Abstract
Context
A vast body of published literature examines and evaluates the properties of bone restorative materials in combination with other biomaterials or as stand-alone applications. If we exclude the studies investigating the effectiveness of regenerative therapy with enamel matrix derivative (EMD), in all other cases, bone regenerative materials are placed on a "pedestal." Therefore, the study we have initiated covers methods whose protocol does not use bone-repair materials. The clinical and radiographic results obtained are compared to determine which of these methods is the most reliable. The most important goal we set out was to determine if periodontal regenerative therapy would be effective without the use of bone graft restorative materials.
Aim
This study aimed to investigate, analyze, and compare the outcomes of four groups of patients with vertical bone defects (infrabony defects (IBDs)) who were treated using regenerative methods without the involvement of bone repair materials.
Materials and methods
Forty-eight cases that fulfilled all participation criteria for the study were selected. The O'Leary plaque index (PI) and Ainamo and Bay gingival index (GI) were assessed at the reassessment visit after the Hygiene Phase, the current periodontal status was recorded, and at least one IBD was identified. Cone beam computed tomography (CBCT) was ordered, and the size of each defect was measured by three parameters. In this study, all IBDs were randomly allocated to four groups. The first category encompasses IBDs, wherein regenerative therapy utilizing autogenous, platelet-rich plasma (PRP) is implemented. The second group comprises IBDs, which undergo regenerative therapy utilizing EMD. The third category encompasses IBDs in which guided tissue regeneration (GTR) is conducted using solely a barrier membrane. The fourth group encompasses IBDs, wherein GTR utilizing a barrier membrane and PRP took place. Six months after regenerative therapy, regardless of which of the four methods was used, all patients were reassessed clinically by CBCT. Statistical methods were used to evaluate, analyze, and compare the results in the four groups.
Results
A statistically significant decrease in the "probing pocket depth" indication, a statistically significant clinical attachment level gain, and a statistically significant decrease in the CBCT indicators "A" and "B" were observed in all four groups of patients under study. When it comes to the CBCT indicator "C," the results for each of the four groups of patients under study fall somewhere between statistical significance and non-significant.
Conclusions
Regardless of the regenerative therapy technique used, all patients under examination showed improvements in imaging and clinical markers. The four patient groups' results did not differ in any way that was statistically significant.
Keywords: autogenous platelet-rich plasma, barrier membrane, enamel matrix derivative, guided tissue regeneration, periodontology, platelet-rich plasma (prp), regenerative therapy, vertical bone defects
Introduction
Periodontitis is among the most prevalent disease in the oral cavity. The disease is essentially infectious and inevitably leads to inflammation and destruction affecting all components of the periodontium [1,2]. Therefore, in periodontal treatment, two fundamental stages stand out today. The first stage is responsible for controlling and eliminating the inflammatory process (hygienic phase). The second stage of treatment is related to the restoration of the periodontal components that have been destroyed (corrective phase) [3].
The first areas to be affected in the development of periodontal disease are the distal interdental areas. These locations significantly hinder patients' ability to maintain personal dental hygiene. Unless timely measures are taken to motivate and educate patients to clean these areas more diligently and with better quality, the development of a periodontal problem is inevitable [4].
Goldman and Cohen classified defects as supraosseous and infraosseous [5]. Infrabony defects (IBDs) develop as a result of vertical bone resorption and are characterized by their base positioned apically to the residual alveolar ridge [5,6].
Diagnosis of these defects is done clinically and radiographically. Due to its significant advantages over conventional 2D radiographs, today, cone beam computed tomography (CBCT) is the preferred method for making an accurate diagnosis, as well as for assessing the size and morphology of the bone defect [7,8]. For qualitative and quantitative bone evaluation, CBCT along with bone histology and histomorphometry are the methods of choice [9,10,11].
It is an undeniable fact that IBDs can be treated. The reason for this lies in the remarkable possibilities of regenerative methods, restoring both the soft and hard components of the periodontium [12].
Although periodontal regenerative therapy has good predictive values, it still poses a huge challenge for dentists. It is no coincidence that new, more sophisticated biomaterials are being developed every day in order to improve outcome data [13,14,15,16,17]. A vast amount of the published literature investigates and evaluates the properties of bone regenerative materials in combination with other biomaterials or as stand-alone applications. If we exclude the studies investigating the effectiveness of regenerative therapy with self-administration of enamel matrix derivatives (EMDs), then in all other cases, bone regenerative materials are placed on a "pedestal" [18,19,20].
Therefore, the study we have initiated covers methods whose protocol does not use bone repair material. Both clinical and CBCT parameters are being investigated to achieve greater precision in the conclusions. The results are compared to determine which of these methods is most reliable. However, the most important goal we set out was to establish whether periodontal regenerative therapy would be an effective method without the use of a bone graft.
Materials and methods
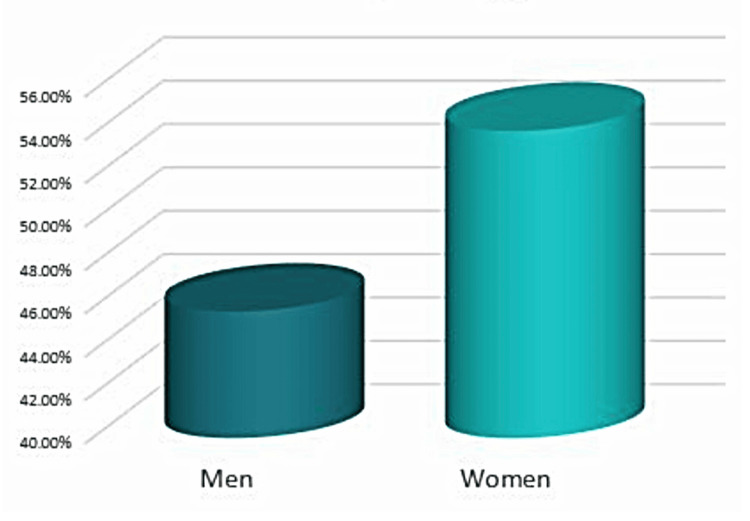
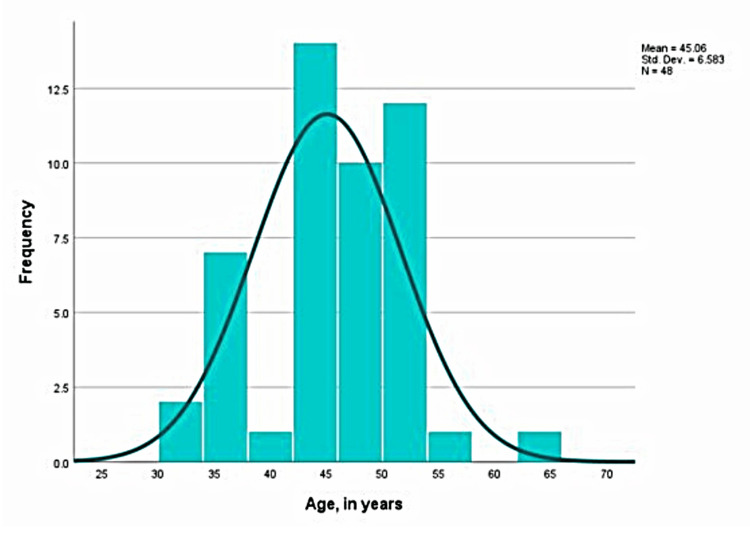
The University Medical and Dental Center, part of the Medical University Varna, Faculty of Dental Medicine, was utilized for this research from August 2022 until July 2023. Forty-eight cases from a total of 30 male and female patients, aged 31 to 63 years (Figures 1, 2), who fulfilled all participation criteria for the study were selected - high degree of motivation to maintain excellent oral hygiene on the part of the patient, completed and signed informed consent and questionnaire on current health status excluding existing systemic diseases. A standard age range between 18 and 65 years was introduced.
Figure 1. Allocation of the patients by gender.
Figure 2. Allocation of the patients by age.
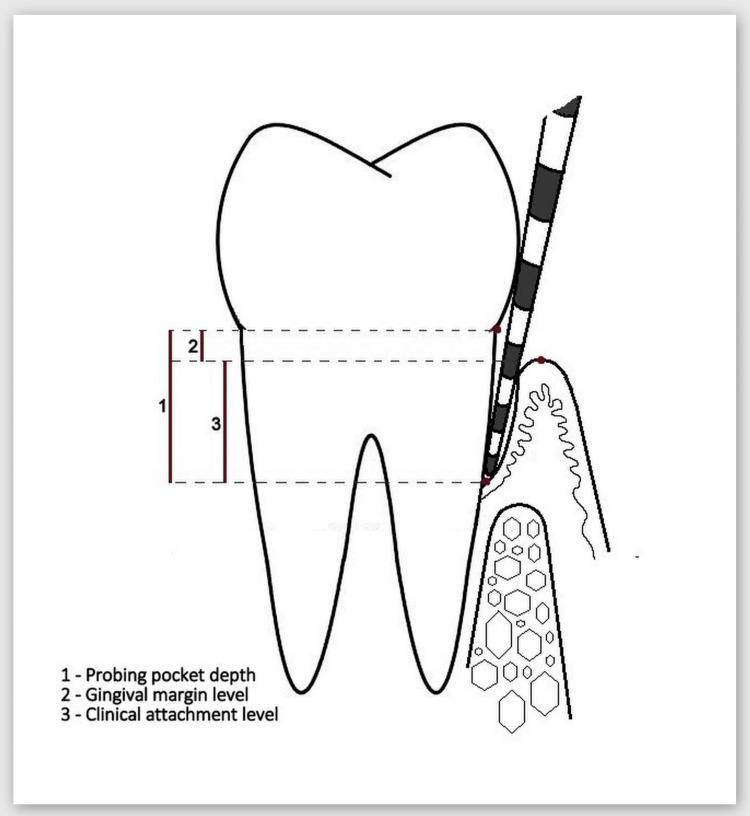
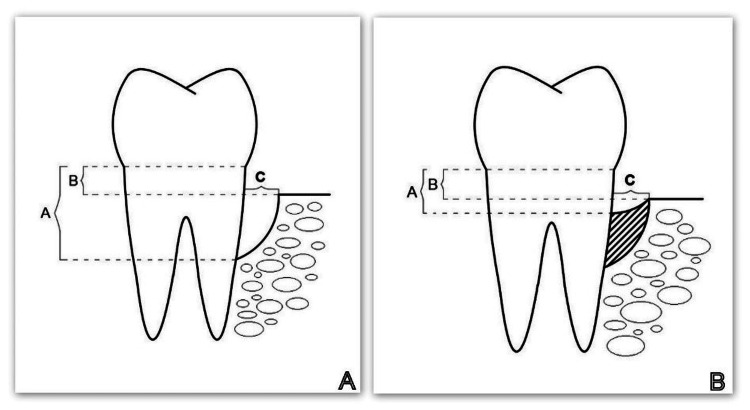
Between six and eight weeks after completion of the hygiene phase, a reassessment visit was scheduled for each study patient. The O'Leary plaque index (PI) and Ainamo and Bay gingival index (GI) were assessed, and the current periodontal status was recorded (clinical parameters examined were probing pocket depth (PPD), gingival margin level (GML), and clinical attachment level (CAL) (Figure 3)), and at least one IBD was identified. In all patients found to have an infrabony defect indicated for surgical treatment, a CBCT scan was ordered and three parameters (described in Figure 4A) assessing the size and morphology of the defect were measured.
Figure 3. Examined clinical parameters under this study.
Image credits: Author Gerova-Vatsova T
Figure 4. Examined radiographic parameters (A, B, and C).
A: CBCT parameters studied before the regenerative therapy; B: CBCT parameters studied six months after the regenerative therapy.
Parameter A: the distance from the cemento-enamel junction (CEJ) to the bottom of the bone defect. Parameter B: the distance from the CEJ to the apex of the bone defect. Parameter C: the width of the defect.
CBCT: cone beam computed tomography
Image credits: Author Gerova-Vatsova T
After completion of the evaluation and analysis of the initial clinical and radiological data, a date for the surgical intervention was scheduled.
In this study, all IBDs were randomly allocated to four groups. The first category encompasses IBDs, wherein regenerative therapy utilizing autogenous, platelet-rich plasma (PRP) is implemented. The second group comprises IBDs, which undergo regenerative therapy utilizing EMD. The third category encompasses IBDs in which guided tissue regeneration (GTR) is conducted using a collagen barrier membrane (Botiss Jason membrane, Berlin, Germany). The fourth group encompasses IBDs, wherein GTR utilizing a collagen barrier membrane (Botiss Jason membrane, Berlin, Germany) and PRP took place (Figure 5).
Figure 5. The four study groups in the study.
Image credits: Author Gerova-Vatsova T
At six months after the regenerative therapy, regardless of which of the four methods were used, clinical and CBCT parameters were measured again in all patients (Figure 4B). Statistical methods were used to evaluate, analyze, and compare the results in the four groups.
Results
Initially, this study must exhibit and compare the PI and GI acquired from the participants across all four groups to ascertain the mutual validity of our results.
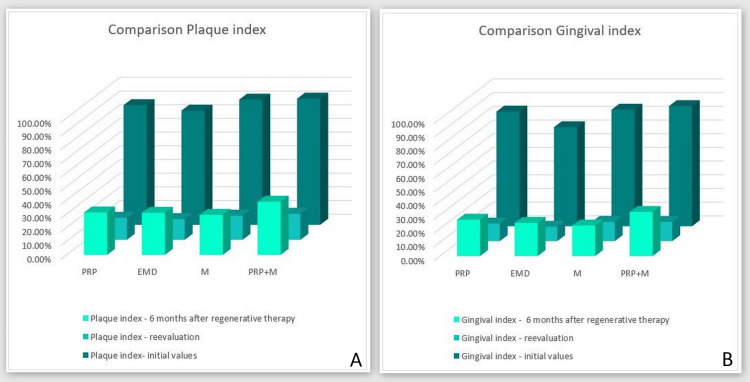
Figure 6 distinctly illustrates the mean percentages of the PI and GI for patients across each group during three reporting time frames: 1) immediately before the beginning of the periodontal treatment; 2) reassessment following the hygiene phase; and 3) six months subsequent to the execution of regenerative therapy. A clear link exists between PI and GI across the three time frames of periodontal therapy among the subjects.
Figure 6. PI and GI acquired from the participants across all four groups .
PI: O'Leary plaque index, GI: Ainamo and Bay gingival index, PRP: platelet-rich plasma, EMD: enamel matrix derivative, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane
It is evident that the plaque and gingival indices before the initiation of periodontal therapy were markedly elevated in each participant, indicating that all subjects in the study commenced with inadequate personal oral hygiene and a significant percentage of gingival inflammation. During the reassessment phase, there was a notable reduction in the values for both indices across all four groups, indicating the substantial cooperation of every individual during periodontal therapy. At the six-month post-regenerative therapy phase, there was an elevation in both the PI and GI scores, which may be attributed to a decline in the patients' individual oral hygiene practices. The PI values at stage 3 across the four groups were essentially equivalent, so permitting the exclusion of PI and GI as modifiers in the outcomes of any of the groups.
Probing pocket depth
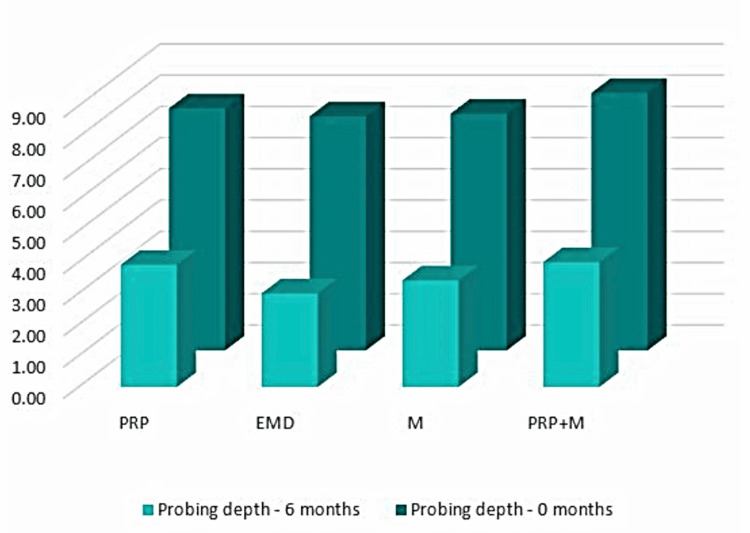
Figure 7 illustrates that during the hygienic phase, the PPD parameter exhibited a notably elevated mean value across all four patient groups (first group = 7.75 mm; second group = 7.50 mm; third group = 7.58 mm; fourth group = 8.25 mm). In the six-month post-regenerative therapy, a notable decrease in PPD was seen relative to baseline measurements (first group = 3.92 mm; second group = 3.00 mm; third group = 3.42 mm; fourth group = 4.00 mm). The comprehensive results are displayed in Table 1.
Table 1. Comparison of the clinical and radiographic parameters at 0 and six months.
PRP: autogenous platelet-rich plasma, EMD: enamel matrix derivatives, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane, PPD: probing pocket depth, GML: gingival margin level, CAL: clinical attachment level
| Method | PPD - 0 months | GML - 0 months | CAL - 0 months | PPD - 6 months | GML - 6 months | CAL- 6 months | A - 0 months | B - 0 months | C - 0 months | A - 6 months | B - 6 months | C - 6 months |
| PRP | 7.75 | 0.08 | -7.67 | 3.92 | 0.17 | -3.75 | 6.37 | 3.19 | 2.37 | 4.68 | 2.69 | 2.01 |
| EMD | 7.50 | -0.08 | -7.58 | 3.00 | -0.58 | -3.58 | 5.85 | 2.64 | 2.07 | 4.34 | 2.14 | 1.84 |
| M | 7.58 | -0.33 | -7.92 | 3.42 | -0.67 | -4.08 | 7.14 | 3.61 | 1.89 | 5.46 | 3.12 | 1.78 |
| PRP+M | 8.25 | -0.58 | -8.83 | 4.00 | -0.33 | -4.33 | 7.15 | 3.31 | 2.37 | 5.74 | 2.93 | 1.86 |
Figure 7. PPD comparison between 0 and six months.
PPD: probing pocket depth, PRP: platelet-rich plasma, EMD: enamel matrix derivative, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane
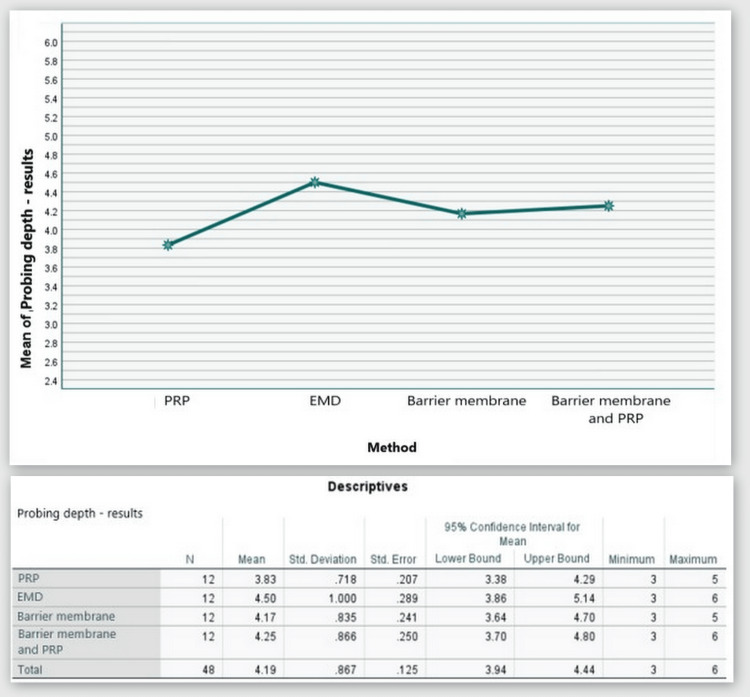
Based on the statistical analyses, it was found that PPD was decreased by 3.83 mm on average in Group 1. In Group 2, it was decreased by 4.50 mm. In Group 3, it was decreased by 4.17 mm on average. In Group 4, it was decreased by 4.25 mm on average (Figure 8).
Figure 8. Statistical analysis results and graphics for the PPD.
PPD: probing pocket depth
The ANOVA test demonstrated that the findings for PPD at six months post-regenerative therapy were not statistically significant among all four patient groups, F(3,44) = 1.228, p = 0.311 > 0.05 (Appendix A).
Gingival margin level
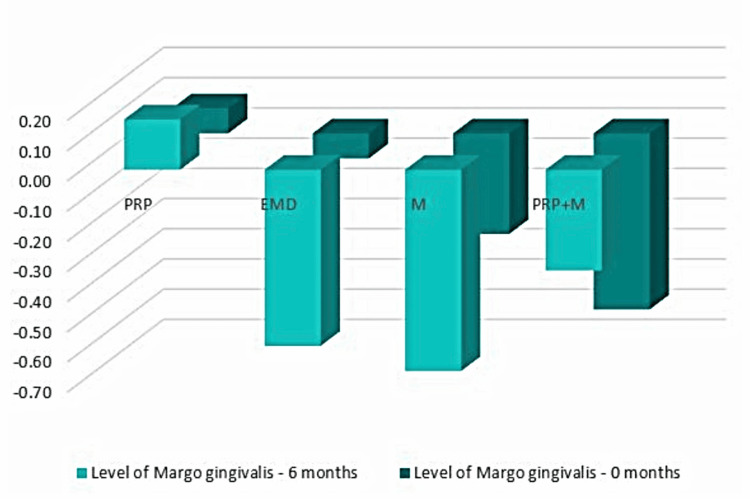
Figure 9 illustrates that throughout the hygienic phase, the mean values of the GML parameter were 0.08 mm for Group 1, -0.08 mm for Group 2, -0.33 mm for Group 3, and -0.58 mm for Group 4. At six months post-regenerative therapy, there was a relative retention of values compared to the baseline measurements (Group 1 = 0.17 mm; Group 2 = -0.58 mm; Group 3 = -0.67 mm; Group 4 = -0.33 mm). This indicates that clinically, there was virtually no apical or coronal migration of the gingival margin seen. The findings are elaborated upon in Table 1.
Figure 9. GML comparison at 0 and six months.
GML: gingival margin level, PRP: platelet-rich plasma, EMD: enamel matrix derivative, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane
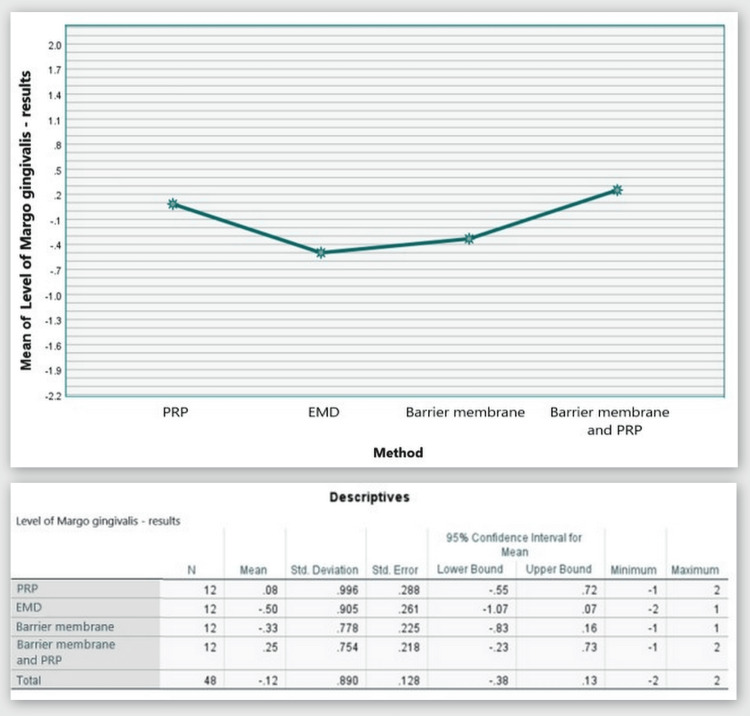
Based on the statistical analyses, Groups 1 and 4 showed a shift of the gingival margin in the coronal direction by an average of 0.08 mm and 0.25 mm, respectively, while the other two groups showed a shift of the gingival margin in the apical direction by an average of 0.5 mm and 0.33 mm (Figure 10).
Figure 10. Statistical analysis results and graphics for GML.
GML: gingival margin level
The ANOVA test demonstrated that the findings for GML at six months post-regenerative therapy were not statistically significant among all four groups: F(3, 44) = 1.973, p = 0.132 > 0.05 (Appendix B).
Clinical attachment level
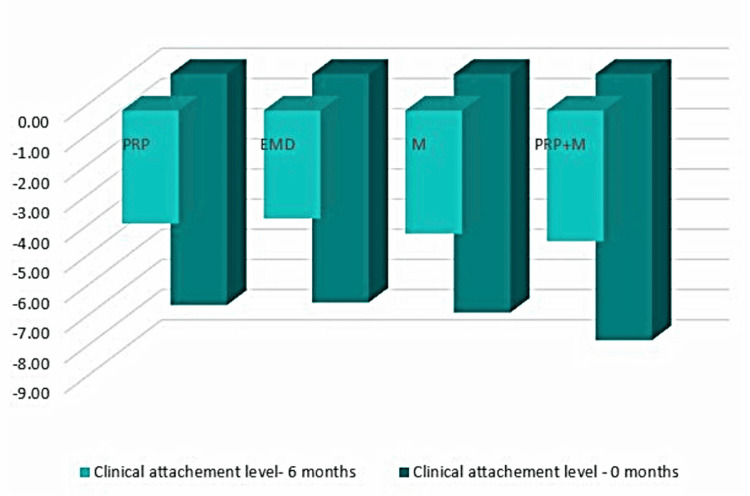
Figure 11 shows that throughout the hygienic phase, Group 1 patients had a CAL measurement of 7.67 mm, Group 2 - 7.58 mm, Group 3 - 7.92 mm, and Group 4 - 8.83 mm. Six months after the regeneration therapy, all methods showed a significant reduction in these values, indicating CAL gain (Group 1, 3.75 mm; Group 2, 3.58 mm; Group 3, 4.08 mm; and Group 4, 4.33 mm on average).
Figure 11. CAL comparison at 0 and six months.
CAL: clinical attachment level, PRP: platelet-rich plasma, EMD: enamel matrix derivative, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane
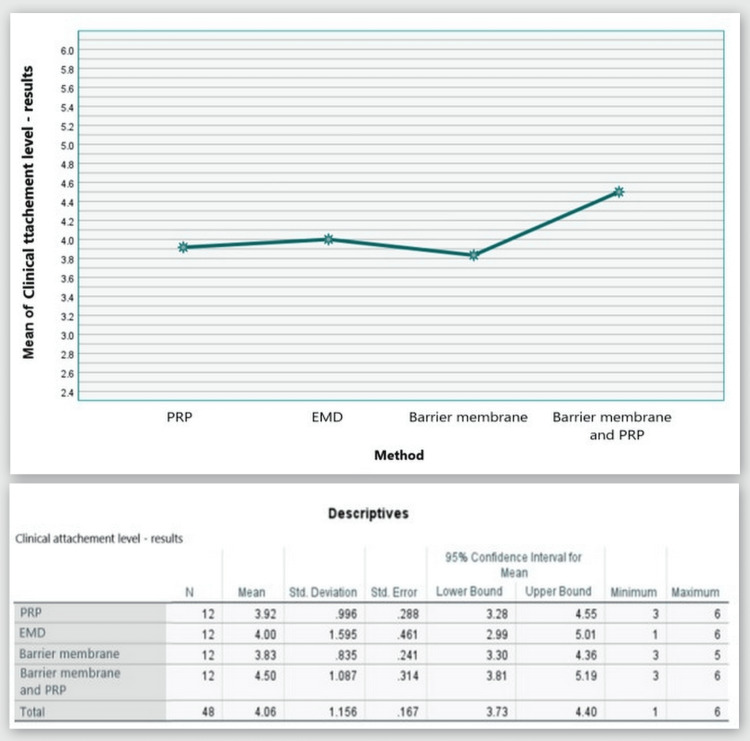
As visible in Figure 12, CAL gain was observed in all four groups with mean values of 3.92, 4.00, 3.83, and 4.50 mm, respectively.
Figure 12. Statistical analysis results and graphics for the CAL.
CAL: clinical attachment level
The ANOVA test demonstrated that the findings for CAL at six months post-regenerative therapy were not statistically significant among all four groups: F(3, 44) = 0.795, p = 0.503 > 0.05 (Appendix C).
А: the distance from the cementoenamel junction (CEJ) to the base of the bone defect (by CBCT)
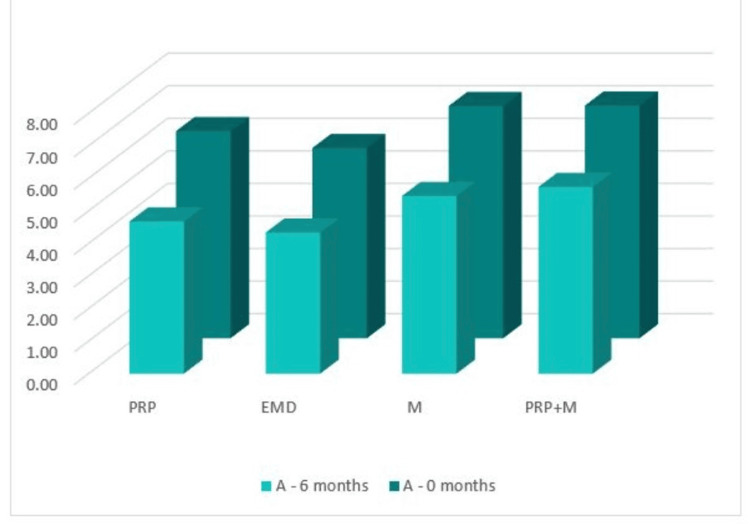
Figure 13 shows that in the CBCT study, the "A" index prior to surgery was 6.37 mm in Group 1, 5.85 mm in Group 2, 7.14 mm in Group 3, and 7.15 mm in Group 4. The "A" index decreased from the starting values six months following regeneration therapy (Group 1 = 4.68 mm; Group 2 = 4.34 mm; Group 3 = 5.46 mm; Group 4 = 5.74 mm). Table 1 details the outcomes.
Figure 13. Parameter A comparison at 0 and six months.
PRP: platelet-rich plasma, EMD: enamel matrix derivative, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane
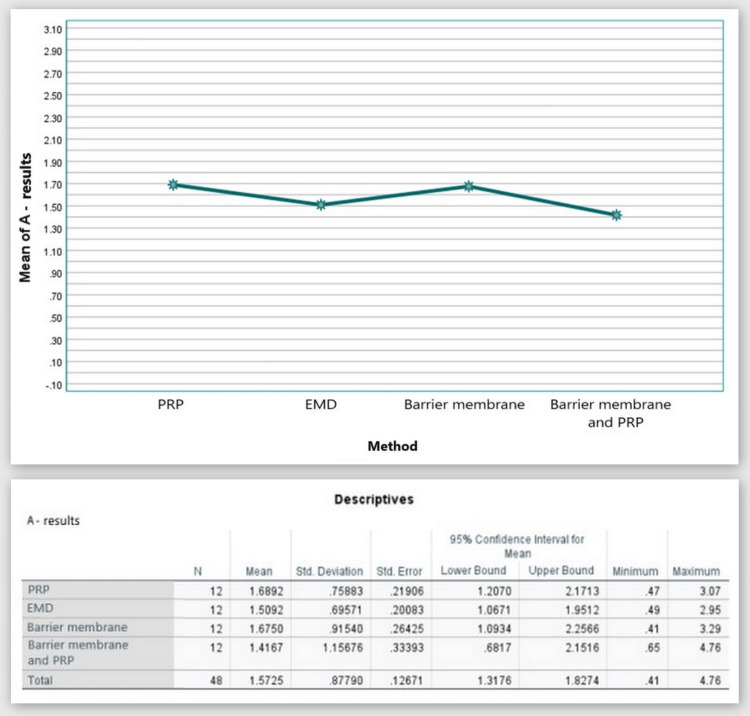
As visible in Figure 14, reduction in the distance from the CEJ to the base of the bone defect was observed in all four groups with mean values over six months of 1.69, 1.51, 1.68, and 2.14 mm, respectively.
Figure 14. Statistical analysis results and graphics for parameter A.
The ANOVA test demonstrated that the findings for index “A” at six months post-regenerative therapy were not statistically significant among all four groups: F(3, 44) = 0.259, p = 0.854 > 0.05 (Appendix D).
В: the distance from the CEJ to the bone crest (on the CBCT)
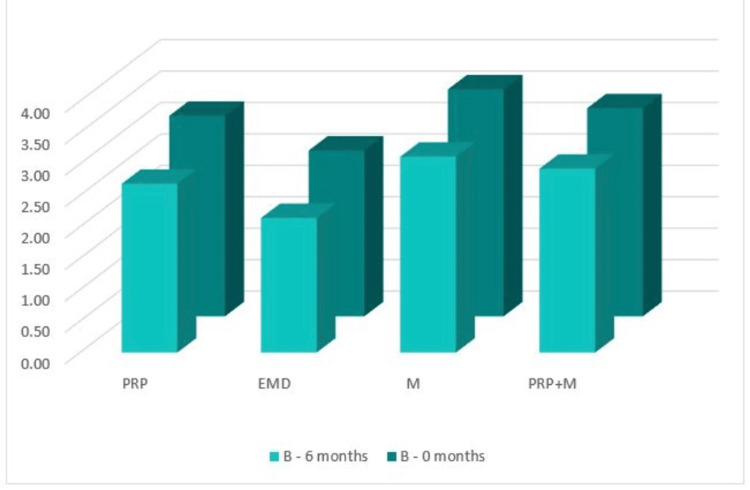
Figure 15 shows that in the CBCT study, the "B" index prior to surgery was 3.19 mm in Group 1, 2.64 mm in Group 2, 3.61 mm in Group 3, and 3.31 mm in Group 4. The "B" index decreased from the starting values six months following regeneration therapy (Group 1 = 2.69 mm; Group 2 = 2.14 mm; Group 3 = 3.12 mm; and Group 4 = 2.93 mm). Table 1 details the outcomes.
Figure 15. Parameter B comparison at 0 and six months.
PRP: platelet-rich plasma, EMD: enamel matrix derivative, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane
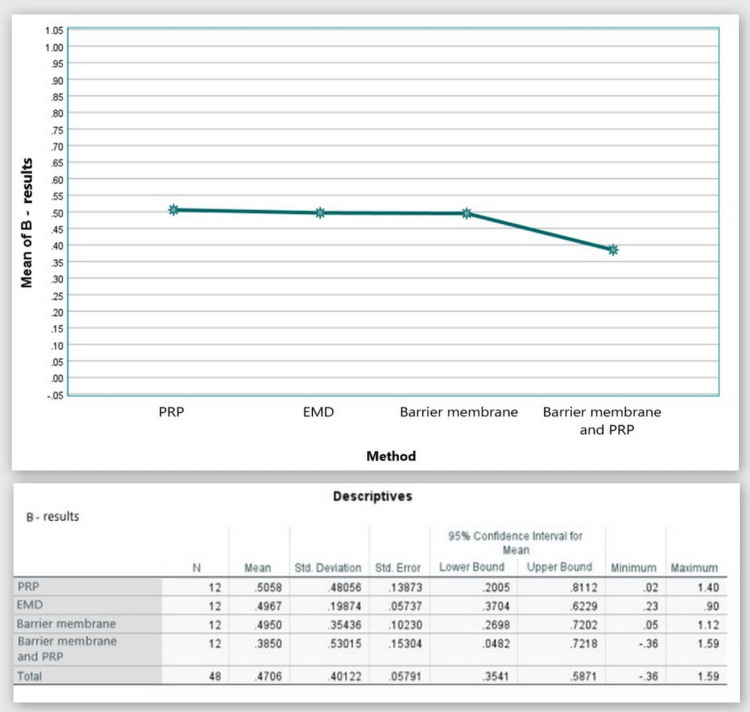
As visible in Figure 16, a reduction in the distance from the CEJ to the bone crest was observed in all four groups with mean values over six months of 0.51 mm, 0.50 mm, 0.50 mm, and 0.39 mm, respectively.
Figure 16. Statistical analysis results and graphics for parameter B.
The ANOVA test demonstrated that the findings for index “B” at six months post-regenerative therapy were not statistically significant among all four groups: F(3, 44) = 0.233, p = 0.873 > 0.05 (Appendix E).
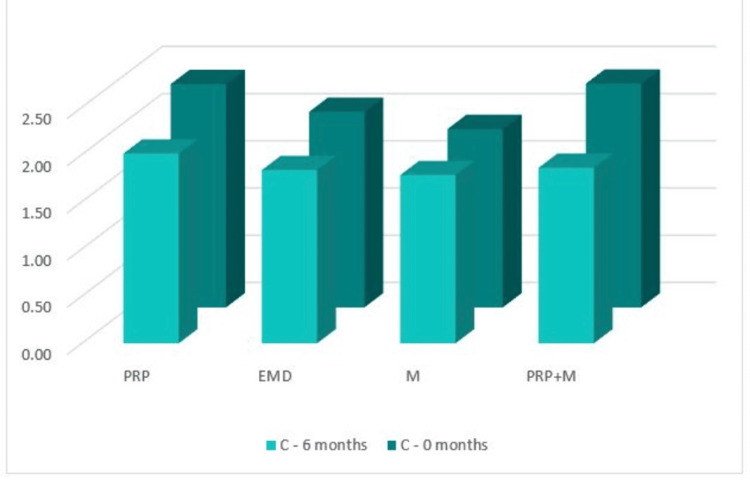
С: width of the bone defect (by CBCT)
Figure 17 shows that in the CBCT study, the "C" index prior to surgery was 2.37 mm in Group 1, 2.074 mm in Group 2, 1.89 mm in Group 3, and 2.37 mm in Group 4. The "C" index decreased from the starting values six months following regeneration therapy (Group 1 = 2.01 mm; Group 2 = 1.84 mm; Group 3 = 1.78 mm, and Group 4 = 1.86 mm). Table 1 details the outcomes.
Figure 17. Parameter C comparison at 0 and six months.
PRP: platelet-rich plasma, EMD: enamel matrix derivative, M: barrier membrane, PRP+M: autogenous platelet-rich plasma and barrier membrane
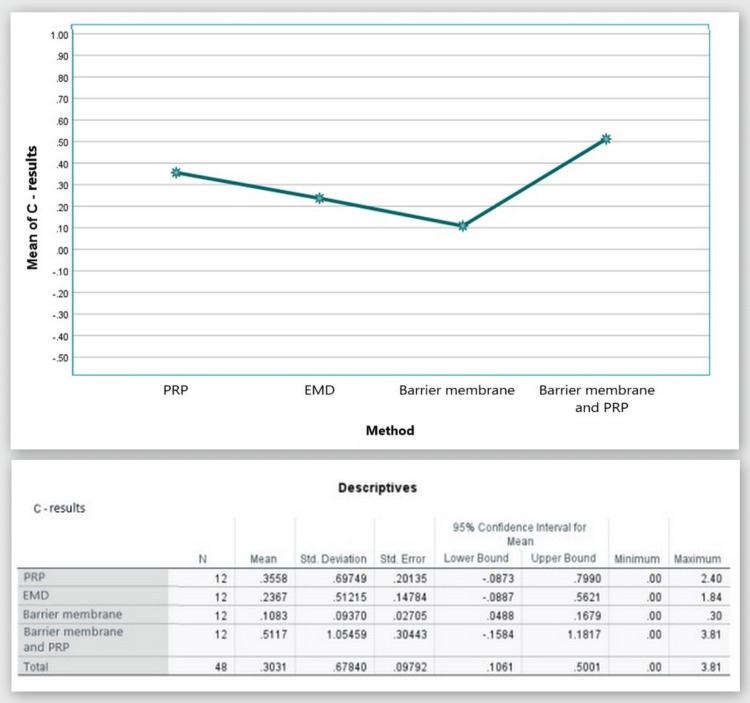
As visible in Figure 18, reduction in the width of the bone defect was observed in all four groups with mean values over six months of 0.36 mm, 0.24 mm, 0.11 mm, and 0.51 mm, respectively.
Figure 18. Statistical analysis results and graphics for parameter C.
The ANOVA test demonstrated that the findings for index “C” at six months post-regenerative therapy were not statistically significant among all four groups: F(3, 44) = 0.758, p = 0.523 > 0.05 (Appendix F).
Discussion
The prevalence of interproximal infraosseous defects was found to increase dramatically with age. In 2012, Eke et al. published their study assessing the incidence, severity, and broadness of periodontal disease in the US aged residents. It appears that periodontitis is a disease that occurs more frequently in men than in women [21]. In contrast to these data, in the present study, a greater number of patients were female.
In 2017, an epidemiological study estimated the prevalence of infraosseous defects in 329 adults by clinical and radiological examinations [22]. The study showed that IBDs predominate in the area of the second molars of the mandible. The outcomes of the present study corroborate those of Najim et al. (2017).
IBDs greatly raise the chance that the condition may worsen. A prompt and precise diagnosis is necessary for this. These days, CBCT is used to corroborate the clinical diagnosis of such problems. CBCT is the preferred imaging technique for capturing our results since it offers numerous benefits over two-dimensional X-ray examinations.
Regenerative therapy of periodontal IBDs results in decreased PPD and CAL gain, resulting in extended tooth life from five to 20 years with good supportive therapy [23,24,25].
The results of the clinical indicators "PPD" and "CAL" in this study are comparable to multiple studies conducted to date, demonstrating that regenerative therapy, regardless of the method used, results in a dramatic reduction in periodontal pocket probing depth and a significant gain in clinical attachment level [26,27,28,29,30].
Regarding the CBCT indicators, we have no basis for comparison at this stage, as these parameters have not been investigated so far.
Numerous surgical methods and materials have been developed over time to help regenerate periodontal bone deficiencies. In these days, the emphasis is on creating better biomaterials (barrier membranes, bone grafts, growth factors, and a combination of the aforementioned) to deliver even better outcomes in regenerative medicine [31,32,33]. This study validates the indisputable properties of some of the biomaterials that we often utilize in our clinic, such as barrier membranes and EMD.
The clinical and radiographic results of the four groups did not differ statistically significantly, according to our current investigation. We can therefore draw the conclusion that materials like PRP and EMD perform just as well as GTR. Here, it is important to make clear that the surgical procedure for using PRP and EMD in periodontal regenerative therapy is far simpler than that of GTR and that postoperative problems are also less common.
Limitations
As a major limitation of this study, it is important to note the characteristics of the bone defects (depth and width) as a possible factor influencing the results. Some of the bone defects were entirely two-walled, others were entirely three-walled, and still others were combined (two-walled and three-walled). This could somewhat influence the final results obtained.
Other limitations could be the short follow-up period of the present study and the rather limited sample of participants included in the study.
As guidelines for future studies, we can identify a larger sample of participants and a longer follow-up period.
Conclusions
Regardless of the regenerative therapy technique used, all patients under examination showed improvements in imaging and clinical markers. The four patient groups' results did not differ in any way that was statistically significant.
Here, it is important to make clear that the surgical procedure for using PRP and EMD in periodontal regenerative therapy is far simpler than that of GTR, and that postoperative problems are also less common.
Concerning the methods of regenerative therapy with EMD and with the independent use of PRP, it should be noted that their surgical protocol is identical, but obtaining the PRP material is a harder procedure, more time-consuming and is associated with possible errors in methodology. The most significant advantage of the PRP method over the EMD method is the more affordable cost.
Appendices
Appendix A
Table 2. ANOVA test with Tukey's HSD for PPD.
PPD: probing pocket depth, EMD: enamel matrix derivative, ANOVA: analysis of variance, HSD: honestly significant difference, PRP: platelet-rich plasma
| Dependent variable | PPD | Mean | 95% confidence interval | |||
| (1) Method | (J) Method | Difference (I-J) | Std. error | Sig. | Lower bound | Upper bound |
| PRP | EMD | -667 | .351 | 244 | -1.60 | .27 |
| Barrier membrane | -333 | .351 | 779 | -1.27 | .60 | |
| Barrier membrane and PRP | -417 | .351 | .639 | -1.35 | .52 | |
| EMD | PRP | 667 | .351 | 244 | -.27 | 1.60 |
| Barrier membrane | .333 | .351 | 779 | -.60 | 1.27 | |
| Barrier membrane and PRP | 250 | 351 | 892 | -.69 | 1.19 | |
| Barrier membrane | PRP | 333 | 351 | 779 | -.60 | 1.27 |
| EMD | -333 | 351 | 779 | -1.27 | .60 | |
| Barrier membrane and PRP | -083 | 351 | 995 | -1.02 | 85 | |
| Barrier membrane and PRP | PRP | 417 | 351 | 639 | -.52 | 1.35 |
| EMD | -250 | 351 | 892 | -1.19 | 69 | |
| Barrier membrane | .083 | 351 | 995 | -.85 | 1.02 | |
Appendix B
Table 3. ANOVA test with Tukey's HSD for GML.
GML: gingival margin level, EMD: enamel matrix derivative, ANOVA: analysis of variance, HSD: honestly significant difference, PRP: platelet-rich plasma
| Dependent variable | GML | Mean | 95% confidence interval | |||
| (1) Method | (J) Method | Difference (I-J) | Std. Error | Sig. | Lower bound | Upper bound |
| PRP | EMD | 583 | .353 | .360 | -.36 | 1.52 |
| Barrier membrane | 417 | .353 | .642 | -.52 | 1.36 | |
| Barrier membrane and PRP | -.167 | .353 | .965 | -1.11 | .77 | |
| EMD | PRP | -.583 | .353 | .360 | -1.52 | .36 |
| Barrier membrane | -.167 | 353 | 965 | -1.11 | .77 | |
| Barrier membrane and PRP | -750 | .353 | .161 | -1.69 | .19 | |
| Barrier membrane | PRP | -417 | 353 | 642 | -1.36 | .52 |
| EMD | 167 | 353 | 965 | -77 | 1.11 | |
| Barrier membrane and PRP | -,583 | 353 | 360 | -1.52 | 36 | |
| Barrier membrane and PRP | PRP | 167 | 353 | 965 | -77 | 1.11 |
| EMD | 750 | 353 | .161 | -19 | 1.69 | |
| Barrier membrane | 583 | 353 | 360 | -.36 | 1.52 | |
Appendix C
Table 4. ANOVA test with Tukey's HSD for CAL.
CAL: clinical attachment level, EMD: enamel matrix derivative, ANOVA: analysis of variance, HSD: honestly significant difference, PRP: platelet-rich plasma
| Dependent variable | CAL | Mean | 95% confidence interval | |||
| (1) Method | (J) Method | Difference (I-J) | Std. Error | Sig. | Lower bound | Upper bound |
| PRP | EMD | -083 | 475 | 998 | -1.35 | 1.19 |
| Barrier membrane | .083 | 475 | 998 | -1.19 | 1.35 | |
| Barrier membrane and PRP | -583 | 475 | 613 | -1.85 | .69 | |
| EMD | PRP | .083 | 475 | 998 | -1.19 | 1.35 |
| Barrier membrane | .167 | 475 | 985 | -1.10 | 1.44 | |
| Barrier membrane and PRP | -.500 | 475 | .720 | -1.77 | .77 | |
| Barrier membrane | PRP | -.083 | .475 | .998 | -1.35 | 1.19 |
| EMD | -.167 | .475 | .985 | -1.44 | 1.10 | |
| Barrier membrane and PRP | -.667 | .475 | .504 | -1.94 | .60 | |
| Barrier membrane and PRP | PRP | 583 | .475 | .613 | -69 | 1.85 |
| EMD | .500 | .475 | .720 | -77 | 1.77 | |
| Barrier membrane | .667 | 475 | .504 | -.60 | 1.94 | |
Appendix D
Table 5. ANOVA test with Tukey's HSD for Parameter A.
EMD: enamel matrix derivative, ANOVA: analysis of variance, HSD: honestly significant difference, PRP: platelet-rich plasma
| Dependent variable | Parameter A | Mean | 95% Confidence interval | |||
| (1) Method | (J) Method | Difference (I-J) | Std. error | Sig. | Lower bound | Upper bound |
| PRP | EMD | .18000 | 36719 | 961 | -8004 | 1.1604 |
| Barrier membrane | 01417 | 36719 | 1.000 | -9662 | 9946 | |
| Barrier membrane and PRP | 27250 | 36719 | 880 | -7079 | 1.2529 | |
| EMD | PRP | -18000 | 36719 | 961 | -1.1604 | 8004 |
| Barrier membrane | -16583 | 36719 | 969 | -1.1462 | 8146 | |
| Barrier membrane and PRP | 09250 | 36719 | 994 | -8879 | 1.0729 | |
| Barrier membrane | PRP | -01417 | 36719 | 1.000 | -9946 | 9662 |
| EMD | .16583 | 36719 | 969 | -8146 | 1.1462 | |
| Barrier membrane and PRP | 25833 | 36719 | 895 | -7221 | 1.2387 | |
| Barrier membrane and PRP | PRP | -27250 | 36719 | 880 | -1.2529 | 7079 |
| EMD | -09250 | 36719 | 994 | -1.0729 | 8879 | |
| Barrier membrane | -25833 | 36719 | 895 | -1.2387 | 7221 | |
Appendix E
Table 6. ANOVA test with Tukey's HSD for parameter B.
EMD: enamel matrix derivative, ANOVA: analysis of variance, HSD: honestly significant difference, PRP: platelet-rich plasma
| Dependent variable | Parameter B | Mean | 95% confidence interval | |||
| (1) Method | (J) Method | Difference (I-J) | Std. error | Sig. | Lower bound | Upper bound |
| PRP | EMD | 00917 | 16796 | 1.000 | -4393 | 4576 |
| Barrier membrane | 01083 | .16796 | 1.000 | -4376 | 4593 | |
| Barrier membrane and PRP | 12083 | .16796 | 889 | -3276 | 5693 | |
| EMD | PRP | -00917 | .16796 | 1.000 | -4576 | 4393 |
| Barrier membrane | 00167 | .16796 | 1.000 | -4468 | .4501 | |
| Barrier membrane and PRP | 11167 | 16796 | 910 | -3368 | 5601 | |
| Barrier membrane | PRP | -01083 | 16796 | 1.000 | -4593 | 4376 |
| EMD | -00167 | 16796 | 1.000 | -4501 | 4468 | |
| Barrier membrane and PRP | 11000 | 16796 | 913 | -3385 | 5585 | |
| Barrier membrane and PRP | PRP | -12083 | 16796 | 889 | -5693 | 3276 |
| EMD | -11167 | 16796 | 910 | -5601 | 3368 | |
| Barrier membrane | -11000 | 16796 | 913 | -5585 | 3385 | |
Appendix F
Table 7. ANOVA test with Tukey's HSD for parameter C.
EMD: enamel matrix derivative, ANOVA: analysis of variance, HSD: honestly significant difference, PRP: platelet-rich plasma
| Dependent variable | Parameter C | Mean | 95% confidence interval | |||
| (1) Method | (J) Method | Difference (I-J) | Std. error | Sig. | Lower bound | Upper bound |
| PRP | EMD | 11917 | 27912 | 974 | -6261 | 8644 |
| Barrier membrane | 24750 | 27912 | 812 | -4977 | 9927 | |
| Barrier membrane and PRP | -15583 | 27912 | 944 | -9011 | 5894 | |
| EMD | PRP | -11917 | 27912 | 974 | -8644 | 6261 |
| Barrier membrane | 12833 | 27912 | 967 | -6169 | 8736 | |
| Barrier membrane and PRP | -27500 | 27912 | 759 | -1.0202 | 4702 | |
| Barrier membrane | PRP | -24750 | 27912 | 812 | -9927 | 4977 |
| EMD | -12833 | 27912 | 967 | -8736 | 6169 | |
| Barrier membrane and PRP | -40333 | 27912 | 479 | -1.1486 | 3419 | |
| Barrier membrane and PRP | PRP | 15583 | 27912 | 944 | -5894 | 9011 |
| EMD | 27500 | 27912 | 759 | -4702 | 1.0202 | |
| Barrier membrane | 40333 | 27912 | 479 | -3419 | 1.1486 | |
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Commission on Ethics of Research at Medical University – Varna issued approval 118/23.06.22. This trial was registered (NCT06639555) at Clinicaltrials.gov.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Tsvetalina Gerova-Vatsova
Acquisition, analysis, or interpretation of data: Tsvetalina Gerova-Vatsova
Drafting of the manuscript: Tsvetalina Gerova-Vatsova
Critical review of the manuscript for important intellectual content: Tsvetalina Gerova-Vatsova
References
- 1.Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. J Am Dent Assoc. 2018;149:576–588. doi: 10.1016/j.adaj.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correlation between oral health and some common mucocutaneous conditions. Yotsova R, Stoyanova Z. Scr Sci Med Dentalis. 2022;8:41–49. [Google Scholar]
- 3.Periodontal regeneration. Wang HL, Greenwell H, Fiorellini J, et al. J Periodontol. 2005;76:1601–1622. doi: 10.1902/jop.2005.76.9.1601. [DOI] [PubMed] [Google Scholar]
- 4.Imaging methods in periodontology. Mol A. Periodontol 2000. 2004;34:34–48. doi: 10.1046/j.0906-6713.2003.003423.x. [DOI] [PubMed] [Google Scholar]
- 5.The infrabony pocket: classification and treatment. Goldman HM, Cohen DW. J Periodontol. 1958;1:272–291. [Google Scholar]
- 6.Prevalence and distribution of bone defects in moderate and advanced adult periodontitis. Vrotsos JA, Parashis AO, Theofanatos GD, Smulow JB. J Clin Periodontol. 1999;26:44–48. doi: 10.1034/j.1600-051x.1999.260108.x. [DOI] [PubMed] [Google Scholar]
- 7.Accuracy of cone beam computed tomography for periodontal defect measurements. Misch KA, Yi ES, Sarment DP. J Periodontol. 2006;77:1261–1266. doi: 10.1902/jop.2006.050367. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic validity (or acuity) of 2D CCD versus 3D CBCT-images for assessing periodontal breakdown. Vandenberghe B, Jacobs R, Yang J. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:395–401. doi: 10.1016/j.tripleo.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Bone histomorphometry as a diagnostic tool. A review article. Yotsova R, Peev S, Ivanova N. Scr Sci Med Dent. 2023;9:7–23. [Google Scholar]
- 10.A modified protocol for staining of undecalcified bone samples using toluidine blue—a histological study in rabbit models. Peev S, Parushev I, Yotsova R. Applied Sciences. 2024;14:461. [Google Scholar]
- 11.Histomorphometric analysis of osseointegrated intraosseous dental implants using undecalcified specimens: a scoping review. Peev S, Yotsova R, Parushev I. Biomimetics. 2024;9:672. doi: 10.3390/biomimetics9110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: a systematic review and meta-analysis. Nibali L, Koidou VP, Nieri M, Barbato L, Pagliaro U, Cairo F. J Clin Periodontol. 2020;47 Suppl 22:320–351. doi: 10.1111/jcpe.13237. [DOI] [PubMed] [Google Scholar]
- 13.Biological properties and medical applications of carbonate apatite: a systematic review. Yotsova R, Peev S. Pharmaceutics. 2024;16 doi: 10.3390/pharmaceutics16020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bone grafts in periodontics. Balaji V R, Manikandan D, Ramsundar A. Matrix Sci Med. 2020;4:57–63. [Google Scholar]
- 15.Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: a review. Sheikh Z, Hamdan N, Ikeda Y, Grynpas M, Ganss B, Glogauer M. Biomater Res. 2017;21:9. doi: 10.1186/s40824-017-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitosan as a barrier membrane material in periodontal tissue regeneration. Xu C, Lei C, Meng L, Wang C, Song Y. J Biomed Mater Res B Appl Biomater. 2012;100:1435–1443. doi: 10.1002/jbm.b.32662. [DOI] [PubMed] [Google Scholar]
- 17.Carbonate apatite as a bone substitute material. A review. Yotsova R, Peev S. Scr Sci Med Dent. 2023;9:33–42. [Google Scholar]
- 18.Enamel matrix protein derivatives: role in periodontal regeneration. Rathva VJ. Clin Cosmet Investig Dent. 2011;3:79–92. doi: 10.2147/CCIDEN.S25347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healing of two and three wall intrabony periodontal defects following treatment with an enamel matrix derivative combined with autogenous bone. Yilmaz S, Cakar G, Yildirim B, Sculean A. J Clin Periodontol. 2010;37:544–550. doi: 10.1111/j.1600-051X.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 20.Evaluating change of marginal bone height with cone-beam computed tomography following surgical treatment with guided tissue regeneration (bone grafting) or access flap alone: a retrospective study. Lee IK, Choi HS, Jeong SH, Lee JT. Medicina (Kaunas) 2021;57 doi: 10.3390/medicina57090869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevalence of periodontitis in adults in the United States: 2009 and 2010. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 22.Prevalence of intrabony defects in a Swedish adult population. A radiographic epidemiological study. Najim U, Norderyd O. Acta Odontol Scand. 2017;75:123–129. doi: 10.1080/00016357.2016.1265665. [DOI] [PubMed] [Google Scholar]
- 23.Infrabony defects 20 years after open flap debridement and guided tissue regeneration. Petsos H, Ratka-Krüger P, Neukranz E, Raetzke P, Eickholz P, Nickles K. J Clin Periodontol. 2019;46:552–563. doi: 10.1111/jcpe.13110. [DOI] [PubMed] [Google Scholar]
- 24.Medium- and long-term clinical benefits of periodontal regenerative/reconstructive procedures in intrabony defects: systematic review and network meta-analysis of randomized controlled clinical studies. Stavropoulos A, Bertl K, Spineli LM, Sculean A, Cortellini P, Tonetti M. J Clin Periodontol. 2021;48:410–430. doi: 10.1111/jcpe.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Periodontal regeneration: is it still a goal in clinical periodontology? Cirelli JA, Fiorini T, Moreira CH, Molon RS, Dutra TP, Sallum EA. Braz Oral Res. 2021;35:0. doi: 10.1590/1807-3107bor-2021.vol35.0097. [DOI] [PubMed] [Google Scholar]
- 26.Effect of platelet-rich plasma on the healing of intra-bony defects treated with a natural bone mineral and a collagen membrane. Döri F, Huszár T, Nikolidakis D, Arweiler NB, Gera I, Sculean A. J Clin Periodontol. 2007;34:254–261. doi: 10.1111/j.1600-051X.2006.01044.x. [DOI] [PubMed] [Google Scholar]
- 27.Effect of platelet-rich plasma on the healing of intrabony defects treated with Beta tricalcium phosphate and expanded polytetrafluoroethylene membranes. Döri F, Huszár T, Nikolidakis D, et al. J Periodontol. 2008;79:660–669. doi: 10.1902/jop.2008.070473. [DOI] [PubMed] [Google Scholar]
- 28.Treatment of non-contained infrabony defects with enamel matrix derivative alone or in combination with biphasic calcium phosphate bone graft: a 12-month randomized controlled clinical trial. Losada M, González R, Garcia ÀP, Santos A, Nart J. J Periodontol. 2017;88:426–435. doi: 10.1902/jop.2016.160459. [DOI] [PubMed] [Google Scholar]
- 29.Twenty years of enamel matrix derivative: the past, the present and the future. Miron RJ, Sculean A, Cochran DL, et al. J Clin Periodontol. 2016;43:668–683. doi: 10.1111/jcpe.12546. [DOI] [PubMed] [Google Scholar]
- 30.Combined use of platelet-rich plasma and bone grafting with or without guided tissue regeneration in the treatment of anterior interproximal defects. Yassibag-Berkman Z, Tuncer O, Subasioglu T, Kantarci A. J Periodontol. 2007;78:801–809. doi: 10.1902/jop.2007.060318. [DOI] [PubMed] [Google Scholar]
- 31.Application of platelet-rich plasma for alveolar ridge preservation. A review article. Yotsova R, Peev S, Kolarov R. SScr Sci Med Dent. 2022;8:18–37. [Google Scholar]
- 32.Socket preservation using dense polytetrafluoroethylene membranes and platelet-rich plasma. Yotsova R. Cureus. 2024;16 doi: 10.7759/cureus.72265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alveolar ridge preservation using dense polytetrafluoroethylene membranes. A review article. Yotsova R, Peev S, Georgiev T. Scr Sci Med Dent. 2021;7:31–38. [Google Scholar]