Abstract
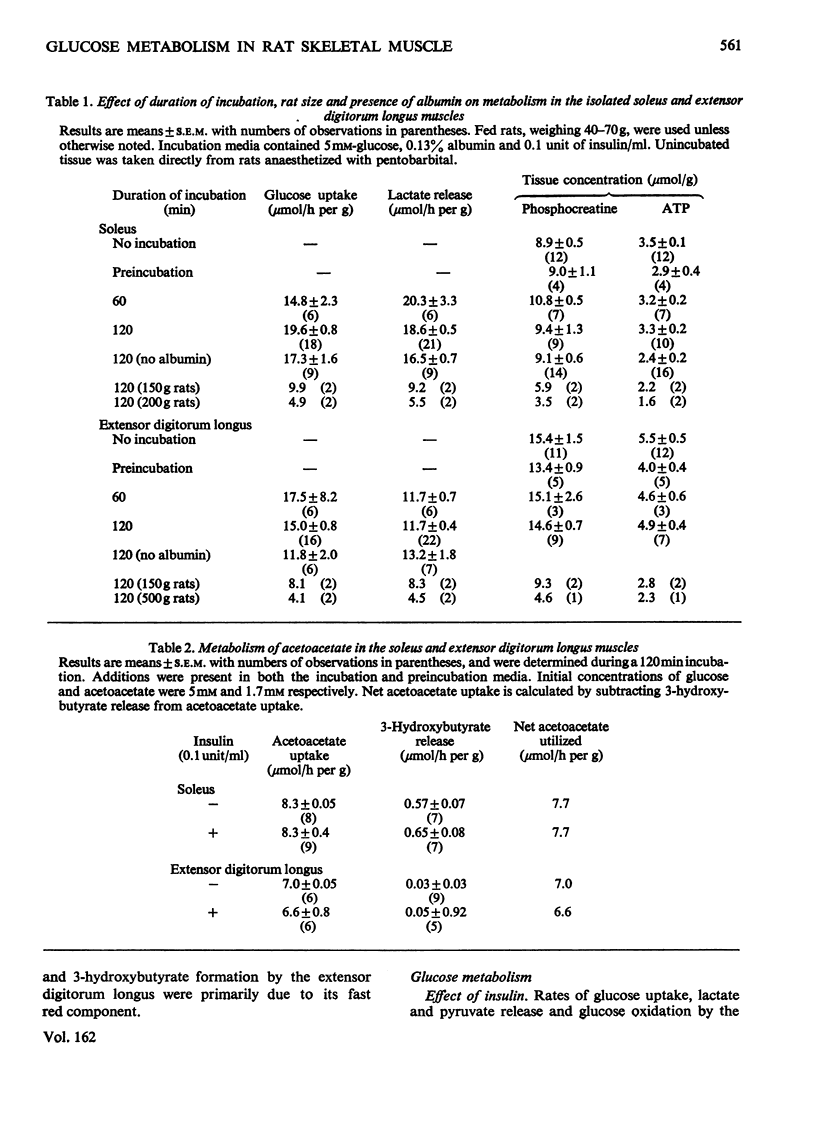
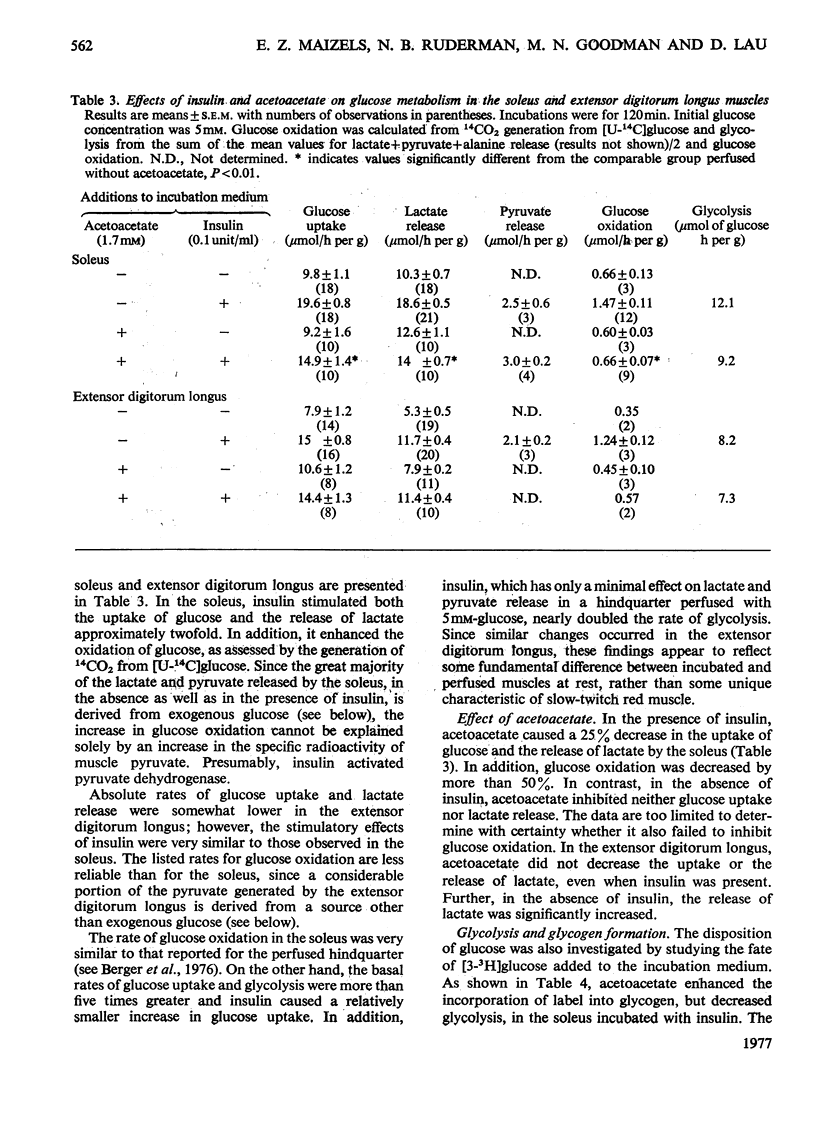
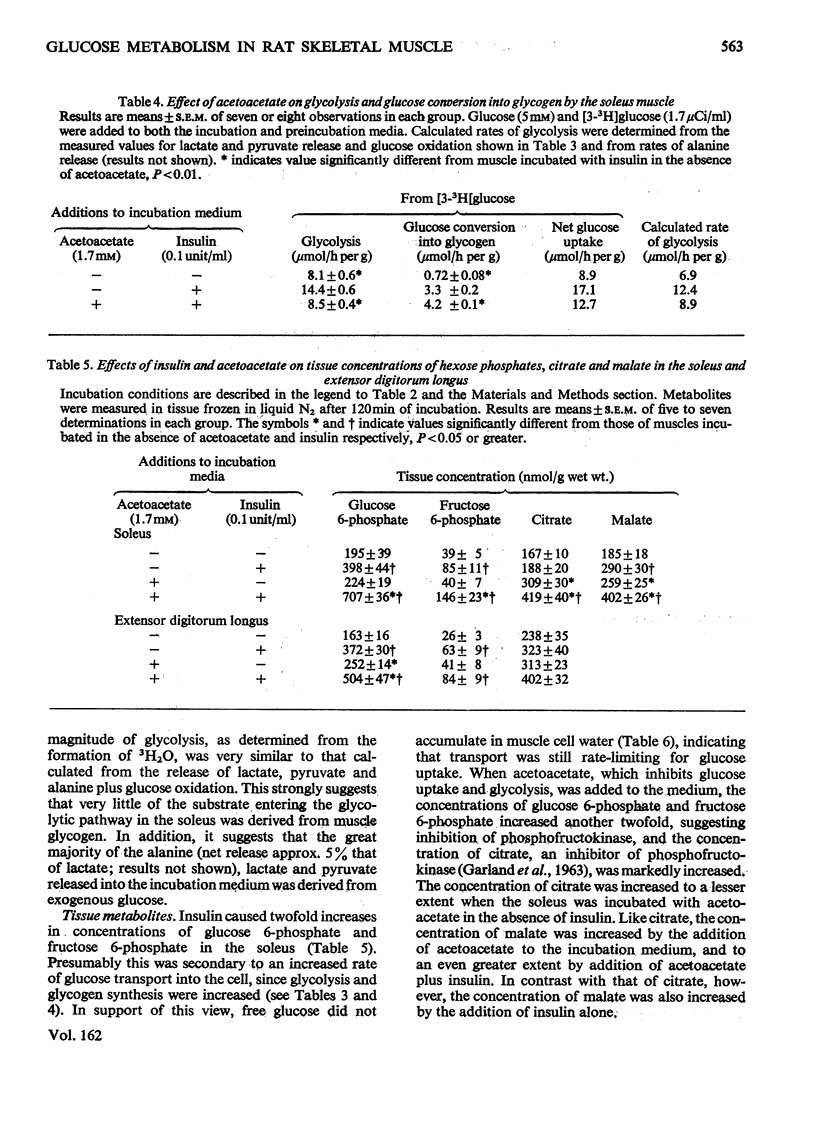
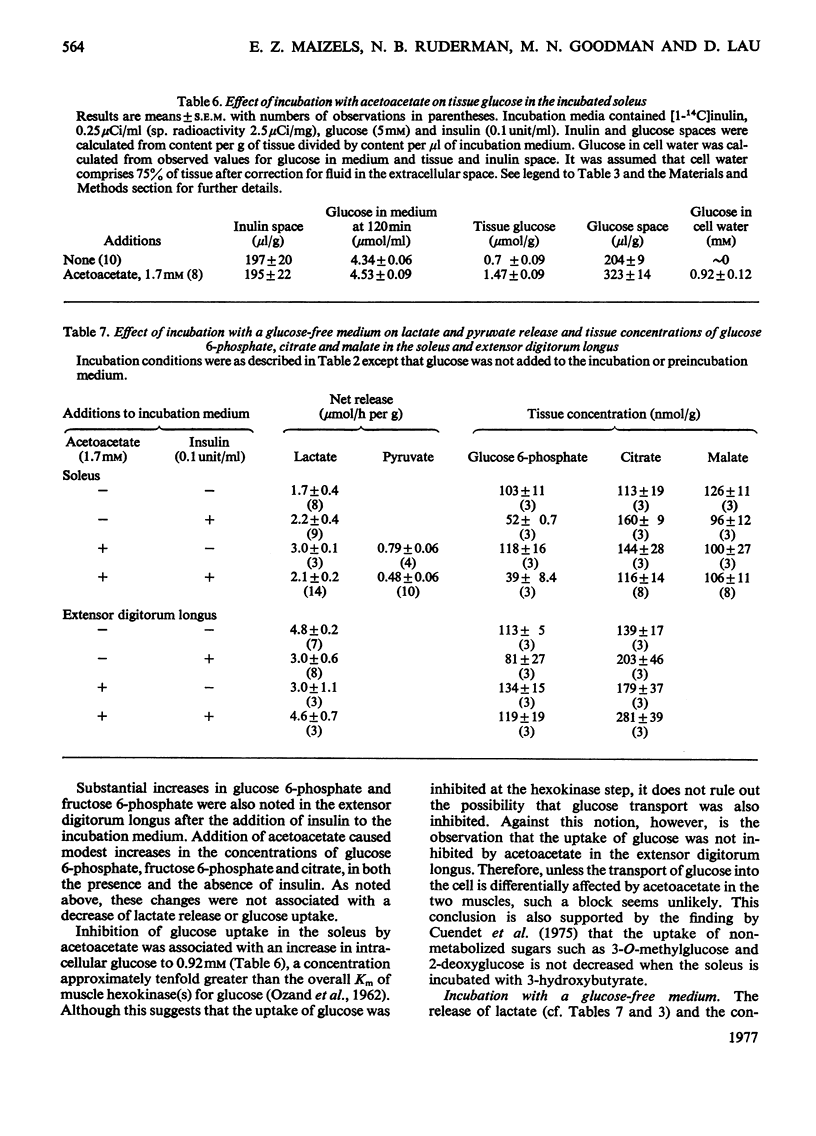
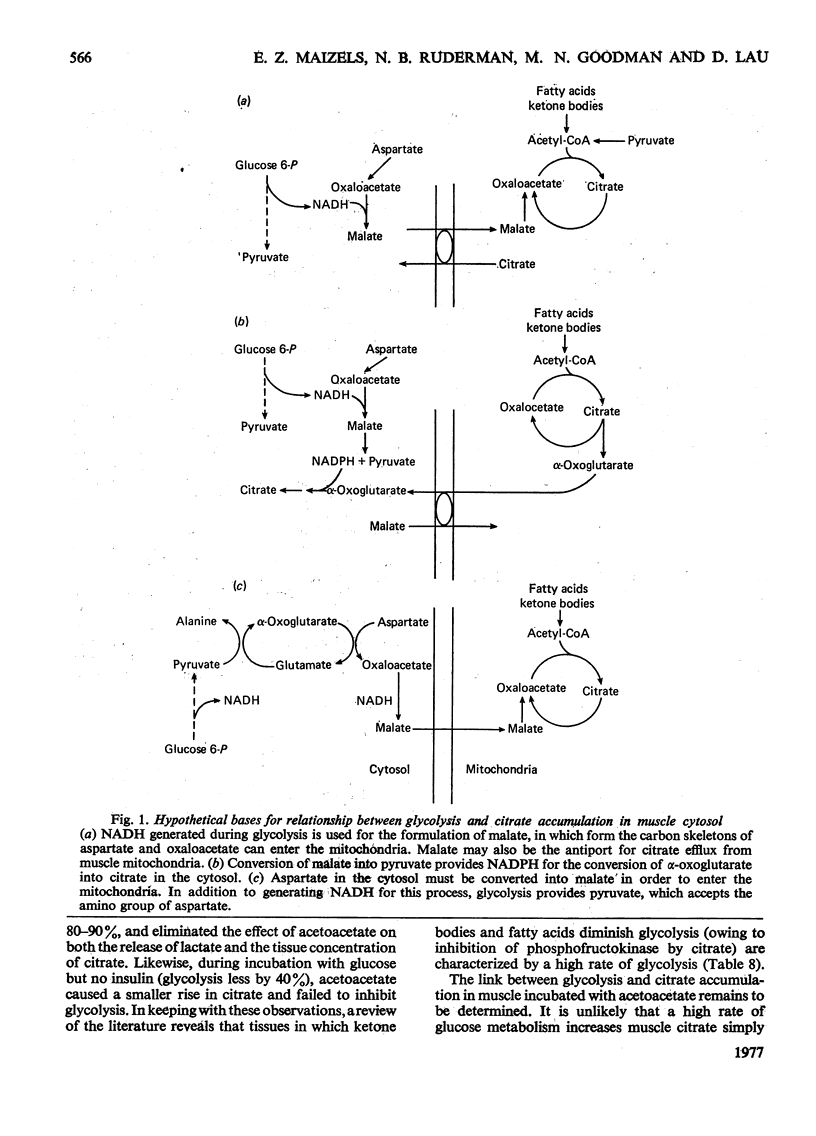
1. The effect of acetoacetate on glucose metabolism was compared in the soleus, a slow-twitch red muscle, and the extensor digitorum longus, a muscle composed of 50% fast-twitch red and 50% white fibres. 2. When incubated for 2h in a medium containing 5 mM-glucose and 0.1 unit of insulin/ml, rates of glucose uptake, lactate release and glucose oxidation in the soleus were 19.6, 18.6 and 1.47 micronmol/h per g respectively. Acetoacetate (1.7 mM) diminished all three rates by 25-50%; however, it increased glucose conversion into glycogen. In addition, it caused increases in tissue glucose, glucose 6-phosphate and fructose 6-phosphate, suggesting inhibition of phosphofructokinase. The concentrations of citrate, an inhibitor of phosphofructokinase, and of malate were also increased. 3. Rates of glucose uptake and lactate release in the extensor digitorum longus were 50-80% of those in the soleus. Acetoacetate caused moderate increases in tissue glucose 6-phosphate and possibly citrate, but it did not decrease glucose uptake or lactate release. 4. The rate of glycolysis in the soleus was approximately five times that previously observed in the perfused rat hindquarter, a muscle preparation in which acetoacetate inhibits glucose oxidation, but does not alter glucose uptake or glycolysis. A similar rate of glycolysis was observed when the soleus was incubated with a glucose-free medium. Under these conditions, tissue malate and the lactate/pyruvate ratio in the medium were decreased, and acetoacetate did not decrease lactate release or increase tissue citrate or glucose 6-phosphate. An intermediate rate of glycolysis, which was not decreased by acetoacetate, was observed when the soleus was incubated with glucose, but not insulin. 5. The data suggest that acetoacetate glucose inhibits uptake and glycolysis in red muscle under conditions that resemble mild to moderate exercise. They also suggest that the accumulation of citrate in these circumstances is linked to the rate of glycolysis, possibly through the generation of cytosolic NADH and malate formation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrouny G. A. Differential patterns of glycogen metabolism in cardiac and skeletal muscles. Am J Physiol. 1969 Sep;217(3):686–693. doi: 10.1152/ajplegacy.1969.217.3.686. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Winder W. W., Terjung R. L., Holloszy J. O. Glycolytic enzymes in different types of skeletal muscle: adaptation to exercise. Am J Physiol. 1973 Oct;225(4):962–966. doi: 10.1152/ajplegacy.1973.225.4.962. [DOI] [PubMed] [Google Scholar]
- Beatty C. H., Bocek R. M. Interrelation of carbohydrate and palmitate metabolism in skeletal muscle. Am J Physiol. 1971 Jun;220(6):1928–1934. doi: 10.1152/ajplegacy.1971.220.6.1928. [DOI] [PubMed] [Google Scholar]
- Berger M., Hagg S. A., Goodman M. N., Ruderman N. B. Glucose metabolism in perfused skeletal muscle. Effects of starvation, diabetes, fatty acids, acetoacetate, insulin and exercise on glucose uptake and disposition. Biochem J. 1976 Aug 15;158(2):191–202. doi: 10.1042/bj1580191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Stimulation of phosphorylation and inactivation of pyruvate dehydrogenase by physiological inhibitors of the pyruvate dehydrogenase reaction. Nature. 1975 Oct 30;257(5529):808–809. doi: 10.1038/257808a0. [DOI] [PubMed] [Google Scholar]
- DAWSON D. M., ROMANUL F. C. ENZYMES IN MUSCLES. II. HISTOCHEMICAL AND QUANTITATIVE STUDIES. Arch Neurol. 1964 Oct;11:369–378. doi: 10.1001/archneur.1964.00460220031004. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Amino acid metabolism in exercising man. J Clin Invest. 1971 Dec;50(12):2703–2714. doi: 10.1172/JCI106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J. Fuel homeostasis in exercise. N Engl J Med. 1975 Nov 20;293(21):1078–1084. doi: 10.1056/NEJM197511202932107. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J., NEWSHOLME E. A. CITRATE AS AN INTERMEDIARY IN THE INHIBITION OF PHOSPHOFRUCTOKINASE IN RAT HEART MUSCLE BY FATTY ACIDS, KETONE BODIES, PYRUVATE, DIABETES, AND STARVATION. Nature. 1963 Oct 12;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Martel S. B., Kushmerick M. J. In vitro preparations of the diaphragm and other skeletal muscles. Methods Enzymol. 1975;39:82–94. doi: 10.1016/s0076-6879(75)39012-5. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- Hagg S. A., Taylor S. I., Ruberman N. B. Glucose metabolism in perfused skeletal muscle. Pyruvate dehydrogenase activity in starvation, diabetes and exercise. Biochem J. 1976 Aug 15;158(2):203–210. doi: 10.1042/bj1580203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Pernow B., Jones N. L. Uptake and release of free fatty acids and other metabolites in the legs of exercising men. J Appl Physiol. 1967 Jul;23(1):90–99. doi: 10.1152/jappl.1967.23.1.90. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. Identification in skeletal muscle of a distinct extracellular pool of amino acids, and its role in protein synthesis. Biochem J. 1971 Mar;121(5):817–827. doi: 10.1042/bj1210817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. The effect of insulin on free amino acid pools and protein synthesis in rat skeletal muscle in vitro. Biochem J. 1971 Dec;125(3):751–756. doi: 10.1042/bj1250751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Koehler J. O., Morgan H. E. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci U S A. 1972 Apr;69(4):816–820. doi: 10.1073/pnas.69.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. H., Walton J. L., Krebs H. A., Williamson D. H. Metabolic fuels during and after severe exercise in athletes and non-athletes. Lancet. 1969 Aug 30;2(7618):452–455. doi: 10.1016/s0140-6736(69)90164-0. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of tritiated glucose by rat adipose tissue. J Biol Chem. 1966 Aug 10;241(15):3600–3610. [PubMed] [Google Scholar]
- Kerbey A. L., Randle P. J., Cooper R. H., Whitehouse S., Pask H. T., Denton R. M. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Biochem J. 1976 Feb 15;154(2):327–348. doi: 10.1042/bj1540327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- MacDonald M., Neufeldt N., Park B. N., Berger M., Ruderman N. Alanine metabolism and gluconeogenesis in the rat. Am J Physiol. 1976 Aug;231(2):619–626. doi: 10.1152/ajplegacy.1976.231.2.619. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Hawkins R. A., Veech R. L. Decreased rate of glucose utilization by rat brain in vivo after exposure to atmospheres containing high concentrations of CO2. J Neurochem. 1975 Nov;25(5):553–558. doi: 10.1111/j.1471-4159.1975.tb04367.x. [DOI] [PubMed] [Google Scholar]
- OZAND P., NARAHARA H. T., CORI C. F. Studies of tissue permeability. VIII. The effect of anaerobiosis on glucose uptake in frog sartorius muscle. J Biol Chem. 1962 Oct;237:3037–3043. [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr Human forearm metabolism during progressive starvation. J Clin Invest. 1971 Jul;50(7):1536–1545. doi: 10.1172/JCI106639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain V. M., Manchester K. L. The influence of electrical stimulation in vitro on protein synthesis and other metabolic parameters of rat extensor digitorum longus muscle. Biochem J. 1970 Jun;118(2):209–220. doi: 10.1042/bj1180209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Roche T. E., Reed L. J. Function of calcium ions in pyruvate dehydrogenase phosphatase activity. Biochem Biophys Res Commun. 1972 Oct 17;49(2):563–571. doi: 10.1016/0006-291x(72)90448-2. [DOI] [PubMed] [Google Scholar]
- Randle P. J., England P. J., Denton R. M. Control of the tricarboxylate cycle and its interactions with glycolysis during acetate utilization in rat heart. Biochem J. 1970 May;117(4):677–695. doi: 10.1042/bj1170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Garland P. B., Hales C. N., Newsholme E. A., Denton R. M., Pogson C. I. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Winder W. W., Holloszy J. O. A sparing effect of increased plasma fatty acids on muscle and liver glycogen content in the exercising rat. Biochem J. 1976 Jun 15;156(3):647–655. doi: 10.1042/bj1560647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Berger M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem. 1974 Sep 10;249(17):5500–5506. [PubMed] [Google Scholar]
- Ruderman N. B., Goodman M. N. Regulation of ketone body metabolism in skeletal muscle. Am J Physiol. 1973 Jun;224(6):1391–1397. doi: 10.1152/ajplegacy.1973.224.6.1391. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Houghton C. R., Hems R. Evaluation of the isolated perfused rat hindquarter for the study of muscle metabolism. Biochem J. 1971 Sep;124(3):639–651. doi: 10.1042/bj1240639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Ross P. S., Berger M., Goodman M. N. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem J. 1974 Jan;138(1):1–10. doi: 10.1042/bj1380001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]
- Safer B., Williamson J. R. Mitochondrial-cytosolic interactions in perfused rat heart. Role of coupled transamination in repletion of citric acid cycle intermediates. J Biol Chem. 1973 Apr 10;248(7):2570–2579. [PubMed] [Google Scholar]
- Stauffacher W., Renold A. E. Effect of insulin in vivo on diaphragm and adipose tissue of obese mice. Am J Physiol. 1969 Jan;216(1):98–105. doi: 10.1152/ajplegacy.1969.216.1.98. [DOI] [PubMed] [Google Scholar]
- Terjung R. L., Baldwin K. M., Winder W. W., Holloszy J. O. Glycogen repletion in different types of muscle and in liver after exhausting exercise. Am J Physiol. 1974 Jun;226(6):1387–1391. doi: 10.1152/ajplegacy.1974.226.6.1387. [DOI] [PubMed] [Google Scholar]
- Thompson M. P., Williamson D. H. Metabolic interactions of glucose, acetoacetate and adrenaline in rat submaxillary gland in vitro. Biochem J. 1975 Mar;146(3):635–644. doi: 10.1042/bj1460635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder W. W., Baldwin K. M., Holloszy J. O. Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem. 1974 Sep 16;47(3):461–467. doi: 10.1111/j.1432-1033.1974.tb03713.x. [DOI] [PubMed] [Google Scholar]


