Abstract
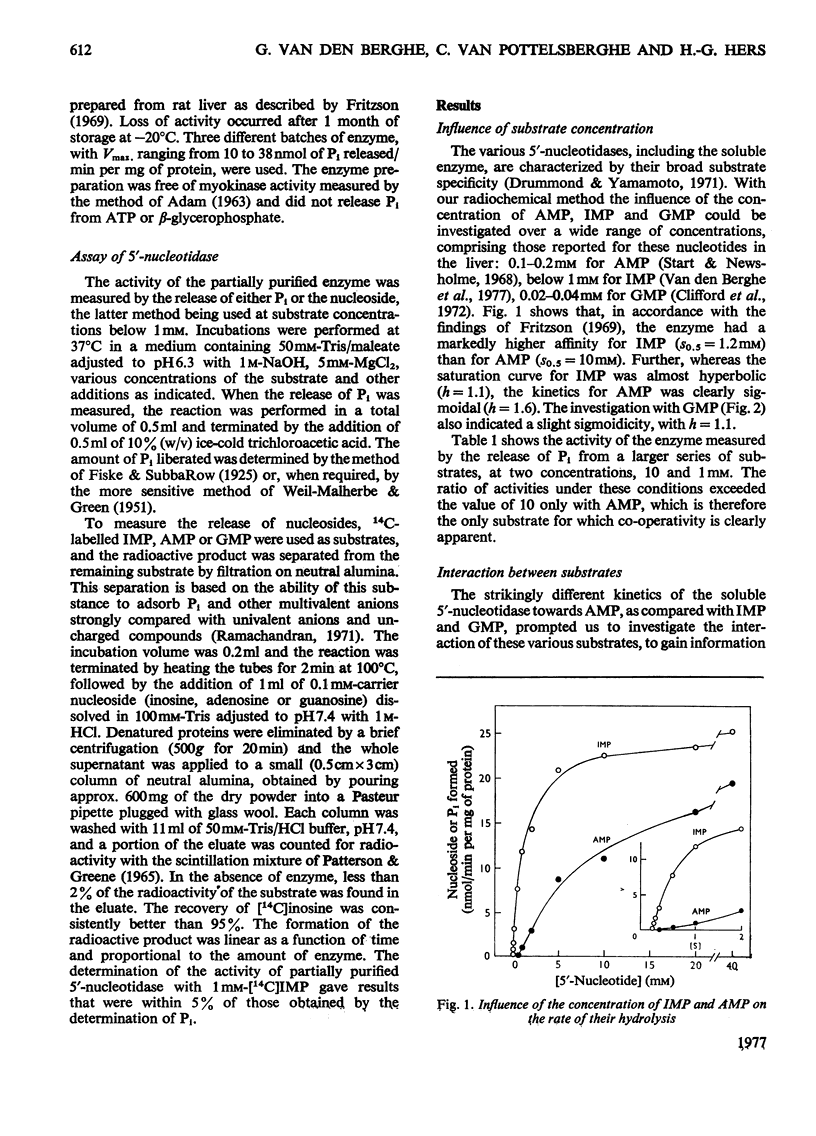
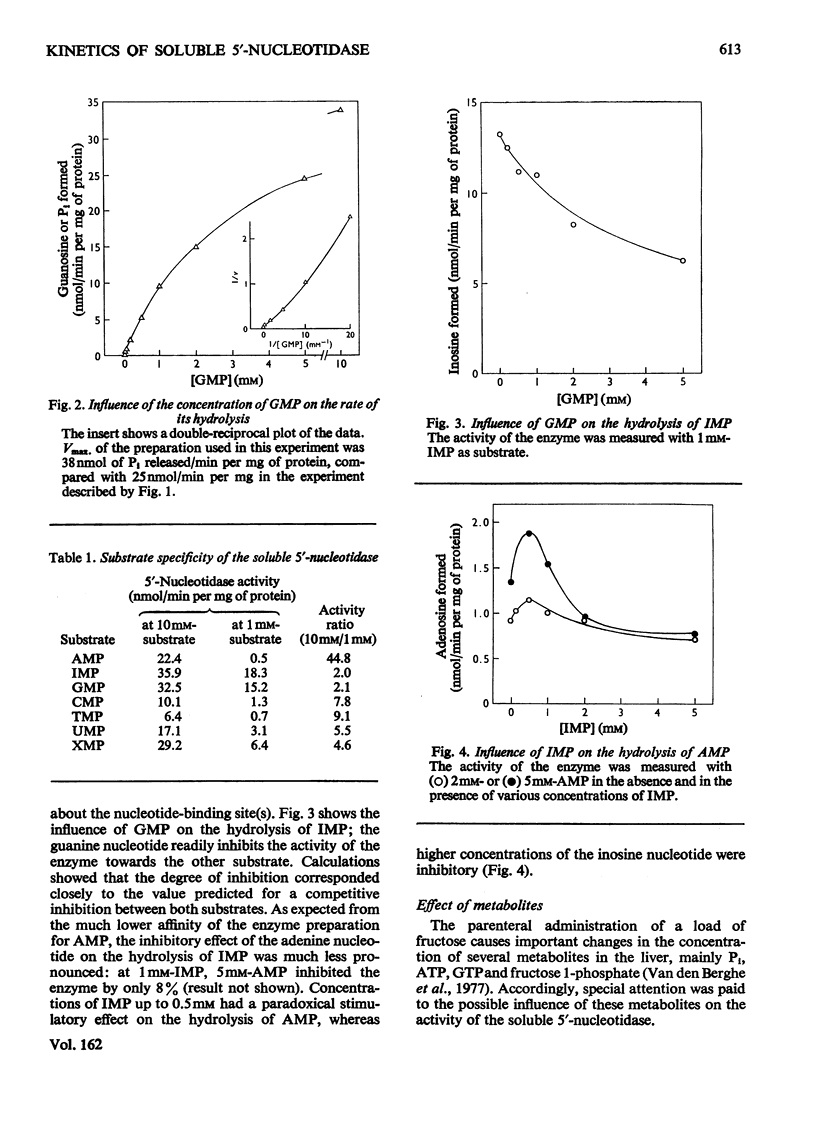
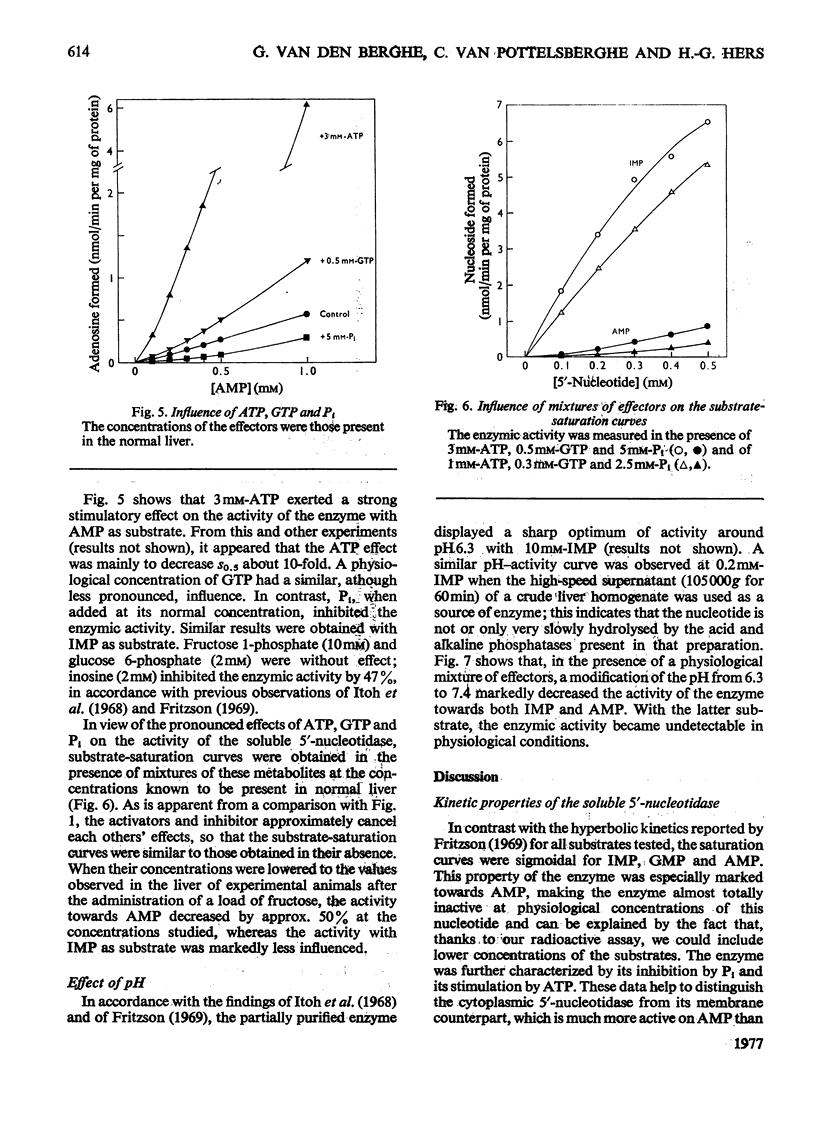
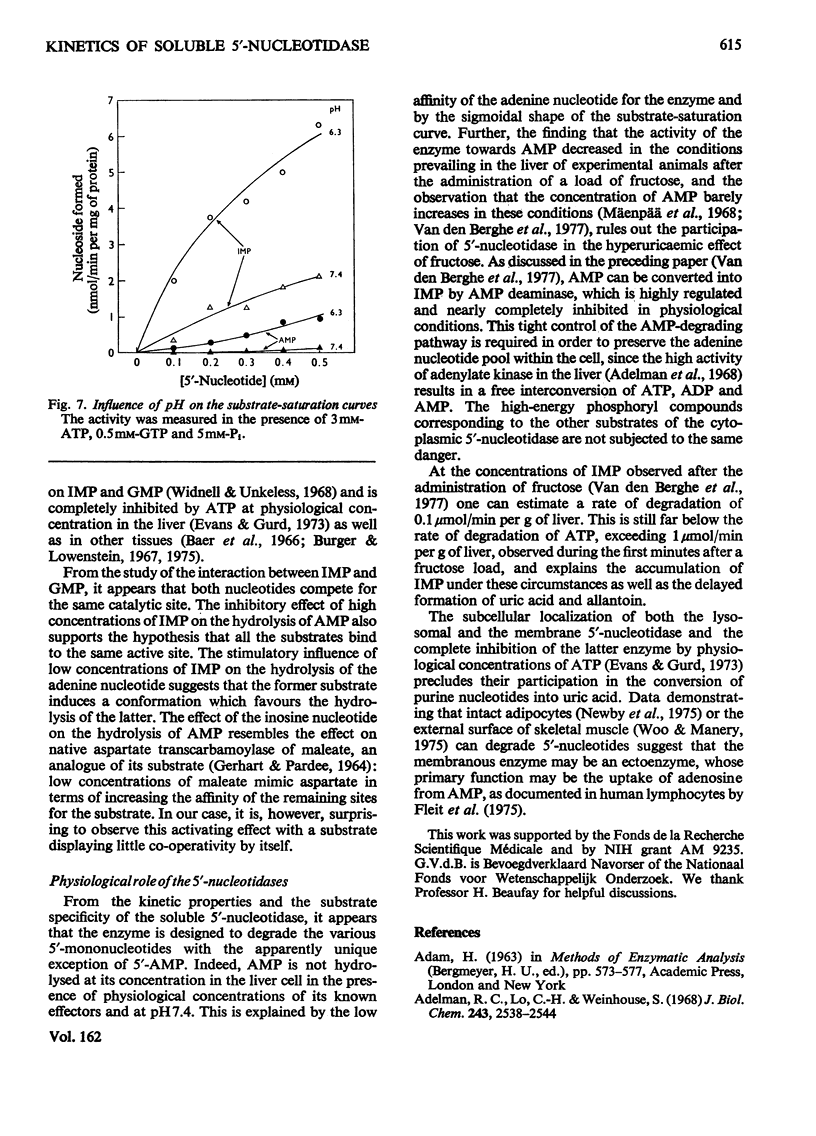
1. The kinetic properties of the 5'-nucleotidase (EC 3.1.3.5) present in the cytosol of rat liver were investigated in relation to the conversion of adenine nucleotides into uric acid, with particular reference to the stimulation of this process by fructose. The enzyme was assayed by the release of Pi and by a new and more sensitive radiochemical procedure. 2. When IMP was used as substrate, the partially purified enzyme displayed almost hyperbolic kinetics (h = 1.1) with S0.5 = 1.2 mM. Similar kinetics were observed with GMP and other nucleoside 5'-monophosphates, except AMP. 3. Vmax. of the enzyme for AMP was about the same as for IMP, but the kinetics were sigmoidal (h = 1.6) with S 0.5 = 10 mM. 4. The hydrolysis of IMP was inhibited competitively by GMP. IMP, at concentrations up to 0.5 mM, had a paradoxical stimulatory action on the hydrolysis of 2-5 mM-AMP and was inhibitory at higher concentrations. 5. The activity of the enzyme towards AMP and IMP was stimulated by ATP and GTP, and inhibited by Pi. Activators and inhibitor approximately cancelled each others' effects. At pH 7.4, the enzymic activity with 0.2 mM-AMP was undetectable under physiological conditions. 6. It is concluded that, in the liver cell, AMP is not hydrolysed by the soluble 5'-nucleotidase, but that its degradation requires prior deamination to IMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R. C., Lo C. H., Weinhouse S. Dietary and hormonal effects on adenosine triphosphate: adenosine monophosphate phosphotransferase activity in rat liver. J Biol Chem. 1968 May 25;243(10):2538–2544. [PubMed] [Google Scholar]
- Baer H. P., Drummond G. I., Duncan E. L. Formation and deamination of adenosine by cardiac muscle enzymes. Mol Pharmacol. 1966 Jan;2(1):67–76. [PubMed] [Google Scholar]
- Burger R. M., Lowenstein J. M. 5'-Nucleotidase from smooth muscle of small intestine and from brain. Inhibition of nucleotides. Biochemistry. 1975 Jun 3;14(11):2362–2366. doi: 10.1021/bi00682a014. [DOI] [PubMed] [Google Scholar]
- Burger R., Lowenstein J. M. Adenylate deaminase. 3. Regulation of deamination pathways in extracts of rat heart and lung. J Biol Chem. 1967 Nov 25;242(22):5281–5288. [PubMed] [Google Scholar]
- Clifford A. J., Riumallo J. A., Baliga B. S., Munro H. N., Brown P. R. Liver nucleotide metabolism in relation to amino acid supply. Biochim Biophys Acta. 1972 Sep 14;277(3):443–458. doi: 10.1016/0005-2787(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H., Conklyn M., Stebbins R. D., Silber R. Function of 5'-nucleotidase in the uptake of adenosine from AMP by human lymphocytes. J Biol Chem. 1975 Dec 10;250(23):8889–8892. [PubMed] [Google Scholar]
- Fritzson P. Nucleotidase activities in the soluble fraction of rat liver homogenate. Partial purification and properties of a 5'-nucleotidase with pH optimum 6.3. Biochim Biophys Acta. 1969 May 27;178(3):534–541. doi: 10.1016/0005-2744(69)90222-8. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- Ito R., Mitsui A., Tsushima K. Properties of 5'-nucleotidase from hepatic tissue of higher animals. J Biochem. 1968 Feb;63(2):165–169. doi: 10.1093/oxfordjournals.jbchem.a128757. [DOI] [PubMed] [Google Scholar]
- Itoh R., Mitsui A., Tsushima K. 5'-nucleotidase of chicken liver. Biochim Biophys Acta. 1967 Sep 12;146(1):151–159. doi: 10.1016/0005-2744(67)90081-2. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Luzio J. P., Hales C. N. The properties and extracellular location of 5'-nucleotidase of the rat fat-cell plasma membrane. Biochem J. 1975 Mar;146(3):625–633. doi: 10.1042/bj1460625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Ramachandran J. A new simple method for separation of adenosine 3',5'-cyclic monophosphate from other nucleotides and its use in the assay of adenyl cyclase. Anal Biochem. 1971 Sep;43(1):227–239. doi: 10.1016/0003-2697(71)90128-x. [DOI] [PubMed] [Google Scholar]
- Start C., Newsholme E. A. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. doi: 10.1042/bj1070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Unkeless J. C. Partial purification of a lipoprotein with 5'-nucleotidase activity from membranes of rat liver cells. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1050–1057. doi: 10.1073/pnas.61.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Y. T., Manery J. F. 5'-nucleotidase: an ecto-enzyme of frog skeletal muscle. Biochim Biophys Acta. 1975 Jul 27;397(1):144–152. doi: 10.1016/0005-2744(75)90188-6. [DOI] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]


