has high mortality rates, longer hospital stays, and usually causes suppurative infections, such as brain abscess, liver abscesses, and thoracic empyema.6,17,18 Several virulence factors, such as antigens I/II surface proteins, hyaluronidase, and polysaccharidic capsule have been known as contributors of abscess formation by S. intermedius.6 This may be the reason that S. intermedius-induced CNS infections usually present as brain abscess rather than meningitis. Although, several cases of S. intermedius brain abscesses have been documented, only four cases of S. intermedius meningitis or ventriculitis have been previously reported (Table 2). Four of the five involved individuals were over the age of 60, while the fourth case was a 6-year-old boy. Among them, there was no specific risk factors for S. intermedius meningitis or ventriculitis been recognized, such as dental operation, sinusitis, or diabetes mellitus.6 CSF cultures yielded negative results in two of the reported five cases. The percentage of CSF culture positive was two in these five cases. The S. intermedius were identified by PCR amplification or mNGS in the culture-negative cases. As previous documented that SAGs are susceptible to β-lactam antibiotics and display less antibiotic resistance,19 all the reported patients survived after 2 to 6 weeks duration of β-lactam antibiotics treatment.20–23
Table 2.
Summary of Analyzed Streptococcus intermedius Meningitis/Ventriculitis Case Reports
| Clinical Profile | Symptoms | Imaging Results | CSF Analysis and Pathogen Identification | Diagnosis | Outcome |
|---|---|---|---|---|---|
| Case No1: 63-year-old healthy British male22 | Headache for 1 week, feeling intermittently hot and sweaty, and having clumsiness and unsteadiness, and diarrhea | Brain CT showed enlargement of the right lateral ventricle with increased density collection in the anterior horn and body of the right lateral ventricle, which suggests ventriculitis | CSF analysis: WBC:350 /μLwith 90% neutrophil; protein: 4466 mg/dL CSF culture negative. 16s rDNA PCR of CSF revealed positive for S. intermedius. |
S. intermedius ventriculitis | After 6-week cefotaxime administration and ventricular drainage, recovery with left-sided homonymous hemianopia remains. |
| Case No2: 6-year-old healthy Polish boy20 | Fever, headache, neck pain, right ear pain, and altered mental status | Brain CT showed thickened mucosa and foamy discharge in the right compartment of the sphenoid sinus as well as the single airless cells of the right mastoid process. | CSF analysis: WBC: 7197 /μL with 88% neutrophil; Protein: 130.4 mg/dL; Glucose: 2 mg/dL; Lactate: 11.1 mmol/L CSF culture: S. intermedius |
S. intermedius meningitis | Recovery after 14-day ceftriaxone administration. |
| Case No3: 62-year-old American male with history of hypertension, hyperlipidemia, previous ischemic stroke, and obstructive sleep apnea21 | Progressive vomiting, malaise, lightheadedness, headaches, acute onset disorientation, confusion, and eye rolling | Brain MRI showed diffuse ependymal enhancement that was not displayed throughout the ventricular system with debris present, which is most consistent with ventriculitis. | CSF analysis: WBC: 250,000 /μL; Protein: 1103 mg/dL; glucose: 6 mg/dL CSF culture: S. intermedius |
S. intermedius ventriculitis | After 4 dose of intrathecal vancomycin, 6-week ceftriaxone combined with ampicillin and ventricular drainage, recovery and kept rehabilitation. |
| Case No4: 64-year-old healthy Finnish male23 | Headache, fever, imbalance, blurred vision, and general slowness. | Brain MRI showed the ependyma of the right lateral ventricle and cavum septi pellucidi enhanced intensively— a sign of ventriculitis. | CSF analysis: WBC: 940 /μL; Protein: 1696 mg/dL; Glucose: 16.2 mg/dL CSF culture negative. 16s rRNA PCR of CSF revealed positive result of S. intermedius. |
S. intermedius ventriculitis | After 3-week antibiotic administration with caxone and following cefotaxime and ventricular drainage, recovery with slight left-sided hemiparesis. |
| Our case: 56-year-old Taiwanese male with history of hypertension and dyslipidemia | Headache, chills, nuchal rigidity, and nausea over 3 days | Brain MRI showed leptomeningeal enhancement in the bilateral cerebral sulci and brainstem surface, suggestive of meningitis and subependymal enhancement with sedimentation in the bilateral lateral ventricles | CSF analysis: WBC:1687 /μLwith 68% neutrophil; Protein: 772 mg/dL; Glucose: <10 mg/dL CSF culture negative. mNGS of CSF yielded S. intermedius. |
S. intermedius meningitis and ventriculitis | Recovery after antibiotic administration with 2-week ceftriaxone and Vancomycin following 4-week ceftriaxone, and underwent ventriculo-peritoneal shunt implementation |
Abbreviations: CSF, cerebrospinal fluid; CT, computed tomography; PCR, polymerase chain reaction; MRI, magnetic resonance imaging.
Optimal and adequate sampling for pathogen identification before antibiotic administration is important in the management of CNS infections. While conventional microbiologic testing is insufficient to detect all pathogens, mNGS exhibits satisfactory diagnostic performance in CNS infections and has a higher detection rate than conventional culture techniques.24 The application of mNGS can improve the diagnosis of CNS infections, with a sensitivity and specificity of 73 and 99%, respectively.25 Furthermore, DNA or RNA sequencing of pathogens via mNGS accelerates turnaround time in testing, which ranges from approximately 6 h to 7 d (average of 2 d).
Conclusion
We report a rare case of S. intermedius infection presenting with meningitis and cerebral ventriculitis. In this case, no pathogen was identified using conventional diagnostic methods, including CSF culture, pathogen Ag testing, and a Filmarray multiplex PCR panel. Using the mNGS technique, CSF samples discovered DNA sequences of S. intermedius, confirming the diagnosis; thus, verifying the therapeutic strategy. As a cutting-edge technology, mNGS has a significantly high-detection sensitivity, and can be applied for the detection of unusual pathogens in CNS infections.
Acknowledgments
This work was supported by grants from Tri-Service General Hospital (TSGH-E-112252, TSGH-E-113286), Taoyuan Armed Forces General Hospital (TYAFGH_E_112039, TYAFGH_E_113059) and National Science and Technology Council (NSTC-108-2314-B-016-029, NSTC-111-2314-B-016-032). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical Statement and Informed Consent
The study has reviewed and approved by the Institutional Review Board of the Tri-Service General Hospital (TSGHIRB No.: C202415030) and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent to have the case details and any accompanying potentially identifiable images or data published has been obtained by the patient.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial Meningitis in the United States, 1998–2007. N Engl J Med. 2011;364(21):2016–2025. doi: 10.1056/NEJMoa1005384 [DOI] [PubMed] [Google Scholar]
- 2.Glimåker M, Sjölin J, Åkesson S, et al. Lumbar Puncture Performed Promptly or After Neuroimaging in Acute Bacterial Meningitis in Adults: a Prospective National Cohort Study Evaluating Different Guidelines. Clin Infect Dis. 2018;66(3):321–328. doi: 10.1093/cid/cix806 [DOI] [PubMed] [Google Scholar]
- 3.Wilson MR, Sample HA, Zorn KC, et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi: 10.1056/NEJMoa1803396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Men X, Zhao G, Zhao W, et al. Pathogen identification in culture-negative cerebrospinal fluid specimens of patients with purulent meningitis using next-generation sequencing technology. Int J Clin Exp Pathol. 2020;13(9):2427–2438. [PMC free article] [PubMed] [Google Scholar]
- 5.Claridge JE, Attorri S, Musher DM, et al. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32(10):1511–1515. doi: 10.1086/320163 [DOI] [PubMed] [Google Scholar]
- 6.Issa E, Salloum T, Tokajian S. From Normal Flora to Brain Abscesses: a Review of Streptococcus intermedius. Front Microbiol. 2020;11:826. doi: 10.3389/fmicb.2020.00826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Cui P, Zhang H-C, et al. Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult central nervous system infection. J Transl Med. 2020;18(1):199. doi: 10.1186/s12967-020-02360-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing X-W, Xavier SCDC, Roque ALR, et al. Metagenomic Next-Generation Sequencing for Diagnosis of Infectious Encephalitis and Meningitis: a Large, Prospective Case Series of 213 Patients. Front Cell Infect Microbiol. 2020;10:10. doi: 10.3389/fcimb.2020.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan L, Lv Z, Zhang Y, et al. Use of Metagenomic Next-Generation Sequencing to Identify Pathogens Involved in Central Nervous System Infections. Infect Drug Resist. 2024;17(null):3605–3615. doi: 10.2147/IDR.S474410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wunrow HY, Bender RG, Vongpradith A, et al. Global, regional, and national burden of meningitis and its aetiologies, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023;22(8):685–711. doi: 10.1016/S1474-4422(23)00195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beek DVD, de Gans J, Spanjaard L, et al. Clinical Features and Prognostic Factors in Adults with Bacterial Meningitis. N Engl J Med. 2004;351(18):1849–1859. doi: 10.1056/NEJMoa040845 [DOI] [PubMed] [Google Scholar]
- 12.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice Guidelines for the Management of Bacterial Meningitis. Clinl Infect Dis. 2004;39(9):1267–1284. doi: 10.1086/425368 [DOI] [PubMed] [Google Scholar]
- 13.Bodilsen J, Dalager-Pedersen M, Schønheyder HC, et al. Time to antibiotic therapy and outcome in bacterial meningitis: a Danish population-based cohort study. BMC Infect Dis. 2016;16(1):392. doi: 10.1186/s12879-016-1711-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodilsen J, Brandt CT, Sharew A, et al. Early versus late diagnosis in community-acquired bacterial meningitis: a retrospective cohort study. Clin Microbiol Infect. 2018;24(2):166–170. doi: 10.1016/j.cmi.2017.06.021 [DOI] [PubMed] [Google Scholar]
- 15.Mishra AK, Fournier PE. The role of Streptococcus intermedius in brain abscess. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32(4):477–483. doi: 10.1007/s10096-012-1782-8 [DOI] [PubMed] [Google Scholar]
- 16.Pilarczyk-Zurek M, Sitkiewicz I, Koziel J. The Clinical View on Streptococcus anginosus Group - Opportunistic Pathogens Coming Out of Hiding. Front Microbiol. 2022;13:956677. doi: 10.3389/fmicb.2022.956677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sibley CD, Church DL, Surette MG, et al. Pyrosequencing reveals the complex polymicrobial nature of invasive pyogenic infections: microbial constituents of empyema, liver abscess, and intracerebral abscess. Eur J Clin Microbiol Infect Dis. 2012;31(10):2679–2691. doi: 10.1007/s10096-012-1614-x [DOI] [PubMed] [Google Scholar]
- 18.Sinha D, Sun X, Khare M, et al. Pangenome analysis and virulence profiling of Streptococcus intermedius. BMC Genomics. 2021;22(1):522. doi: 10.1186/s12864-021-07829-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracy M, Wanahita A, Shuhatovich Y, et al. Antibiotic Susceptibilities of Genetically Characterized Streptococcus milleri Group Strains. Antimicrob Agents Chemother. 2001;45(5):1511–1514. doi: 10.1128/AAC.45.5.1511-1514.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tkacz K, Piwowarczyk A, Podsiadły E, et al. Streptococcus intermedius Acute Meningitis in an Immunocompetent Child. Pediatr Infect Dis J. 2022;41(10):e428–e429. doi: 10.1097/INF.0000000000003613 [DOI] [PubMed] [Google Scholar]
- 21.Daly T, et al. Primary pyogenic ventriculitis caused by Streptococcus intermedius. Am J Pharma Pharm Sci. 2022;2022;1. [Google Scholar]
- 22.Vajramani GV, Akrawi H, Jones G, et al. Primary ventriculitis caused by Streptococcus intermedius. Br. J. Neurosurg. 2007;21(3):293–296. doi: 10.1080/02688690701246129 [DOI] [PubMed] [Google Scholar]
- 23.Allonen S, Aittoniemi J, Vuorialho M, et al. Streptococcus intermedius causing primary bacterial ventriculitis in a patient with severe periodontitis - a case report. BMC Neurol. 2024;24(1):112. doi: 10.1186/s12883-024-03604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan L, Zhu XY, Lai LM, et al. Clinical application and evaluation of metagenomic next-generation sequencing in pathogen detection for suspected central nervous system infections. Sci Rep. 2024;14(1):16961. doi: 10.1038/s41598-024-68034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller S, Naccache SN, Samayoa E, et al. Laboratory validation of a clinical metagenomic sequencing assay for pathogen detection in cerebrospinal fluid. Genome Res. 2019;29(5):831–842. doi: 10.1101/gr.238170.118 [DOI] [PMC free article] [PubMed] [Google Scholar]