Abstract
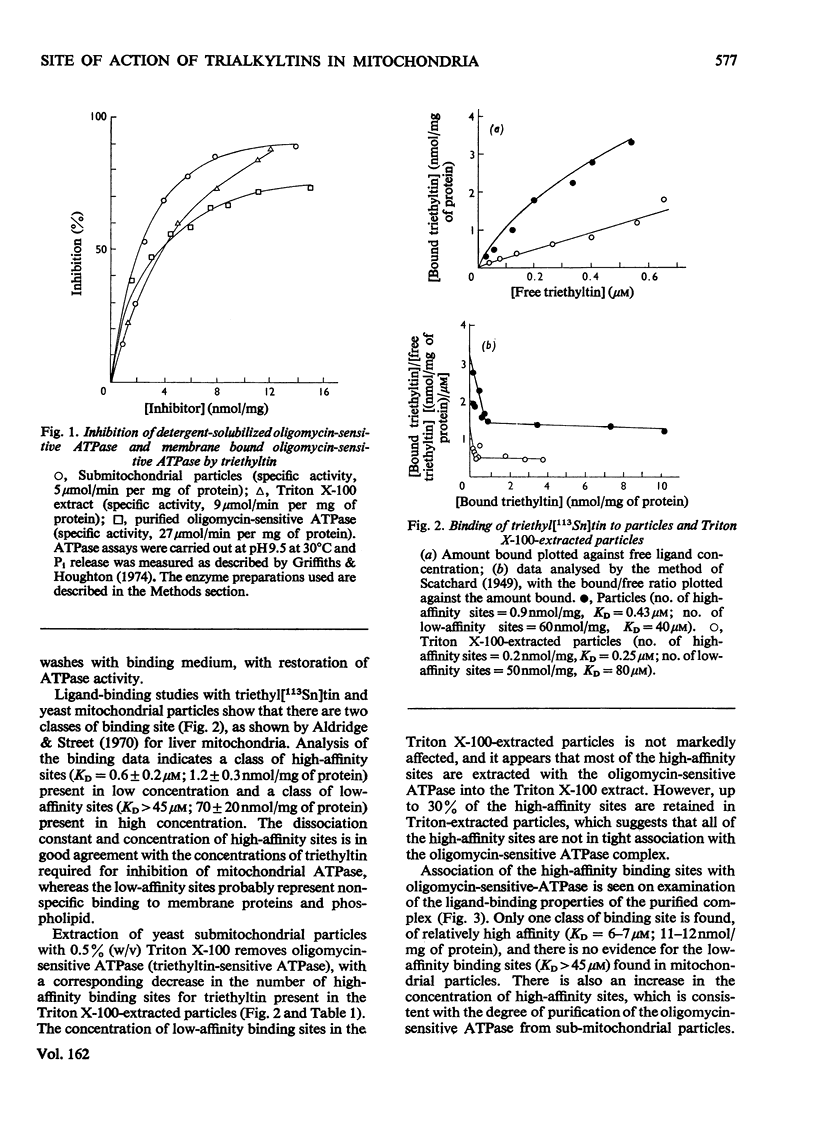
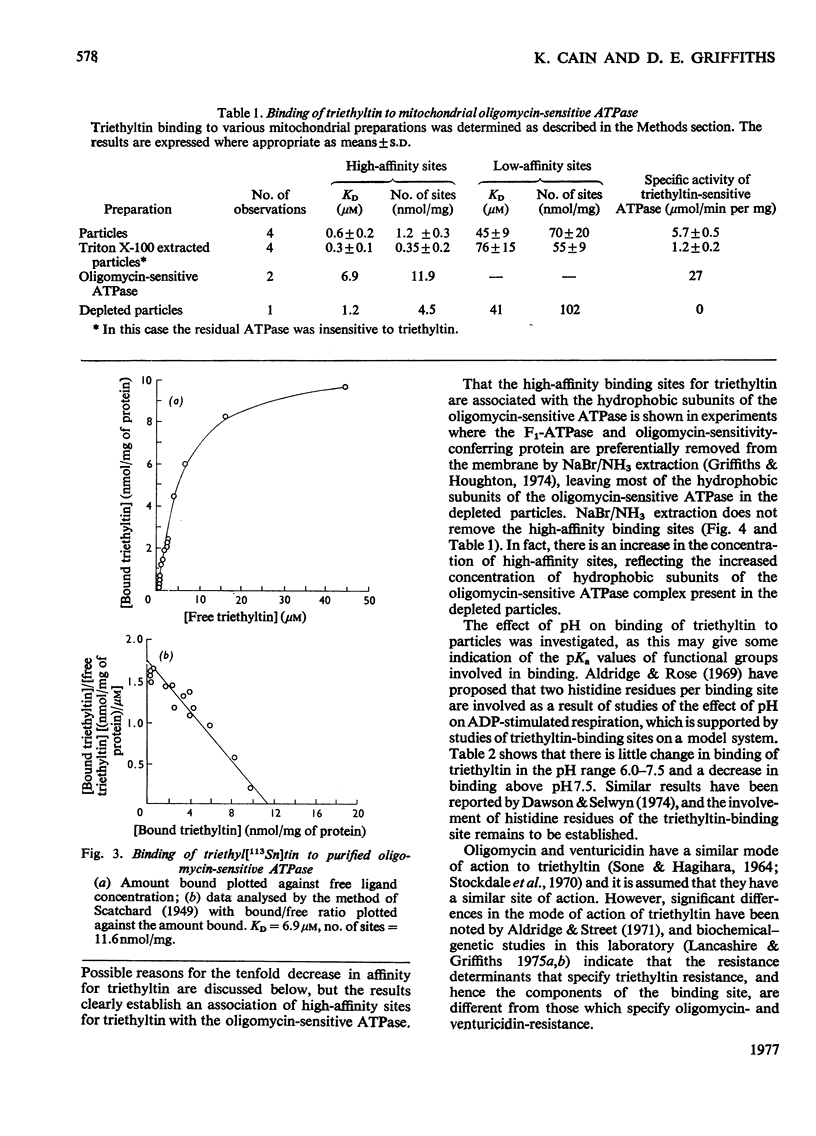
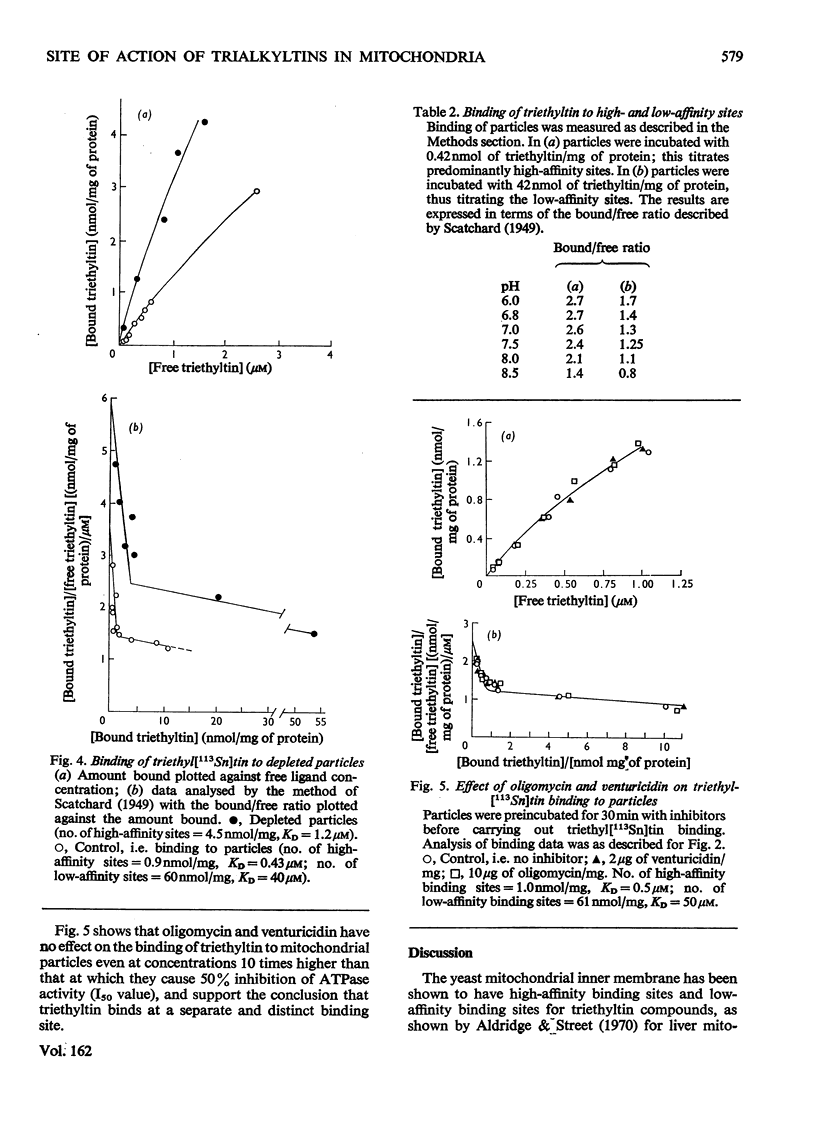
Ligand-binding studies with labelled triethyltin on yeast mitochondrial membranes showed the presence of high-affinity sites (KD = 0.6 micronM; 1.2 +/- 0.3 nmol/mg of protein) and low-affinity sites (KD less than 45 micronM; 70 +/- 20 nmol/mg of protein). The dissociation constant of the high-affinity site is in good agreement with the concentration of triethyltin required for inhibition of mitochondrial ATPase (adenosine triphosphatase) and oxidative phosphorylation. The high-affinity site is not competed for by oligomycin or venturicidin, indicating that triethyltin reacts at a different site from these inhibitors of oxidative phosphorylation. Fractionation of the mitochondrial membrane shows a specific association of the high-affinity sites with the ATP synthase complex. During purification of ATP synthase (oligomycin-sensitive ATPase) there is a 5-6-fold purification of oligomycin- and triethyltin-sensitive ATPase activity concomitant with a 7-9-fold increase in high-affinity triethyltin-binding sites. The purified yeast oligomycin-sensitive ATPase complex contains approximately six binding sites for triethyltin/mol of enzyme complex. It is concluded that specific triethyltin-binding sites are components of the ATP synthase complex, which accounts for the specific inhibition of ATPase and oxidative phosphorylation by triethyltin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge W. N., Rose M. S. The mechanism of oxidative phosphorylation A hypothesis derived from studies of trimethyltin and triethyltin compounds. FEBS Lett. 1969 Jul;4(2):61–68. doi: 10.1016/0014-5793(69)80197-3. [DOI] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. Biochemical effects and properties of trialkyltins. Biochem J. 1964 May;91(2):287–297. doi: 10.1042/bj0910287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. The relation between the specific binding of trimethylytin and triethyltin to mitochondria and their effects on various mitochondrial functions. Biochem J. 1971 Aug;124(1):221–234. doi: 10.1042/bj1240221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. The specific binding of trimethyltin and triethyltin to rat liver mitochondria. Biochem J. 1970 Jun;118(1):171–179. doi: 10.1042/bj1180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byington K. H. Effects of triphenyltin compounds on the adenosine triphosphatase activity of beef heart submitochondrial particles. Biochem Biophys Res Commun. 1971 Jan 8;42(1):16–22. doi: 10.1016/0006-291x(71)90355-x. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Selwyn M. J. The action of trialkyltin compounds on mitochondrial respiration. The effect of pH. Biochem J. 1974 Mar;138(3):349–357. doi: 10.1042/bj1380349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Selwyn M. J. The action of tributyltin on energy coupling in coupling-factor-deficient submitochondrial particles. Biochem J. 1975 Nov;152(2):333–339. doi: 10.1042/bj1520333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths D. E., Houghton R. L., Lancashire W. E., Meadows P. A. Studies on energy-linked reactions: isolation and properties of mitochondrial venturicidin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1975 Feb 21;51(2):393–402. doi: 10.1111/j.1432-1033.1975.tb03939.x. [DOI] [PubMed] [Google Scholar]
- Griffiths D. E., Houghton R. L. Studies on energy-linked reactions: modified mitochondrial ATPase of oligomycin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1974 Jul 1;46(1):157–167. doi: 10.1111/j.1432-1033.1974.tb03608.x. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- Lancashire, Griffiths D. E. Studies on energy-linked reactions: genetic analysis of venturicidin-resistant mutants. Eur J Biochem. 1975 Feb 21;51(2):403–413. doi: 10.1111/j.1432-1033.1975.tb03940.x. [DOI] [PubMed] [Google Scholar]
- Lancashire W. E., Griffiths D. E. Studies on energy-linked reactions: isolation, characterisation and genetic analysis of trialkyl-tin-resistant mutants of Saccharomyces cerevisiae. Eur J Biochem. 1975 Feb 21;51(2):377–392. doi: 10.1111/j.1432-1033.1975.tb03938.x. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Rose M. S., Aldridge W. N. Oxidative phosphorylation. The effect of anions on the inhibition by triethyltin of various mitochondrial functions, and the relationship between this inhibition and binding of triethyltin. Biochem J. 1972 Mar;127(1):51–59. doi: 10.1042/bj1270051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONE N., HAGIHARA B. INHIBITORY ACTION OF TRIALKYLTIN COMPOUNDS ON OXIDATIVE PHOSPHORYLATION IN MITOCHONDRIA. J Biochem. 1964 Aug;56:151–156. doi: 10.1093/oxfordjournals.jbchem.a127972. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Stockdale M., Dawson A. P., Selwyn M. J. Effects of trialkyltin and triphenyltin compounds on mitochondrial respiration. Eur J Biochem. 1970 Aug;15(2):342–351. doi: 10.1111/j.1432-1033.1970.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. IV. Role of mitochondrial and cytoplasmic protein synthesis in the biosynthesis of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1971 May 10;246(9):3050–3056. [PubMed] [Google Scholar]
- Tzagoloff A., Maclennan D. H., Byington K. H. Studies on the mitochondrial adenosine triphosphatase system. 3. Isolation from the oligomycin-sensitive adenosine triphosphatase complex of the factors which bind F-1 and determine oligomycin sensitivity of bound F-1. Biochemistry. 1968 Apr;7(4):1596–1602. doi: 10.1021/bi00844a049. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assembly of the mitochondrial membrane system. V. Properties of a dispersed preparation of the rutamycin-sensitive adenosine triphosphatase of yeast mitochondria. J Biol Chem. 1971 Dec 10;246(23):7328–7336. [PubMed] [Google Scholar]
- Tzagoloff A., Rubin M. S., Sierra M. F. Biosynthesis of mitochondrial enzymes. Biochim Biophys Acta. 1973 Feb 12;301(1):71–104. doi: 10.1016/0304-4173(73)90013-x. [DOI] [PubMed] [Google Scholar]
- Watling A. S., Selwyn M. J. Effect of some organometallic compounds on the permeability of chloroplast membranes. FEBS Lett. 1970 Oct 5;10(3):139–142. doi: 10.1016/0014-5793(70)80437-9. [DOI] [PubMed] [Google Scholar]



