Abstract
Objective
To delineate the trend of use of botulinum toxin, including onabotulinum toxinA (OTA), in active military personnel and veterans with the diagnoses of migraine and post-traumatic headache (PTH) and describe the efficacy of botulinum toxin administration.
Background
Service members and veterans represent a unique population in the medical management of headache disorders, particularly migraine. They exhibit higher susceptibility to pain of greater intensity and longer durations, possibly due to their history of exposure to combat, trauma, and the associated psychological stresses. Given the burden and morbid nature of these headache disorders, prophylactic measures to reduce migraine attacks and disability are imperative. Specifically, the use of OTA for migraine prophylaxis has been well validated in chronic migraine.
Methods
The scoping review conformed to guidelines delineated by Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). The databases Medline, Embase, and Google Scholar were accessed for our literature search, and the time frame of the search was set from database inception to April 1, 2024.
Results
A total of 8 articles meeting the inclusion criteria were obtained after screening a total of 43 papers. Studies were primarily conducted in the United States (87.5%), with a single article published on veterans from Taiwan. Study types were mainly retrospective chart reviews with the exception of 2 randomized controlled trials. Chronic migraine was the most common headache diagnosis examined, being assessed in 6 studies, followed by PTH, which was represented in the remaining 2 studies.
Conclusion
The occupational exposure of service members appears to result in a higher incidence of headache disorders such as chronic migraine and PTH, which are amenable to preventative management such as that with botulinum toxin. Despite its effectiveness, the use of botulinum toxin in treating headaches and craniofacial pain in service members remains under-researched, warranting further exploration in this population, specifically.
Keywords: onabotulinum toxin A, botulinum toxin, scoping review, veterans, servicemen, facial pain, chronic pain, headache
Introduction
The population of past and present service members represents a unique and challenging group when it comes to medical management of headache and craniofacial pain. It is recognized that they exhibit higher susceptibility to pain of higher intensity and longer durations due to their history of exposure to combat, trauma, and the associated psychological stresses.1,2 Post-concussion syndrome, new persistent daily headache (NPDH), and migraine are all widely prevalent in this population, with many patients meeting criteria for chronic migraine.3,4
From 2002 to 2007, the incidence of migraine in US male active duty service members increased dramatically by almost 60%, likely associated with active military deployment in Iraq.5 Subsequently, from 2008 to 2019, more than 5% of the study population seen at Veterans Health Administration facilities were diagnosed with migraine, amounting to over half a million patients.3 In US army officer trainees, the prevalence of migraine headaches was reported to be as high as 14% in males and 31% in females.6 In contrast, in the civilian population, the prevalence is estimated to be 6% in males and 18% in females.7–9 In addition, 19.5% of nearly two million military personnel deployed in Iraq and Afghanistan experienced traumatic brain injury (TBI), in which post-traumatic headache (PTH) was deemed the defining symptom.4 These findings substantiate the markedly higher prevalence of headache disorders in service members in relation to the general population.10,11
The significant disabilities experienced by individuals and the social and economic costs accrued often necessitate implementation of prophylactic measures to preserve individual functionality and limit productivity lost. The annual burden on the economy due to migraine alone amounted to $78 billion USD when calculated in 2014,12 and the impact on productivity was estimated to be up to 686,000 workdays lost annually.13 As delineated in the Global Burden of Disease study 2019 (GBD 2019), the global incidence of migraine sits at 87.6 million (97% UI: 76.6, 98.7), which is 40.1% higher compared to 1990.14 As a result, various measures of prophylaxis have been established, ranging from the conservative management of environmental and dietary triggers to the utilization of pharmaceutical agents ranging from β-blockers to neuropathic pain medications, and more recently CGRP targeting treatments.15
The use of onabotulinum toxin A (OTA) for migraine prophylaxis has been well established in the management of individuals with headache meeting the definition of chronic migraine according to the International Classification of Headache Disorders, 3rd edition (ICHD-3). The pivotal PREEMPT I and II trials demonstrated that in individuals with chronic migraine, OTA significantly reduced mean headache days per month (−9.0 OTA vs −6.7 placebo, p < 0.001), moderate/severe headache days/month (−8.5 OTA vs −5.8 placebo, p < 0.001), and monthly cumulative headache hours (−132.4 OTA vs −90.0 placebo, p < 0.001) when administered in 12-weekly intervals per protocol.16,17 Our scoping review seeks to delineate the trend of use of botulinum toxin in active military personnel and veterans with the diagnoses of chronic migraine and PTH and describe the efficacy of botulinum toxin in the context of the defined conditions.
Methods
This scoping review conformed to guidelines laid out by Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).18 The databases Medline, Embase, and Google Scholar were accessed for our literature search, and the time frame of the search was set from database inception to April 1, 2024. The search strategy was collectively chosen by two authors (QR and CR) with deliberate inclusion of terms “headache”, “facial pain”, and “migraine” to capture the maximum number of articles utilizing botulinum toxin in the population of active and ex-service members. The overall search strategy was reviewed and approved by all authors following appropriate amendments. The two authors, QR and CR, screened the articles for inclusion in the review. In the event of a disagreement, a third author, DP, was consulted. In recognition that critical appraisal of each evidence source is not mandatory in accordance with the checklist under PRISMA Extension for Scoping Reviews (PRISMA-ScR), this was not performed in our study.18
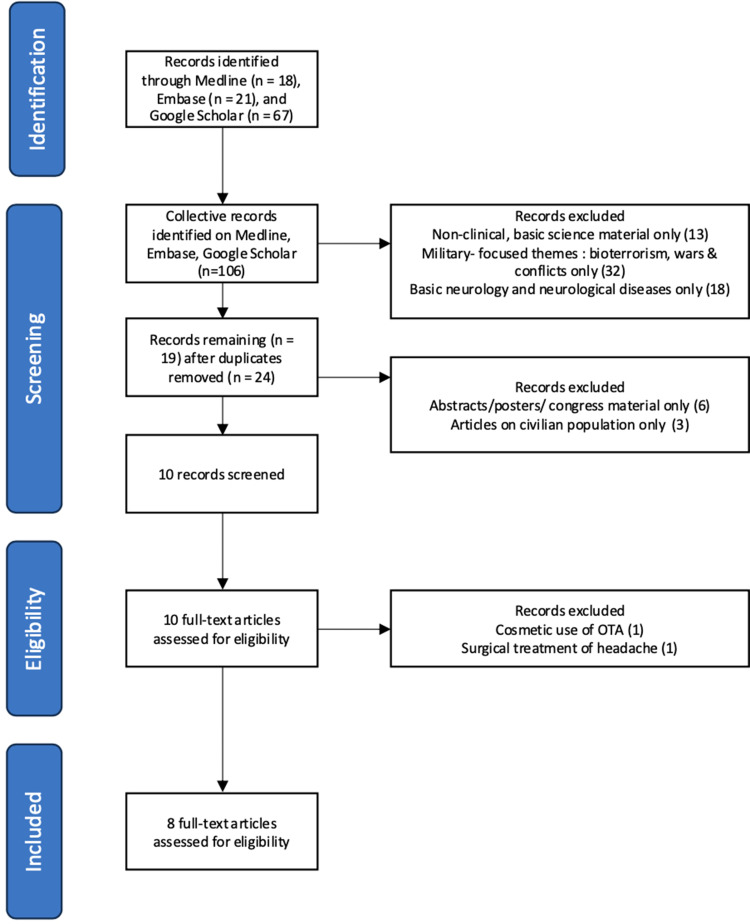
A comprehensive query was conducted on April 1, 2024, on PubMed utilizing the following search strategy: (“veteran” OR “military” OR “armed forces” OR “navy” OR “army” OR “servicemen”) AND (“botulinum toxin” OR “botox”) AND (“headache” OR “facial pain” OR “migraine” OR “post traumatic headache”). A similar strategy was adopted for the search on Embase and subsequently Google Scholar, and the results are collectively reflected in Figure 1.
Figure 1.
Flowchart overview of the scoping review analysis.
Inclusion Criteria
Inclusion criteria for studies used were the following: 1. Title, article and abstract available in English. 2. Peer-reviewed article inferring International Classification of Headache Disorders-3 (ICHD-3) criteria in defining chronic migraine and PTH. 3. Studies including individuals ≥18 years of age. 4. Studies using botulinum toxin as the primary preventative modality in the management of migraine and other headache disorders 5. Papers in the form of case reports, case series, reviews, and expert opinions due to the rarity of literature, with the aim of capturing the broadest scope for our review of OBA use in various types of headaches in military personnel.
Exclusion Criteria
Exclusion criteria used were the following: 1. Literature not in English. 2. Non-human studies. 3. Individuals ≤18 years of age 18. 4. Abstracts without full-text articles published in journal supplementary sections.
Results
A total of 106 articles were identified, and 8 articles meeting the above inclusion criteria were obtained following extensive screening according to Figure 1, with the oldest dating back to 2007.19–27 Studies were most commonly conducted in the United States (87.5%),19,21,22,24–27 with a single article published on the veterans in Asia, from Taiwan.20 Study types were generally retrospective chart reviews19,20,24,26 with two randomized controlled trials with subjects numbering 32 and 40, respectively.25,27 Chronic migraine was the most common headache pathology examined, being assessed in 6 studies,20–22,24–26 followed by PTH, which was represented in the remaining 2 studies.19,27 Relevant data from the selected articles were summarized in Table 1.
Table 1.
Study Demographics and Outcomes of Botulinum Toxin Use in Service Members
| Year | Author | Country | Study Type | Headache Type | Botox Type | n | M:F | Age | Service Member Type | Study Site | Results | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | Yerry et al19 | United States | Retrospective chart review | Post-traumatic Headache | Onabotulinum toxin A | 64 | 63:1 | 31.3 ± 7.5 | Undefined | Womack Army Medical Center | 3 lost to follow up but 41 (64.1%) had GEC improvement | Neck pain and worsened headache in 2 patients who discontinued OTA treatment |
| 2015 | Kazerooni et al21 | United States | Case series | Chronic Migraine | Incobotulinumtoxin A | 21 | 10:11 | 40 | Undefined | Veterans Affairs San Diego Healthcare System | Significant reduction in headache days per month using OTA (19.1 vs 9.1 days; p< 0.001) and headache intensity (8.3 vs 4.1; p< 0.001) | No significant adverse effects |
| 2013 | Lin et al20 | Taiwan | Retrospective chart review | Chronic Migraine | Onabotulinum toxin A | 94 | 15:79 | 47.6 ± 13.6 | Undefined | Taipei Veterans General Hospital | Significant improvement in median migraine MIDAS at 12 weeks versus baseline (p< 0.001), responders defined as >50% improvement of MIDAS | 19.1% lateral eyebrow elevation, 5.3% neck soreness, 4.3% ptosis |
| 2019 | Diel et al26 | United States | Retrospective chart review | Chronic Migraine | Onabotulinum toxin A | 72 | 43:29 | 48 (SD 10.1) | Undefined | Miami VA Medical Center | VLSQ-8 (especially questions 2,3 and 4) and interictal photophobia NRS significantly improved following PTA (p< 0.05) | Unreported |
| 2013 | Grogan et al22 | United States | Retrospective chart review | Chronic Migraine | Rimabotulinum Toxin B | 128 | 27:101 | 19–90 (mean 42) | Undefined | San Antonio Military Medical Center | “Imploding” - and “ocular-directed” headaches were more likely to be responders to RTB (p <0.0025); patients with aura were more likely to be responders to RTB (p = 0.0007) | Transient injection site stinging (82%), dry mouth (15%), cervical muscle stiffness and tenderness (4%) |
| 2019 | Williams et al24 | United States | Retrospective chart review | Chronic Migraine, Occipital Neuralgia, TBI, neck trauma | Onabotulinum toxin A | 30 | 20:10 | Age range 27 to 55 | Veterans | Central Texas Veterans Health Care System | 41% lower probability for a headache day following OTA intervention versus pre-intervention period (p < 0.001) over 28 days | Unreported |
| 2007 | Vo et al25 | United States | Randomized Controlled Trial | Chronic Migraine | Onabotulinum toxin A | 32 | 5:27 | 44.3 ± 11.3 in OTA; 40.7 ± 4.2 in control | Undefined | Walter Reed Army Medical Center | Being in OTA versus control groups not influencing periods for frequency of headaches (p= 0.63), headache severity (p= 0.415), and headache index (p= 0.533) | No significant adverse effects |
| 2021 | Zirovich et al27 | United States | Randomized Controlled Trial | Post-traumatic Headache | Onabotulinum toxin A | 40 | 38:2 | 34.3 (SD 8.6) | Veterans | Greater Los Angeles VA System | Headaches and headache days per week in OTA group reduced by 1.6 (95% CI, 0.6 to 2.6) and 1.4 (95% CI, 0.9 to 1.9) versus control which increased by 0.3 (95% CI, −0.6 to 1.5) and 0.1 (−0.6 to 0.4) respectively, with p values 0.48 and 0.005 respectively; Pain severity in OTA group reduced by 0.06 (95% CI, 0.1 to 0.11) versus an increase of 0.04 (95% CI, −0.01 to 0.08) with p= 0.006 | Pain, forehead paresthesia, itching, sinusitis; No difference in rates between OTA and control groups, p=0.23 |
Abbreviations: GEC, global evaluation of change; OTA, onabotulinumtoxin A; MIDAS, migraine disability assessment score; VLSQ-8, visual light sensitivity questionnaire 8; NRS, numerical rating scale; RTB, rimabotulinumtoxin B.
Migraine Management with Botulinum Toxin
The migraine-centric articles focused solely on preventative application of botulinum toxin injection via the migraine PREEMPT protocol16,17 in achieving measurable endpoints of symptom improvement. In veterans attending the Veterans Affairs San Diego Health System, investigators found that there was a significant reduction in mean headache days per month (19.1 vs 9.1; p < 0.001) as well as a reduction in headache intensity measured by the numerical rating scale (NRS) (8.3 vs 4.1; p < 0.001).21 This was corroborated in a separate study that demonstrated mean headache days per month reduced from 29.3 days to 6.5 days (p < 0.001).20 A study utilizing rimabotulinum toxin B in treatment-resistant episodic and chronic migraine patients achieved at least 50% symptom improvement in 79% (102/128) of individuals.22 In this group of general responders, 57% (58/102) were considered highly responsive to the treatment with >75% symptom relief, while 76% (44/58) of subjects considered sustained responders with symptom control for over 12 months while receiving regular botulinum toxin injections.22
Migraine-induced photophobia and dry eyes were examined in the context of OTA therapy administered in the management of chronic migraine.26 Although only pre-treatment migraine severity symptoms scores were collected for pain, the study did demonstrate improvements in photophobia and dry eyes, which were key symptoms of distress in the ailment.26 From the selected studies, only a single group failed to report benefits with OTA therapy in the evaluation of headache frequency (p = 0.63), headache severity (p = 0.415) and headache index encompassing frequency and intensity (p = 0.533).25 However, the control group demonstrated significantly worse headache index scores over the 3-month follow-up (p = 0.020), and this trend was not replicated in the treatment group that received OTA.25
In the 6 migraine-centric studies, only one evaluated a combination of chronic migraine and occipital neuralgia, which was then treated with a combination therapy of migraine protocol using OTA injections and occipital nerve block performed with local anesthetics.24 The dual therapy was found to be significantly efficacious in reducing the number of headache days per month (p < 0.0001) in post 9/11 combat veterans with a history of neck trauma or TBI.24
Post-Traumatic Headache Management with Botulinum Toxin
A retrospective case series illustrated the role of OTA in the treatment of PTH in the setting of mild traumatic brain injury.19 Of the 64 participants in the study, 63 were males with 1 female. The percentage of individuals who suffered from blast injuries was 56.3% (36/64), which was deemed to be the most frequent traumatic exposure, and 11% (7/64) reported a history of headache prior to the injury. The percentage of individuals endorsing more than one type of headache (chronic tension-type headache, hemicrania continua, craniofacial dystonia, other terminal branch neuralgia) was 56.3% (36/64), and 75% (48/64) reported continuous pain. Mixed continuous headaches with migraine features were identified as the most common headache diagnosis (40.6%, 26/64), while 25% (16/64) described classic chronic migraine symptoms. Specific injection protocols were employed in response to the type(s) of headache(s) presented (FSFD - fixed site fixed dose, FTP - follow-the-pain, and CD - cervical dystonia), and when appropriate, more than one protocol was applied to the same patient.19 The average interval between the injury and the initial treatment with the chronic migraine injection protocol was 10.8+21.9 months, and 64% (41/64) individuals responded positively to OTA injections, while 2 reported side effects of neck pain and worsened headaches, warranting discontinuation of OTA injections. The study supports the preventative potential of OTA in individuals affected by PTH.
Botulinum Toxin Safety Profile
Six studies attempted to capture adverse events associated with therapeutic botulinum toxin, with most being mild and of minimal clinical consequence. Two articles demonstrated no complications at all,21,25 while the most common side effects described included local “stinging” at the site of injection, neck soreness, dry mouth and ptosis.20,22 Reported minor negative effects, such as pain, paresthesia, pruritus, and sinusitis, were not significantly different between treatment and placebo groups (p = 0.23).27
Discussion
Our literature search on botulinum toxin utilization in the population of service members captured 2 major categories of headaches, namely PTH and migraine, which appeared to manifest with a higher degree of prevalence in this unique community. A 17-year cross-sectional study indicated, following adjustment for demographics, a higher prevalence of severe headache or migraine (24.2% relative increase) and facial pain (69.4% relative increase) over the same period in younger male servicemen compared to the civilian population.28 Furthermore, these numbers are thought to be underestimated as many affected by the condition do not actively seek medical treatment, and oftentimes, self-medicate to obtain symptom control.29 These statistics support the use of more efficacious preventative modalities such as OTA to address the condition of headache and craniofacial pain in this population.
The efficacy of OTA in the management of chronic migraine headache has been well established since its 2011 approval by the United States Food and Drug Administration (FDA). It has been consistently reported in the literature that the use of OTA as a prophylactic agent effectively reduces the frequency of migraine episodes and the intensity of each attack and improves the quality of life of individuals most negatively affected by the disease.16 Given the complex relationship of the multiple headache disorders experienced by veterans as a likely result of their occupational exposures, the astute and responsible use of OTA in this unique population of patients should be strongly considered given the potential for significant benefits. This largely coincides with our findings in this paper in which studies demonstrated the superiority of OTA in reducing headache days per month, headache intensity, and headache-associated disability scores, as well as migraine-induced photophobia.20–22,24,26 One study proposed that in coexistent presentations of chronic migraine with occipital neuralgia, dual therapy of migraine protocol and occipital nerve blockade achieved positive therapeutic results.24
Traumatic brain injury (TBI), however minor, may result in a host of post-concussion symptoms including headache, fatigue, irritability, and cognitive decline.30–32 The headache incidence in this group of polytraumatized populations with TBI is estimated to be as high as 90%, and up to 33% of all service members suffering from mild TBI could require specialist neurology input for headache symptom management.30 Despite the preventive treatment, 15–53% of this group could experience PTH.33 Therefore, it is unsurprising to find a high degree of associations between headache and TBI. The constellation of symptoms propagated by TBI forms a component of a syndrome termed the “polytrauma clinical triad”, with post-traumatic stress disorder and chronic pain comprising the other 2 elements of the triad.2,34 It is widely reported that the more severe the initial TBI insult, the higher the probability of observing more severe headache symptoms and neurological manifestations in keeping with migraine-like features.35 The odds of reporting migraine-like symptoms are also significantly higher in subjects who had previously suffered from moderate TBI as opposed to mild TBI.35
In the PTH population displaying symptoms of headache consistent with chronic migraine described above, for cervical dystonia or mixed symptoms, targeted OTA injection protocols were employed, termed “FSFD” (chronic migraine protocol of 31 injections), “FTP” (additional parietal injections) or “CD” techniques.19 Despite some degree of variability in injection sites and methods, as well as mixed symptomatology, 64% of the sample reported improvement in symptoms following OTA therapy.
An earlier narrative review defined acute PTH as a headache developing within 7 days following a known head injury or after gaining consciousness from the initial insult. The group noted that dosing and injection technique had not been systematically established in the context of PTH.36 However, in acute PTH 15–90 days from initial diagnosis deemed unresponsive or intolerant to conventional oral therapy, OTA could be considered as an appropriate acute therapy.36 Given that service members with histories of migraine could very well be subjected to traumatic concussions in combat, the exact pathology of the headache could be challenging to decipher.37 Furthermore, PTH in service members could have a migraine phenotype, bearing all diagnostic criteria of migraine clearly presenting following a trauma to the head.38,39 Therefore, in chronic PTH bearing features of chronic migraine, botulinum toxin treatment could be beneficial by extrapolation of known data in the civilian population and even be considered as a prophylactic agent against PTH.36 In 2021, the benefit of botulinum toxin in PTH was further demonstrated in a randomized controlled, cross-over study.27 A total of 40 subjects meeting defined criteria for chronic PTH were randomized to receive either abobotulinumtoxinA (387.5 units) or normal saline injections administered in 31 facial and cervical muscle sites. The results revealed a significant reduction in mean headache days per week by 0.14 (−3.5%, p < 0.001) compared to baseline, which was accompanied by a significant weekly decrease of 0.06 in pain severity score versus the placebo group (p = 0.001).27
In the preclinical context, this has been corroborated by a murine model study in which mice that received the administration of OTA shortly following traumatic stimulation led to the prevention of both acute and long-term development of chronic headache secondary to neural adaptations.40 In humans, the use of botulinum toxin in a randomized controlled trial of 40 subjects reduced headache frequency per week by 43.3% (p < 0.001) and headache frequency per day by 44.4% (p < 0.001) at the 16-week mark.27 Headache intensity was also reduced significantly by 0.06 with botulinum toxin treatment compared to an increase of 0.04 in the placebo group, achieving significant intergroup difference parameters (p = 0.006).27
Conclusion
The occupational exposure of service members results in a higher incidence of headache disorders, which are likely amenable to preventative management such as with botulinum toxin. The potential benefit is significant on review of the current peer-reviewed articles, but the overall paucity of literature as demonstrated in our literature search, specifically placebo-controlled trials, targeting this uniquely high-risk population reveals an opportunity for more rigorous investigations in migraine and PTH. In addition to clinical trials, subsequent research could potentially look into the volume of delivery of OTA for headaches in the military setting, willingness of therapy uptake, as well as community care absorption of therapy that failed to materialize in the military setting. When the military search terms were replaced with their civilian equivalents on Medline, the strategy yielded over 1,000 articles, highlighting the immense discrepancy in research performed on the civilian population versus that on service members and veterans. Botulinum toxin use in the treatment of headache and various craniofacial pain, specifically in the service members population, remains a subject worthy of further in-depth exploration.
Disclosure
Dr Rohan Jotwani reports personal fees from Mary Ann Liebert, during the conduct of the study. Dr Sean Li reports Consultants from Abbott, Avanos, Averitas Pharma, Biotronik, Boston Scientific, Nalu, PainTeq, Presidio, Saluda, SPR Therapeutic, Vertos; grants from Avanos, Averitas Pharma, Nevro, Presidio, Saluda, SPR Therapeutic; stock from Nalu stock and NeuroOne, outside the submitted work. Dr Michael Schatman reports Senior Medical Advisor from Apurano Pharma, outside the submitted work. Dr Sait Ashina reports personal fees from AbbVie, Lundbeck, Teva, Pfizer, Eli Lilly, Satsuma, Theranica, Impel Neuropharma, Linpharma, and Tonix, outside the submitted work, and is a trustee of the IHS Board.
References
- 1.Thomas MM, Harpaz-Rotem I, Tsai J, Southwick SM, Pietrzak RH. Mental and physical health conditions in US combat veterans: results from the National Health and Resilience in Veterans Study. Prim Care Companion CNS Disord. 2017;19(3). doi: 10.4088/PCC.17m02118 [DOI] [PubMed] [Google Scholar]
- 2.Iljazi A, Ashina H, Al-Khazali HM, et al. Post-traumatic stress disorder after traumatic brain injury-A systematic review and meta-analysis. Neurol Sci. 2020;41(10):2737–2746. doi: 10.1007/s10072-020-04458-7 [DOI] [PubMed] [Google Scholar]
- 3.Finkel AG. Headaches in veterans: different or the same? Neurology. 2022;99(18):779–780. doi: 10.1212/WNL.0000000000201081 [DOI] [PubMed] [Google Scholar]
- 4.Bryan CJ, Hernandez AM. Predictors of post-traumatic headache severity among deployed military personnel. Headache. 2011;51(6):945–953. doi: 10.1111/j.1526-4610.2011.01887.x [DOI] [PubMed] [Google Scholar]
- 5.Department of Defence. Medical Surveillance Monthly Report May 2008. 2008.
- 6.Helseth EK, Erickson JC. The prevalence and impact of migraine on US Military officer trainees. Headache. 2008;48(6):883–889. doi: 10.1111/j.1526-4610.2007.00962.x [DOI] [PubMed] [Google Scholar]
- 7.Lipton RB, Bigal ME. The epidemiology of migraine. Am J Med. 2005;118(Suppl 1):3S–10S. doi: 10.1016/j.amjmed.2005.01.014 [DOI] [PubMed] [Google Scholar]
- 8.Breslau N, Rasmussen BK. The impact of migraine: epidemiology, risk factors, and co-morbidities. Neurology. 2001;56(6 Suppl 1):S4–12. doi: 10.1212/wnl.56.suppl_1.s4 [DOI] [PubMed] [Google Scholar]
- 9.Lyngberg AC, Rasmussen BK, Jørgensen T, Jensen R. Incidence of primary headache: a Danish epidemiologic follow-up study. Am J Epidemiol. 2005;161(11):1066–1073. doi: 10.1093/aje/kwi139 [DOI] [PubMed] [Google Scholar]
- 10.Gasperi M, Schuster NM, Franklin B, Nievergelt CM, Stein MB, Afari N. Migraine prevalence, environmental risk, and comorbidities in men and women veterans. JAMA Netw Open. 2024;7(3):e242299. doi: 10.1001/jamanetworkopen.2024.2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theeler BJ, Flynn FG, Erickson JC. Chronic daily headache in U.S. soldiers after concussion. Headache. 2012;52(5):732–738. doi: 10.1111/j.1526-4610.2012.02112.x [DOI] [PubMed] [Google Scholar]
- 12.Buse DC, Yugrakh MS, Lee LK, Bell J, Cohen JM, Lipton RB. Burden of illness among people with migraine and ≥ 4 monthly headache days while using acute and/or preventive prescription medications for migraine. J Manag Care Spec Pharm. 2020;26(10):1334–1343. doi: 10.18553/jmcp.2020.20100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yucel A, Thach A, Kumar S, Loden C, Bensink M, Goldfarb N. Estimating the economic burden of migraine on US employers. Am J Manag Care. 2020;26(12):e403–e408. doi: 10.37765/ajmc.2020.88547 [DOI] [PubMed] [Google Scholar]
- 14.Fan L, Wu Y, Wei J, et al. Global, regional, and national time trends in incidence for migraine, from 1990 to 2019: an age-period-cohort analysis for the GBD 2019. J Headache Pain. 2023;24(1):79. doi: 10.1186/s10194-023-01619-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17–24. [PubMed] [Google Scholar]
- 16.Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793–803. doi: 10.1177/0333102410364676 [DOI] [PubMed] [Google Scholar]
- 17.Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804–814. doi: 10.1177/0333102410364677 [DOI] [PubMed] [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 19.Yerry JA, Kuehn D, Finkel AG. Onabotulinum toxin A for the treatment of headache in service members with a history of mild traumatic brain injury: a cohort study. Headache. 2015;55(3):395–406. doi: 10.1111/head.12495 [DOI] [PubMed] [Google Scholar]
- 20.Lin KH, Chen SP, Fuh JL, Wang YF, Wang SJ. Efficacy, safety, and predictors of response to botulinum toxin type A in refractory chronic migraine: a retrospective study. J Chin Med Assoc. 2014;77(1):10–15. doi: 10.1016/j.jcma.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 21.Kazerooni R, Lim J, Ashley Blake PD, Lessig S. IncobotulinumtoxinA for migraine: a retrospective case series. Clin Ther. 2015;37(8):1860–1864. doi: 10.1016/j.clinthera.2015.05.509 [DOI] [PubMed] [Google Scholar]
- 22.Grogan PM, Alvarez MV, Jones L. Headache direction and aura predict migraine responsiveness to rimabotulinumtoxin B. Headache. 2013;53(1):126–136. doi: 10.1111/j.1526-4610.2012.02288.x [DOI] [PubMed] [Google Scholar]
- 23.Busse JW, Casassus R, Carrasco-Labra A, et al. Management of chronic pain associated with temporomandibular disorders: a clinical practice guideline. BMJ. 2023;383:e076227. doi: 10.1136/bmj-2023-076227 [DOI] [PubMed] [Google Scholar]
- 24.Williams KA, Lawson RM, Perurena OH, Coppin JD. Management of chronic migraine and occipital neuralgia in post 9/11 combat veterans. Mil Med. 2019;184(7–8):E207–E211. doi: 10.1093/milmed/usy405 [DOI] [PubMed] [Google Scholar]
- 25.Vo AH, Satori R, Jabbari B, et al. Botulinum toxin type-a in the prevention of migraine: a double-blind controlled trial. Aviat Space Environ Med. 2007;78(5 Suppl):B113–8. [PubMed] [Google Scholar]
- 26.Diel RJ, Hwang J, Kroeger ZA, et al. Photophobia and sensations of dryness in patients with migraine occur independent of baseline tear volume and improve following botulinum toxin A injections. Br J Ophthalmol. 2019;103(8):1024–1029. doi: 10.1136/bjophthalmol-2018-312649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zirovich MD, Pangarkar SS, Manh C, et al. Botulinum toxin type A for the treatment of post-traumatic headache: a randomized, placebo-controlled, cross-over study. Mil Med. 2021;186(5–6):493–499. doi: 10.1093/milmed/usaa391 [DOI] [PubMed] [Google Scholar]
- 28.Taylor KA, Kapos FP, Sharpe JA, Kosinski AS, Rhon DI, Goode AP. Seventeen-year national pain prevalence trends among U.S. military veterans. medRxiv. 2023. doi: 10.1101/2023.03.27.23287408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolodner K, Lipton RB, Lafata JE, et al. Pharmacy and medical claims data identified migraine sufferers with high specificity but modest sensitivity. J Clin Epidemiol. 2004;57(9):962–972. doi: 10.1016/j.jclinepi.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 30.Gauntlett-Gilbert J, Wilson S. Veterans and chronic pain. Br J Pain. 2013;7(2):79–84. doi: 10.1177/2049463713482082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashina H, Eigenbrodt AK, Seifert T, et al. Post-traumatic headache attributed to traumatic brain injury: classification, clinical characteristics, and treatment. Lancet Neurol. 2021;20(6):460–469. doi: 10.1016/S1474-4422(21)00094-6 [DOI] [PubMed] [Google Scholar]
- 32.Ashina H, Porreca F, Anderson T, et al. Post-traumatic headache: epidemiology and pathophysiological insights. Nat Rev Neurol. 2019;15(10):607–617. doi: 10.1038/s41582-019-0243-8 [DOI] [PubMed] [Google Scholar]
- 33.Sico JJ, Seng EK, Wang K, et al. Characteristics and gender differences of headache in the veterans health administration: a national cohort study, fiscal year 2008–2019. Neurology. 2022;99(18):e1993–e2005. doi: 10.1212/WNL.0000000000200905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46(6):697–702. doi: 10.1682/jrrd.2009.01.0006 [DOI] [PubMed] [Google Scholar]
- 35.Coffman C, Reyes D, Hess MC, et al. Relationship between headache characteristics and a remote history of TBI in veterans: a 10-year retrospective chart review. Neurology. 2022;99(2):e187–e198. doi: 10.1212/WNL.0000000000200518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theeler BJ, Erickson JC. Posttraumatic headache in military personnel and veterans of the Iraq and Afghanistan conflicts. Curr Treat Options Neurol. 2012;14(1):36–49. doi: 10.1007/s11940-011-0157-2 [DOI] [PubMed] [Google Scholar]
- 37.Department of Defence. Risk factors for migraine after OEF/OIF deployment, active component, U.S. Armed Forces. Medical Surveillance Monthly Report December 2009. 2009;16(12):10–13. [Google Scholar]
- 38.Theeler BJ, Flynn FG, Erickson JC. Headaches after concussion in US soldiers returning from Iraq or Afghanistan. Headache. 2010;50(8):1262–1272. doi: 10.1111/j.1526-4610.2010.01700.x [DOI] [PubMed] [Google Scholar]
- 39.Ruff RL, Ruff SS, Wang XF. Headaches among Operation Iraqi Freedom/Operation Enduring Freedom veterans with mild traumatic brain injury associated with exposures to explosions. J Rehabil Res Dev. 2008;45(7):941–952. doi: 10.1682/jrrd.2008.02.0028 [DOI] [PubMed] [Google Scholar]
- 40.Navratilova E, Oyarzo J, Anderson T, et al. Preclinical assessment of onabotulinumtoxinA for the treatment of mild traumatic brain injury-related acute and persistent post-traumatic headache. Cephalalgia. 2022;42(11–12):1194–1206. doi: 10.1177/03331024221099841 [DOI] [PMC free article] [PubMed] [Google Scholar]