Abstract
Aim
In order to better understand the incidence of IBD in China, we conducted a retrospective study to analyze the clinical information of IBD patients in Shanghai, China.
Methods
From January 2014 to December 2021, patients diagnosed with IBD and hospitalized were enrolled. The demographic, clinical features, symptoms, laboratory tests and treatment data of the patients were retrospectively analyzed.
Results
This study included 454 patients with UC and 333 patients with CD. The rate of hospitalization for IBD showed an escalating trend throughout the period, the number of hospitalizations was significantly higher in CD patients than in UC patients. The male patients had more complications than the female patients (p < 0.05). Definitive diagnosis of IBD in older patients was difficult (p < 0.05), and misdiagnosis was common. The incidence of complications and extraintestinal manifestations in elderly IBD patients was lower, but the incidence of intestinal obstruction was higher (p < 0.05). There was a significant correlation between the disease activity grades of IBD and fibrinogen, hemoglobin, albumin. Elderly IBD patients presented with lower rates of immunosuppressant, biologics, surgery or enteral nutrition.
Conclusion
This study analyzed the incidence, characteristics and treatment of IBD patients in Shanghai, and provided evidence-based evidence for doctors to more effectively diagnose and treat IBD in the future.
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, clinical features, treatment
Introduction
Inflammatory bowel disease (IBD) is characterized by recurrent, chronic, nonspecific intestinal inflammation with an unknown etiology, which can be divided into Crohn’s disease (CD) and ulcerative colitis (UC).1,2 There have been more than 6.8 million IBD cases reported worldwide in 2017, representing a growing global burden of the disease. Total years lived with disability (YLDs) due to IBD had increased from 0.56 million in 1990 to 1.02 million in 2017, which rose from fifth to fourth among the disease in the Global Burden of Disease, Injuries, and Risk Factors Study (GBD) digestive disease category.3 In the 30 years between 1990 and 2019, the prevalence, incidence, disability-adjusted life years (DALYs) and YLDs of IBD continued to increase.4 Hospitalization rates for IBD are relatively stable in countries in the compound epidemic phase, whereas hospitalization rates soar rapidly in newly industrialized countries in the second phase.5
The peak age group of UC was 30–40 years and CD was 20–40 years, thereafter, a second peak occurred between 60 and 70 years of age. The main clinical symptoms of IBD are diarrhea, abdominal pain, anemia, loss of weight, and mucous blood stool, which have a serious adverse impact on work and quality of life. The diagnosis of IBD is mainly based on clinical symptoms and related laboratory and special examination results, and it is necessary to rule out other non-infectious and infectious enteritis.
The current treatment of IBD is mainly divided into drug therapy and surgery. At present, the main clinical applications of drugs include 5-aminosalicylates (5-ASA), corticosteroids, immunomodulators, biological agent, and small molecule drugs. Surgical resection is often used as salvage therapy in patients with severe IBD who do not respond to medical treatment or in those with severe complications, as well as for patients who cannot tolerate adverse drug reactions. Other therapeutic means such as fecal microbiota transplantation, leukocytapheresis (LCAP), as new adjuvant therapies have gradually been introduced into the clinic.
In recent decades, with the industrialization and urbanization of underdeveloped countries and regions such as Asia, South America, and the Middle East, emerging industrialized countries have also begun to experience IBD, which has now become a global disease. It was estimated that the number of IBD patients in China would reach 1.5 million in 2025.6 The prevalence of IBD in China will continue to increase rapidly in the next 10 years. Physicians should fully understand IBD and provide more efficient and economical diagnosis and treatment for patients. Consequently, this study retrospectively analyzed the basic information and clinical characteristics of IBD patients treated in our hospital from January 2014 to December 2021, such as age, gender, clinical manifestations, symptoms, laboratory test, and treatment. Based on clinical data, our study analyzed the clinical characteristics, diagnosis and treatment of IBD patients in Shanghai, hoping to provide evidence for better diagnosis and treatment of IBD in China.
Methods
Patient Selection
Based on the medical records of Huadong Hospital affiliated to Fudan University, we collected and analyzed the clinical history and demographic data of IBD patients admitted to the Department of Gastroenterology from January 2014 to December 2021. We included IBD patients attending inpatient or outpatient clinics, and the diagnostic criteria for both CD and UC were met the “Chinese Consensus on Diagnosis and Treatment in Inflammatory Bowel Disease (2018, Beijing)”.7 We excluded patients with intestinal tuberculosis, Behcet’s disease, and HIV infection.
Data Collection
Specifically, we collected the time of admission, gender, age at diagnosis of IBD, interval time between onset to diagnosis, urban and rural distribution, living habits, type and location of diseases, Mayo score activity status of UC, clinical behavior and Crohn’s Disease Activity Index (CDAI) of CD, symptoms, extra-intestinal manifestations, concomitant diseases, clinical lab indexes, complications, and treatment.
Statistical Methods
This study has undergone biostatistics review. The results were analyzed with the statistical software SPSS 26. Continuous variable data were expressed as mean ± standard deviation (x ± SD). Two groups of continuous variables with normal distribution were analyzed by two-sample independent t test, and one-way ANOVA was used for comparison between multiple groups, while non-parametric tests were used to compare continuous variables that were not normally distributed. Categorical variables were analyzed by chi-square test. p <0.05 for the difference to be statistically significant. Pearson correlation analysis or Spearman rank correlation analysis was used for data correlation analysis, and p <0.05 indicated a correlation between the data.
Ethical Considerations
The study protocol was approved by the Ethics Committee of Huadong Hospital (No. ISRCTN: 2023K002). Informed consent was obtained from all patients prior to the study in accordance with the Declaration of Helsinki.
Results
Patient Demographic Information
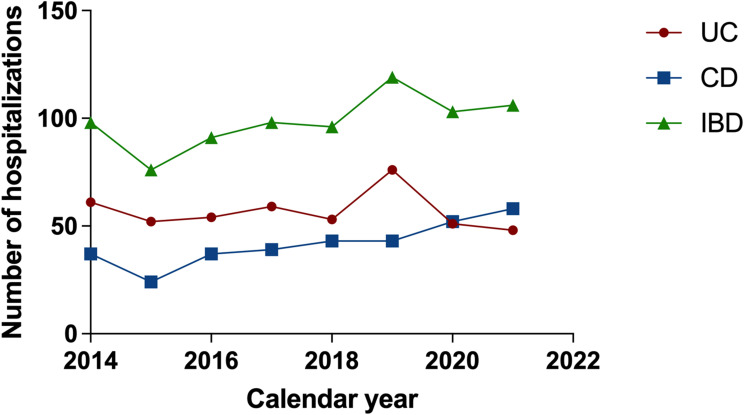
A total of 787 patients were enrolled in this study, including 454 UC patients and 333 CD patients. During the period from 2014 to 2021, the number of hospitalized cases of CD had an increasing trend year by year (r = 0.876, p = 0.004); meanwhile, there was no significant change in the number of UC inpatients (r = −0.119, p = 0.780) (Figure 1).
Figure 1.
Time distribution of IBD hospitalization rates in Huadong hospital from 2014 to 2021.
A total of 787 patients were included in our study, including 325 female patients and 462 male patients. The incidence of CD and UC were mainly in male patients (CD: 65.2% vs 34.8%, p < 0.01; UC: 54.0% vs 46.0%, p < 0.01). Meanwhile, the median age of onset of UC patients was 52 years, which was significantly higher than that of CD patients (38 years). The majority of CD patients were 20–40 years old (46.2%), and the majority of UC patients were aged between 40 and 60 years (45.4%). In our study, patients over 70 years of age diagnosed with IBD were very rare, and patients with UC were more common than patients with CD (9.7% vs 1.2%, p < 0.01). The average interval time (IT) of CD was longer than UC (2.58 ± 4.24 years vs 1.31 ± 3.23 years, p < 0.01). The IT is longer in aged UC patients than in non-aged UC patients (2.13 ± 5.30 years vs 1.04 ± 1.82 years, p < 0.05); however, there was no significant difference between the aged and non-aged CD patients (2.60 ± 5.53 years vs 2.60 ± 3.92 years, p > 0.05). The prevalence of IBD in urban areas was higher than that in rural areas. Smoking and alcohol consumption were not significantly associated with IBD (Table 1).
Table 1.
Demographic Information of the IBD Patients
| Ulcerative Colitis (N=454) | Crohn’s Disease (N=333) | p value | |
|---|---|---|---|
| Gender | 0.002 | ||
| Male | 245 (54.0%) | 217 (65.2%) | |
| Female | 209 (46.0%) | 116 (34.8%) | |
| Settlement | <0.01 | ||
| Rural area | 105(23.1%) | 115(34.5%) | |
| Urban area | 349(76.9%) | 218(65.5%) | |
| Smoking history | 69(15.2%) | 58(17.4%) | >0.05 |
| Drinking history | 29(6.4%) | 18 (5.4%) | >0.05 |
| Age (y) | |||
| Diagnosis median age | 52(37, 61) | 38(26.5, 56) | <0.01 |
| 14–19 | 8(1.8%) | 20(6.0%) | 0.001 |
| 20–29 | 48(10.6%) | 81(24.3%) | <0.001 |
| 30–39 | 66(14.5%) | 73(21.9%) | 0.007 |
| 40–49 | 82(18.1%) | 34(10.2%) | 0.002 |
| 50–59 | 124(27.3%) | 67(20.1%) | 0.020 |
| 60–69 | 82(18.1%) | 54(16.2%) | 0.499 |
| 70–79 | 40(8.7%) | 3(0.9%) | <0.001 |
| ≥80 | 4(0.9%) | 1(0.3%) | 0.403 |
Analysis of lesion location of CD patients revealed that more than half were ileocolonic lesions (L3: 50.5%, Table 2). Behavior (B1) was common (65.2%) among the CD affected patients. The proportion of male patients with stricturing or penetrating disease was significantly higher than that of female patients (41.0% vs 23.3%, p < 0.01). The male patients also had higher proportion of perianal disease than female patients (26.3% vs 14.7%, p < 0.05). Colonic lesions are the most common in elderly CD patients (L2: 37.9%) followed by the ileocolon (L3: 32.8%) and ileum (L1: 29.3%). Meanwhile, in non-elderly CD patients, ileocolon is the most common lesion site (L3: 54.2%). There was no significant difference in the proportion of upper gastrointestinal involvement between the non-aged and aged CD patients (p > 0.05). The proportion of aged CD patients with stricturing or penetrating disease was lower than that of non-aged CD patients (22.4% vs 37.5%, p < 0.05). Perianal lesion was less common in aged CD patients (3.4% vs 26.2%, p < 0.01). Among the UC patients, pancolitis UC (E3: 47.6%) was commonly observed. The phenotype of UC patients of different genders and different ages was mainly dominated by E3, with no significant difference between the phenotypes (p > 0.05) (Table 3).
Table 2.
The Phenotypic of Crohn’s Disease
| Phenotype | Male N=217 | Female N=116 | p value | Aged N=58 | Non-aged N=275 | p value |
|---|---|---|---|---|---|---|
| Age at diagnosis | <0.001 | |||||
| A1(≤16 years) | 3(1.4%) | 2(1.7%) | ||||
| A2(17–40 years) | 149(68.6%) | 31(26.7%) | ||||
| A3(<40 years) | 65(30.0%) | 83(71.6%) | ||||
| Location | 0.510 | 0.002 | ||||
| L1(terminal ileum) | 61(28.1%) | 32(27.6%) | 17(29.3%) | 76(27.6%) | ||
| L2(colon) | 40(18.4%) | 29(25.0%) | 22(37.9%) | 47(17.1%) | ||
| L3(ileocolon) | 114(52.5%) | 54(46.6%) | 19(32.8%) | 149(54.2%) | ||
| L4(upper gastrointestinal) | 2(0.9%) | 1(0.9%) | 0(0.0%) | 3(1.1%) | ||
| Behavior | ||||||
| B1(inflammatory) | 128(59.0%) | 89(76.7%) | 0.001 | 45(77.6%) | 172(62.5%) | 0.029 |
| B2(stricturing)/ B3(penetrating) | 89(41.0%) | 27(23.3%) | 13(22.4%) | 103(37.5%) | ||
| P: perianal | 57(26.3%) | 17(14.7%) | 0.015 | 2(3.4%) | 72(26.2%) | <0.001 |
Table 3.
The Phenotypic of Ulcerative Colitis
| Phenotype | Male N=245 | Female N=209 | p value | Aged N=126 | Non-aged N=328 | p value |
|---|---|---|---|---|---|---|
| Location | 0.793 | 0.248 | ||||
| E1(proctitis) | 65(26.5%) | 53(25.4%) | 39(31.0%) | 79(24.1%) | ||
| E2(distal) | 67 (27.4%) | 53(25.4%) | 34(27.0%) | 86(26.2%) | ||
| E3(extensive) | 113(46.1%) | 103 (49.2%) | 53(42.0%) | 163(49.7%) |
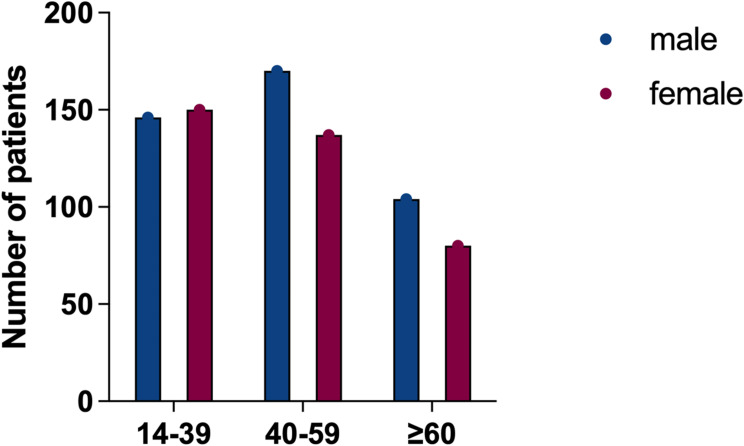
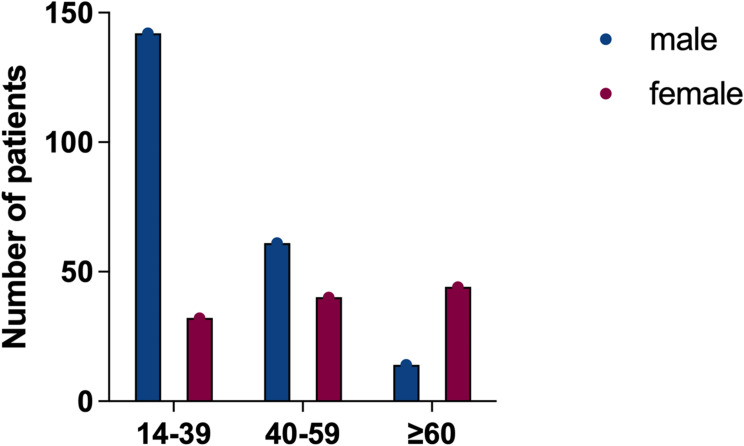
The fraction of individuals with CD was significantly different between males and females among different ages, but there was no significant difference in UC patients (p > 0.05). Subgroup analysis revealed that more men than women with CD below 40 years, while among elderly CD patients over 60 years old, women were more than men (p < 0.01) (Figures 2 and 3).
Figure 2.
Number of UC patients by gender and age group.
Figure 3.
Number of CD patients by gender and age group.
Comparison of Symptoms and Complications
As shown in Table 4, the most common symptom in UC patients was bloody stool (68.9%), followed by abdominal pain (51.1%), and diarrhea (39.9%), but there was no significant correlation with age (Table 5). The most common clinical symptoms in CD patients was abdominal pain (52.6%), diarrhea (28.2%), nausea and vomiting (12.3%). Bloody stool (27.6% vs 5.5%, p < 0.01) and asymptomatic (10.3% vs 1.8%, p < 0.01) were more common in elderly CD patients than in non-elderly, and perianal lesions were lower in elderly (1.7% vs 19.6%, p < 0.01) (Table 6). Moreover, CD patients had a higher incidence of complications such as intestinal stenosis (27.3%), intestinal obstruction (7.2%), bowel perforation (5.4%), fistula (10.2%), celiac abscess (1.8%), and perianal lesion (22.2%), which was significantly higher than UC patients (intestinal stenosis: 3.5%; intestinal obstruction: 0.4%; fistula: 0.0%; bowel perforation: 0.0%; celiac abscess: 0.0%; perianal lesion: 0.0%; p < 0.01). The proportion of extraintestinal manifestations (EIMs) in CD was twice that in UC (8.7% vs 4.4%, p < 0.05), and the most common EIMs in IBD were oral ulcers (Table 4).
Table 4.
Comparison of Symptoms and Complications
| Symptoms | Ulcerative Colitis(%) N=454 |
Crohn’s Disease(%) N=333 |
p value |
|---|---|---|---|
| Diarrhea | 181(39.9%) | 94(28.2%) | 0.001 |
| Bloody stool | 313(68.9%) | 31(9.3%) | <0.001 |
| Mucus stool | 116(25.6%) | 2(0.6%) | <0.001 |
| Abdominal pain | 232(51.1%) | 175(52.6%) | 0.687 |
| Abdominal bloating | 47(10.4%) | 34(10.2%) | 0.948 |
| Nausea, vomiting | 56(12.3%) | 41(12.3%) | 0.992 |
| Fever, weight loss | 21(4.6%) | 15(4.5%) | 0.936 |
| No symptoms | 9(2.0%) | 11(3.3%) | 0.245 |
| Extraintestinal manifestations | 20(4.4%) | 29(8.7%) | 0.014 |
| Oral ulcer | 12(2.6%) | 21(6.3%) | 0.011 |
| Joint pain | 9(2.0%) | 8(2.4%) | 0.689 |
| Rash | 3(0.7%) | 5(1.5%) | 0.294 |
| Eye lesion | 1(0.2%) | 0(0.0%) | 0.391 |
| Intestinal stenosis | 16(3.5%) | 91(27.3%) | <0.001 |
| Intestinal obstruction | 2(0.4%) | 24(7.2%) | <0.001 |
| Bowel perforation | 0(0.0%) | 18(5.4%) | <0.001 |
| Fistula | 0(0.0%) | 34(10.2%) | <0.001 |
| Celiac abscess | 0(0.0%) | 6(1.8%) | 0.006 |
| Massive gastrointestinal hemorrhage | 2(0.4%) | 3(0.9%) | 0.655 |
| Canceration | 2(0.4%) | 0(0.0%) | 0.511 |
| Perianal lesion | 0(0.0%) | 74(22.2%) | <0.001 |
Table 5.
Comparison of Symptoms and Complications with UC Patients of Different Age
| Symptoms | Aged(%) N=126 |
Non-aged(%) N=328 |
p value |
|---|---|---|---|
| Diarrhea | 48(38.1%) | 133(40.5%) | 0.633 |
| Bloody stool | 87(69.0%) | 226(68.9%) | 0.976 |
| Mucus stool | 32(25.4%) | 84 (25.6%) | 0.963 |
| Abdominal pain | 71(56.3%) | 161(49.1%) | 0.166 |
| Abdominal bloating | 17(13.5%) | 30(9.1%) | 0.174 |
| Nausea, vomiting | 16(12.7%) | 40(12.2%) | 0.884 |
| Fever, weight loss | 7(5.6%) | 14(4.3%) | 0.559 |
| No symptoms | 4(3.2%) | 5(1.5%) | 0.271 |
| Rash, joint pain | 1(0.8%) | 5(1.5%) | 0.683 |
| Intestinal stenosis | 6(4.8%) | 10(3.0%) | 0.398 |
| Intestinal obstruction | 1(0.8%) | 1(0.3%) | 0.478 |
| Massive gastrointestinal hemorrhage | 0(0.0%) | 2(0.6%) | 0.598 |
| Canceration | 0(0.0%) | 2(0.6%) | 0.598 |
Table 6.
Comparison of Symptoms and Complications with CD Patients of Different Age
| Symptoms | Aged(%) N=58 | Non-aged(%) N=275 | p value |
|---|---|---|---|
| Diarrhea | 15(25.9%) | 79(28.7%) | 0.660 |
| Bloody stool | 16(27.6%) | 15(5.5%) | <0.001 |
| Abdominal pain | 27(46.6%) | 148(53.8%) | 0.314 |
| Abdominal bloating | 7(12.1%) | 27(9.8%) | 0.607 |
| Nausea, vomiting | 5(8.6%) | 36(13.1%) | 0.346 |
| Fever, weight loss | 2(3.4%) | 13(4.7%) | 0.752 |
| No symptoms | 6(10.3%) | 5(1.8%) | 0.005 |
| Rash, joint pain | 2(3.4%) | 5(1.8%) | 0.352 |
| Intestinal stenosis | 11(19.0%) | 80(29.1%) | 0.116 |
| Intestinal obstruction | 8(13.8%) | 16(5.8%) | 0.047 |
| Bowel perforation | 0(0.0%) | 18(6.5%) | 0.051 |
| Fistula | 3(5.2%) | 31(11.3%) | 0.163 |
| Celiac abscess | 1(1.7%) | 5(1.8%) | 0.961 |
| Perianal lesion | 2(3.4%) | 72(26.2%) | <0.001 |
| Massive gastrointestinal hemorrhage | 1(1.7%) | 2(0.7%) | 0.438 |
Comparison of Concomitant Diseases
As shown in Table 7, the proportion of concomitant diseases was significantly higher in UC patients than in CD patients (86.6% vs 79.6%, p < 0.01), especially those with two or more diseases (73.3% vs 58.9%, p < 0.01). Hypertension, diabetes, colorectal polyps, and reflux esophagitis was more common in patients with UC than in CD (23.8% vs 14.7%, 7.7% vs 4.2%, 20.9% vs 8.1%, 10.6% vs 5.1%, p < 0.05). Psoriasis (3.3%), EB virus infection (6.9%), and rheumatic diseases (5.7%) including Sjogren’s syndrome, rheumatoid arthritis, lupus erythematosus-like syndrome was more common in CD patients.
Table 7.
Comparison of Concomitant Diseases
| Concomitant diseases | Ulcerative Colitis(%) N=454 | Crohn’s Disease(%) N=333 | p value |
|---|---|---|---|
| Yes | 393(86.6%) | 265(79.6%) | 0.009 |
| With two or more | 333(73.3%) | 196(58.9%) | <0.001 |
| Rheumatic diseases | 8(1.8%) | 19(5.7%) | 0.003 |
| Hypertension | 108(23.8%) | 49(14.7%) | 0.002 |
| Pulmonary nodules | 28(6.2%) | 14(4.2%) | 0.226 |
| Cerebral infarction | 23(5.1%) | 12(3.6%) | 0.325 |
| Diabetes | 35(7.7%) | 14(4.2%) | 0.044 |
| Hyperlipidemia | 12(2.6%) | 8(2.4%) | 0.832 |
| Osteoporosis | 27(5.9%) | 25(7.5%) | 0.384 |
| Thyroid dysfunction | 14(3.1%) | 6(1.8%) | 0.259 |
| Colorectal polyps | 95(20.9%) | 27(8.1%) | <0.001 |
| Reflux esophagitis | 48(10.6%) | 17(5.1%) | 0.006 |
| Peptic ulcer | 34(7.5%) | 30(9.0%) | 0.441 |
| Fatty liver | 70(15.4%) | 43(12.9%) | 0.322 |
| Autoimmune liver disease | 0(0.0%) | 1(0.3%) | 0.423 |
| Old pulmonary tuberculosis | 20(4.4%) | 7(2.1%) | 0.079 |
| Hepatitis B | 9(2.0%) | 9(2.7%) | 0.504 |
| EB virus infection | 7(1.5%) | 23(6.9%) | <0.001 |
| T-SPOT positive | 5(1.1%) | 5(1.5%) | 0.750 |
| Upper gastrointestinal cancers | 1(0.2%) | 2(0.6%) | 0.787 |
| Colorectal cancer | 9(2.0%) | 1(0.3%) | 0.051 |
| Other tumors | 18(4.0%) | 16(4.8%) | 0.567 |
Laboratory Examination and Its Application in the Assessment of IBD
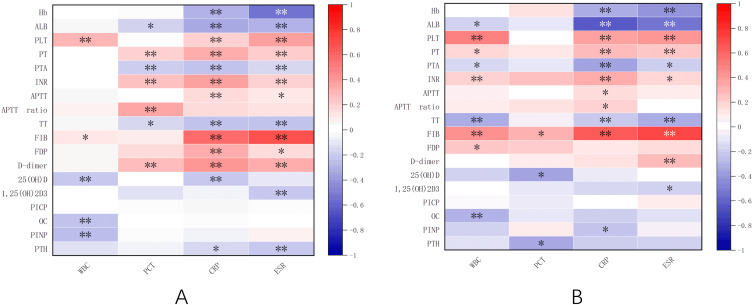
IBD activity was assessed by laboratory indicators such as hemoglobin (Hb), albumin (ALB), platelet counts (PLT), prothrombin activity (PTA), prothrombin time (PT), activated partial thromboplastin time (APTT), APTT ratio, thrombin time (TT), international normalized index of prothrombin time (INR), fibrinogen degradation products (FDP), fibrinogen (FIB), D-dimer (DDI), 25-hydroxyvitamin D, 1.25-dihydroxyvitamin D, osteocalcin, C-telopeptide of type I collagen, N-terminal propeptide of type I collagen, parathyroid hormone (PTH), white blood cells (WBC), procalcitonin (PCT), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) were examined and analyzed by heat map visualization. As shown in Figure 4, PLT, PT, INR, APTT, APTT ratio, FIB, FDP and DDI were positively correlated with inflammatory indexes in IBD patients, while Hb, ALB, PTA, TT, 25-hydroxyvitamin D, osteocalcin, 1.25-dihydroxyvitamin D, PTH and N-terminal propeptide of type I collagen were negatively correlated with inflammatory indexes in IBD. Among them, FIB and Hb were strongly correlated with inflammatory indexes of UC, FIB and ALB were strongly correlated with inflammatory indexes of CD.
Figure 4.
Heat map of correlation between laboratory parameters and inflammatory markers in UC (A) and CD (B). *p<0.01; **p<0.001.
Comparison of Drugs for the Treatment of IBD
Commonly used drugs for the treatment of IBD included 5-aminosalicylic acid (5-ASA), immunomodulators, steroids and biological therapy, and enteral nutrition is increasingly used for supportive therapy. In our study, the proportion of patients with UC and CD treated with 5-ASA was 87.2% and 62.8%, respectively. Compared with CD patients, UC patients were less likely to receive biological therapy (21.9% vs 1.3%) and surgical treatment (14.1% vs 3.7%) (Table 8). Enteral nutrition was administered to 2.2% of UC patients and 28.2% of CD patients. Similar numbers of patients with UC (12.6%) and CD (14.7%) received glucocorticoids. In the current study, immunomodulators (4.3% vs 11.1%, p < 0.01) and biological therapy (3.3% vs 12.1%, p < 0.01) was significantly less used in elderly IBD patients than in non-elderly patients, while enteral nutrition (8.7% vs 14.6%, p < 0.05) and operability (4.3% vs 9.3%, p < 0.05) was less applied in elderly IBD patients (Table 9).
Table 8.
Summary of the Treatment for UC and CD
| Medicine | Ulcerative Colitis(%) N=454 |
Crohn’s Disease(%) N=333 |
p value |
|---|---|---|---|
| 5-ASA | 396(87.2%) | 209(62.8%) | <0.001 |
| Steroid | 57(12.6%) | 49(14.7%) | 0.381 |
| Immune suppressor | 13(2.9%) | 62(18.6%) | <0.001 |
| Biological therapy | 6(1.3%) | 73(21.9%) | <0.001 |
| Immune suppressor combined with steroid | 7(1.5%) | 20(6.0%) | 0.001 |
| Enteral nutrition | 10(2.2%) | 94(28.2%) | <0.001 |
| Surgery | 17(3.7%) | 47(14.1%) | <0.001 |
Table 9.
Summary of the Treatment for IBD with Different Age
| Medicine | Aged(%) N=184 | Non-aged(%) N=603 | p value |
|---|---|---|---|
| 5-ASA | 148(80.4%) | 457(75.8%) | 0.191 |
| Steroid | 29(15.8%) | 77(12.8%) | 0.298 |
| Immune suppressor | 8(4.3%) | 67(11.1%) | 0.006 |
| Biological therapy | 6(3.3%) | 73(12.1%) | <0.001 |
| Immune suppressor combined with steroid | 4(2.2%) | 23(3.8%) | 0.285 |
| Enteral nutrition | 16(8.7%) | 88(14.6%) | 0.039 |
| Surgery | 8(4.3%) | 56(9.3%) | 0.032 |
Discussion
IBD is a chronic and persistent disease, which first appeared in Western countries. Sporadic cases of IBD began to appear in newly industrialized countries including China in the 1950s. Due to the differences in investigation methods, data sources and population background, the incidence of IBD varied greatly among different countries and regions.8 It has been suggested that IBD severity, susceptibility and progression were associated with sex hormone levels.9–11 The prevalence of male was higher in both UC and CD. And consistent with the results of previous studies on age difference in IBD patients,12 this study also found that the average age of onset of CD patients was younger than that of UC patients.
Laboratory indicators such as CRP, ESR, PCT and PLT have been widely studied in the occurrence and development of IBD. However, there are great differences in the sensitivity and specificity of these parameters in evaluating CD and UC. For example, CRP has a better role in assessing disease activity in CD than that in UC.13–15 ESR, like CRP, can also be used to assess the disease status of IBD; however, an increase in CRP or ESR may be associated with age or sex.16 Patients with active IBD often have a hypercoagulable state and are prone to complications such as thromboembolism. Anemia in patients with IBD may result from depletion of iron stores due to malabsorption and chronic bleeding, or from inadequate utilization of iron stores due to persistent inflammation. Bone health is essential for IBD patients because malabsorption, malnutrition, steroid use and inflammation can easily lead to bone loss, while vitamin D deficiency can also have serious adverse effects on the health of IBD patients.17 In the current study, we found that PLT and coagulation function indexes were positively correlated with inflammatory indexes in UC and CD patients, while Hb, ALB and bone metabolism indexes were negatively correlated with inflammatory indexes in UC and CD patients. Among them, FIB and Hb are strongly correlated with inflammatory indicators of UC patients, FIB and ALB are strongly correlated with inflammatory indicators of CD patients. Endoscopic examination with mucosal biopsy is an important method for the diagnosis and monitoring of IBD. UC is characterized endoscopically by continuous mucosal hyperemia, erosion, spontaneous mucosal bleeding, and ulceration starting from the rectum. The typical endoscopic and pathological features of CD are Aphthous ulcer, longitudinal ulcer, cobblestone-like appearance, non-caseating granuloma. The results of our study indicate that extensive colonic involvement is the most common disease subtype in UC. In CD patients, ileocolonic involvement was the main lesion, and the disease behavior was mainly non-penetrating and non-stricturing.
Treatment strategies for IBD largely depend on previous treatment and the severity of the disease. At present, it is increasingly emphasized that the goal of IBD treatment is not only to achieve clinical symptom relief but also to achieve endoscopic mucosal and tissue healing. Biological agents play an increasingly important role in the treatment of IBD, and infliximab can effectively treat fistulous CD and other perianal diseases.18–20 According to our current study, more than half of UC and CD patients still received 5-ASA as the first-line treatment. Probably because of cost, only about one-fifth of the CD patients received biologics. If conservative treatment is ineffective, surgical treatment may also be considered. A large cohort study of CD patients over 15 years showed that more than one-fifth of CD patients underwent surgery for disease activity or complexity within 15 years of diagnosis, with approximately 10% of these patients undergoing multiple surgeries and 7% of severe patients undergoing permanent enterostomy.21 In this study, 14% of CD patients had surgery, whereas a lower percentage of UC patients underwent surgery, about less than 4%. Not only medical drugs and surgical treatment but also enteral nutrition support therapy is extremely essential for CD patients, especially for patients with malnutrition or the management of CD in children during the growth and development period.22 The proportion of CD patients receiving enteral nutrition was about 10 times higher than that of UC patients (28.2% vs 2.2%). Many studies have shown that comorbidities, more adverse drug reactions after the use of immunosuppressors or biologics, and the decline of liver and kidney physiological functions are the reasons for the low level of immunosuppressive agents or biological agents for elderly patients with IBD.23–25
Our study has limitations. First of all, the data source of this study was a single-center and the sample size was small, the main objects of our hospital were middle-aged and elderly patients, and there were few underage inpatients and few nationwide multi-center research data in primary hospitals. A large number of studies have shown that smoking is a risk factor for CD, and smoking is closely related to the increased risk of postoperative recurrence of CD.26,27 Nevertheless, this study showed that IBD disease activity was not significantly associated with smoking and alcohol consumption. Due to the small sample size included, this may have an impact on the results of the study. At the same time, this study is a retrospective study, which may have recall bias to a certain extent. The proportion of CD patients with psoriasis and rheumatic diseases was significantly higher than that of UC patients, which may be due to the higher application rate of TNF antagonists in CD patients than in UC patients. The use of TNF antagonists can cause immune-mediated complications such as psoriasis and lupus-like syndromes.28 However, as some IBD patients in this study were accompanied by other chronic diseases, these confounding factors would also affect the hematological parameters. Therefore, the reliability and sensitivity of FIB, Hb and ALB as auxiliary reference indexes for judging IBD activity need to be further studied and confirmed.
At present, the epidemiological research of IBD, especially in the elderly, is limited. This study systematically sorted out and analyzed the clinical data of IBD inpatients in our hospital from the basic data and clinical characteristics. In addition, the basic data such as the interval time between onset and diagnosis, gender composition and clinical characteristics such as clinical phenotype, symptoms, complications and treatment of IBD in elderly patients were summarized. In addition, this study also found that FIB, Hb and ALB were strongly correlated with inflammatory indicators in IBD patients, which could be used as auxiliary indicators to judge the activity of UC or CD. It is hoped that the results of this study will help clinicians to better understand the disease classification and characteristics of IBD, and to diagnose and treat CD and UC patients more effectively.
Acknowledgments
We would like to express our heartfelt gratitude to the participants for their contributions and experience sharing.
Funding Statement
This study was supported in part by the Key Specializend Disease Project of Huadong Hospital (ZDZB2221), and the Key disciplines of Huadong Hospital (ZDXK2213).
Co-First Authors
Lin Mi and Ke Wang.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2 PMID: 27914657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roda G, Ng SC, Kotze PG, et al. Crohn’s disease. Nat Rev Dis Primers. 2020;6(1):22. doi: 10.1038/s41572-020-0183-z PMID: 32433463. [DOI] [PubMed] [Google Scholar]
- 3.GBD. Inflammatory bowel disease collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017, Lancet Gastroenterol Hepatol. 2020;5(1):17–30. PMID: 31648971. doi: 10.1016/S2468-1253(19)30333-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Dong Z, Wan X. Global, regional, and national burden of inflammatory bowel disease and its associated anemia, 1990 to 2019 and predictions to 2050: an analysis of the global burden of disease study 2019. Autoimmun Rev. 2024;23(3):103498. doi: 10.1016/j.autrev.2023.103498 PMID: 38052263. [DOI] [PubMed] [Google Scholar]
- 5.Buie MJ, Quan J, Windsor JW, et al.,;. Global hospitalization trends for crohn’s disease and ulcerative colitis in the 21st century: a systematic review with temporal analyses. Clin Gastroenterol Hepatol. 2023;21(9):2211–2221. PMID: 35863682. doi: 10.1016/j.cgh.2022.06.030 [DOI] [PubMed] [Google Scholar]
- 6.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150 PMID: 26323879. [DOI] [PubMed] [Google Scholar]
- 7.Group IB. Chinese Medical Association. Beijing: chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). Chin J Dig. 2018;38(5):292–311. doi: 10.1111/1751-2980.12994 PMID: 33905603. [DOI] [Google Scholar]
- 8.Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56–66. doi: 10.1038/s41575-020-00360-x PMID: 33033392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah SC, Khalili H, Gower-Rousseau C, et al. Sex-based differences in incidence of inflammatory bowel diseases-pooled analysis of population-based studies from Western Countries. Gastroenterology. 2018;155(4):1079–1089e1073. doi: 10.1053/j.gastro.2018.06.043 PMID: 29958857. [DOI] [PubMed] [Google Scholar]
- 10.Khalili H, Granath F, Smedby KE, et al. Association between long-term oral contraceptive use and risk of Crohn’s disease complications in a nationwide study. Gastroenterology. 2016;150(7):1561–1567e1561. doi: 10.1053/j.gastro.2016.02.041 PMID: 26919969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortizo R, Lee SY, Nguyen ET, Jamal MM, Bechtold MM, Nguyen DL. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur J Gastroenterol Hepatol. 2017;29(9):1064–1070. doi: 10.1097/MEG.0000000000000915 PMID: 28542115. [DOI] [PubMed] [Google Scholar]
- 12.Duricova D, Burisch J, Jess T, Gower-Rousseau C, Lakatos PL. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis. 2014;8(11):1351–1361. doi: 10.1016/j.crohns.2014.05.006 PMID: 24951261. [DOI] [PubMed] [Google Scholar]
- 13.Fagan EA, Dyck RF, Maton PN, et al. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur J Clin Invest. 1982;12(4):351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x PMID: 6814926. [DOI] [PubMed] [Google Scholar]
- 14.Solem CA, EV Loftus Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707–712. doi: 10.1097/01.mib.0000173271.18319.53 PMID: 16043984. [DOI] [PubMed] [Google Scholar]
- 15.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029 PMID: 17681163. [DOI] [PubMed] [Google Scholar]
- 16.Siemons L, Ten Klooster PM, Vonkeman HE, van Riel PL, Glas CA, van de Laar MA. How age and sex affect the erythrocyte sedimentation rate and C-reactive protein in early rheumatoid arthritis. BMC Musculoskelet Disord. 2014;15:368. doi: 10.1186/1471-2474-15-368 PMID: 25373740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abegunde AT, Muhammad BH, Ali T. Preventive health measures in inflammatory bowel disease. World J Gastroenterol. 2016;22(34):7625–7644. doi: 10.3748/wjg.v22.i34.7625 PMID: 27678347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;10:CD000067. doi: 10.1002/14651858.CD000067.pub3 PMID: 26517527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;8:CD006884. doi: 10.1002/14651858.CD006884.pub3 PMID: 25157445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350(9):876–885. doi: 10.1056/NEJMoa030815 PMID: 14985485. [DOI] [PubMed] [Google Scholar]
- 21.Cosnes J, Bourrier A, Nion-Larmurier I, Sokol H, Beaugerie L, Seksik P. Factors affecting outcomes in Crohn’s disease over 15 years. Gut. 2012;61(8):1140–1145. doi: 10.1136/gutjnl-2011-301971 PMID: 22387526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179–1207. doi: 10.1016/j.crohns.2014.04.005 PMID: 24909831. [DOI] [PubMed] [Google Scholar]
- 23.Jeuring SF, van den Heuvel TR, Zeegers MP, et al. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age-an increasing distinct entity? Inflamm Bowel Dis. 2016;22(6):1425–1434. doi: 10.1097/MIB.0000000000000738 PMID: 26933752. [DOI] [PubMed] [Google Scholar]
- 24.Simian D, Fluxá D, Flores L, et al. Inflammatory bowel disease: a descriptive study of 716 local Chilean patients. World J Gastroenterol. 2016;22(22):5267–5275. doi: 10.3748/wjg.v22.i22.5267 PMID: 27298570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturm A, Maaser C, Mendall M, et al. European crohn’s and colitis organisation topical review on IBD in the elderly. J Crohns Colitis. 2017;11(3):263–273. doi: 10.1093/ecco-jcc/jjw188 PMID: 27797918. [DOI] [PubMed] [Google Scholar]
- 26.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81(11):1462–1471. doi: 10.4065/81.11.1462 PMID: 17120402. [DOI] [PubMed] [Google Scholar]
- 27.Vuitton L, Koch S, Peyrin-Biroulet L. Preventing postoperative recurrence in Crohn’s disease: what does the future hold? Drugs. 2013;73(16):1749–1759. doi: 10.1007/s40265-013-0128-x PMID: 24132799. [DOI] [PubMed] [Google Scholar]
- 28.Hou JK, Feagins LA, Waljee AK. Characteristics and behavior of elderly-onset inflammatory bowel disease: a multi-center us study. Inflamm Bowel Dis. 2016;22(9):2200–2205. doi: 10.1097/MIB.0000000000000849 PMID: 27482973. [DOI] [PubMed] [Google Scholar]