Abstract
Three genetic pathways promote flowering of Arabidopsis under long photoperiods. These pathways are represented by the genes CO, FCA, and GA1, which act in the long-day, autonomous, and gibberellin pathways, respectively. To test whether these are the only pathways that promote flowering under long photoperiods, the co-2 fca-1 ga1-3 triple mutant was constructed. These plants never flowered under long- or short-day conditions, indicating that the three pathways impaired by these mutations are absolutely required for flowering under these conditions. The triple mutant background represents a “vegetative ground state” enabling the roles of single pathways to be described in the corresponding double mutants. The phenotypes of plants carrying all eight combinations of wild-type and mutant alleles at the three loci were compared under long- and short-day conditions. This analysis demonstrated that under long photoperiods the long-day pathway promoted flowering most effectively, whereas under short photoperiods the gibberellin pathway had the strongest effect. The autonomous pathway had a weak effect when acting alone under either photoperiod but appeared to play an important role in facilitating the promotion of flowering by the other two pathways. The vegetative phenotype of the triple mutant could be overcome by vernalization, suggesting that a fourth pathway promoted flowering under these conditions. These observations are discussed in light of current models describing the regulation of flowering time in Arabidopsis.
Many mutations that delay flowering of Arabidopsis have been isolated, but none of them prevent flowering under all conditions (Koornneef et al., 1998a; Simpson et al., 1999; Michaels and Amasino, 2000; Reeves and Coupland, 2000). Genetic and physiological analysis suggests that at least three independent pathways promote flowering. These are the long-day, autonomous, and gibberellin (GA)-dependent pathways. Mutations affecting the long-day pathway delay flowering under long but not short days, whereas mutations affecting the autonomous pathway delay flowering irrespective of photoperiod (Koornneef et al., 1991, 1998b). The autonomous pathway probably promotes flowering by reducing the expression of the FLC gene that encodes a repressor of flowering (Michaels and Amasino, 1999a, 2001; Sheldon et al., 1999). Mutations affecting GA synthesis delay flowering under long and short days, but have their strongest effect under short days (Wilson et al., 1992; Blázquez et al., 1998; Nilsson et al., 1998).
Vernalization, extended exposure to low temperatures soon after germination, can also promote flowering. This response probably uses a different pathway from those described above (Chandler et al., 1996; Simpson et al., 1999), but in common with the autonomous pathway leads to repression of FLC expression (Michaels and Amasino, 1999b; Sheldon et al., 1999). The FRI gene confers a vernalization response on naturally occurring varieties, and promotes elevated levels of FLC expression (Michaels and Amasino, 1999a; Sheldon et al., 1999; Johanson et al., 2000). Combination of dominant FRI and FLC alleles with a mutation impairing GA biosynthesis generated a genotype that did not flower unless vernalized (Michaels and Amasino, 1999b).
Double mutant analysis has been used to establish relationships between individual genes involved in the promotion of flowering (Putterill et al., 1995; Koornneef et al., 1998b; for review, see Simpson et al., 1999). Although the results of such analyses are often complex, they have led to the formulation of detailed models of genetic interactions (Koornneef et al., 1998a). These experiments broadly indicate that the three pathways described above promote flowering under standard long-day conditions. Most of the mutations that cause late flowering were placed in one of the pathways, by demonstrating that double mutants carrying two mutations within the same pathway do not flower later than the most severe of the single mutants. These genetic experiments were extended by the construction of transgenic plants overexpressing flowering time genes, and describing the effect of these on the late-flowering phenotype caused by mutations affecting each pathway (Kardailsky et al., 1999; Kobayashi et al., 1999; Onouchi et al., 2000). Many of the conclusions derived from these experiments were supported by analysis of gene expression in wild-type and mutant backgrounds (Kardailsky et al., 1999; Kobayashi et al., 1999; Michaels and Amasino, 1999a; Sheldon et al., 1999; Lee et al., 2000; Samach et al., 2000). Although the GA, long-day, and autonomous pathways can act independently to promote flowering, they converge on common target genes that act in the early stages of flower development. For example, the GA and long-day pathways both promote expression of LEAFY (Blázquez and Weigel, 2000), and the autonomous and long-day pathways both promote expression of SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1)/AGL20 (Borner et al., 2000; Lee et al., 2000; Samach et al., 2000).
Here, we describe a triple mutant in which the activities of the long-day, autonomous, and GA-dependent pathways are impaired. These plants do not flower under long or short days, suggesting that under these conditions no other flowering time pathway can promote flowering in this background. We compare the flowering time of this triple mutant with the three double mutants and three single mutants, and interpret their phenotypes by reference to current models for the promotion of flowering in wild-type plants.
RESULTS
co-2 fca-1 ga1-3 Plants Do Not Flower under Long or Short Days
The co-2, fca-1, and ga1-3 mutations affect the long-day, autonomous, and GA-dependent floral promotion pathways, respectively. These are the primary pathways that promote flowering in early flowering varieties of Arabidopsis such as Landsberg erecta (La-er; see above; for review, see Koornneef et al., 1998a; Simpson et al., 1999). The three mutations represent severe mutant alleles at each locus (“Materials and Methods”). The triple mutant co-2 fca-1 ga1-3 was constructed (“Materials and Methods”) to test whether these pathways are essential for flowering to occur, or whether a further pathway can promote flowering when the function of all three pathways is impaired. Under both long and short days these triple mutant plants never flowered (Table I; Fig. 1). Over 90 rosette leaves were scored under long days and over 100 under short days, and the plants were then transferred to a long-day greenhouse. After 6 months, the majority of plants had died without flowering, whereas approximately 30% of the population continued to produce new leaves without having formed floral buds.
Table I.
The flowering time of wild-type and single, double, and triple mutant combinations of co-2, fca-1, and gal-3
| Genotype | Long Days
|
Short Days
|
||||
|---|---|---|---|---|---|---|
| Rosette leaves | Cauline leaves | Total leaf no. | Rosette leaves | Cauline leaves | Total leaf no. | |
| La-er | 5.6 ± 0.6 | 3.8 ± 0.7 | 9.4 ± 1.2 | 25.3 ± 4.4 | 9.4 ± 2.3 | 34.6 ± 6.1 |
| co-2 | 13.4 ± 1.1 | 6.3 ± 1.3 | 19.7 ± 2.2 | 22.6 ± 2.2 | 8.3 ± 1.8 | 30.9 ± 3.5 |
| fca-1 | 23.4 ± 2.6 | 7.4 ± 1.3 | 30.8 ± 3.4 | 48.5 ± 4.3 | 10.0 ± 2.1 | 58.5 ± 5.3 |
| gal-3 | – | – | 15.5 ± 1.0 | – | – | 68.8 ± 7.5 |
| co-2 fca-1 | 32.8 ± 1.8 | 14.3 ± 1.3 | 47.0 ± 2.6 | 44.0 ± 5.6 | 10.4 ± 1.0 | 54.4 ± 6.1 |
| co-2 gal-3 | – | – | 67.9 ± 13.8 (70%) | – | – | 89.0 ± 14.2 (50%) |
| fca-1 gal-3 | – | – | 35.3 ± 3.0 | – | – | 91.0 ± 6.7 (50%) |
| co-2 fca-1 gal-3 | – | – | >90 | – | – | >100 |
| co-2 fca-1 gal-3 (+vernalization) | – | – | 50.0 ± 7.6 | – | – | N.D.a |
N.D., Not determined.
Figure 1.
Photographs illustrating the phenotypes of plants carrying all eight combinations of the co-2, fca-1, and ga1-3 mutations grown under long days. A, Six-week-old wild-type (left), co-2 (middle), and fca-1 (right) plants. Only the wild type is flowering. B, Six-week-old ga1-3 plant. The plant is not flowering. C, Eight-week-old co-2 (left), fca-1 (middle), and co-2 fca-1 (right) mutant plants. All plants are flowering, but the double mutant is delayed compared to the others. D, Eight-week-old ga1-3 mutant plant. The arrow indicates the position of floral buds. E, Nine-week-old fca-1 ga1-3 (left), co-2 ga1-3 (middle), and co-2 fca-1 ga1-3 (right) mutant plants. Only the fca-1 ga1-3 plant is flowering, and the arrow indicates the position of floral buds. F, Twelve-week-old co-2 ga1-3 (left) and co-2 fca-1 ga1-3 (right) plants. Older leaves have senesced. The co-2 ga1-3 plant is flowering, whereas the fca-1 co-2 ga1-3 plant remains vegetative. The arrow indicates the position of floral buds.
co-2 fca-1 ga1-3 Plants Retain a Response to Vernalization
Vernalization promotes flowering of plants carrying mutations in the autonomous pathway, and of certain late-flowering ecotypes (Martínez-Zapater and Somerville, 1990; Chandler et al., 1996; for review, see Koornneef et al., 1998a; Simpson et al., 1999). To test whether the co-2 fca-1 ga1-3 triple mutant plants would flower in response to vernalization, they were given a 7-week vernalization treatment and then transferred to long days (“Materials and Methods”). All of the co-2 fca-1 ga1-3 plants flowered after vernalization, producing an average of approximately 50 leaves (Table I). Therefore, combining mutations affecting all three pathways does not prevent promotion of flowering by vernalization.
Study of Double Mutants Enables Analysis of the Role of Single Pathways
As described above, in the triple mutant co-2 fca-1 ga1-3 three flowering time pathways are impaired and the plants never flowered under long or short days. Therefore, analysis of double mutants should allow the effectiveness of a single pathway to be determined in a background in which the other two pathways are impaired. The flowering times of the three double mutants, co-2 ga1-3, co-2 fca-1, and fca-1 ga1-3, were compared under the same conditions. The plants were grown under long and short days with the three single mutants as controls, and the flowering times of all genotypes were scored.
Under long days, the co-2, fca-1, and ga1-3 single mutants flowered later than wild type (Table I; Fig. 1). The latest flowering of these mutants was fca-1, which produced a total of 31 leaves, compared with 20 for co-2 and 16 for ga1-3. The earliest flowering of the double mutants was fca-1 ga1-3, which produced around 35 leaves and was only slightly later than fca-1. The co-2 fca-1 double mutants showed a more dramatic late-flowering phenotype, flowering after the production of 47 leaves. However, the latest flowering double mutant was co-2 ga1-3, which produced around 68 leaves before flowering, and 30% of the population did not flower during the 4.5 months of the experiment.
Under short days, only the fca-1 and ga1-3 mutations caused late flowering, whereas the co-2 mutant flowered slightly earlier than wild type (Table I). In these conditions ga1-3 was the latest flowering single mutant, producing around 69 leaves before flowering, compared with 59 for fca-1, 31 for co-2, and 35 for La-er. The co-2 fca-1 double mutant was the earliest flowering double mutant genotype. These plants produced around 54 leaves, similar to the number of leaves produced by fca-1. The latest flowering double mutant genotypes were co-2 ga1-3 and fca-1 ga1-3. These two double mutants flowered after producing around 90 leaves, and 50% of the plants in each genotype did not flower during the 5 months of the experiment.
DISCUSSION
The CO, FCA, and GA1 genes act in the long-day, autonomous, and GA flowering time pathways, respectively. Construction of the triple mutant co-2 fca-1 ga1-3 demonstrated that these plants do not flower under long or short days, and therefore that the three major pathways affected by these mutations are essential for flowering to occur under long or short days. However, all of the triple mutant plants flowered when vernalized, indicating that vernalization can promote flowering even when these three pathways are impaired. Therefore, flowering of the triple mutant has an absolute requirement for vernalization. A recent report similarly demonstrated that plants carrying ga1-3 and dominant alleles of FRI and FLC did not flower unless vernalized (Michaels and Amasino, 1999b; discussed further below).
The Role of Different Floral Promotion Pathways Assessed in Double Mutants
The triple mutant background represents a vegetative “ground state” in which the activity of single pathways can be studied by restoring the activity of one pathway in appropriate double mutants. Therefore, double mutant plants in which the functions of two separate flowering time pathways are compromised can be used to study the function of the third pathway. A similar approach was used in the elaboration of the ABC model of flower development in Arabidopsis by constructing triple mutant plants that formed leaf-like structures rather than floral organs in all whorls (Bowman et al., 1991). In the analysis of flowering time, for example, the autonomous and GA-dependent pathways are disrupted in an fca-1 ga1-3 double mutant, and so the flowering time of this mutant will largely depend on the activity of the long-day pathway. Likewise, in a co-2 ga1-3 double mutant, where the long-day and GA-dependent pathways are disrupted, flowering time will be determined by the activity of the autonomous pathway. Using this approach, the ability of individual pathways to promote flowering was assessed from the severity of the phenotypes of the three double mutants (Table I).
Using this logic, the long-day pathway was the most effective in promoting flowering under long days because fca-1 ga1-3 was the earliest flowering of the three double mutants. The autonomous pathway similarly was the weakest because co-2 ga1-3 was the latest flowering double mutant, and 30% of these plants did not flower under long days, suggesting that in some individuals the autonomous pathway is incapable of promoting flowering in the absence of the other two pathways.
The double mutant analysis demonstrated that the autonomous pathway has a relatively weak effect in promoting flowering in co-2 ga1-3 plants in which the other two pathways are impaired. However, the fca-1 single mutant is the latest flowering of all of the single mutants, suggesting that in wild-type plants the autonomous pathway has an important role in promoting early flowering. Therefore, the phenotypes of the fca-1 single mutant and the co-2 ga1-3 double mutants lead to apparently different interpretations as to the importance of the autonomous pathway. However, these can be reconciled if the role of the autonomous pathway is not to directly promote flowering, but rather to facilitate the promotion of flowering by the long-day and GA pathways. This is consistent with the previous proposal that the autonomous pathway interacts with both the long-day and GA pathways to promote flowering (Nilsson et al., 1998). The autonomous pathway was shown more recently to repress the expression of the floral inhibitor FLC, and FLC expression was elevated in an fca mutant (Michaels and Amasino, 1999a; Sheldon et al., 1999). In addition, the late flowering of several autonomous pathway mutants has an absolute requirement for FLC, suggesting that the autonomous pathway promotes flowering only through its effects on FLC regulation (Michaels and Amasino, 2001). Therefore, the autonomous pathway may facilitate the promotion of flowering by the long-day and GA pathways by reducing the inhibitory effect of FLC. This is consistent with the observation that CO and FLC have antagonistic effects on the expression of downstream target genes such as SUPPRESSOR OF OVEREXPRESSION OF CO 1 and FT (Borner et al., 2000; Lee et al., 2000; Samach et al., 2000). In contrast, in a co-2 ga1-3 double mutant, the autonomous pathway would not promote flowering significantly because repression of FLC expression in a background in which the major promotive pathways are impaired would be insufficient to promote flowering. The striking enhancement of the late-flowering phenotype of the co-2 or ga1-3 mutants in the co-2 ga1-3 double mutant suggests that the long-day and GA pathways show some redundancy, as was proposed previously (Putterill et al., 1995; Michaels and Amasino, 1999b). Therefore, the comparison of these double and triple mutants suggests the model shown in Figure 2 for the promotion of flowering under long days.
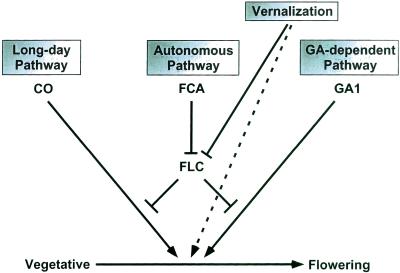
Figure 2.
Schematic illustration of Arabidopsis flowering time pathways. The long-day, autonomous, and GA pathways promote flowering of Arabidopsis under standard long-day conditions, and are represented respectively by the CO, FCA, and GA1 genes that are discussed in the text. The autonomous pathway acts by repressing expression of the floral inhibitor FLC. The genetic data presented here indicate that these three pathways are required for flowering to occur, and suggests that the autonomous pathway accelerates flowering by facilitating the activity of the long-day and GA pathways. Vernalization promotes flowering by repressing expression of the floral inhibitor FLC, but detailed comparison of the flowering times of vernalized co-2 fca-1 ga1-3 triple mutants and co-2 ga1-3 double mutants suggests that vernalization may also promote flowering independently of FLC, as represented by the dotted line.
Under short days, the relative importance of the three pathways in the promotion of flowering differs from under long days. The relatively early flowering phenotype of the co-2 fca-1 double mutant, compared with that of fca-1 ga1-3 and co-2 ga1-3 double mutants, suggests that the GA-dependent promotion pathway has the strongest effect under these conditions. On their own, the long-day promotion and autonomous promotion pathways have very little effect under short days, based upon the phenotypes of extremely late-flowering fca-1 ga1-3 and co-2 ga1-3 double mutants. Thus, in comparison with long days, the GA pathway has a greater importance, the autonomous pathway remains relatively ineffective in the absence of the other two pathways, and the importance of the long-day pathway is dramatically reduced.
The co-2 fca-1 ga1-3 Triple Mutant Flowers in Response to Vernalization
Although the triple mutant did not flower under inductive long-day conditions, it did flower after vernalization. This supports the earlier demonstration that fca-1 ga1-3 and FRI FLC ga1-3 plants show a normal response to vernalization (Michaels and Amasino, 1999b; Chandler et al., 2000). The autonomous pathway and vernalization act by related mechanisms involving reduction in expression of FLC. Therefore, vernalization of the triple mutant may be expected to produce a similar flowering time phenotype as the co-2 ga1-3 double mutant, in which both genotypes would be impaired in the long-day and GA pathways and have low levels of expression of FLC. However, 30% of co-2 ga1-3 double mutants never flowered and the others flowered extremely late, whereas 100% of the population of vernalized triple mutants flowered and they did so earlier than the co-2 ga1-3 double mutants. Therefore, vernalization of the triple mutant is more effective in promoting flowering than restoring the activity of the autonomous pathway by constructing a co-2 ga1-3 double mutant. This observation suggests that vernalization may promote flowering by additional mechanisms as well as repressing FLC expression, or may more thoroughly repress FLC expression than the autonomous pathway acting through FCA. The recent demonstration that an flc null mutant still shows a vernalization response supports the first of these possibilities (Michaels and Amasino, 2001).
MATERIALS AND METHODS
Plant Material
Mutant seed stocks were all in the Arabidopsis ecotype La-er. fca-1 and co-2 mutants were provided by Maarten Koornneef (Wageningen University, The Netherlands), ga1-3 mutants were obtained from the Nottingham Stock Center (UK), co-2 ga1-3 mutants were as described by Putterill et al. (1995), and fca-1 ga1-3 double mutants were kindly provided by Dr. Caroline Dean (John Innes Centre, Norwich, UK).
The sequence of the ga1-3 allele suggests that this is likely to be a null mutant (Sun and Kamiya, 1994). However, the ga1-3 mutant contains a small amount of GA, despite the complete absence of the ent-kaurene synthetase gene that is required for an early step in GA biosynthesis (Zeevaart and Talon, 1992; Sun and Kamiya, 1994). The fca-1 and co-2 mutations cause strong phenotypes relative to other alleles at these loci, suggesting that they are likely to be strong loss of function alleles (Macknight et al., 1997; F. Robson and G. Coupland, unpublished data). Sequence analysis of fca-1and co-2 demonstrated that these alleles are missense mutations (Putterill et al., 1995; Macknight et al., 1997).
To obtain co-2 fca-1 and co-2 fca-1 ga1-3 plants, an fca-1 ga1-3 plant was crossed to a co-2 plant. The genotypes of the F2 plants were checked using a combination of PCR-based and phenotypic markers. The presence of the fca-1 and ga1-3 mutations was analyzed using cleaved-amplified polymorphic sequence markers that could distinguish between mutant and wild-type alleles. The ga1-3 mutation was also identified by the dark-green dwarf appearance of homozygous mutant plants. A cleaved-amplified polymorphic sequence marker was not available to test the co-2 mutation, so this was determined by the presence of a linked (approximately 3.3 cM) transparent testa 4 mutation that affects anthocyanin accumulation in the seed coat (Putterill et al., 1995).
PCR Markers
FCA consisted of 5′ AGA GGA ACC ACG TTT CTC ACC 3′ and 5′ CCA GGC ACC CTT GCA GAA TC 3′. After amplification, the DNA is digested with MseI. This produces fragment sizes of 317, 239, 112, and 58 bp in wild type (La-er) and fragment sizes of 317, 239, 95, 58, and 17 bp in fca-1.
For GA1, wild type consisted of 5′ TTT GCG CCA ACA CAC AAA CCT T 3′ and 5′ AAG CTT CGA ACT CCA GGT TCT A 3′, and ga1-3 consisted of 5′ TGT ATG CAC GTT AAC GAT CAA T 3′ and 5′ TTT CTT CAT ACC ACC TGC GTT C 3′. The wild type primers amplify an approximately 1.2-kb fragment from wild type (La-er) but will not make a product with ga1-3 DNA. The ga1-3 primers amplify an approximately 0.8-kb fragment from ga1-3, but will not amplify wild-type DNA. Using all four primers in a single PCR reaction, it is possible to distinguish between ga1-3/ga1-3, ga1-3/+, and +/+ genotypes.
Growth Conditions and Measurements of Flowering Time
Plants were grown in compost composed of, by volume, 3 John Innes no.1: 2 vermiculite: 2 grit. Seed dormancy was broken by incubating seeds on moist filter paper in the dark at 4°C for 4 d prior to transfer to compost. Flowering time was measured under defined conditions by growing plants in Controlled Environment rooms (Sanyo Gallenkemp, Loughborough, UK) at 20°C. Short days comprised a photoperiod of 10 h lit with 400-W metal halide power star lamps supplemented with 100-W tungsten halide lamps (photosynthetically active radiation [PAR] 113.7 μmol m−2 s−1, red to far-red [R/FR] ratio 2.41). A similar cabinet and lamps were used for long days. The conditions were the same as short days for the first 10 h, and then extended for a further 6 h using only the tungsten halide lamps (PAR 14.27 μmol m−2 s−1, R/FR ratio 0.66). At least 10 plants were used to examine the flowering time of each genotype. This was measured as mean rosette and cauline leaf number, together with the sd of the mean (Koornneef et al., 1991).
To examine the flowering times of plants carrying the ga1-3 mutation, seeds were germinated without applying exogenous GA. Seeds were soaked overnight in water at 4°C. Embryos were dissected out of their seed coats under sterile conditions by applying gentle pressure with number 5 forceps, and transferred to germination medium plates. The germination medium plates were moved to the appropriate growth room, left for 2 d, and undamaged seedlings were transferred to soil.
To determine the vernalization response of the co-2 fca-1 ga1-3 triple mutant, seeds were germinated by dissecting the embryos out of their seed coats as described above. Plants were vernalized immediately after sowing on soil. Vernalization was carried out for 7 weeks at 5°C in short-day conditions (fluorescent light, PAR 9.5 μmol m−2 s−1, R/FR ratio 3.9).
ACKNOWLEDGMENTS
We are grateful to Caroline Dean for providing fca-1 ga1-3 seeds and primers for genotyping the fca-1 mutation. We would also like to thank Tai-Ping Sun for providing primers for genotyping the ga1-3 mutation prior to publication.
Footnotes
This work was supported by a Biotechnology and Biological Sciences Research Council studentship (to P.H.R.).
LITERATURE CITED
- Blázquez MA, Weigel D. Integration of floral inductive signals in Arabidopsis. Nature. 2000;404:889–892. doi: 10.1038/35009125. [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleissner R, Wisman E, Apel K, Melzer S. A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J. 2000;24:591–599. doi: 10.1046/j.1365-313x.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. Genetic interactions among floral homeotic genes of Arabidopsis. Development. 1991;112:1–20. doi: 10.1242/dev.112.1.1. [DOI] [PubMed] [Google Scholar]
- Chandler J, Martínez-Zapater JM, Dean C. Mutations causing defects in the biosynthesis and response to gibberellins, abscisic acid and phytochrome B do not inhibit vernalization in Arabidopsis fca-1. Planta. 2000;210:677–682. doi: 10.1007/s004250050059. [DOI] [PubMed] [Google Scholar]
- Chandler J, Wilson A, Dean C. Arabidopsis mutants showing an altered response to vernalization. Plant J. 1996;10:637–644. doi: 10.1046/j.1365-313x.1996.10040637.x. [DOI] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science. 2000;290:344–347. doi: 10.1126/science.290.5490.344. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Peeters AJM, Soppe W. Genetic control of flowering time in Arabidopsis. Annu Rev Plant Physiol Plant Mol Biol. 1998a;49:345–370. doi: 10.1146/annurev.arplant.49.1.345. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, de Vries HB, Hanhart CJ, Peeters AJ. Genetic interactions among late-flowering mutants of Arabidopsis. Genetics. 1998b;148:885–892. doi: 10.1093/genetics/148.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000;14:2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight R, Bancroft I, Page T, Lister C, Schmidt R, Love K, Westphal L, Murphy G, Sherson S, Cobbet C. FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell. 1997;89:737–745. doi: 10.1016/s0092-8674(00)80256-1. [DOI] [PubMed] [Google Scholar]
- Martínez-Zapater JM, Somerville CR. Effect of light quality and vernalization on late flowering mutants of Arabidopsis thaliana. Plant Physiol. 1990;92:770–776. doi: 10.1104/pp.92.3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999a;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. The gibberellic acid biosynthesis mutant ga1-3 of Arabidopsis thaliana is responsive to vernalization. Dev Genet. 1999b;25:194–198. doi: 10.1002/(SICI)1520-6408(1999)25:3<194::AID-DVG2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. Memories of winter: vernalization and the competence to flower. Plant Cell Environ. 2000;23:1145–1153. [Google Scholar]
- Michaels SD, Amasino RM. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations, but not responsiveness to vernalization. Plant Cell. 2001;13:935–941. doi: 10.1105/tpc.13.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O, Lee I, Blázquez MA, Weigel D. Flowering-time genes modulate the response to LEAFY activity. Genetics. 1998;150:403–410. doi: 10.1093/genetics/150.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onouchi H, Igeño MI, Perilleux C, Graves K, Coupland G. Mutagenesis of plants over-expressing CONSTANS demonstrates novel interactions among Arabidopsis flowering-time genes. Plant Cell. 2000;12:885–900. doi: 10.1105/tpc.12.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Reeves PH, Coupland G. Response of plant development to environment: control of flowering by daylength and temperature. Curr Opin Plant Biol. 2000;3:37–42. doi: 10.1016/s1369-5266(99)00041-2. [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer ZS, Yanofsky MF, Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock WJ, Dennis ES. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell. 1999;11:445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Gendall AR, Dean C. When to switch to flowering. Annu Rev Cell Dev Biol. 1999;99:519–550. doi: 10.1146/annurev.cellbio.15.1.519. [DOI] [PubMed] [Google Scholar]
- Sun T, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Talon M. Gibberellin mutants in Arabidopsis thaliana. In: Karssen CM, van Loon LC, Vreugdenhil D, editors. Current Plant Sciences and Biotechnology in Agriculture. XIII, Progress in Plant Growth Regulation. Amersterdam: Kluwer; 1992. pp. 34–42. [Google Scholar]