Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) refers to the liver pathological changes caused by excessive fat accumulation in hepatocytes owing to various reasons, which has become an emerging health challenge. Erhong Jiangzhi Decoction (EHJD) is a traditional Chinese medicine decoction. This study aims to investigate the therapeutic effect of EHJD on NAFLD.
Methods
NAFLD model was constructed by high-fat diet (HFD)-induced mice and oleic acid-induced HepG2 cells. Mice were intragastrically administered with EHJD and HepG2 cells were treated with EHJD drug-containing serum. The effects of EHJD on NAFLD were explored in vivo and in vitro. Histological assessment was performed by hematoxylin-eosin and oil red O staining. ELISA was exploited to detect the expression of lipid accumulation, liver function, inflammation, and oxidative stress related indicators. The expression of Nrf2/HO-1 pathway was detected by qRT-PCR and Western blot.
Results
In HFD-induced NAFLD mice, the body weight was increased, and liver/weight, inguinal fat/weight, and epididymal fat/weight were higher, while EHJD reduced them. Staining results exhibited that EHJD decreased inflammatory cell infiltration and oil red lipid droplets in HFD-induced mice. In addition, EHJD treatment suppressed TC, TG, ALT and AST levels; TNF-α, IL-1β, IL-6 and MDA levels were inhibited by EHJD, while GSH-Px, CAT and T-AOC levels were increased in NAFLD through the in vivo and in vitro experiments. The suppression of Nrf2 weakened the inhibitory effect of EHJD on lipid metabolism, liver injury, inflammation and oxidative stress.
Conclusion
EHJD had a protective effect on NAFLD by alleviating lipid accumulation and liver injury, inhibiting inflammation, and oxidative stress, which was achieved by the restoration of Nrf2.
Keywords: Erhong Jiangzhi Decoction, nonalcoholic fatty liver disease, lipid accumulation, Nrf2/HO-1 signaling pathway, oxidative stress
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease that is intimately correlated with type II diabetes and obesity, and has become an emerging health challenge.1 Globally the prevalence of NAFLD is gradually raising, and by 2020, the global prevalence rate is up to 25%.2,3 The disease has a high probability of being accompanied by metabolic syndrome,4 and is capable of progressing to nonalcoholic steatohepatitis (NASH).5 Although progress has been made in the study of the complex pathogenesis of the disease, there are still major challenges. The ongoing search and development of new drugs remains a current priority.
Liver is a crucial organ for lipid metabolism, which acts as a central regulator of lipid homeostasis.6 Therefore, regulation of lipid metabolic homeostasis is an effective means to alleviate NAFLD. It has been reported that some potential targets can regulate lipid accumulation to improve NAFLD. LB100, a serine/threonine protein phosphatase 2A inhibitor, regulates lipogenesis and fatty acid oxidation in the liver through the AMPK/Sirt1 pathway to ameliorate hepatic steatosis,7 which may be a possible therapeutic agent for NAFLD. Deficiency of P2Y2R ameliorates hepatic steatosis by enhancing fatty acid β-oxidation via AMPK signaling and PGC-1α pathway.8 In NAFLD, Allyl isothiocyanate suppresses the NF-κB pathway and promotes the Sirt1/AMPK pathway to ameliorate inflammation and hepatic steatosis through promoting.9
Some natural active substances have been proven to have a positive effect on the improvement of NAFLD. Bioactive proteins and antioxidant peptides extracted from Litsea cubeba fruit meal can improve high-fat diet (HFD)-induced NAFLD by regulating lipid metabolism, oxidative stress, and inflammatory responses.10 Pterostilbene (PTE), an active compound extracted from blueberries and grapes, alleviates oxidative stress damage induced by excessive lipid accumulation in hepatocytes through the AMPK/mTOR signaling pathway and by promoting Nrf2 autophagy, thereby improving NAFLD.11 Cordycepin exerts significant protective effects on hepatic steatosis and liver injury in NASH mice by activating the AMPK signaling pathway.12 Additionally, traditional Chinese medicine (TCM) has been shown to be greatly effective in NAFLD. For centuries, TCM has been extensively applied in Asia for the therapy of liver diseases.13 Erhong Jiangzhi Decoction (EHJD) is a TCM, and its main effective components include red yeast rice and Rhodiola rosea L. Red yeast rice has been reported to be effective in treating high cholesterol and dyslipidemia and reducing cardiovascular risk.14,15 In NAFLD mice, red yeast rice improves disease by inhibiting lipid synthesis and NF-κB/NLRP3-mediated liver inflammation.16 Salidroside, a major glycoside extracted from Rhodiola rosea L., is proved to suppress oxidative stress and ameliorate abnormal lipid metabolism to improve NAFLD/NASH.17 However, the specific mechanism of EHJD on NAFLD treatment has not been fully understood.
NF-E2-related factor 2 (Nrf2) is capable to regulate downstream anti-oxidative stress genes like heme oxygenase (HO-1), affecting metabolic and anti-oxidant defenses.18,19 Normally, Nrf2 can be ubiquitinated by Kelch-like ECH-related protein 1 (Keap1), leading to proteasomal degradation.20,21 When encountering oxidative stress, Nrf2 activates the transcription of antioxidant response element.22 In this study, investigate the effects of EHJD on NAFLD by constructing mouse and cell models, and the effects of EHJD on Nrf2 pathway were also explored. Our study aims to scientifically validate the efficacy of EHJD and elucidate the underlying mechanisms by which it exerts its beneficial effects on NAFLD. This validation not only bridges the gap between traditional medicine and modern scientific research but also offers a novel therapeutic approach that could be integrated into contemporary medical practice, potentially paving the way for the development of new, effective, and safe treatments for NAFLD based on natural compounds.
Materials and Methods
Materials and Reagents
The normal diet (18% calories from fat) was obtained from Trophic Animal Feed High-tech Co., Ltd. (Nantong, China). The herbal granules of EHJD were obtained from the First Affiliated Hospital of Hainan Medical University. The HFD (60% calories from fat) was supplied by HFK Bioscience (Beijing, China). Primary antibodies, including anti-Nrf2 (ab62352), anti-HO-1 (ab90492), and anti-GAPDH (ab9485), goat anti-rabbit IgG secondary antibody (ab205718), were bought from Abcam (Cambridge, UK). SYBR Green Master Mix and Pierce BCA Protein Assay Kit were purchased from Ther mo Fisher Scientific (Waltham, MA, USA). Hiscript II QRT Supermix kit was obtained from Vazyme (Nanjing, China). Hematoxylin, eosin, oil red O solution, and oleic acid (OA) were purchased from Sigma-Aldrich (Shanghai, China). HepG2 cell line was obtained from iCell (Shanghai, China). ELISA kits for measuring levels of TC, TG, LDC-C, HDL-C, AST, ALT, IL-6, IL-1β, and TNF-α, MDA, GSH-Px, CAT, and T-AOC were bought from Esebio (Shanghai, China).
Establishment of NAFLD Mice Model
C57BL/6 male mice (6–8 weeks, 18–20g, SPF Biotechnology Co., Ltd, Beijing, China) were housed under pathogen-free conditions at 23 ± 2°C with a 12-h/12-h light/dark cycle, and they had free access to eat and drink. Mice were randomly grouped into Control, HFD, L-EHJD (low-dose EHJD, 10 mL/kg/d) and H-EHJD (high-dose EHJD, 15 mL/kg/d) group (n=6). The Control group was fed with normal diet, the HFD group was fed with HFD, and H-EHJD and L-EHJD groups were fed with HFD and intragastrically administered with EHJD (two red lipid-lowering Chinese medicine 3g powder was dissolved in 15.6 mL of boiling water and used after cooling). The main effective components included red yeast rice and Rhodiola rosea L, supplemented by Lotus Leaves, Alisma orientale, and Salvia miltiorrhiza. The body weight was weighed every 4 days. Four weeks later, the liver, heart, inguinal fat, and epididymal fat of mice were weighed. The liver tissues and serum were taken for subsequent experiments.
Preparation of EHJD Medicated Serum
After adaptive feeding for a week, SD male rats (220g-230g, SPF Biotechnology Co., Ltd) were separated into Control and EHJD group (n=5) at random. In the Control group, rats were given 0.9% normal saline by gavage, and rats in the EHJD group were given 15 mL/kg EHJD by gavage. After continuous administration for 7 days, rats were anesthetized by isoflurane inhalation and sacrificed by cervical dislocation, and blood was obtained from the heart. The separated serum of 5 rats was mixed and inactivated at 56°C for 30 min. Afterwards, the filtered serum was stored at −80°C for cell culture.
In this study, all animal experiments confirmed to the Guide for the Care and Use of Laboratory Animals, and has been approved by the Ethics Committee of Hainan Medical University (HYLL-2021-134).
Cell Culture
HepG2 cells were inoculated and cultured in high-glucose DMEM containing 10% FBS and 1% penicillin-streptomycin solution, and placed in a 5% CO2 incubator at 37°C. Cells were treated with OA23 and different concentrations of EHJD drug containing serum to investigate the effects of EHJD in vitro. Cells were grouped into Control (intervened with normal serum), OA (intervened with 660 μmol/L OA and normal serum), OA+10% drug serum and OA+20% drug serum group. Afterwards, in order to verify the effects of Nrf2, siRNAs targeting Nrf2, including si-Nrf2-1, si-Nrf2-2 and si-Nrf2-3, and corresponding negative control (si-NC) were synthesized from Ribobio (Guangzhou, China). HepG2 cells in each group were transfected with siRNAs for 48 h using Lipofectamine 3000 (Thermo Fisher Scientific).
Hematoxylin–Eosin (HE) and Oli Red O Staining
In vivo, the liver tissues of mice were fixed in 4% paraformaldehyde solution and finally embedded in paraffin. Then the tissues were cut into 4 μm sections for HE staining. The dried liver tissue sections were immersed sequentially in xylene and gradient ethanol solutions. Afterwards, the slices were stained with hematoxylin and eosin and then dehydrated with gradient ethanol.24 At last, the slices were sealed with neutral balsam and examined by a microscope.
Oil red O was applied to stain frozen liver sections to assess lipid droplets. In vitro, HepG2 cells in each group were washed and fixed. Subsequently, they were stained with oil red O for 30 min, and washed with 60% isopropanol and PBS in turn.25 Finally, stained sections and cells were observed using an optical microscope. The area percentage of oil red in cells were quantified by Image J.
Enzyme Linked Immunosorbent Assay (ELISA)
Following the manufacturer’s instructions, blood lipids (TC, TG, LDC-C, and HDL-C) and liver function related indicators (AST, ALT) in serum and cells were measured by ELISA kits, as well as inflammation (IL-6, IL-1β, and TNF-α) and oxidative stress indicators (MDA, GSH-Px, CAT, and T-AOC) in liver tissues and cells. Briefly, 50 μL of diluted sample was added to each well in a 96-well plate. Subsequently, the samples were incubated with 100 μL of horseradish peroxidase (HRP)-labeled antibody for 60 min at 37°C. Within 15 min after incubation, the OD value was measured at 450 nm by a microplate reader.
Cell Counting Kit-8 (CCK-8) Assay
HepG2 cells (1.5×104/well) were seeded in 96-well plates and incubated for 24 h. Subsequently, CCK-8 assay was performed to evaluate proliferation of HepG2 cells in each group. The cells were incubated with 10% CCK-8 solution in the dark at 37°C for 2 h, and the absorbance of the sample was measured at 450 nm by a microplate reader.
Real-Time Fluorescence Quantitative PCR (RT-qPCR)
The total RNA of the cells was extracted with TRIzol, and the concentration of RNA was determined by UV spectrophotometer. The cDNA was synthesized using the Hiscript II QRT Supermix kit. SYBR Green Master Mix was applied to run RT-qPCR. The procedure was: 95°C for 30s, 40 cycles of (92°C for 10s, 60°C for 30s, and 72°C for 10s). GAPDH was set as an internal inference. The primer sequence was presented in Table 1.
Table 1.
Primer Sequences for RT-qPCR
| Name | Sequence (5′–3′) |
|---|---|
| GAPDH-F | CCGGGAAACTGTGGCGTGATGG |
| GAPDH-R | AGGTGGAGGAGTGGGTGTCGCTGTT |
| HO-1-F | CACGCATATACCCGCTACCT |
| HO-1-R | CCAGAGTGTTCATTCGAGCA |
| Nrf2-F | GTCCCAGCAGAGTGATGGTT |
| Nrf2-R | TCACACACTTTCTGCGTGCT |
Western Blot (WB)
The procedure was carried out according to the reported methods.10 Cells/tissues were treated with RIPA to isolate total protein samples. Concentration of proteins was measured by Pierce BCA Protein Assay Kit. Proteins were separated using a 10% SDS-PAGE gel and then transferred to a PVDF membrane. After being sealed in 5% skimmed milk, the membrane was then incubated with primary antibodies at 4°C for a night. Afterwards, it was incubated with the corresponding secondary antibody for 1 h at room temperature. Each experiment was repeated three times. On the Tanon 5200 Chemiluminescent Imaging System incubation plate, ECL solution was used to developing, and the image was exposed and captured.
Statistical Analysis
Data were analyzed by GraphPad Prism 7.0, and expressed as mean ± standard deviation. One-way ANOVA and Tukey’s test were used for comparisons among multiple groups. P < 0.05 indicated a statistically significant difference.
Results
Effects of EHJD on Body Indexes and Liver Histopathology in HFD-Induced Mice
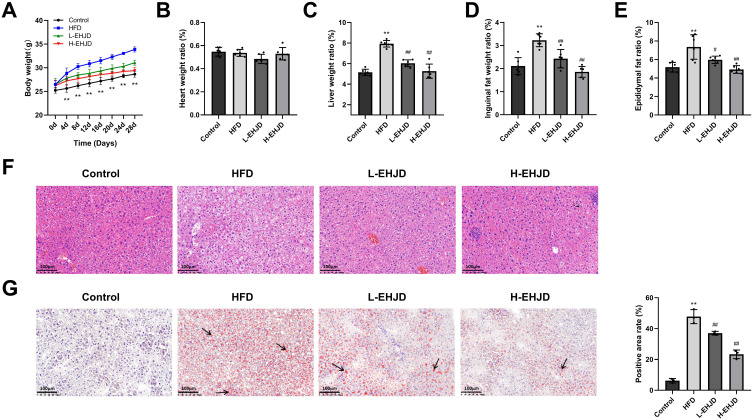
In the HFD-induced NAFLD model group, the body weight of mice was significantly higher than that in the Control group on the 4–28 d. L-EHJD and H-EHJD significantly reduced the body weight of HFD-induced NAFLD mice on the 8–28 d (Figure 1A). There was no significant difference in heart/weight among the four groups of mice (Figure 1B). Higher liver/weight, inguinal fat/weight, and epididymal fat/weight were significantly shown in the HFD group than those in the Control group. L-EHJD and H-EHJD significantly decreased liver/body weight, inguinal fat/weight, and epididymal fat/weight of HFD-induced NAFLD mice (Figure 1C–E).
Figure 1.
Effects of Erhong Jiangzhi Decoction (EHJD) on body weight, related organ indexes and liver histopathology of NAFLD model mice induced by high-fat diet (HFD) (n=6). (A) Changes in body weight of mice. **P<0.01 vs 0d. (B–E) Organ (liver, heart) and adipose tissue (epididymal fat, inguinal fat) weight ratio. (F) Hematoxylin-eosin staining of liver tissues (× 200, 100 μm). (G). Oil red O staining of liver tissues (× 200, 100 μm). Black arrows indicate oil-red droplets. **P<0.01 vs Control #P<0.05 ## P<0.01 vs NAFLD.
HE staining revealed that in the Control group, the liver tissue structure and cell level of mice were clear. Cells were arranged neatly and orderly, and no interstitial edema and inflammatory cell infiltration were observed. Compared with the Control group, there was the interstitial edema in the HFD group, and inflammatory cells were significantly increased, with the slightly disordered cell arrangement and the concentrated and solidified nucleus. In comparison with the HFD group, neatly arranged cells and less inflammatory cells infiltration were observed in the L-EHJD and H-EHJD group (Figure 1F).
Oil red O staining indicated a clear tissue structure and cell level in the Control group, and no oil red lipid droplets were observed. Compared with the Control group, the lipid droplets in the HFD group were obvious and showed oil red. Compared with the HFD group, oil red lipid drops reduced in L-EHJD group and significantly decreased in H-EHJD group, with a lower positive area rate (Figure 1G).
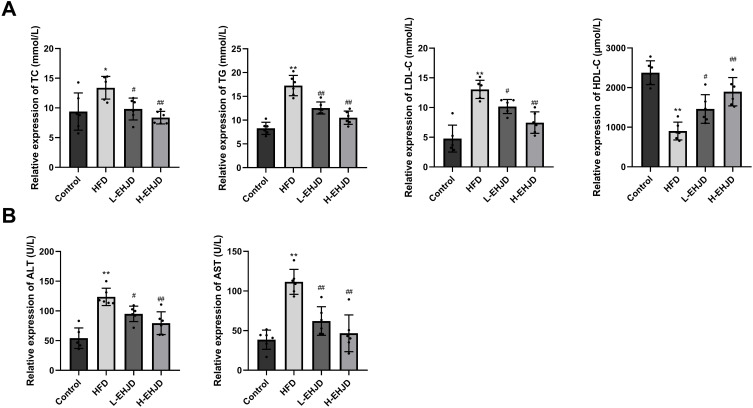
EHJD Alleviated Lipid Accumulation and Liver Injury in HFD-Induced Mice
Contrast with the Control group, the levels of TC, TG, and LDL-C were significantly increased in the HFD group, as well as the decreased HDL-C. Compared with the HFD group, TC, TG, LDL-C levels were significantly decreased and the level of HDL-C was significantly increased in the L-EHJD and H-EHJD groups (Figure 2A). In the HFD group, the levels of ALT and AST were significantly higher than those in the Control group. L-EHJD and H-EHJD significantly decreased levels of ALT and AST in HFD-induced mice (Figure 2B).
Figure 2.
Erhong Jiangzhi Decoction (EHJD) improved lipid accumulation and liver injury in NAFLD model mice induced by high-fat diet (HFD) (n=6). (A) ELISA detection of blood lipid markers (TC, TG, LDL-C, and HDL-C). (B) ELISA detection of liver function markers (ALT, AST). *P<0.05 **P<0.01 vs Control #P<0.05 ## P<0.01 vs NAFLD.
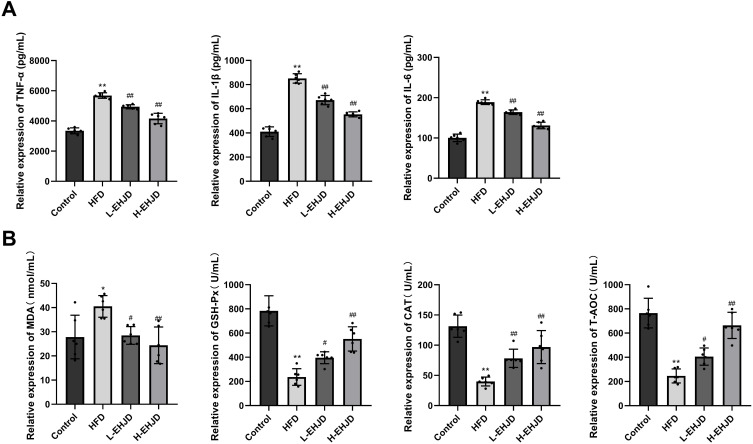
EHJD Inhibited Liver Inflammation and Oxidative Stress in HFD-Induced Mice
A significant increase in the levels of TNF-α, IL-6, and IL-1β was shown in the HFD group compared to the Control group. In the L-EHJD and H-EHJD groups, a significant decrease in the levels of TNF-α, IL-6, and IL-1β expression were observed in HFD-induced NAFLD mice (Figure 3A). In comparison with the Control group, the levels of MDA in the HFD group were significantly increased, as well as the reduced T-AOC, GSH-Px, and CAT. In the L-EHJD and H-EHJD groups, a significantly decreased MDA was observed in HFD-induced NAFLD mice, while the levels of T-AOC, GSH-Px, and CAT were significantly elevated (Figure 3B).
Figure 3.
Erhong Jiangzhi Decoction (EHJD) inhibited liver inflammation and oxidative stress in NAFLD model mice induced by high-fat diet (HFD) (n=6). (A) ELISA detection of the levels of TNF-α, IL-1β, and IL-6. (B) ELISA detection of the levels of antioxidant markers (MDA, GSH-Px, CAT, and T-AOC). *P<0.05 **P<0.01 vs Control #P<0.05 ## P<0.01 vs NAFLD.
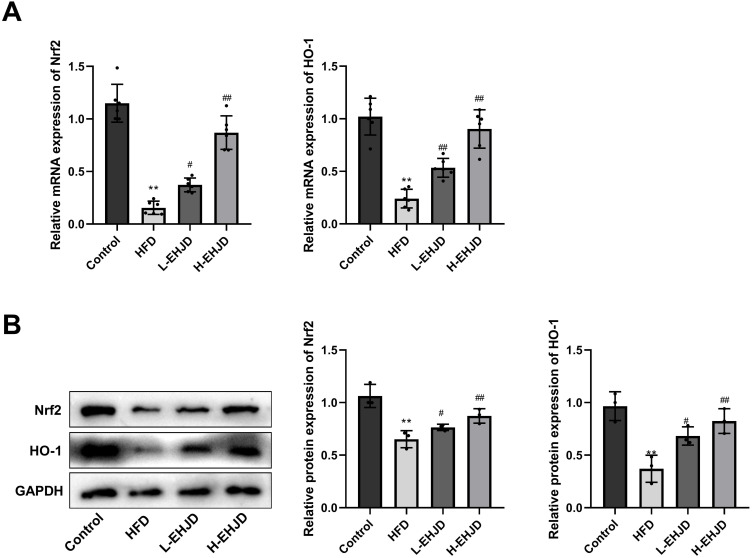
EHJD Activated Nrf2/HO-1 Signaling Pathway in HFD-Induced Mice
Nrf2 signaling pathway is a pivotal defense mechanism against oxidative stress.18,20–22 In comparison with the Control group, the levels of Nrf2 and HO-1 were significantly declined in the HFD group. L-EHJD and H-ENJD significantly elevated the mRNA and protein levels of Nrf2 and HO-1 in HFD-induced NAFLD mice (Figure 4A and B).
Figure 4.
Erhong Jiangzhi Decoction (EHJD) regulated Nrf2/HO-1 signaling pathway in NAFLD model mice induced by high-fat diet (HFD). (A) RT-qPCR detection of the expression of Nrf2 and HO-1 (n=6). (B) Western blot detection of protein expression of Nrf2 and HO-1 (n=3). **P<0.01 vs Control #P<0.05 ## P<0.01 vs NAFLD.
EHJD Drug-Containing Serum Alleviated Lipid Accumulation and Liver Function Injury in OA-Induced HepG2 Cells
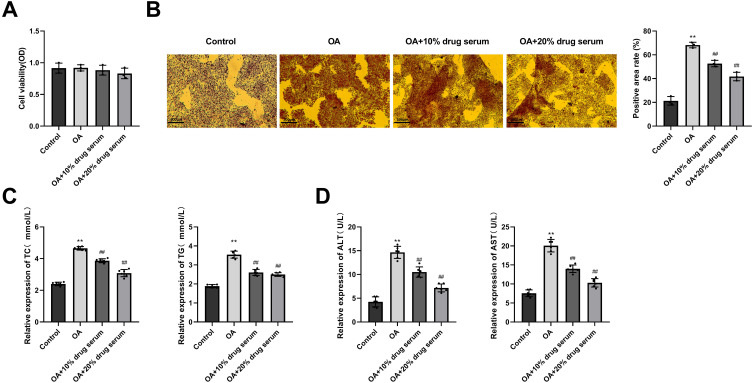
To investigate the effects of EHJD in OA-induced HepG2 cells, cells were treated with different concentrations of EHJD drug containing serum from rats. CCK-8 assay demonstrated that there was no significant difference in cell viability between the four groups (Figure 5A). Compared to the Control group, the red lipid droplets in the OA group significantly increased. Compared with the OA group, significantly less lipid droplets were observed in the OA+10% drug serum group and the OA+20% drug serum group (Figure 5B). ELISA showed that the levels of TC, TG, ALT, and AST in the OA group were significantly higher than those in the Control group. Compared with the OA group, the levels of TC, TG, ALT, and AST in the OA+10% drug serum group and the OA+20% drug serum group were significantly reduced (Figure 5C and D).
Figure 5.
Effects of Erhong Jiangzhi Decoction (EHJD) drug containing serum on viability and lipid accumulation in HepG2 cells induced by oleic acid (OA). (A) CCK-8 assay was performed to detect cell viability. (B) Oil red O staining of HepG2 cells (×200, 100 μm). (C) ELISA was used to detect the levels of lipid markers (TC, TG). (D) ELISA was used to detect the levels of liver function markers (ALT, AST) (n=6). **P<0.01 vs Control ## P<0.01 vs OA.
EHJD Drug-Containing Serum Inhibited Inflammation and Oxidative Stress in OA-Induced HepG2 Cells
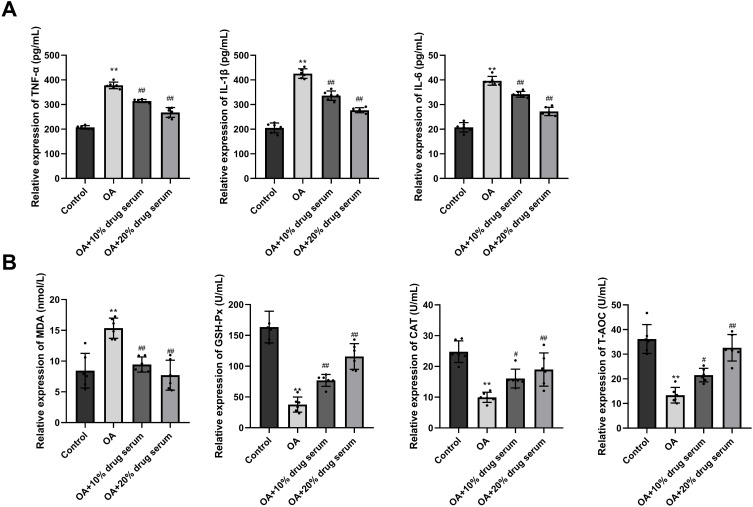
In Comparison with the Control group, there was a significant elevation in the levels of TNF-α, IL-6 and IL-1β in the OA group. In the OA+10% drug serum group and the OA+20% drug serum group, TNF-α, IL-6 and IL-1β were significantly lower than those in the OA group (Figure 6A). Compared with the Control group, the level of MDA in the OA group was significantly increased, while the levels of T-AOC, GSH-Px, and CAT were significantly decreased. In the OA+10% drug serum group and the OA+20% drug serum group, the levels of MDA were significantly decreased than that in the OA group, as well as the increased T-AOC, GSH-Px, and CAT (Figure 6B).
Figure 6.
Effects of Erhong Jiangzhi Decoction (EHJD) drug containing serum on inflammatory factors and antioxidant markers in HepG2 cells induced by oleic acid (OA) (n=6). (A) ELISA was used to detect the levels of inflammatory factors (TNF-α, IL-1β and IL-6). (B) ELISA was used to detect the levels of antioxidant markers (MDA, GSH-Px, CAT, and T-AOC). **P<0.01 vs Control #P<0.05 ## P<0.01 vs OA.
EHJD Drug-Containing Serum Activated Nrf2/HO-1 Signaling Pathway in OA-Induced HepG2 Cells
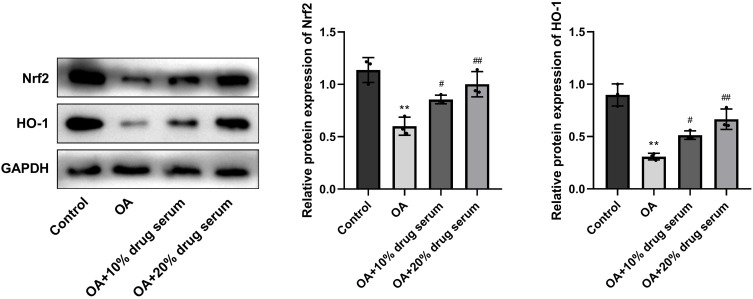
Compared to the Control group, the levels of Nrf2 and HO-1 in the OA group were significantly decreased. In the OA+10% drug serum and OA+20% drug serum groups, there was a significant raise in the levels of Nrf2 and HO-1, which was contrast with the OA group (Figure 7).
Figure 7.
Erhong Jiangzhi Decoction (EHJD) drug containing serum regulated Nrf2/HO-1 signaling pathway in HepG2 cells induced by oleic acid (OA) (n=3). **P<0.01 vs Control #P<0.05 ## P<0.01 vs OA.
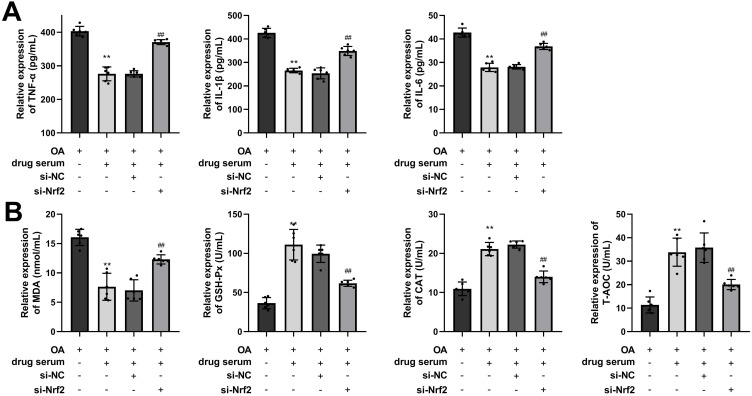
EHJD Drug-Containing Serum Inhibited Lipid Accumulation, Inflammation, and Oxidative Stress in OA-Induced HepG2 Cells Through Nrf2/HO-1 Pathway
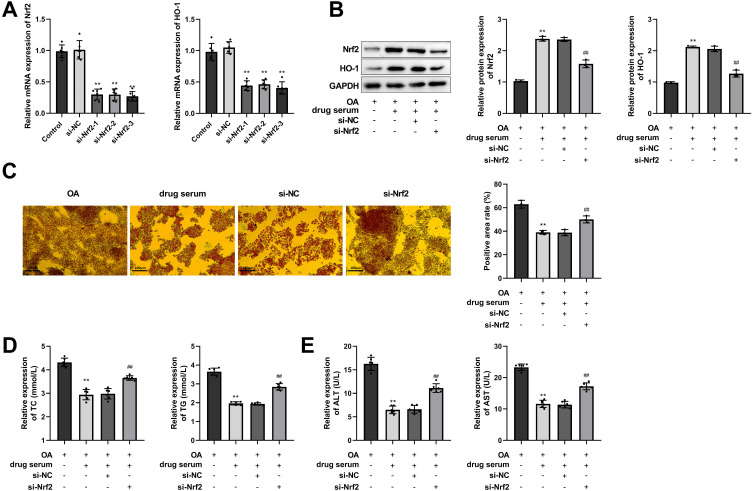
Nrf2 was silenced in HepG2 cells, and the efficiency of Nrf2 silencing was verified by RT-qPCR, showing that the levels of Nrf2 and the downstream gene HO-1 were significantly down-regulated (Figure 8A). si-Nrf2-3 with a relatively high silencing efficiency was applied for following experiments. As the results showed, Nrf2/HO-1 pathway was activated in the drug serum (EHJD) group, while si-Nrf2 inhibited the pathway (Figure 8B). Furthermore, oil red O staining results demonstrated that EHJD drug-containing serum significantly suppressed lipid accumulation in OA-induced HepG2 cells, while knockdown of Nrf2 reversed it (Figure 8C). TC, TG, ALT, and AST were also suppressed by EHJD treatment, and si-Nrf2 raised them (Figure 8D and E). Moreover, inflammatory factors (TNF-α, IL-1β, IL-6) and MDA levels were inhibited by EHJD treatment, while the levels of antioxidant indicators GSH-Px, CAT, and T-AOC were elevated. Suppression of Nrf2 reversed the effects of EHJD treatment on inflammation and oxidative stress in OA-induced HepG2 cells (Figure 9A and B). Therefore, it was proved that EHJD treatment alleviated lipid accumulation and liver function injury in OA-induced HepG2 cells through Nrf2/HO-1 pathway, as well as the inhibition of inflammation and oxidative stress.
Figure 8.
EHJD drug containing serum alleviated lipid accumulation and liver function injury in oleic acid (OA)-induced HepG2 cells through Nrf2/HO-1 pathway. (A) RT-qPCR was performed to verify the silencing efficiency of Nrf2/HO-1 pathway (n=6). **P<0.01 vs Control. (B) Western blot was used to detect the expression of Nrf2 and HO-1 (n=3). (C) Oil red O staining results of OA-induced HepG2 cells (×200, 100 μm). (D–E). ELISA was executed to measure the levels of blood lipid markers (TC, TG) and liver function markers (ALT, AST) (n=6). **P<0.01 vs OA ##P<0.01 vs si-NC.
Figure 9.
EHJD drug containing serum inhibited inflammation and oxidative stress in oleic acid (OA)-induced HepG2 cells through Nrf2/HO-1 pathway. (A and B) ELISA was executed to measure the levels of inflammatory factors (TNF-α, IL-1β and IL-6) and antioxidant markers (MDA, GSH-Px, CAT, and T-AOC) (n=6). **P<0.01 vs OA ##P<0.01 vs si-NC.
Discussion
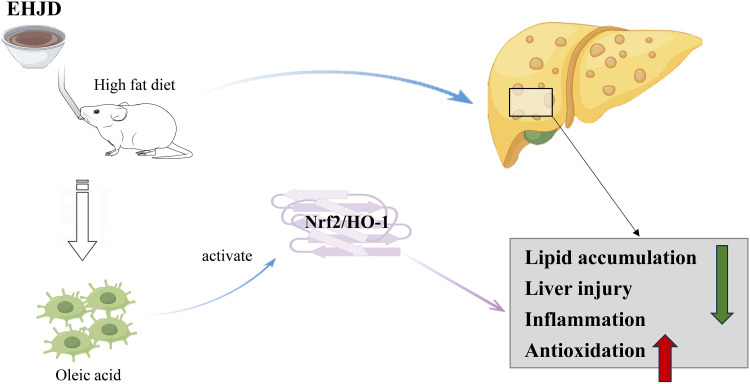
NAFLD is one of the most common causes of liver disease worldwide and its pathogenesis is complicated.26 Drugs are commonly used in the treatment of this disease, which are still being developed intensely and rapidly.5 In our study, we constructed HFD-induced NAFLD mice and OA-induced HepG2 cells. Through the in vivo and in vitro experiments, EHJD treatment significantly restrained lipid accumulation and liver injury, and inhibited oxidative stress and inflammation by activating the Nrf2/HO-1 pathway to mitigate NAFLD, providing a potential option for the therapy of NAFLD (Figure 10).
Figure 10.
The mechanism diagram of Erhong Jiangzhi Decoction (EHJD) on nonalcoholic fatty liver disease (NAFLD).
TCM and Chinese patent medicine have emerged as a promising strategy for managing NAFLD. Chitosan Oligosaccharide reduces lipid accumulation in C57BL/6 mice, and also promotes the Nrf2 pathway and up-regulates the levels of antioxidant enzymes to inhibit hepatic oxidative stress, leading to amelioration of NAFLD.27 Aucubin exerts anti-inflammatory and hepatoprotective effects by inhibiting oxidative stress and lipid accumulation via the AMPK and Nrf2/HO-1 pathways.28 Hesperetin, a citrus flavonoid, can alleviate liver steatosis, inflammatory cell infiltration and fibrosis, and improve oxidative stress in liver through the PI3 K/AKT-Nrf2 pathway, thereby alleviating NAFLD.29 Chicoric acid, the main nutrient component of chicory, takes a preventive part in improving oxidative stress by the AMPK/Nrf2/NFκB axis, and shapes the gut microbiota to protect liver cells from injury.30 Ginkgolide B ameliorates NAFLD by promoting Nrf2 signaling pathway to inhibit ferroptosis.31
In addition to the single active ingredient of Chinese herbal medicine, some complex TCM decoctions also have therapeutic effects on NAFLD. Linggui Zhugan decoction reduces hepatic steatosis by SOCS6 modification via N2-methyladenosine in NAFLD.32 Hedansanqi Tiaozhi Tang was protective against NAFLD in vitro and in vivo through activation of the Nrf2/HO-1 antioxidant pathway, while increasing antioxidant capacity and lipolysis, and improving hepatic function and pathologic features.33 Shuangyu Tiaozhi decoction exerts a variety of anti-NAFLD mechanisms, including improving insulin resistance, inflammation, and lipid deposition.34 Similar to above results, in our study, EHJD significantly improved pathologic features of liver, and alleviated lipid accumulation and liver injury, as well as the inhibition of oxidative stress and inflammation in the HFD-induced NAFLD mice model. The same results were shown in the OA-induced HepG2 cells, indicating that EHJD drug-containing serum significantly improved the progression of NAFLD by alleviating lipid accumulation and liver injury, and inhibiting inflammation and oxidative stress. When it comes to the effects of EHJD on NAFLD, red yeast rice and Rhodiola rosea L. play a pivotal role. Red yeast rice is generated by the fermentation of the Monascus purpureus mold, which has been widely used in TCM and is recognized as being able to reduce cholesterol.35 Moreover, evidence has shown that red yeast rice can lower the level of blood lipid, which is used as an effective treatment for hyperlipidemia.36 Rhodiola rosea L. is an adaptogenic plant rich in polyphenols, and studies have shown its neuroprotective and antioxidant effects.37,38 In cigarette smoke and LPS induced rats, Rhodiola rosea L. inhibits inflammation, oxidative stress, and fibrosis to alleviate chronic obstructive pulmonary disease.39 Furthermore, Salidroside extracted from Rhodiola rosea L. is reported to effectively alleviate lipid accumulation and liver inflammation in HFD induced mice.40 It is due to the combination of multiple medications that EHJD has made a better therapeutic effect on NAFLD.
The Nrf2 signaling pathway is of importance in the protection of NAFLD. Nrf2 is capable to suppress hepatic steatosis and attenuate NASH and hepatic fibrosis by inhibiting inflammation and oxidative stress.41 SQSTM1/p62 protects mouse liver from lipotoxicity by NFE2L2/NRF2, which is activated through ULK1-mediated autophagic KEAP1 degradation.42 Carbon monoxide releasing molecule-A1 improves tissue damage in steatosis liver through Nrf2 activation and improvement of mitochondrial function, thereby improving NAFLD.43 Knockdown of GCN2 attenuates hepatic steatosis and oxidative stress in obese mice, which is a novel regulator of NRF2.44 For the first time, Zhong et al directly evidence the function of Nrf2 in regulating lipogenic metabolism through transcriptional activation of PPARγ.45 Nrf2 may inhibit the progression of NASH by alleviating lipotoxicity, inflammation, endoplasmic reticulum stress, and iron overload.46 These findings confirm that Nrf2 pathway is of vital importance for NAFLD. Herein, si-Nrf2 weakened the inhibitory effects of EHJD drug-containing serum from rats on OA-induced HepG2 cells, proving that EHJD treatment may inhibit lipid accumulation, inflammation, and oxidative stress to alleviate NAFLD through the restoration of Nrf2.
This study supplies a scientific basis to support the clinical application of EHJD in the treatment of NAFLD and a reliable reference for exploring the pharmacological mechanism of EHJD. However, there are some limitations in this study; our study used only two concentrations of EHJD to explore the dose-dependent effect of EHJD on NAFLD. Further studies should include the establishment of a concentration gradient of EHJD to explore the dose-effect relationship. The absence of a positive control group is also a shortcoming, and future studies will prioritize the inclusion of positive controls to provide a robust benchmark for evaluating the therapeutic potential of EHJD in managing NAFLD. Additionally, the effects of EHJD on NAFLD can be deeply explored in the next studies using methods such as sequencing or mass spectrometry.
Conclusion
To summarize, this study verified the effects of EHJD on NAFLD by the in vitro and in vivo experiments, indicating that EHJD can suppress lipid accumulation and oxidative stress, reduce inflammation and liver injury to alleviate NAFLD by Nrf2 restoration under obesity.
Funding Statement
Nature Foundation of Hainan Province (No: 821MS0831).
Data Sharing Statement
All data in the manuscript is available through the responsible corresponding author.
Ethical Approval and Consent to Participate
All methods are reported in accordance with ARRIVE guidelines for the reporting of animal experiments. The animal experiments conformed to the Guide for the Care and Use of Laboratory Animals. Animal study has been approved by the Ethics Committee of Hainan Medical University (HYLL-2021-134, 2021.04.13). All methods were performed in accordance with Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest to disclose for this work.
References
- 1.Pafili K, Roden M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol Metabol. 2021;50:101122. doi: 10.1016/j.molmet.2020.101122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033 [DOI] [PubMed] [Google Scholar]
- 3.Cotter TG, Rinella M. Nonalcoholic Fatty Liver Disease 2020: the State of the Disease. Gastroenterology. 2020;158(7):1851–1864. doi: 10.1053/j.gastro.2020.01.052 [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metabol. 2021;50:101238. doi: 10.1016/j.molmet.2021.101238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen XY, Cai CZ, Yu ML, et al. LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway. World J Gastroenterol. 2019;25(45):6607–6618. doi: 10.3748/wjg.v25.i45.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusabimana T, Park EJ, Je J, et al. P2Y2R Deficiency Ameliorates Hepatic Steatosis by Reducing Lipogenesis and Enhancing Fatty Acid β-Oxidation through AMPK and PGC-1α Induction in High-Fat Diet-Fed Mice. Int J Mol Sci. 2021;22(11):5528. doi: 10.3390/ijms22115528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CX, Gao JG, Wan XY, et al. Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J Gastroenterol. 2019;25(34):5120–5133. doi: 10.3748/wjg.v25.i34.5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Wang YM, Zeng XY, et al. Bioactive proteins and antioxidant peptides from Litsea cubeba fruit meal: preparation, characterization and ameliorating function on high-fat diet-induced NAFLD through regulating lipid metabolism, oxidative stress and inflammatory response. Int J Biol Macromol. 2024;280(Pt 4):136186. [DOI] [PubMed] [Google Scholar]
- 11.Shen B, Wang Y, Cheng J, et al. Pterostilbene alleviated NAFLD via AMPK/mTOR signaling pathways and autophagy by promoting Nrf2. Phytomedicine. 2023;109:154561. doi: 10.1016/j.phymed.2022.154561 [DOI] [PubMed] [Google Scholar]
- 12.Lan T, Yu Y, Zhang J, et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology. 2021;74(2):686–703. doi: 10.1002/hep.31749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai X, Feng J, Chen Y, et al. Traditional Chinese Medicine in nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Chin Med. 2021;16(1):68. doi: 10.1186/s13020-021-00469-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cicero AFG, Fogacci F, Zambon A. Red Yeast Rice for Hypercholesterolemia: JACC Focus Seminar. J Am Coll Cardiol. 2021;77(5):620–628. doi: 10.1016/j.jacc.2020.11.056 [DOI] [PubMed] [Google Scholar]
- 15.Banach M, Catapano AL, Cicero AFG, et al. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: a position paper of the International Lipid Expert Panel. Pharmacol Res. 2022;183:106370. doi: 10.1016/j.phrs.2022.106370 [DOI] [PubMed] [Google Scholar]
- 16.Zou J, Yan C, Wan JB. Red yeast rice ameliorates non-alcoholic fatty liver disease through inhibiting lipid synthesis and NF-κB/NLRP3 inflammasome-mediated hepatic inflammation in mice. Chin Med. 2022;17(1):17. doi: 10.1186/s13020-022-00573-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu B, Liu X, Liang Y, Zheng K, Zhang C, Lu L. Salidroside in the Treatment of NAFLD/NASH. Chem Biodivers. 2022;19(12):e202200401. doi: 10.1002/cbdv.202200401 [DOI] [PubMed] [Google Scholar]
- 18.Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas C, Shah N, Muthu M, et al. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. J Biol Chem. 2014;289(39):26882–26894. doi: 10.1074/jbc.M114.567685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865(5):721–733. doi: 10.1016/j.bbamcr.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Kasai S, Shimizu S, Tatara Y, Mimura J, Itoh K. Regulation of Nrf2 by Mitochondrial Reactive Oxygen Species in Physiology and Pathology. Biomolecules. 2020;10(2):320. doi: 10.3390/biom10020320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev. 2018;98(3):1169–1203. doi: 10.1152/physrev.00023.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen B, Feng H, Cheng J, et al. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. J Cell & Mol Med. 2020;24(9):5097–5108. doi: 10.1111/jcmm.15139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long JK, Dai W, Zheng YW, Zhao SP. miR-122 promotes hepatic lipogenesis via inhibiting the LKB1/AMPK pathway by targeting Sirt1 in non-alcoholic fatty liver disease. Mol Med. 2019;25(1):26. doi: 10.1186/s10020-019-0085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu MF, Xi QH, Sheng Y, et al. Antioxidant Peptides from Monkfish Swim Bladders: ameliorating NAFLD In Vitro by Suppressing Lipid Accumulation and Oxidative Stress via Regulating AMPK/Nrf2 Pathway. Mar Drugs. 2023;21(6):360. doi: 10.3390/md21060360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassir F. NAFLD: mechanisms, Treatments, and Biomarkers. Biomolecules. 2022;12(6):824. doi: 10.3390/biom12060824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao W, Sun W, Liu L, et al. Chitosan Oligosaccharide Attenuates Nonalcoholic Fatty Liver Disease Induced by High Fat Diet through Reducing Lipid Accumulation, Inflammation and Oxidative Stress in C57BL/6 Mice. Mar Drugs. 2019;17(11):645. doi: 10.3390/md17110645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen B, Zhao C, Wang Y, et al. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J Cell & Mol Med. 2019;23(6):4063–4075. doi: 10.1111/jcmm.14293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Wang T, Liu P, et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021;12(9):3898–3918. doi: 10.1039/D0FO02736G [DOI] [PubMed] [Google Scholar]
- 30.Ding X, Jian T, Li J, et al. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxid Med Cell Longev. 2020;2020:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Chen J, Gao Q, Shan X, Wang J, Lv Z. Study on the attenuated effect of Ginkgolide B on ferroptosis in high fat diet induced nonalcoholic fatty liver disease. Toxicology. 2020;445:152599. doi: 10.1016/j.tox.2020.152599 [DOI] [PubMed] [Google Scholar]
- 32.Dang Y, Xu J, Yang Y, et al. Ling-gui-zhu-gan decoction alleviates hepatic steatosis through SOCS2 modification by N6-methyladenosine. Biomed Pharmacothe. 2020;127:109976. doi: 10.1016/j.biopha.2020.109976 [DOI] [PubMed] [Google Scholar]
- 33.Qiu M, Xiao F, Wang T, et al. Protective effect of Hedansanqi Tiaozhi Tang against non-alcoholic fatty liver disease in vitro and in vivo through activating Nrf2/HO-1 antioxidant signaling pathway. Phytomedicine. 2020;67:153140. doi: 10.1016/j.phymed.2019.153140 [DOI] [PubMed] [Google Scholar]
- 34.Yin G, Liang H, Sun W, et al. Shuangyu Tiaozhi decoction alleviates non-alcoholic fatty liver disease by improving lipid deposition, insulin resistance, and inflammation in vitro and in vivo. Front Pharmacol. 2022;13:1016745. doi: 10.3389/fphar.2022.1016745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen T, Karl M, Santini A. Red Yeast Rice. Foods. 2017;6(3):19. doi: 10.3390/foods6030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang H, Deng P, Ma YF, et al. Advances in Experimental and Clinical Research of the Gouty Arthritis Treatment with Traditional Chinese Medicine. eCAM. 2021;2021:8698232. doi: 10.1155/2021/8698232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agapouda A, Grimm A, Lejri I, Eckert A. Rhodiola Rosea Extract Counteracts Stress in an Adaptogenic Response Curve Manner via Elimination of ROS and Induction of Neurite Outgrowth. Oxid Med Cell Longev. 2022;2022:5647599. doi: 10.1155/2022/5647599 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Zhang S, Jiang S, Deng N, Zheng B, Li T, Liu RH. Phytochemical Profiles, Antioxidant Activity and Antiproliferative Mechanism of Rhodiola rosea L. Phenolic Extract. Nutrients. 2022;14(17):3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui H, Liu X, Zhang J, et al. Rhodiola rosea L. Attenuates Cigarette Smoke and Lipopolysaccharide-Induced COPD in Rats via Inflammation Inhibition and Antioxidant and Antifibrosis Pathways. Evid Based Complement Alternat Med. 2021;2021:6103158. doi: 10.1155/2021/6103158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Cai J, Fan P, et al. Salidroside protects mice from high-fat diet-induced obesity by modulating the gut microbiota. Int Immunopharmacol. 2023;120:110278. doi: 10.1016/j.intimp.2023.110278 [DOI] [PubMed] [Google Scholar]
- 41.Sharma RS, Harrison DJ, Kisielewski D, et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). CMGH. 2018;5(3):367–398. doi: 10.1016/j.jcmgh.2017.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee DH, Park JS, Lee YS, et al. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy. 2020;16(11):1949–1973. doi: 10.1080/15548627.2020.1712108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upadhyay KK, Jadeja RN, Vyas HS, et al. Carbon monoxide releasing molecule-A1 improves nonalcoholic steatohepatitis via Nrf2 activation mediated improvement in oxidative stress and mitochondrial function. Redox Biol. 2020;28:101314. doi: 10.1016/j.redox.2019.101314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan J, Yu Z, Gao J, et al. Inhibition of GCN2 alleviates hepatic steatosis and oxidative stress in obese mice: involvement of NRF2 regulation. Redox Biol. 2022;49:102224. doi: 10.1016/j.redox.2021.102224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong CC, Zhao T, Hogstrand C, Chen F, Song CC, Luo Z. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J Nutr Biochem. 2022;100:108883. doi: 10.1016/j.jnutbio.2021.108883 [DOI] [PubMed] [Google Scholar]
- 46.Bathish B, Robertson H, Dillon JF, Dinkova-Kostova AT, Hayes JD. Nonalcoholic steatohepatitis and mechanisms by which it is ameliorated by activation of the CNC-bZIP transcription factor Nrf2. Free Radic Biol Med. 2022;188:221–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in the manuscript is available through the responsible corresponding author.